Introduction
The resection of large liver tumors is a frequent
intractable problem for liver surgeons (1). A larger hepatectomy can lead to serious
complications, including post-hepatectomy liver failure and
small-for-size syndrome (2,3). Portal vein ligation (PVL) (4) or portal vein embolization (PVE)
(5) have been widely used to
increase the future liver volume (FLV) and reduce complication risk
of patients with marginal FLVs. Over the 4–8 week waiting period
for an adequate FLV, the tumor may continue to progress and as such
is a shortcoming of this procedure (5). Associating liver partition and portal
vein ligation for staged hepatectomy (ALPPS) has been used for the
hepatectomy of large liver tumors since 2007, when Schnitzbauer
et al (6) described the
technique. ALPPS increases FLV in a much shorter time than PVL or
PVE (5,7,8).
However, according to preliminary reports, this improvement comes
at the cost of increased postoperative morbidity and mortality,
which justifies further investigation into technique modification
(9), particularly in patients with
end-stage liver tumors and/or cirrhosis (10,11).
Currently, ~50% of new global liver cancer cases
occur in China (12), where
hepatocellular carcinoma (HCC) is the third leading cause of
cancer-associated death. In China, the prognosis of patients with
HCC complicated by liver cirrhosis is poor (13). D'Haese et al (10) reported that with strict indications
for surgery, patients with liver cancer complicated with hepatic
fibrosis may undergo ALPPS surgery. It was also demonstrated that
the degree of liver fibrosis and FLV growth rate were negatively
correlated (8). However, the precise
mechanism and indication of ALPPS in patients with cirrhosis are
unclear (10). Research using animal
models is therefore required. Current ALPPS animal models in the
reported literature are based on normal livers and do not
appropriately simulate conditions of liver cirrhosis (14–18).
Therefore, the data regarding the feasibility and safety of ALPPS
in livers with fibrosis or cirrhosis remains poor. In the present
study, an ALPPS model was developed in a highly reproducible animal
model of cirrhosis to assess the mechanism of ALPPS, refine the
procedure and identify ways to further improve ALPPS outcomes.
Materials and methods
Animal models
A total of 76 male Sprague-Dawley (SD) rats (age,
6–8 weeks; weight, 220–250 g) were obtained from Dashuo Laboratory
Animal Co., Ltd. Rats were housed in cages at a temperature of
21–25°C and a humidity of 45–55%. Animals were also exposed to an
artificial 12 h light/dark cycle with ad libitum access to
food and water. All procedures were performed according to the
guidelines and with the approval of the Animal Care and Ethics
committee of the West China Hospital of Sichuan University
(Sichuan, China; approval no. 2017001A).
Experimental design
One group of 10 rats (training group) was used to
determine basic data which were used to determine normal liver
weights and normal liver enzyme range. Rats in the training group
received open surgery without liver surgery or model drug
injection. After obtaining normal liver tissue samples and serum
samples, animals were sacrificed. In the experimental groups, rats
were randomly divided into a liver cirrhosis group (group A) and a
normal control group (group B). Animals were sacrificed at
different time points (1, 2, 3, 7 and 14 days; n=6 animals per
group per time point).
Anatomical exploration
The SD rat liver is divided into five sections,
which include: The right lobe (RL), the right median lobe (RML),
the left median lobe (LML), the left lateral lobe (LLL) and the
caudal lobe (CL; Fig. 1A). According
to previous experimental studies (17,19,20),
liver sections account for the following total liver volumes: LML,
10%; LLL, 30%; RML, 30%; RL, 22%; and CL, 8%. The median lobe is
supplied by two portal branches: The right branch and the left
branch. This experimental model of ALPPS was developed to maintain
the LML, CL and LLL as the FLV (~50%; Fig. 1B-D). Microcomputed tomography and
3-dimensional reconstructions were used to observe individual lobes
and hepatic veins (Fig. 1E).
Arterial circulation and biliary duct branches were maintained in
all rats.
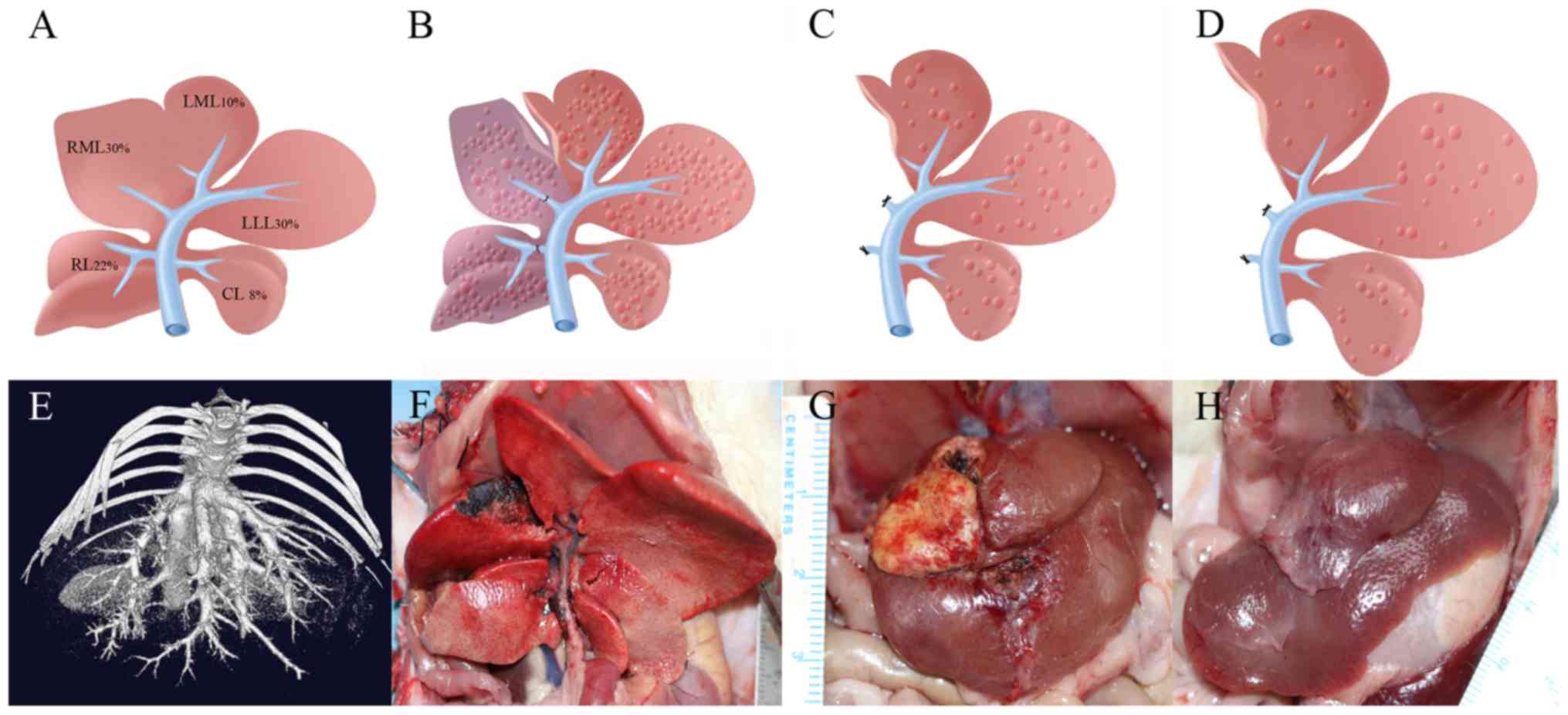 | Figure 1.Construction of the ALPPS model in
rats with cirrhosis. (A) A schematic of the anatomical structures
in the normal rat liver and the approximate volumes of the liver
lobes. (B) A schematic of liver cirrhosis after ligation of the
portal veins of the RL and RML. After ligation, the liver darkened
in color and the liver was separated along the middle ischemic
line. (C) Day 7 after the first phase, the ligated region was
excised and (D) Postoperative day 14, the remaining liver volume
increased. (E) Preoperative microcomputed tomography assessment of
hepatic veins and distribution characteristics. (F) The rat model
of liver cirrhosis with ALPPS after surgery (after sacrifice). (G)
Day 7 after step I, the ligated liver lobes appeared necrotic
(yellow color). (H) Postoperative day 14, the LLL and LML had grown
and the volume increased. ALPPS, Associating liver partition and
portal vein ligation for staged hepatectomy; RL, right lobe; RML,
right median lobe; LML, left median lobe; LLL, left lateral lobe;
CL, caudal lobe. |
Induction of liver cirrhosis
In group A, liver cirrhosis was induced via a
subcutaneous injection of 50% (v/v) carbon tetrachloride
(CCl4 dissolved in olive oil; Chengdu Kelong Chemical
Reagent Factory) administered at 1.0 ml/100 g of body weight 2
times a week for a total of 12 weeks as previously described
(21). In group B, the same volume
of 0.9% sodium chloride solution was injected twice a week for a
total of 12 weeks based on rat weight (1.0 ml/100 g). After
assessing rat weight, the volume of model drugs was calculated and
subcutaneous injections were performed at different sites in the
abdomen. On the 1st day of week 13, surgical procedure was
performed. Rats were subsequently humanely sacrificed via
exsanguination under anesthesia on postoperative days 1, 2, 3, 7 or
14.
Surgical procedure
All surgical procedures were performed under
anesthesia with 2–4% isoflurane mixed with pure oxygen at a flow
rate of 0.5 l/min (Shenzhen Ruiwode Life Technology Co., Ltd.).
Rats underwent a laparotomy via a transverse upper-abdominal
incision. The mobilization and dissection of portal veins was
performed under an operating microscope (Zeiss GmBH; magnification,
×10–25). The branch of the portal vein and branches of the lobes
were exposed and prepared for ligation to remove the peripheral
ligaments of the liver. A bulldog clamp was briefly applied near
the hepatic pedicle (pringle maneuver) (20) to reduce the amount of blood loss
during the parenchymal transection. After the operation, rats were
re-warmed with an electric blanket at 36°C until they awakened and
were then returned to their cages in the laboratory.
Parenchymal transection
Occlusion of 50% of the liver mass was performed via
ligation of the portal veins, which supply the RML and RL. Hepatic
transection was performed by placing a clamp stepwise along the
transection plane, which was marked left of the demarcation line on
the median lobe following the left PVL. The middle median hepatic
vein was maintained and a minimal distance of 0.5 cm was kept from
the vena cava. Bleeding was prevented using a choice of
compression, ligation or electrocoagulation. For the 14-day group,
the ligated liver lobes (RL+RML) were excised on the 7th day after
first stage surgery, and then sacrificed on the 7th days after
second stage surgery (Fig. 1C and
G). Daily monitoring was undertaken and body weights were
recorded.
Liver acquisition and sampling
At 1 h prior to surgery, blood was collected in
serum tubes (serum Z/1.2 ml; Wuxi Nest Biotechnology Co., Ltd.) via
puncture of the right femoral artery and stored at −20°C until
further use. Serum aspartate aminotransferase (AST), alanine
aminotransferase (ALT), albumin (ALB) and total bilirubin (TBIL)
levels were determined using an automated chemical analyzer (Bayer
Advia 1650; Bayer AG). After the ligation of the RML and RL, ~0.5 g
of liver tissue was placed in a −20°C frozen slicer (Leica CM1900;
Leica Microsystems GmbH) to produce frozen sections for the early
diagnosis of liver fibrosis. Hematoxylin and eosin staining were
performed after embedding sections in paraffin (4 mm thick) to
assess the degree of liver fibrosis for final diagnosis. Samples
from group B were obtained using the same procedures. Liver lobes
were weighed to calculate the liver weight/body weight ratio using
the following formula: Liver weight of the individual lobe (g)/body
weight (g) ×100%. Furthermore, the rate of residual liver
hyperplasia was used to calculate proliferation (residual liver
hyperplasia rate=(postoperative liver weight-predicting
preoperative liver weight)/predicted preoperative liver weight
×100%). For example, the LML hyperplasia rate=(LML weight-0.004×
preoperative body weight)/(0.004× preoperative body weight) ×100%.
Liver tissues from the RML and LML were fixed with 10% buffered
formalin for 48 h at 25°C. Fibrotic and necroinflammation features
were evaluated in sections (5 µm thick) stained with standard
Masson's trichrome (23–25°C for 5–10 min) and hematoxylin-eosin
(HE; hematoxylin, 23–25°C for 10–20 min; eosin, 23–25°C for 3–5
min), respectively, using Scheuer's scoring system (22): Stage 0, no fibrosis; stage I,
expansion of the portal tracts without linkage; stage II, portal
expansion with portal linkage; stage III, extensive portal to
portal and focal portal to central linkage; and stage IV,
cirrhosis. Other pathological features of liver tissue were
evaluated using the Ishak grading system (23) and practice guidelines (24) for non-alcoholic fatty liver disease.
Digital images of all slides were captured using a slide scanner
(Axio Imager A2; Carl Zeiss Microscopy AG).
Immunohistochemical staining and
determination of cytokine levels
The aforementioned liver specimens were dehydrated
and embedded in paraffin wax (6 mm thick) for immunohistochemical
staining. Primary anti-Ki-67 (cat. no. ab156956; Abcam) and
anti-proliferating cell nuclear antigen (PCNA) antibodies (cat. no.
ab18197; Abcam) were used to detect the respective proteins using
standard immunohistochemical methods (17) in accordance with the manufacturer's
protocol. The proliferation index (PI) was expressed as the
fraction of proliferating hepatocyte nuclei to the total number of
hepatocyte nuclei (accurate to 0.1%), as described previously
(25). The numbers of Ki-67-positive
and PCNA-positive hepatocytes were determined in three random
visual fields under a digital slide scanner (magnification, ×100;
Axio Imager A2). All histological analyses were performed in a
blinded fashion with respect to the experimental groups. Plasma
tumor necrosis factor alpha (TNF-α; cat. no. xl-Er0359; Xinle
Biological Technology Co., Ltd.), hepatocyte growth factor (HGF;
cat. no. xl-Er0153; Xinle Biological Technology Co., Ltd.) and
interleukin-6 (IL-6; xl-Er0196; Xinle Biological Technology Co.,
Ltd.) levels were determined using ELISA which was performed in
accordance with the manufacturer's protocol.
Statistical analysis
Statistical analyses were performed using software
packages (GraphPad Prism version 6; GraphPad Software, Inc.; SPSS
22.0 for Windows software; IBM Corp.). P<0.05 was considered to
indicate a statistically significant result. The sizes of the
groups (n=6) were calculated to establish a statistical power of
83.4% (G*Power; version 3.1.9.2; http://www.gpower.hhu.de/), expecting moderately high
differences between medians based on previous studies of FLV
hypertrophy using these techniques (17,26). All
quantitative variables that were normally distributed were
presented as the mean ± standard deviation and were compared using
an independent Student's t-test. The median or minimum-maximum
value was used to represent data that did not conform to the normal
distribution. A Mann-Whitney U non-parametric test was used.
Results
Model and preoperative evaluation
In group A, 4 rats of the 36 used (11.11%; 30 were
used per group, 6 additional rats used following model failure)
exhibited slower absorption of modeling drugs (drug accumulation
under the skin of the abdomen). Symptoms were relieved by
puncturing and extruding oil. No abnormalities were observed during
the modeling of group B rats. At the end of the 12th week, after an
assessment of preoperative body weight between the two groups
(347.56±43.22 vs. 350.81±51.22 g; P=0.45; Table I), both groups of rats were used in
the experiment. Three rats of the 36 used (8.3%; 30 were used per
group, 6 additional rats used following model failure) in group A
exhibited moderate ascites and were therefore excluded.
Furthermore, 3 other rats exhibited serious adhesions in stage II,
causing excess blood loss and death. These were replaced with 6
rats with cirrhosis that were induced in the current study as
aforementioned (subsequent experiments had no complications or
accidents). The operations performed on rats in group B in the
first and second stages were successful and no surgical incidents
occurred. Postoperative recovery (determined by the regain of
consciousness, limb movement and sensitivity to light) in rats with
liver cirrhosis was slower than in the group B and the restoration
of postoperative activity was delayed, with limb movement and
normal speed returning 5–6 h after surgery. The preliminary fast
paraffin sections obtained during surgery indicated that all rats
in group A exhibited cirrhotic nodules and were diagnosed with
cirrhosis (stage IV). Liver cirrhosis and liver fibrosis were not
observed in group B.
 | Table I.Observational markers for rat ALPPS
surgery. |
Table I.
Observational markers for rat ALPPS
surgery.
| Parameters | Group A | Group B | P-value |
|---|
| Number | 36 | 30 |
|
| Preoperative body
weight (g) | 347.56±43.22 | 350.81±51.22 | 0.45 |
| Modeling and
surgical complications n (%) |
|
|
|
| Slower
absorption of modeling drugs | 4 (11.11) | 0 (0) |
|
|
Ascites | 3 (8.33) | 0 (0) |
|
| Serious
adhesions in stage II or death | 3 (8.33) | 0 (0) |
|
| Stage I ALPPS |
|
|
|
|
Operative time (min) | 43.53±12.37 | 36.32±10.66 | 0.02 |
| Blood
loss (ml) | 5.33±3.09 | 2.36±5.33 | 0.04 |
| Stage II ALPPS |
|
|
|
|
Operative time (min) | 56.14±5.84 | 51.00±3.92 | 0.07 |
| Blood
loss (ml) | 7.93±1.30 | 8.43±1.72 | 0.55 |
| Rate of hyperplasia
in the LML |
|
|
|
| POD
2 | 48.86±0.26% | 58.76±0.19% | 0.004 |
| POD
7 | 30.53±0.31% | 22.30±0.64% | <0.001 |
| POD
14 | 32.56±0.27% | 33.41±0.31% | 0.072 |
| ALT POD
1 (µmol/l) | 282.3–721.0 | 77.0–380.2 | 0.014 |
| AST POD
1 (µmol/l) | 219.5–1102.0 | 256.4–752.4 | 0.036 |
| Proportion of
Ki-67-positive cells POD 7 | 0.2616±0.0082 | 0.1664±0.0048 | 0.003 |
| Proportion of
Ki-67-positive cells POD 14 | 0.2804±0.0018 | 0.1195±0.0007 | <0.001 |
| Proportion of
PCNA-positive cells POD 7 | 0.2151±0.0232 | 0.2526±0.0085 | 0.077 |
| Proportion of
PCNA-positive cells POD 14 | 0.2555±0.0155 | 0.1950±0.0060 | 0.058 |
Surgical details
The operative time for stage I was significantly
longer in the group A than in group B (43.53±12.37 vs. 36.32±10.66
min; P=0.02). Intraoperative blood loss was assessed indirectly by
weighing swabs. The blood loss in stage I was 5.33±3.09 ml in group
A and 2.36±5.33 ml in group B (P=0.04), indicating that greater
blood loss occurred in group A than in group B. At the second
stage, there was no statistical difference in operation time
(P=0.07) and blood loss (P=0.55) between group A and group B
(Table I).
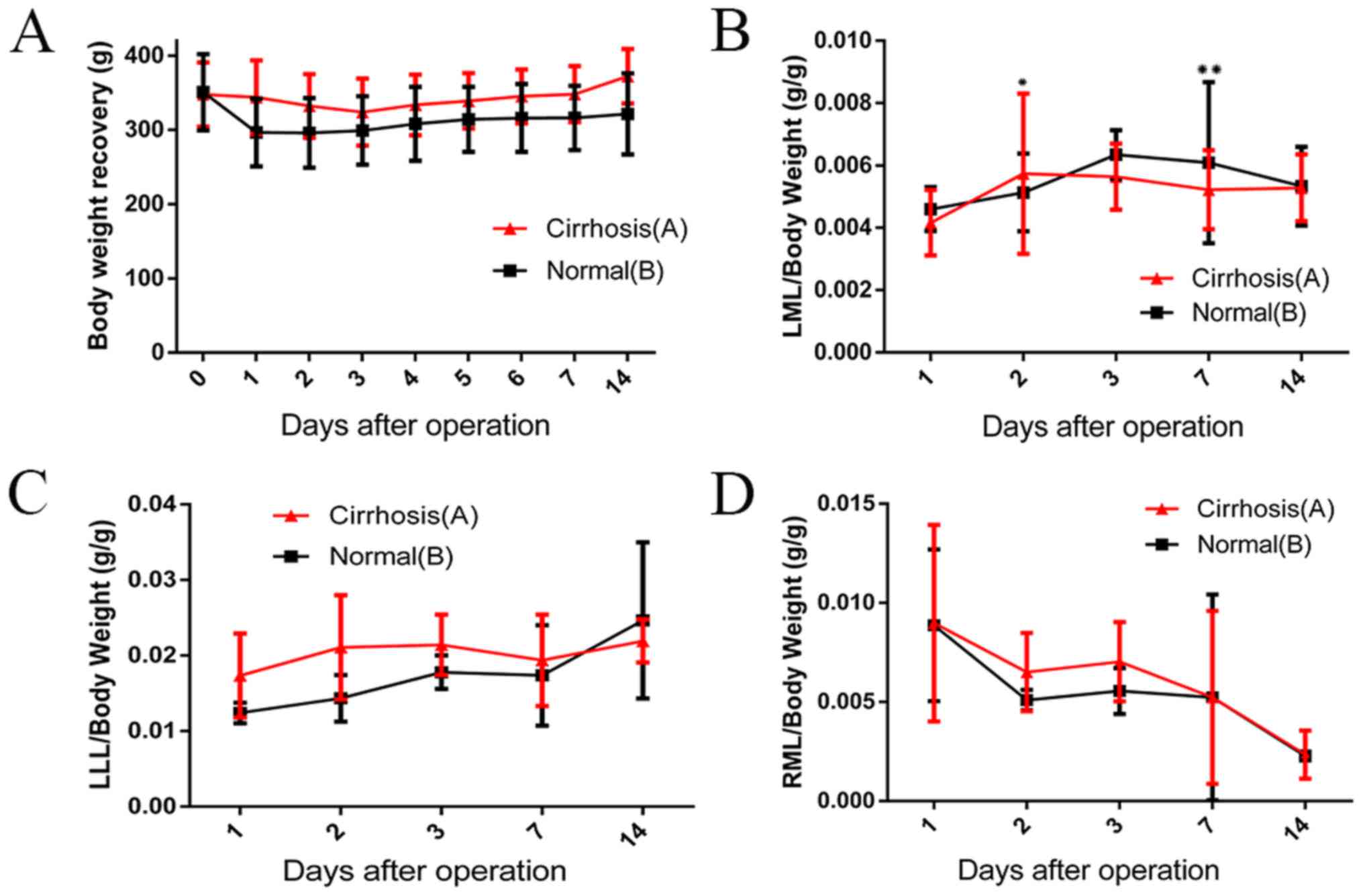
Liver weight evaluation
In the training group, the average LML, LLL and LML
liver lobe:body weight ratios were 0.004, 0.014 and 0.010,
respectively (data not shown). On the third postoperative day, the
body weight of group A rats decreased to the lowest value and then
began to steadily increase. Group A body weights increased to a
value greater than the preoperative weight by the 7th day after the
operation. A similar increasing trend was observed in group B. The
changes in hyperplasia were significant on the second day after
surgery (Fig. 2B). On the
postoperative days 2, 7 and 14, the rates of hyperplasia of the LML
in the group A were 48.86±0.26, 30.53±0.31 and 32.56±0.27%,
respectively. The rates of hyperplasia in the LML of group B were
increased by 58.76±0.19% on day 2 (P=0.004), 22.30±0.64% on day 7
(P<0.001) and 33.41±0.31% on day 14 (P=0.072; Fig. 2B). Although a significant increase in
the ratio of LLL to preoperative weight was observed in the group
B, differences between the two groups were not significant
(Fig. 2C). On day 3 after stage I,
the weight of the atrophic hepatic lobe (RML) decreased. By the
14th day, necrosis or atrophy was gradually revealed and the value
of RML/body weight decreased to the lowest point in both groups due
to the lack of blood supply (Fig.
2D).
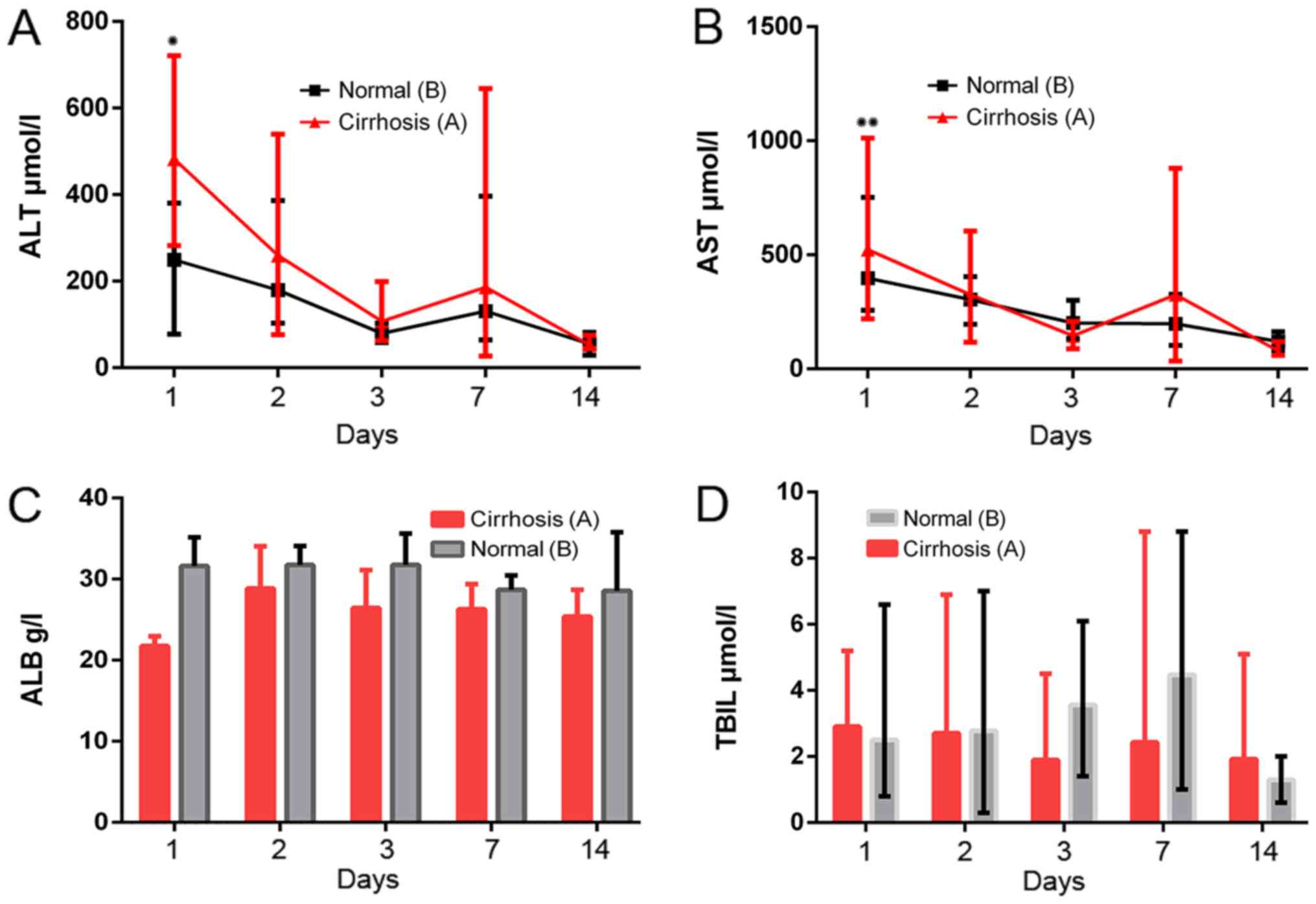
Liver function
Compared with the group B, significantly higher ALT
(282.3–721.0 vs. 77.0–380.2 µmol/l; normal range, 33–98.7 µmol/l)
and AST (219.5–1,102.0 vs. 256.4–752.4 µmol/l; normal range,
69.5–210 µmol/l) levels were recorded 24 h after surgery and a
marked difference was observed between the two groups (P=0.014 and
P=0.036, respectively, Fig. 3A and
B). AST and ALT concentrations gradually returned to normal
levels on postoperative day 3. However, ALB and TBIL values did not
change significantly at 1, 2, 3, 7 or 14 days after surgery. (ALB
normal range, 20–43 g/l; TBIL normal range, 1–20 µmol/l; Fig. 3).
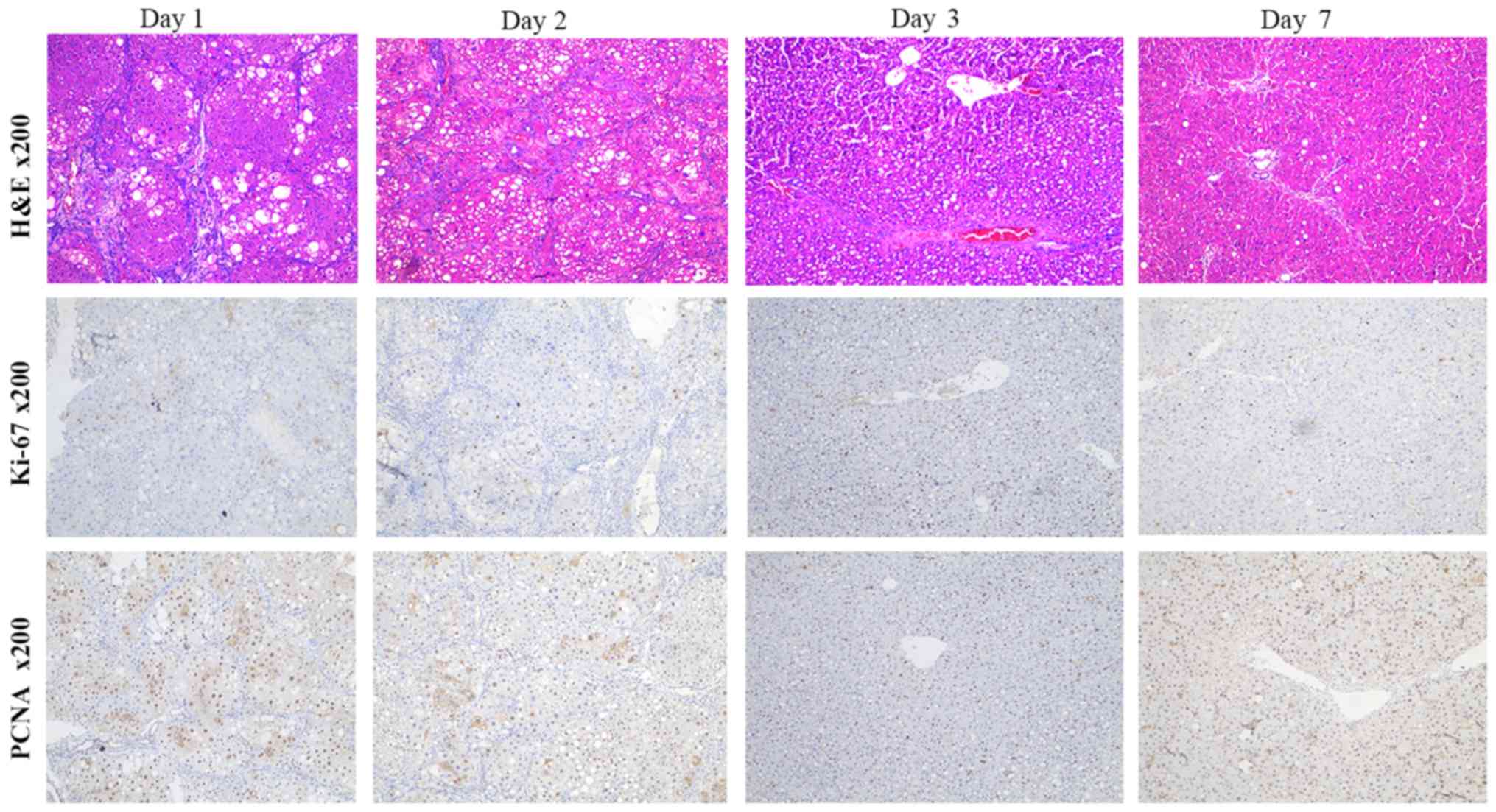
Pathological changes
In the liver group A, typical areas of necrosis and
hyperplasia were observed. New liver cells accumulated around the
small blood vessels. As the proportion of new cells increased,
changes in the fat content, ballooning and fibrosis were gradually
reduced in the visual field. The proportions of Ki-67- and
PCNA-positive (brown-yellow granules within the nucleus) increased
after surgery (Fig. 4). At
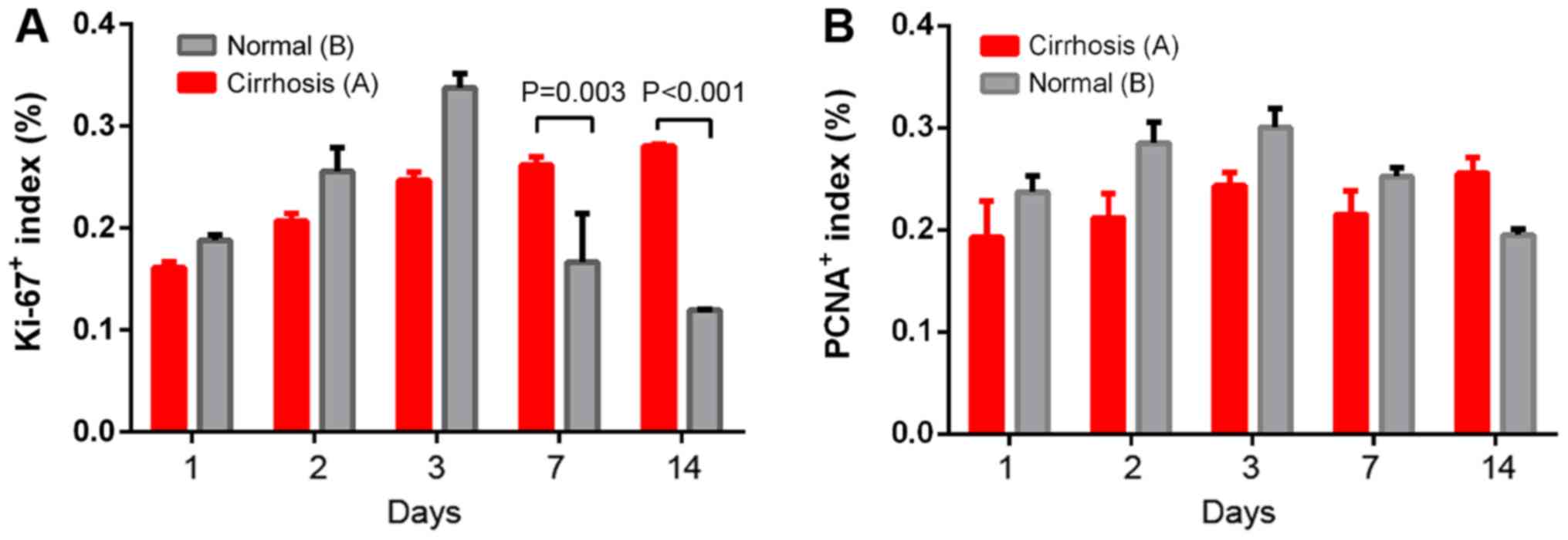
postoperative days 1, 2 and 3, cells in the group B proliferated
more rapidly than in the cirrhotic group. After day 3, the
proliferation rate in the group B began to decrease, but the rate
in the cirrhotic group continued to increase slowly (Fig. 5). After surgery, the proportion of
Ki-67-positive cells was significantly higher in the cirrhotic
group than in the group B at 7 days (0.2616±0.0082 vs.
0.1664±0.0048, P=0.003) and 14 days (0.2804±0.0018 vs.
0.1195±0.0007; P<0.001; Fig. 5A).
However, the PCNA positive cell rate did not reveal significant
differences on day 7 (0.2151±0.0232 vs. 0.2526±0.0085; P=0.077) and
14 (0.2555±0.0155 vs. 0.1950±0.0060; P=0.058) after surgery between
groups A and B. From the characteristics of proliferating cells
(determined via Ki-67 and PCNA-positive cells), the proliferation
patterns of the group B and the cirrhotic group exhibited a trend
to differ.
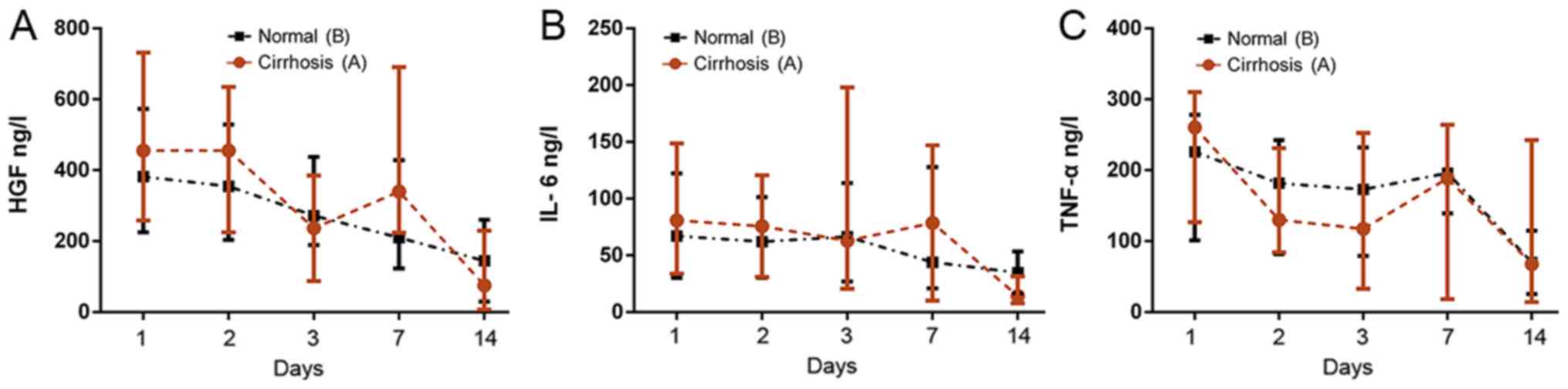
Serum cytokine levels
ELISA revealed different trends for different serum
cytokine levels (Fig. 6). In group
A, the upper limits of HGF and IL-6 levels were generally higher
than in the group B and the fluctuations of the upper and lower
limits were different, but the medians were similar. On the 3rd and
14th postoperative day, median HGF and IL-6 levels in group A were
slightly lower than those in group B, indicating these may be
important for inflammatory and proliferative responses. The
individual differences were larger; while the levels in group B
exhibited a more gradual decrease. Despite the value of TNF-α being
higher than that of group B on the 1st day after surgery, the
overall trend of each group was downward and no statistical
difference was identified. Significant differences in the changes
in cytokine levels were not observed between the two groups on
postoperative days 1, 2, 3, 7 and 14 (Fig. 6).
Discussion
The clinical application of ALPPS in advanced
primary and metastatic liver cancer is an innovative and important,
yet challenging surgical strategy (10,27).
However, complications and mortality in the perioperative period
remain high and the efficacy of this procedure in oncology is
inconclusive (28). Currently, ALPPS
is used in cases of liver metastasis in colon cancer and Barcelona
stage C liver cancer (28,29). Various scholars (9,10,30,31)
are cautious about using ALPPS to treat advanced HCC due to faster
progression, increased liver fibrosis, increased cirrhosis,
insufficient residual hepatic hyperplasia and postoperative liver
failure. A large proportion of newly diagnosed middle- and
late-stage liver cancer cases in China each year are accompanied by
varying degrees of cirrhosis (12,32).
Many patients are unable to undergo surgery due to large tumors.
Palliative surgery including PVL and PVE, is often used as
treatment for HCC which can reduce the size of the tumor, delay
tumor progression and create conditions and opportunities for
treatment such as ALPPS (33). ALPPS
represents a lifeline for patients with large tumors and
hepatocellular carcinoma with multiple masses. R0 [no residual
tumor under the naked eye or under the microscope (negative
margin)] removal can cause many problems and the mechanism of
postoperative residual liver regeneration is unclear, particularly
for livers with hepatitis B after liver cirrhosis (32). A current topic of academic debate is
whether the liver is healthy during short-term regeneration
(10,31,34). The
present study therefore, established an ALPPS model in cirrhotic
rats to further explore this phenomenon.
An ALPPS model was established by ligating the
portal veins of the RL and RML. The RML, CL and LLL account for
~50% of the total liver volume and were therefore used as the
remaining liver in the cirrhotic rats. This ligation method was
used to avoid serious complications caused by wounds and to ensure
surgery was as safe as possible. Due to preliminary experimental
results, the study was unable to mimic 70% of the liver volume in
cirrhotic rats (data not shown). This smaller future liver remnant
(≤30%) may have influenced the outcome of the current study,
however this requires further study. ALPPS surgery in the context
of cirrhosis still maintained significant proliferative capacity
and facilitated second stage hepatectomy in a short period of time.
On a normal liver background, days 1 and 3 after ALPPS constitute
the critical period for liver regeneration, the rate of liver
regeneration slows after 7 days (17,26,33,35).
However, in the present study, slow hyperplasia was observed in the
liver with cirrhosis. Ki-67 and PCNA-positive cells continued to
progress through the cell cycle at days 7 and 14 with no
significant peaks. This outcome however, were not the same as the
proliferation curve in the group B and the pattern of proliferation
differed in cirrhotic livers. Liver regeneration after ALPPS in
rats with liver cirrhosis is slower than in a normal liver, likely
due to an intrahepatic portosystemic shunt blockage after hepatic
parenchymal disconnection (32). The
results of the current study indicated that the regeneration rate
in cirrhotic rats started later but continued for longer than in
normal rats. The remaining liver has a more abundant portal vein
blood supply and aggravates the pressure of the remaining hepatic
portal vein, causing it to harden. In addition, the liver nodules
are affected by blood flow (33). In
the current study, the observation endpoint was 14 days after
surgery. This occurred as weights exceeded those of the
preoperative stage 14 days after surgery, indicating that the
remaining liver function may have returned to normal. It is
hypothesized that after 14 days, the degree of cirrhosis may be
slightly reduced and the proportion of regenerating liver tissue
may gradually increase after ALPPS in the rat model of cirrhosis.
However, how this new liver tissue breaks through the liver fiber
structure requires further research. Currently, the present study
have two hypotheses: One is that the fiber strands are pushed to
one side and the new cells break through the narrow gap to grow;
the other is that the newborn cells or mesenchymal cells secrete
cytokines to induce the dissolution of fiber strips and various
molecules cause cracking and breakage of the fiber strands, giving
the newborn cells enough space to grow. Future experiments will aim
to validate these hypotheses.
Schlegel et al (19) established an ALPPS model in mice with
normal livers to determine the mechanism by which ALPPS promotes
liver regeneration. The FLV growth rate of the ALPPS group was
twice the rate of the PVL group and higher levels of IL-6 and PCNA
were detected than in the controls (29). In the PVL group, additional injury to
other organs (radiofrequency ablation of the spleen, kidney or
lung) was performed and plasma was then harvested and injected into
the PVL group. Finally, the authors observed a similar increase in
FLV to the ALPPS group, indicating that localized trauma or
inflammatory responses might accelerate the induction of hepatocyte
proliferation (19). Almau et
al (36) and Tong et al
(37) established a rat experimental
model based on the ALPPS procedure as described above. Furthermore,
Yao et al (38) revealed that
the reactivation rate of the liver was significantly faster in the
ALPPS group on the 3rd and 7th postoperative days compared with the
PVL group. The mechanism of ALPPS proliferation that was reported
includes massive tissue necrosis and inflammatory responses after
liver disconnection. This stimulates liver regeneration and
upregulation of cytokine expression in regenerated lobes. However,
the ALPPS models reported in the aforementioned studies were all
based on a normal liver background and did not simulate liver
regeneration in the cirrhotic liver. The current study emphasizes
the safety and proliferative capacity of ALPPS after cirrhosis
(stage IV). Compared with liver fibrosis (not all F4 grade), animal
models of cirrhosis are slightly more difficult to establish
(39).
Currently, the methods for clinically assessing the
regeneration of remaining liver tissue depends on CT liver volume
reconstruction, but liver hyperplasia is roughly estimated and may
differ from the actual area (40).
Therefore, doctors suspect that the proliferation of the remaining
liver volume is due to the regeneration of liver cells or
hepatocyte parenchyma edema (34).
The current study measured the weight of each lobe of the liver on
postoperative days 1, 3, 5, 7 and 14 to calculate the remaining
liver growth rate. The present study investigated Ki-67-positive
and PCNA-positive proliferating cells using immunohistochemistry to
measure the number of cells in the regenerating liver that were
undergoing cell proliferation at different time points. The results
indicate that proliferation accompanied by cell division and the
number of cells was significantly increased. However, whether
hepatocyte proliferation is derived from hepatic stem cells or bile
duct-derived cells, needs further investigation. ALPPS represents
one step beyond PVL or PVE but significantly balances time between
liver regeneration and tumor recurrence. The mechanism by which
ALPPS promotes liver regeneration is not yet clear. Previous
studies have indicated that it may be associated with changes in
hepatic blood flow caused by the portal veins of the ligated
hepatic lobe (41), the inflammatory
response and stress response caused by disassociation of the liver
or changes in proliferation-associated factors (17,42).
In the current study, serum levels of TNF-α, IL-6
and HGF were measured to further explore the molecular mechanisms.
Compared with the normal liver group, TNF-α and IL-6 levels in the
1st, 2nd, 7th days after surgery were elevated in the ALPPS group
with cirrhosis. TNF-α and IL-6 serve important roles in the initial
stages of liver regeneration (40).
These two pro-inflammatory cytokines are produced by activated
Kupffer cells in the liver and promote the transition of
hepatocytes from the G0 phase to the G1 phase of the cell cycle
(43). Hepatocytes are sensitive to
growth factors such as HGF and synthesize DNA (44). Therefore, ALPPS may promote the onset
of liver regeneration by upregulating these two pro-inflammatory
cytokines. The group A exhibited elevated cytokine levels,
particularly of HGF and IL-6. The present study indicated that
increased cytokine levels detected in the current experiments may
be associated with pro-inflammatory and stress responses induced by
cirrhosis itself. This cirrhosis may be caused by surgical trauma
and necrosis of the hepatocytes due to decreased microcirculation
in the LML (44). The results
indicate that an inflammatory response is inhibited in the presence
of cirrhosis, but more samples are required to confirm this
hypothesis.
In the current study, the effects of ALPPS on the
FLV-induced rapid liver growth were assessed by the comparison of
cirrhotic and normal liver tissues. FLV growth, liver function
changes and postoperative serum cytokine levels were analyzed. As
the first cirrhosis animal model of ALPPS, the preliminary results
of the current study validated the safety and proliferative
capacity of the surgery. The source of new liver tissue and how the
new cells break through the fiber structure will be studied in
further investigations. The biological behaviors and liver
structure in the human body are more complex than in tumor-free
rats with liver cirrhosis. Therefore, the two models cannot be
accurately compared. Furthermore, when combined with liver cancer,
liver cirrhosis in the context of mechanisms to promote liver
regeneration requires further research.
Acknowledgements
The authors would like to thank Dr Xintao Zeng for
assistance with the revised draft and valuable discussion.
Funding
The current study was supported by the Department of
Science and Technology of Sichuan Province (grant no.
2016FZ0076).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
WW proposed the study. XY and CY performed the
experiments and wrote the first draft. XY collected and analyzed
the data. All authors contributed to the design and interpretation
of the study and to further drafts.
Ethical approval and consent to
participate
All procedures were performed according to the
guidelines and with the approval of the Animal Care and Ethics
Committee of the West China Hospital of Sichuan University
(Permission no: 2017001A).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zaydfudim VM, Vachharajani N, Klintmalm
GB, Jarnagin WR, Hemming AW, Doyle MB, Cavaness KM, Chapman WC and
Nagorney DM: Liver resection and transplantation for patients with
hepatocellular carcinoma beyond milan criteria. Ann Surg.
264:650–658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hemming AW, Reed AI, Langham MJ Jr, Fujita
S and Howard RJ: Combined resection of the liver and inferior vena
cava for hepatic malignancy. Ann Surg. 239:712–721. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chapelle T, Op de Beeck B, Driessen A,
Roeyen G, Bracke B, Hartman V, Huyghe I, Morrison S, Ysebaert D and
Francque S: Estimation of the future remnant liver function is a
better tool to predict post-hepatectomy liver failure than
platelet-based liver scores. Eur J Surg Oncol. 43:2277–2284. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aussilhou B, Lesurtel M, Sauvanet A,
Farges O, Dokmak S, Goasguen N, Sibert A, Vilgrain V and Belghiti
J: Right portal vein ligation is as efficient as portal vein
embolization to induce hypertrophy of the left liver remnant. J
Gastrointest Surg. 12:297–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shindoh J, Vauthey JN, Zimmitti G, Curley
SA, Huang SY, Mahvash A, Gupta S, Wallace MJ and Aloia TA: Analysis
of the efficacy of portal vein embolization for patients with
extensive liver malignancy and very low future liver remnant
volume, including a comparison with the associating liver partition
with portal vein ligation for staged hepatectomy approach. J Am
Coll Surg. 217:126–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schnitzbauer AA, Lang SA, Goessmann H,
Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T,
Goralcyk A, Hörbelt R, et al: Right portal vein ligation combined
with in situ splitting induces rapid left lateral liver lobe
hypertrophy enabling 2-staged extended right hepatic resection in
small-for-size settings. Ann Surg. 255:405–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bertens KA, Hawel J, Lung K, Buac S,
Pineda-Solis K and Hernandez-Alejandro R: ALPPS: Challenging the
concept of unresectability-a systematic review. Int J Surg.
13:280–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sandström P, Røsok BI, Sparrelid E, Larsen
PN, Larsson AL, Lindell G, Schultz NA, Bjørnbeth BA, Isaksson B,
Rizell M and Björnsson B: ALPPS improves resectability compared
with conventional two-stage hepatectomy in patients with advanced
colorectal liver metastasis: Results from a scandinavian
multicenter randomized controlled trial (LIGRO Trial). Ann Surg.
267:833–840. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Linecker M, Björnsson B, Stavrou GA,
Oldhafer KJ, Lurje G, Neumann U, Adam R, Pruvot FR, Topp SA, Li J,
et al: Risk adjustment in ALPPS is associated with a dramatic
decrease in early mortality and morbidity. Ann Surg. 266:779–786.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
D'Haese JG, Neumann J, Weniger M,
Pratschke S, Björnsson B, Ardiles V, Chapman W, Hernandez-Alejandro
R, Soubrane O, Robles-Campos R, et al: Should ALPPS be used for
liver resection in intermediate-stage HCC? Ann Surg Oncol.
23:1335–1343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen JX, Ran HQ and Sun CQ: Associating
microwave ablation and portal vein ligation for staged hepatectomy
for the treatment of huge hepatocellular carcinoma with cirrhosis.
Ann Surg Treat Res. 90:287–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou YM, Sui CJ, Zhang XF, Li B and Yang
JM: Influence of cirrhosis on long-term prognosis after surgery in
patients with combined hepatocellular-cholangiocarcinoma. BMC
Gastroenterol. 17:252017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deal R, Frederiks C, Williams L, Olthof
PB, Dirscherl K, Keutgen X, Chan E, Deziel D, Hertl M and Schadde
E: Rapid liver hypertrophy after portal vein occlusion correlates
with the degree of collateralization between lobes-a study in pigs.
J Gastrointest Surg. 22:203–213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Langiewicz M, Schlegel A, Saponara E,
Linecker M, Borger P, Graf R, Humar B and Clavien PA: Hedgehog
pathway mediates early acceleration of liver regeneration induced
by a novel two-staged hepatectomy in mice. J Hepatol. 66:560–570.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andersen KJ, Knudsen AR, Jepsen BN, Meier
M, Gunnarsson A, Jensen UB, Nyengaard JR, Hamilton-Dutoit S and
Mortensen FV: A new technique for accelerated liver regeneration:
An experimental study in rats. Surgery. 162:233–247. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi H, Yang G, Zheng T, Wang J, Li L,
Liang Y, Xie C, Yin D, Sun B, Sun J, et al: A preliminary study of
ALPPS procedure in a rat model. Sci Rep. 5:175672015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moris D, Vernadakis S, Papalampros A,
Vailas M, Dimitrokallis N, Petrou A and Dimitroulis D: Mechanistic
insights of rapid liver regeneration after associating liver
partition and portal vein ligation for stage hepatectomy. World J
Gastroenterol. 22:7613–7624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schlegel A, Lesurtel M, Melloul E, Limani
P, Tschuor C, Graf R, Humar B and Clavien PA: ALPPS: From human to
mice highlighting accelerated and novel mechanisms of liver
regeneration. Ann Surg. 260:839–847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schadde E, Hertl M, Breitenstein S,
Beck-Schimmer B and Schläpfer M: Rat model of the associating liver
partition and portal vein ligation for staged hepatectomy (ALPPS)
procedure. J Vis Exp:. 126:e558952017.
|
|
21
|
Shaaban AA, Shaker ME, Zalata KR,
El-kashef HA and Ibrahim TM: Modulation of carbon
tetrachloride-induced hepatic oxidative stress, injury and fibrosis
by olmesartan and omega-3. Chem Biol Interact. 207:81–91. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scheuer PJ: Classification of chronic
viral hepatitis: A need for reassessment. J Hepatol. 13:372–374.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishak K, Baptista A, Bianchi L, Callea F,
De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, et al:
Histological grading and staging of chronic hepatitis. J Hepatol.
22:696–699. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chalasani N, Younossi Z, Lavine JE, Diehl
AM, Brunt EM, Cusi K, Charlton M and Sanyal AJ;
AmericanGastroenterological Association; American Association for
the Study of Liver Diseases; American College of Gastroenterologyh,
: The diagnosis and management of non-alcoholic fatty liver
disease: Practice guideline by the American Gastroenterological
Association, American Association for the Study of Liver Diseases,
and American College of Gastroenterology. Gastroenterology.
142:1592–1609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Homeyer A, Schenk A, Dahmen U, Dirsch O,
Huang H and Hahn HK: A comparison of sampling strategies for
histological image analysis. J Pathol Inform. 2 (Suppl):S112011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei W, Zhang T, Zafarnia S, Schenk A, Xie
C, Kan C, Dirsch O, Settmacher U and Dahmen U: Establishment of a
rat model: Associating liver partition with portal vein ligation
for staged hepatectomy. Surgery. 159:1299–1307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moris D, Dimitroulis D, Papalampros A,
Petrou A and Felekouras E: ALPPS procedure for hepatocellular
carcinoma in patients with chronic liver disease: Revealing a terra
incognita. Ann Surg. 266:e106–e107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schnitzbauer AA, Schadde E, Linecker M,
Machado MA, Adam R, Malago M, Clavien PA, de Santibanes E and
Bechstein WO: Indicating ALPPS for colorectal liver metastases: A
critical analysis of patients in the international ALPPS registry.
Surgery. 164:387–394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kambakamba P, Linecker M, Schneider M,
Reiner CS, Nguyen-Kim TDL, Limani P, Romic I, Figueras J, Petrowsky
H, Clavien PA and Lesurtel M: Impact of associating liver partition
and portal vein ligation for staged hepatectomy (ALPPS) on growth
of colorectal liver metastases. Surgery. 163:311–317. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Z, Xu M, Lin N, Pan C, Zhou B, Zhong
Y, Zhong Y and Xu R: Associating liver partition and portal vein
ligation for staged hepatectomy versus conventional two-stage
hepatectomy: A systematic review and meta-analysis. World J Surg
Oncol. 15:2272017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lang H, de Santibanes E, Schlitt HJ,
Malagó M, van Gulik T, Machado MA, Jovine E, Heinrich S, Ettorre
GM, Chan A, et al: 10th Anniversary of ALPPS-Lessons Learned and
quo Vadis. Ann Surg. 269:114–119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai X, Tong Y, Yu H, Liang X, Wang Y,
Liang Y, Li Z, Peng S and Lau WY: The ALPPS in the treatment of
hepatitis B-related hepatocellular carcinoma with cirrhosis: A
single-center study and literature review. Surg Innov. 24:358–364.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsuo K, Murakami T, Kawaguchi D,
Hiroshima Y, Koda K, Yamazaki K, Ishida Y and Tanaka K: Histologic
features after surgery associating liver partition and portal vein
ligation for staged hepatectomy versus those after hepatectomy with
portal vein embolization. Surgery. 159:1289–1298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eshmuminov D, Tschuor C, Raptis DA, Boss
A, Wurnig MC, Sergeant G, Schadde E and Clavien PA: Rapid liver
volume increase induced by associating liver partition with portal
vein ligation for staged hepatectomy (ALPPS): Is it edema,
steatosis, or true proliferation? Surgery. 161:1549–1552. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
García-Pérez R, Revilla-Nuin B, Martínez
CM, Bernabé- García A, Baroja MA and Parrilla PP: Associated liver
partition and portal vein ligation (ALPPS) vs. selective portal
vein ligation (PVL) for staged hepatectomy in a rat model. Similar
regenerative response? PLoS One. 10:e01440962015.PubMed/NCBI
|
|
36
|
Almau Trenard HM, Moulin LE, Padín JM,
Stringa P, Gondolesi GE and Barros SP: Development of an
experimental model of portal vein ligation associated with
parenchymal transection (ALPPS) in rats. Cir Esp. 92:676–681.
2014.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tong YF, Meng N, Chen MQ, Ying HN, Xu M,
Lu B, Hong JJ, Wang YF and Cai XJ: Maturity of associating liver
partition and portal vein ligation for staged hepatectomy-derived
liver regeneration in a rat model. World J Gastroenterol.
24:1107–1119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yao L, Li C, Ge X, Wang H, Xu K, Zhang A
and Dong J: Establishment of a rat model of portal vein ligation
combined with in situ splitting. PLoS One. 9:e1055112014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yifan T, Ming X, Yifan W, Hanning Y,
Guangyi J, Peijian Y, Ke W and Xiujun C: Hepatic regeneration by
associating liver partition and portal vein ligation for staged
hepatectomy (ALPPS) is feasible but attenuated in rat liver with
thioacetamide-induced fibrosis. Surgery. 165:345–352. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schadde E, Raptis DA, Schnitzbauer AA,
Ardiles V, Tschuor C, Lesurtel M, Abdalla EK, Hernandez-Alejandro
R, Jovine E, Machado M, et al: Prediction of mortality after ALPPS
stage-1: An analysis of 320 patients from the international ALPPS
registry. Ann Surg. 262:780–786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schadde E, Ardiles V, Robles-Campos R,
Malago M, Machado M, Hernandez-Alejandro R, Soubrane O,
Schnitzbauer AA, Raptis D, Tschuor C, et al: Early survival and
safety of ALPPS: First report of the International ALPPS Registry.
Ann Surg. 260:829–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Uribe M, Uribe-Echevarría S, Mandiola C,
Zapata MI, Riquelme F and Romanque P: Insight on ALPPS-associating
liver partition and portal vein ligation for staged
hepatectomy-mechanisms: Activation of mTOR pathway. HPB (Oxford).
20:729–738. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schmidt-Arras D and Rose-John S: IL-6
pathway in the liver: From physiopathology to therapy. J Hepatol.
64:1403–1415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kremer M, Son G, Zhang K, Moore SM, Norris
A, Manzini G, Wheeler MD and Hines IN: Smad3 signaling in the
regenerating liver: Implications for the regulation of IL-6
expression. Transpl Int. 27:748–758. 2014. View Article : Google Scholar : PubMed/NCBI
|