Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common cancer worldwide, and is associated with almost half a
million cases of mortality every year (1). In early stage HCC, potential curative
treatments including surgical resection, percutaneous ablation and
liver transplantation are available (2). However, for advanced HCC, curative
treatments are not available and the 5-year survival rate for
advanced stage patients is dismal (3). Therefore, the identification of
potential early biomarkers and discovery of novel therapies are
urgently required, and numerous methods are undergoing development
for this purpose (4).
MicroRNAs (miRNAs/miRs) are a class of endogenous,
single-stranded, small non-coding RNAs that are 18–25 nucleotides
in length, and function as negative regulators of gene expression
(5). miRNAs are able to trigger
either mRNA degradation or translational repression by binding to
the 3′-untranslated region (UTR) of specific mRNAs with perfect or
near-perfect complementarity, respectively (6). Several hundred miRNAs have been
described in humans. The basic role of miRNAs either as oncogenes
or as tumor suppressors in human HCC has been identified (7,8).
However, the specific role of aberrantly expressed miRNAs is yet to
be elucidated. The present hypothesis was that aberrantly expressed
miRNAs may contribute to tumor growth and migration by modulating
the expression of gene products involved in phenotypic
characteristics of cancer cells, including cell growth, migration
and invasion. RNA interference analysis has previously been used in
HCC treatment (9,10). Detectable miRNAs associated with
liver tumor tissue, serum, plasma and urine could provide an
effective means to monitor response to therapies and to establish
prognosis (5).
miR-125a-5p is a miRNA that has been extensively
studied in tumors. In lung cancer, miRNA-125a-5p was revealed to be
a tumor suppressor that directly targets the signal transducer and
activator of transcription 3 and neural precursor cell expressed
developmentally downregulated 9 (11,12).
Natalia, et al (13) reported
that miR-125a-5p could repress cervical cancer cell invasion by
targeting mitogen activated protein kinase 1. Cao et al
(14) suggested that miRNA-125a-5p
inhibited gastric cancer cell invasion and metastasis by regulating
the expression of breast carcinoma metastasis suppressor gene 1.
Furthermore, miRNA-125a-5p has been shown to inhibit cell growth in
hepatitis B virus-associated hepatocellular carcinoma by targeting
the tyrosine protein kinase receptor, ErbB3 (15). However, to the best of our knowledge,
the role and mechanism of miR-125a-5p in hepatocellular carcinoma
remains largely unknown.
The purpose of the current study was to investigate
the role and mechanism of miR-125a-5p in the development of
hepatocellular carcinoma.
Materials and methods
Cell culture
The human hepatocellular carcinoma cell lines
PLC/PRF/5 and MHCC97L, and the human immortalized liver cell line
HL-7702 were purchased from the American Type Culture Collection.
All cells were grown in Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc.) supplemented with 10% (v/v) fetal
bovine serum (FBS), and incubated in a 95% humidified incubator (5%
CO2, 37°C).
Cell transfection
PLC/PRF/5 cells (5×104 cells per well)
were seeded into 6-well plates and incubated in standard conditions
(5% CO2, 37°C), 24 h prior to cell transfection.
Subsequently, miR-125a-5p mimic (5′-UCCCUGAGACCCUUUAACCUGUGA-3′),
mimics control (5′-UUCUCCGAACGUGUCACGUTT-3′), TRIAP1-plasmid,
BCL2L2-plasmid, miR-125a-5p mimic + TRIAP1-plasmid or miR-125a-5p
mimic + BCL2L2-plasmid (Biovector NTCC Inc.) were transfected into
the PLC/PRF/5 cells using 30 µl Lipofectamine 2000™
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. A total of 0.67 µg (50 pmol)
miR-125a-5p mimic or mimic control, 25 µl TRIAP1-plasmid solution
(0.12 g/l) or BCL2L2-plasmid solution (0.12 g/l) was used for
transfection. Following incubation for 48 h, cells were ready for
subsequent experimentation. Cells without any treatment were
considered as the control group (Control).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen™; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
RNAs were reverse transcribed to synthesize the cDNAs using
PrimeScript RTreagent kit (Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocol. Subsequently, qPCR was
performed to analyze the cDNAs using a TaqMan Universal PCR Master
Mix kit (Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: 95°C for 10 min, followed by 38 cycles
of 95°C for 10 sec and 58°C for 60 sec. The primer sequences were
as follows: U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′;
miR-125a-5p forward, 5′-CGATTCCCTGAGACCCTTTAA-3′ and reverse,
5′-TATGGTTTTGACGACTGTGTGAT-3′; TRIAP1 forward,
5′-TATCTTGCAGGAACTGTGTGCTA-3′ and reverse,
5′-AATTTAGGTTCTTCCTCCACAGC-3′; BCL2L2 forward,
5′-TGAGTTCGAGACCCGCTTC-3′ and reverse, 5′-AAAAGTTCATCGGAGACCTG-3′.
GAPDH and U6 were used as the internal controls for mRNA and miRNA
expression, respectively. The 2−ΔΔCq method (16) was used to quantify relative gene
expressions.
Western blot analysis
Cells were harvested and lysed using radio
immunoprecipitation assay buffer containing protease inhibitor
(Abcam). The concentration of protein samples was determined with a
bicinchoninic acid Protein Assay kit (Bio-Rad Laboratories, Inc.).
Equal amount of proteins (30 µg per lane) were separated via
SDS-PAGE on 12% gels then transferred on polyvinylidene fluoride
membranes (Bio-Rad Laboratories, Inc.). After blocking with 5%
non-fat milk at room temperature for 2 h, the membranes were
blotted overnight with the primary antibodies anti-TRIAP1 (cat no.
KL507313; Kanglang Biotechnology Co., Ltd.; 1:2,000) and
anti-BCL2L2 (cat no. ab38629; Abcam; 1:500) at 4°C. The next day,
membranes were incubated with anti-rabbit horseradish
peroxidase-linked IgG secondary antibody (cat no. 7074; Cell
Signaling Technology, Inc.; 1:2,000) at room temperature for 4 h.
Finally, protein bands were visualized using the enhanced
chemiluminescence detection system (Super™ Signal West
Dura Extended Duration substrate; Thermo Fisher Scientific, Inc.)
with the intensity analyzed with Image J software (version 1.8.0;
National Institutes of Health) with GAPDH as the loading
control.
MTT assay
MTT assay was used to assess cell viability.
Briefly, PLC/PRF/5 cells were harvested and then reseeded into
96-well culture plates and cultured at 37°C for 24, 48 or 72 h.
Subsequently, MTT solution (in thiazolyl blue tetrazolium bromide;
Amresco LLC) was added into each culture well and incubated for a
further 4 h. Finally, the optical density values were measured at
570 nm using a Synergy™−2 Multi-function microplate
reader (Bio Tek Instruments, Inc.).
Cell apoptosis assay
Following 48 h of incubation, 3×103
cells/well were seeded into 96-well plates and labeled with Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI; Cell
Signaling Technology, Inc.), as per as the manufacturer's protocol.
The BD LSRFortessa X-20 flow cytometer (BD Biosciences) with FlowJo
software (version 10.0.6; BD Biosciences) was used to analyze cell
apoptosis.
Cell migration assay
The transfected PLC/PRF/5 cells (1×105)
in serum-free medium were placed into the upper chamber of a
24-well plate with 8 mm diameter (Corning, Incorporated). A total
of 500 µl DMEM containing 10% FBS was added in the bottom chamber,
and the cells were subsequently incubated at 37°C for 48 h. The
cells in the upper chamber were removed using a cotton swab, and
the membranes were stained with 0.1% crystal violet for 20 min at
room temperature. Finally, the cells were counted in 10 random
fields under a light microscope (magnification, ×200).
Dual-luciferase reporter assay
TargetScan bioinformatics software (www.targetscan.org) was used to predict the potential
targets of miR-125a-5p, the findings revealed that TRIAP1 and
BCL2L2 were potential targets of miR-125a-5p. To confirm this
prediction, PLC/PRF/5 cells (5×104 cells) were seeded
into each well of a 24-well plate. After 24 h, the cells were
co-transfected with TRIAP1/BCL2L2 3′-UTRpmirGLO plasmid (BioVector
NTCC Inc), containing mutant TRIAP1/BCL2L2 3′-UTR or wild type
TRIAP1/BCL2L2 3′-UTR, and miR-125a-5p mimic or mimic control using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following incubation at 37°C for another 48 h, the luciferase
activity (Luciferase Reporter Assay Kit; AmyJet Scientific, Inc.)
was assessed using the dual-luciferase reporter assay system
(Promega Corporation) and Renilla luciferase was used as the
internal control for the normalization of results.
Statistical analysis
All experiments were repeated at least three times.
Data are presented as the mean ± standard deviation. Statistical
significance was assessed using SPSS 19.0 statistical software (IBM
Corp.). Differences between multiple groups were assessed by one
way analysis of variance followed by Tukey's post hoc test and
differences between two groups were analyzed with Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
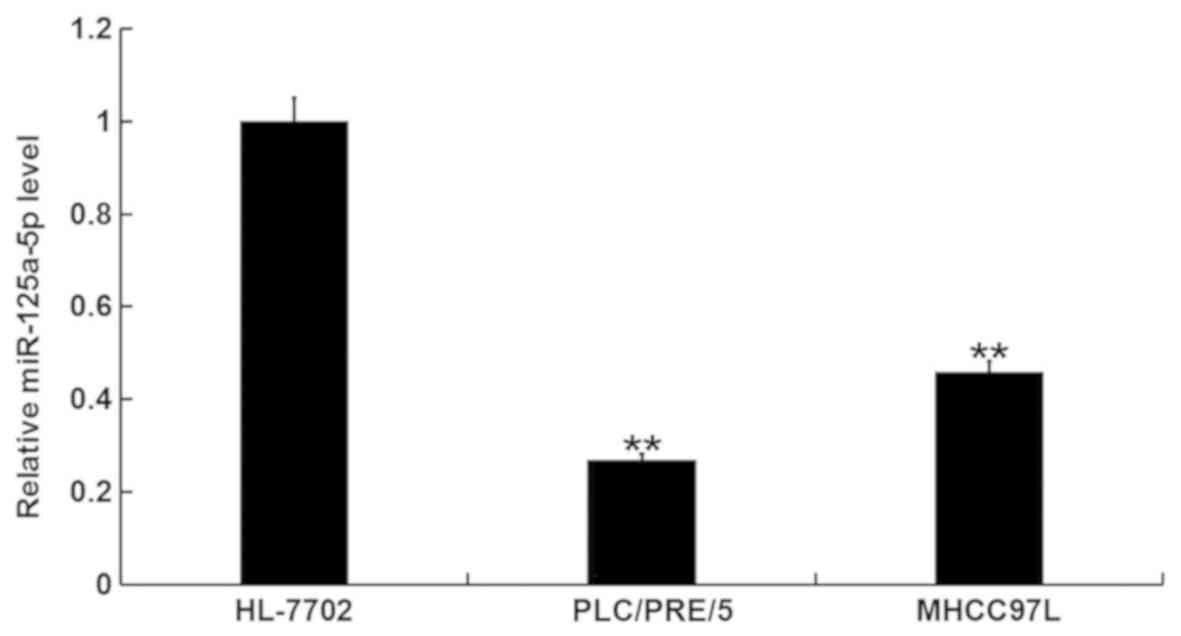
miR-125a-5p is downregulated in human
hepatocellular carcinoma cells
The expression of miR-125a-5p was detected in the
human hepatocellular carcinoma cell line, PLC/PRF/5, and the human
immortalized liver cell line, HL-7702, using RT-qPCR. The results
indicated that, compared with the HL-7702 cells, the level of
miR-125a-5p was markedly downregulated in PLC/PRF/5 cells and
MHCC97L cells. The decrease of miR-125a-5p expression in PLC/PRF/5
cells was greater than in MHCC97L cells (Fig. 1), therefore, PLC/PRF/5 cells were
selected for subsequent experimentation.
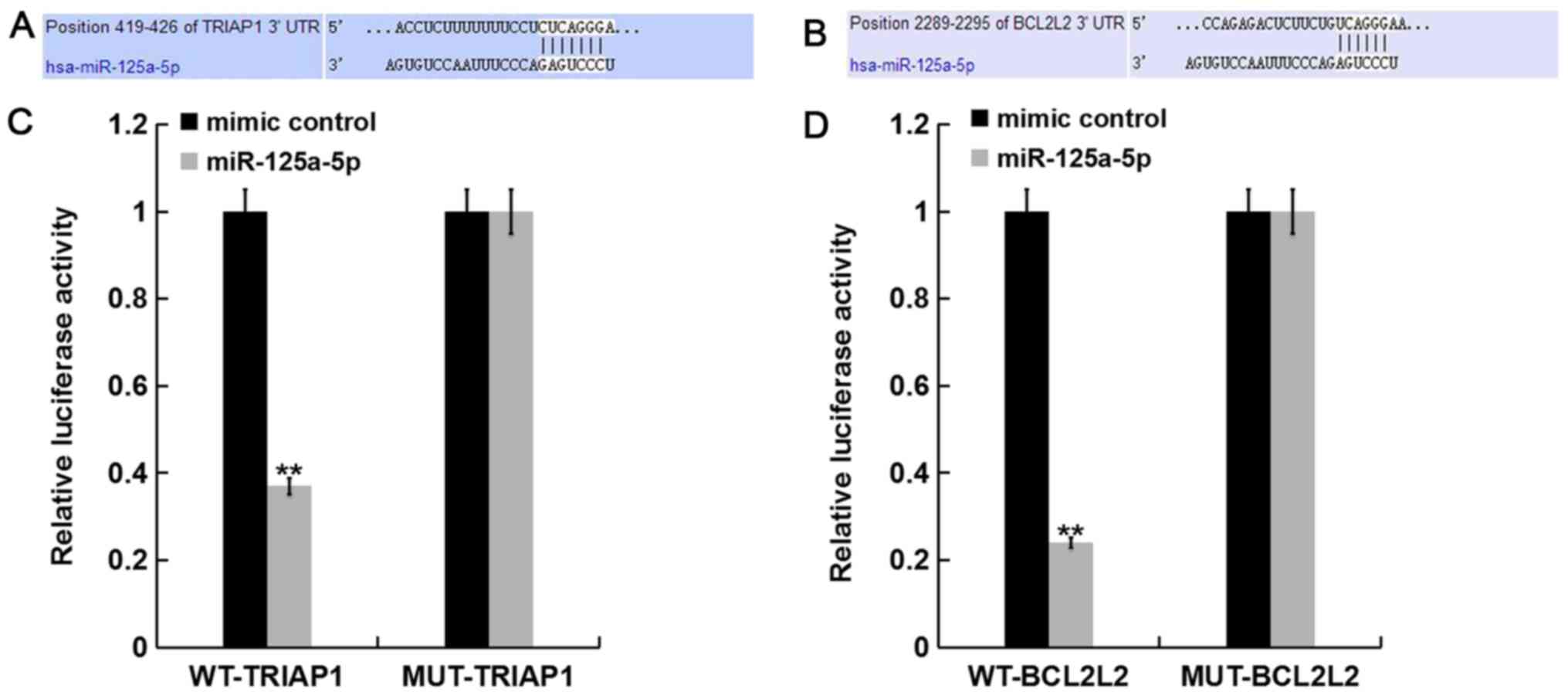
miR-125a-5p directly targets TRIAP1
and BCL2L2
TargetScan database was used to predict the
potential targets of miR-125a-5p. TRIAP1 and BCL2L2 were revealed
to be the predicted targets of miR-125a-5p (Fig. 2A and B). To investigate whether
TRIAP1 and BCL2L2 are direct targets of miR-125a-5p, luciferase
reporter assay was performed. The results indicated that
miR-125a-5p markedly reduced the luciferase activity of
TRIAP1-3′-UTR and BCL2L2-3′-UTR in PLC/PRF/5 cells, but exerted no
effect on the mutant form of TRIAP1-3′-UTR and BCL2L2-3′-UTR
(Fig. 2C and D), indicating that
TRIAP1 and BCL2L2 are direct targets of miR-125a-5p in PLC/PRF/5
cells.
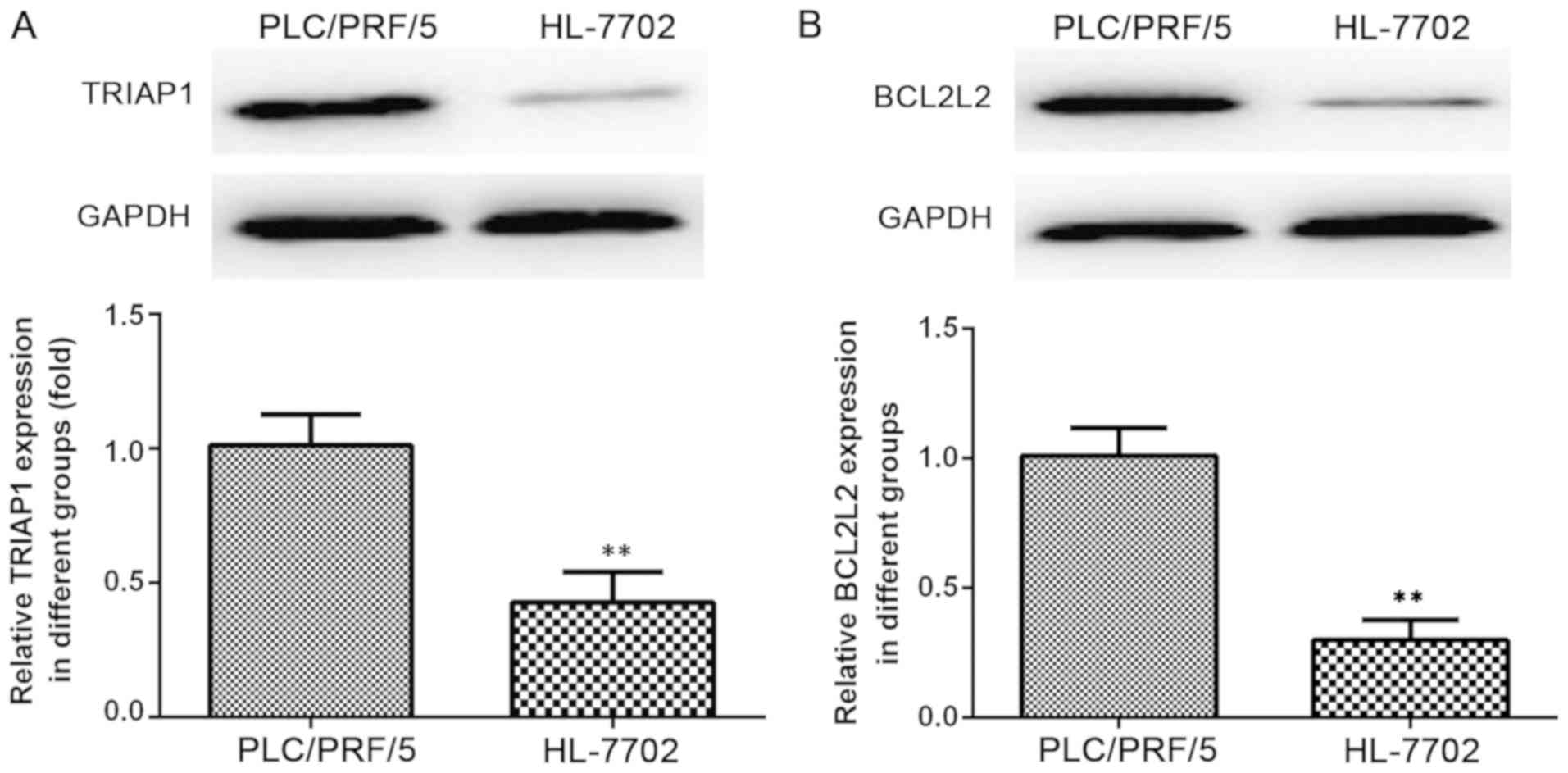
TRIAP1 and BCL2L2 are upregulated in
human hepatocellular carcinoma cells
The human hepatocellular carcinoma cell line,
PLC/PRF/5 and the human immortalized liver cell line, HL-7702 were
analyzed to determine the protein and mRNA expression levels of
TRIAP1 (Fig. 3A) and BCL2L2
(Fig. 3B) using western blot assay
and RT-qPCR respectively. These experiments demonstrated that the
protein expression levels of TRIAP1 and BCL2L2 were markedly higher
in the PLC/PRF/5 cells compared with the HL-7702 cells (Fig. 3A and B).
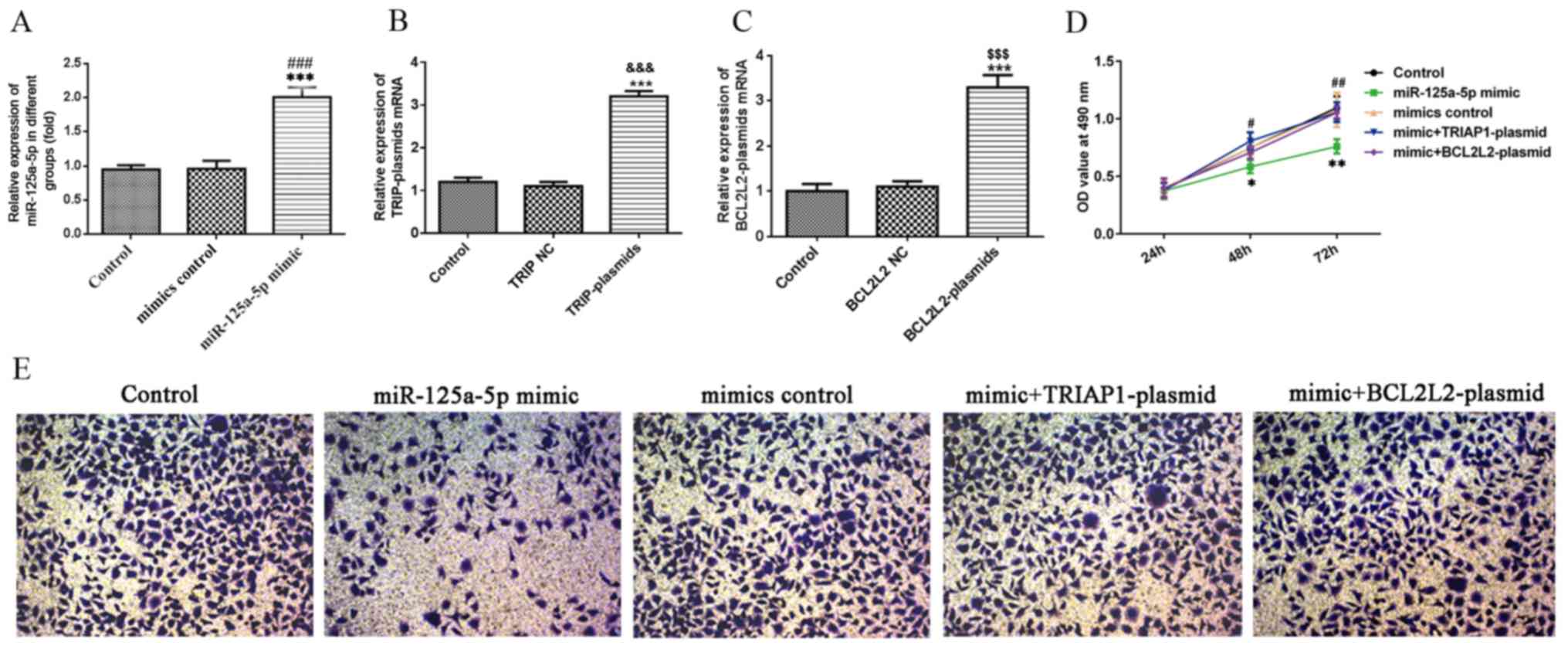
miR-125a-5p inhibits PLC/PRF/5 cell
viability and migration
RT-qPCR analysis determined the upregulation of
miR-125a-5p in PLC/PRF/5 cells, miR-125a-5p was overexpressed using
miR-125a-5p mimic. After 48 h of cell transfection, the effective
upregulation of the mRNA levels of miR-125a-5p as compared with the
non-transfected and control-transfected cells was confirmed as
observed in Fig. 4A. As shown in
Fig. 4B and C, compared with the
control group, the TRIAP1 was successfully overexpressed in TRIAP1
plasmids and BCL2L2 expression was up-regulated in BCL2L2 plasmids.
To determine the effect of miR-125a-5p on hepatocellular carcinoma
cell viability, MTT assay was performed. The results obtained
revealed that the miR-125a-5p notably reduced the cell viability,
but the inhibition was cancelled out upon TRIAP1 or BCL2L2
overexpression (Fig. 4D). Similarly,
Transwell assay revealed that transfection with miR-125a-5p mimic
markedly reduced the migration capacity of the PLC/PRF/5 cells
compared with transfection with the control, but this effect was
reversed when TRIAP1 or BCL2L2 were overexpressed (Fig. 4E).
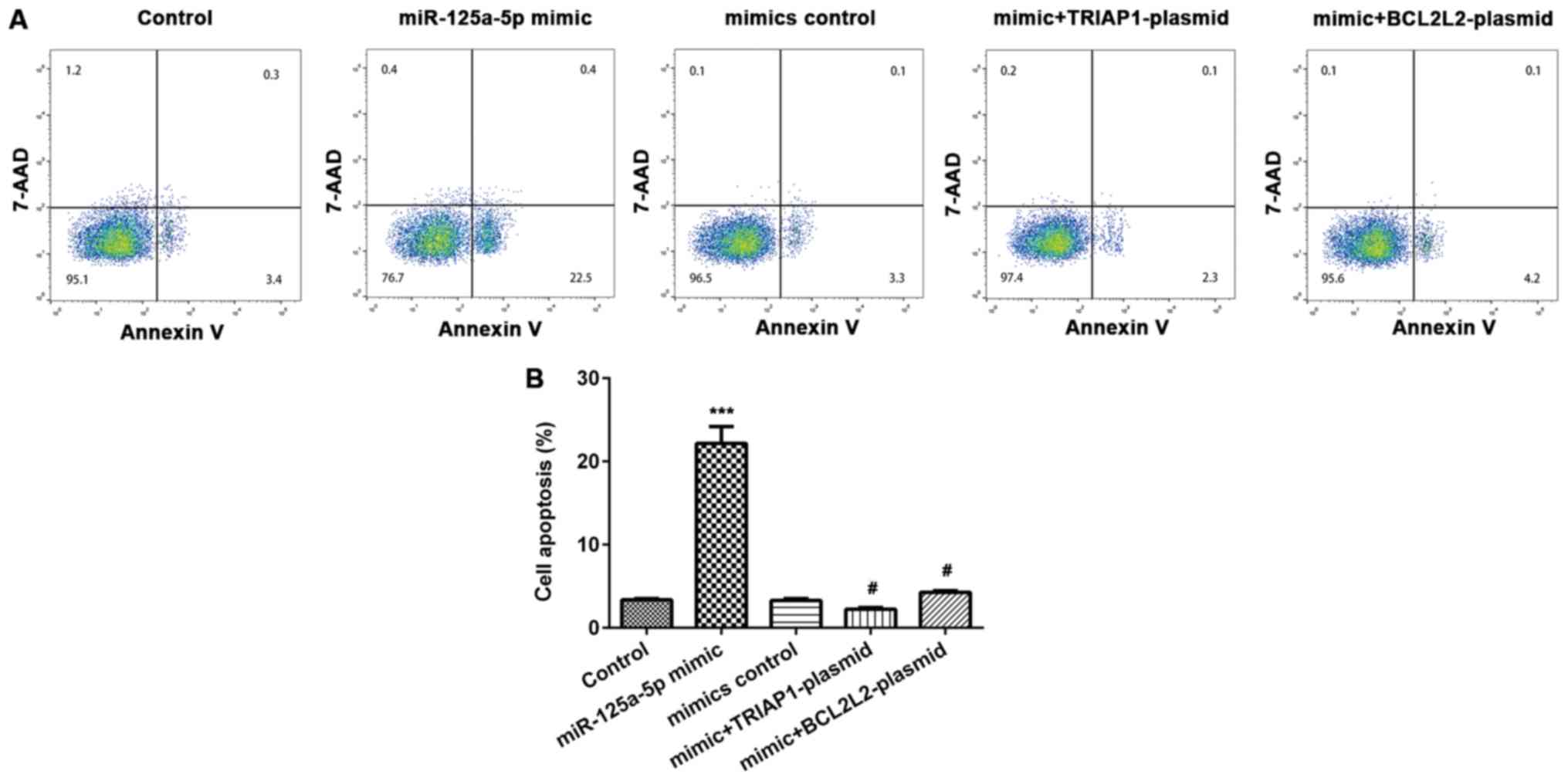
miR-125a-5p induces PLC/PRF/5 cell
apoptosis
Annexin V-FITC/PI apoptosis detection kit was used
to determine the extent of apoptosis of cells in the different
groups. Compared with the control group, the rate of cell apoptosis
was markedly enhanced in the miR-125a-5p mimic transfected
PLC/PRF/5 cells (Fig. 5A).
Furthermore, the effect of miR-125a-5p mimic on PLC/PRF/5 cell
apoptosis was demonstrated to be reversed by TRIAP1 or BCL2L2
overexpression (Fig. 5B).
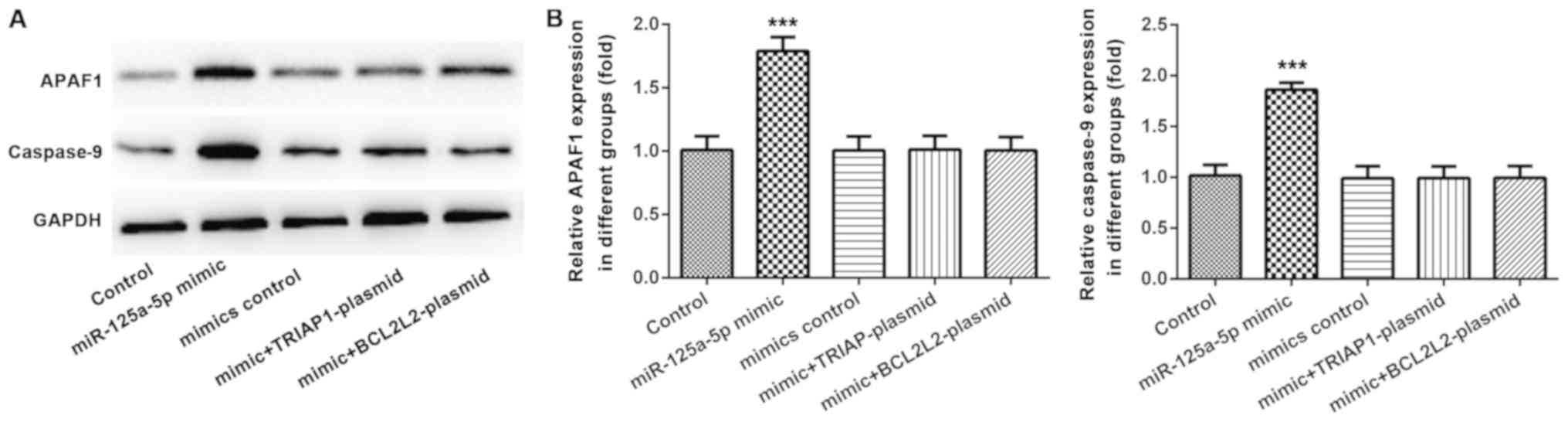
A previous study indicated that TRIAP1 inhibition
could induce cell apoptosis by upregulating caspase-9 and apoptotic
protease-activating factor 1 (APAF1) (17). Therefore, whether caspase-9 and APAF1
were involved in regulating PLC/PRF/5 cell apoptosis by miR-125a-5p
was investigated using western blots. The results demonstrated that
the protein levels of caspase-9 and APAF1 were markedly increased
in miR-125a-5p transfected PLC/PRF/5 cells, and this effect was
inhibited by either TRIAP1 or BCL2L2 overexpression (Fig. 6A and B).
Discussion
The present study demonstrated that miR-125a-5p was
downregulated in the human hepatocellular carcinoma cell lines,
PLC/PRF/5 and MHCC97L, but this effect was more noteworthy in
PLC/PRF/5 cells. Further studies indicated that miR-125a-5p
inhibited PLC/PRF/5 cell proliferation and invasion and induced
cell apoptosis by targeting TRIAP1 and BCL2L2. Therefore, the
findings presented in the present study suggested that miR-125a-5p
may be a novel and promising therapeutic target for treatment of
hepatocellular carcinoma.
Hepatocellular carcinoma is the third leading cause
of cancer- associated mortality in the world (18). Although the treatment methods have
been improved in recent years, the recurrence and mortality rates
associated with hepatocellular carcinoma continue to increase, and
the prognosis of patients is extremely poor (19). The predominant causes of high
mortality in hepatocellular carcinoma are the metastasis and
recurrence of hepatocellular carcinoma. Since the exact mechanism
remains unclear, there continues to be a lack of effective means of
prevention and treatment. Therefore, it is required to find novel
and effective targets for the diagnosis and treatment of
hepatocellular carcinoma.
miRNAs, a class of non-coding small RNAs, have been
revealed to serve a similar role to proto-oncogenes or tumor
suppressor genes in the development and progression of various
types of malignancies (20,21). Increasing evidence has demonstrated
that the abnormal expression of miRNAs is involved in the
occurrence and development of hepatocellular carcinoma (22,23).
miR-125a-5p, an extensively studied miRNA in tumors, has been
identified to serve an inhibitor role in hepatitis B virus-related
hepatocellular carcinoma (15). Jin
et al (24) suggested that
miR-125a-5p is associated with the clinical stages of head and neck
squamous cell carcinoma, and the expression levels in low tumor
stages (stages I and II) were higher compared with that in the high
stages (stages III and IV). Furthermore, miR-125a-5p is a potential
biomarker for acute ischemic stroke (25). However, the role and mechanism of
miR-125a-5p in hepatocellular carcinoma remains largely unclear.
Therefore, the present study was conducted.
Firstly, the expression levels of miR-125a-5p in
human hepatocellular carcinoma cells were detected, and the results
obtained confirmed that miR-125a-5p was downregulated in the human
hepatocellular carcinoma cell line, PLC/PRF/5. Subsequently, in
order to explore the role of miR-125a-5p in hepatocellular
carcinoma, the potential targets of miR-125a-5p were predicted
using the TargetScan database and were confirmed by dual luciferase
reporter assay. These findings suggested that TRIAP1 and BCL2L2
were the targets of miR-125a-5p. Subsequently, the upregulation of
TRIAP1 and BCL2L2 was investigated in PLC/PRF/5 cells. Further
analysis indicated that miR-125a-5p inhibited PLC/PRF/5 cell
proliferation and migration. As TRIAP1 and BCL2L2 are both
apoptosis inhibitory factors (17,26,27),
whether miR-125a-5p exerted an effect on cell apoptosis was
subsequently investigated. As expected, it was shown that
miR-125a-5p overexpression markedly induced PLC/PRF/5 cell
apoptosis. TRIAP1 inhibition has been shown to induce cell
apoptosis by enhancing the expression of caspase-9 and APAF1
(17). Therefore, in the present
study, whether caspase-9 and APAF1 were involved in miR-125a-5p
regulated PLC/PRF/5 cell apoptosis was investigated. The results of
these experiments suggested that the protein levels of caspase-9
and APAF1 were markedly increased in miR-125a-5p transfected
PLC/PRF/5 cells, indicating the involvement of
TRIAP1/caspase9/APAF1 in miR-125a-5p regulated PLC/PRF/5 cell
apoptosis. Furthermore, an important finding of the present study
was that all the effects of miR-125a-5p on PLC/PRF/5 cells were
eliminated upon either TRIAP1 or BCL2L2 overexpression.
In conclusion, to the best of our knowledge, this is
the first study to have revealed that miR-125a-5p may inhibit
hepatocellular carcinoma cell growth and migration by directly
targeting TRIAP1 and BCL2L2. Therefore, miR-125a-5p may be a novel
and promising therapeutic target for the treatment of
hepatocellular carcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science
Fund Program of Xinjiang Uygur Autonomous Region (grant no.
2018D03011; China).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MM conceived and designed the experiments. MY, ML
performed the experiments. ML analyzed the date. MM drafted the
manuscript, MY and MM revised manuscript. The final version of the
manuscript has been read and approved by all authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chiou JF, Tai CJ, Huang MT, Wei PL, Wang
YH, An J, Wu CH, Liu TZ and Chang YJ: Glucose-regulated protein 78
is a novel contributor to acquisition of resistance to sorafenib in
hepatocellular carcinoma. Ann Surg Oncol. 17:603–612. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mazzaferro V, Regalia E, Doci R, Andreola
S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A and
Gennari L: Liver transplantation for the treatment of small
hepatocellular carcinomas in patients with cirrhosis. N Engl J Med.
334:693–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greten TF and Sangro B: Targets for
immunotherapy of liver cancer. J Hepatol. Sep 18–2017.(Epub ahead
of print).
|
|
5
|
Anindo MI and Yaqinuddin A: Insights into
the potential use of microRNAs as biomarker in cancer. Int J Surg.
10:443–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chandrasekaran K, Karolina DS, Sepramaniam
S, Armugam A, Wintour EM, Bertram JF and Jeyaseelan K: Role of
microRNAs in kidney homeostasis and disease. Kidney Int.
81:617–627. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petrovic N, Ergün S and Isenovic ER:
Levels of MicroRNA heterogeneity in cancer biology. Mol Diagn Ther.
21:511–523. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alhoot MA, Wang SM and Sekaran SD: RNA
interference mediated inhibition of dengue virus multiplication and
entry in HepG2 cells. PLoS One. 7:e340602012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin Y, Wang J, Han J, Luo D and Sun Z:
MiR-122 inhibits epithelial-mesenchymal transition in
hepatocellular carcinoma by targeting Snail1 and Snail2 and
suppressing WNT/β-cadherin signaling pathway. Exp Cell Res.
360:210–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong L, Sun S, Shi J, Cao F, Han X and
Chen Z: MicroRNA-125a-5p plays a role as a tumor suppressor in lung
carcinoma cells by directly targeting STAT3. Tumour Biol.
39:10104283176975792017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng H, Wu J, Shi J, Lu C, Wang Y, Sun Q,
Zhang G and Zhao G: miR-125a-5p upregulation suppresses the
proliferation and induces the cell apoptosis of lung adenocarcinoma
by targeting NEDD9. Oncol Rep. 38:1790–1796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Natalia MA, Alejandro GT, Virginia TJ and
Alvarez-Salas LM: MARK1 is a novel target for miR-125a-5p:
Implications for cell migration in cervical tumor cells. Microrna.
7:54–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao Y, Tan S, Tu Y, Zhang G, Liu Y, Li D,
Xu S, Le Z, Xiong J, Zou W, et al: MicroRNA-125a-5p inhibits
invasion and metastasis of gastric cancer cells by targeting BRMS1
expression. Oncol Lett. 15:5119–5130. 2018.PubMed/NCBI
|
|
15
|
Li G, Zhang W, Gong L and Huang X:
MicroRNA 125a-5p inhibits cell proliferation and induces apoptosis
in hepatitis B virus-related hepatocellular carcinoma by
downregulation of ErbB3. Oncol Res. 27:449–458. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fook-Alves VL, de Oliveira MB, Zanatta DB,
Strauss BE and Colleoni GW: TP53 regulated inhibitor of apoptosis 1
(TRIAP1) stable silencing increases late apoptosis by upregulation
of caspase 9 and APAF1 in RPMI8226 multiple myeloma cell line.
Biochim Biophys Acta. 1862:1105–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimada K, Sakamoto Y, Esaki M, Kosuge T,
Morizane C, Ikeda M, Ueno H, Okusaka T, Arai Y and Takayasu K:
Analysis of prognostic factors affecting survival after initial
recurrence and treatment efficacy for recurrence in patients
undergoing potentially curative hepatectomy for hepatocellular
carcinoma. Ann Surg Oncol. 14:2337–2347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao J, Yu W, Hu K, Li M, Chen J and Li Z:
miR-92a promotes tumor growth of osteosarcoma by targeting PTEN/AKT
signaling pathway. Oncol Rep. 37:2513–2521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie F, Yuan Y, Xie L, Ran P, Xiang X,
Huang Q, Qi G, Guo X, Xiao C and Zheng S: miRNA-320a inhibits tumor
proliferation and invasion by targeting c-Myc in human
hepatocellular carcinoma. Onco Targets Ther. 10:885–894. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu HR, Huang RZ, Yu XN, Shi X,
Bilegsaikhan E, Guo HY, Song GQ, Weng SQ, Dong L, Janssen HLA, et
al: Microarray expression profiling of microRNAs reveals potential
biomarkers for hepatocellular carcinoma. Tohoku J Exp Med.
245:89–98. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang T, Thakur A, Chen T, Yang L, Lei G,
Liang Y, Zhang S, Ren H and Chen M: MicroRNA-15a induces cell
apoptosis and inhibits metastasis by targeting BCL2L2 in non-small
cell lung cancer. Tumour Biol. 36:4357–4365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin S, Liu MD, Wu H, Pang P, Wang S, Li
ZN, Sun CF and Liu FY: Overexpression of hsa-miR-125a-5p enhances
proliferation, migration and invasion of head and neck squamous
cell carcinoma cell lines by upregulating C-C chemokine receptor
type 7. Oncol Lett. 15:9703–9710. 2018.PubMed/NCBI
|
|
25
|
Li Y, Tang X, He Q, Yang X, Ren X, Wen X,
Zhang J, Wang Y, Liu N and Ma J: Overexpression of mitochondria
mediator gene TRIAP1 by miR-320b loss is associated with
progression in nasopharyngeal carcinoma. PLoS Genet.
12:e10061832016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tiedt S, Prestel M, Malik R,
Schieferdecker N, Duering M, Kautzky V, Stoycheva I, Böck J,
Northoff BH, Klein M, et al: RNA-Seq identifies circulating
miR-125a-5p, miR-125b-5p, and miR-143-3p as potential biomarkers
for acute ischemic stroke. Circ Res. 121:970–980. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wen Y, Han J, Chen J, Dong J, Xia Y, Liu
J, Jiang Y, Dai J, Lu J, Jin G, et al: Plasma miRNAs as early
biomarkers for detecting hepatocellular carcinoma. Int J Cancer.
137:1679–1690. 2015. View Article : Google Scholar : PubMed/NCBI
|