Introduction
Gastric cancer, a common malignancy, is mainly
caused by unhealthy dietary habits. According to the latest
statistics on the incidence and mortality of gastric cancer, it
ranks second in malignancies worldwide (1). The incidence of gastric cancer in
Vietnam and Philippines is highest (2) and it ranks first in gastrointestinal
malignancies in the two countries. According to the recent
statistics by the World Health Organization (3), the death toll of gastric cancer in 2015
(760,000) ranked fourth in cancer deaths over the world. In the
past two years, the death toll tended to be flush with the second
and the third, and the death is biased towards young age. Radical
gastrectomy (4) refers to the
complete removal of tumor, and then it may be cured, so radical
gastrectomy is also called curative resection of gastric cancer.
The primary sources of gastric cancer mainly include primary
tumors, metastatic lymph nodes, and involve infiltrating tissues.
The current surgical methods for radical gastrectomy include
traditional laparotomy, laparoscope-assisted radical gastrectomy,
full laparoscopic radical gastrectomy and robotic radical
gastrectomy (5).
A previous study found that patients' psychology had
a great impact on the success rate of the operation and the
postoperative prognosis, and postoperative excessive pain had a
strong negative impact on patient recovery (6), so the choice and dose of analgesics was
crucial. Clinical studies have shown that remifentanil is a good
postoperative tranquilizer (7–10). Due
to its unique chemical structure - ester bond, remifentanil is
easily hydrolyzed by non-specific cholinesterase in the body, and
these hydrolyzed sites are mainly located in human tissues and
plasma, so the elimination of remifentanil in the human body mainly
relies on them rather than liver and kidney function. Therefore,
remifentanil has the advantages of rapid onset of analgesia, strong
analgesic effect, rapid drug effect, easy adjustment, no
accumulation in the body and rapid elimination, which enables the
patient to recover quickly after drug withdrawal. Nalbuphine also
has superior advantages in anesthesia and analgesia (11,12),
mainly due to the fact that it has a unique pharmacological
property - antagonistic part of the µ-receptor that inhibits
adverse reactions such as respiratory depression, nausea, cough,
and drowsiness, which are caused by this receptor excitement. At
present, it has not been studied or discussed by scholars to apply
the combination of remifentanil and nalbuphine with proper ratio in
clinical analgesia. Therefore, this study mainly investigated the
clinical analgesic effect of different doses of remifentanil
combined with nalbuphine on postoperative gastric cancer patients,
to improve postoperative analgesia regime.
Patients and methods
Patient data
One hundred cases of gastric cancer patients were
treated from December 2014 to December 2016 in the Xiangyang No. 1
People's Hospital (Xiangyang, China), including 74 males and 26
females, aged from 40 to 68 years, with an average age of 51±6.22
years. The enrolled patients were divided into group A and B, with
50 cases in each group, according to the choice of patient
postoperative analgesia regime. The regime in group A was 0.2 mg/kg
of nalbuphine (Carbone Scientific Co., Ltd.) plus 0.2 µg/kg of
remifentanil (Yaodu Jingwei Information Technology Co., Ltd., SFDA
approval no.: H20143314); in group B it was 0.3 mg/kg of nalbuphine
plus 0.1 µg/kg of remifentanil.
The basic clinical data of the patients were
collected, including demographic data, operation time and vital
signs. Τhis study was approved by the Ethics Committee of the
Xiangyang No. 1 People's Hospital. Patients who participated in
this research had complete clinical data. The signed informed
consents were obtained from the patients or the guardians.
Inclusion and exclusion criteria
Inclusion criteria (13,14): All
enrolled patients met the requirements of the American Society of
Anesthesiologists (ASA) (levels I–II); all gastric cancer patients
admitted were confirmed as positive by clinical diagnosis; both
enrolled patients and their family members were informed and agreed
before treatment. Exclusion criteria: Patients who were allergic to
analgesic drugs, who had a history of drug abuse, patients
undergoing chemotherapy and radiotherapy one month before
operation, patients unwilling to cooperate with the treatment or
with disabilities, were excluded from the study.
Analgesic methods
After entering the operating room, patients' basic
vital signs were measured. An intravenous channel was opened,
oxygen was given, with ECG monitoring. Radical gastrectomy was
performed in strict accordance with the relevant operating
specifications throughout the entire process. All patients
underwent postoperative analgesia using patient-controlled
intravenous analgesia (PCIA). In group A, 0.2 µg/kg of remifentanil
plus 0.2 mg/kg of nalbuphine plus 0.9% of sodium chloride solution
were used to 100 ml for analgesia pump. In group B, 0.1 µg/kg of
remifentanil plus 0.3 mg/kg of nalbuphine plus 0.9% of sodium
chloride solution were used to 100 ml for analgesia pump. The
background dose of the analgesic pump was 2 ml/h, the
self-administered dose was 2 ml, and the locking time was 10
min.
Operation methods
All patients chose to have a supine position, and
the position could be adjusted according to the needs of the
operation. Laparoscopic radical surgery was performed with a
five-hole approach. The surgeon made an incision about 2 cm below
the navel. Trocar (10 mm) was inserted to establish the
pneumoperitoneum. The pressure was controlled between 12 and 15
mmHg. The other four holes were punctured in the left, right upper
abdomen, left and right abdomen. The left upper abdomen was set up
as the main operating hole. After the first assistant pulled the
membrane, the surgeon cut the membrane from the transverse colon
with an ultrasonic scalpel, opened the membrane cavity, and
separated and cut the gastroduodenal artery in the colonic liver
region. Then the gastroenteric artery and vein were found near the
posterior wall of the stomach, and the root of the artery and vein
were clamped, and the distal part of the membrane was cut off and
the sixth group of lymph nodes was removed. After the pancreatic
envelope was separated, the left gastric artery and vein were
exposed. The same method was used to clamp the left gastric artery
and vein on the root, then the distal part was cut off, and the
seventh and eighth groups of lymph nodes were removed completely.
After hepatoduodenal ligament capsulotomy, the right gastric artery
was exposed, and the distal end was cut off after root clamp, and
the third and twelfth lymph nodes were removed. Finally, the lymph
nodes in the spleen area were removed, the stomach was
short-acting, the root of the vein was clamped and the distal end
was cut, and the posterior gastric venous and venous and the
ligaments around the stomach were cut off. The first and second
sets of lymph nodes were removed and the cardia was freed. Total
gastrectomy was performed, and the esophageal jejunum Roux-Y
anastomosis or Bi-type anastomosis was performed. Grade I care was
given after surgery, and conventional antibiotics were used. After
the patient was ventilated, the fastening was released. All
patients underwent the same surgical procedure and the number of
lymph nodes removed was determined by the patient's condition at
the time of surgery.
Methods of observation
The clinical experience of the patients in all
groups at 2, 6, 12, 24 and 48 h after operation was observed and
recorded using visual analogue scale (VAS) and Brinell comfort
score (BCS) (15–18). The VAS scores range from 0 to 10 and
the pain increases with the increase of the number. The BCS scores
range from 1 to 4, and the comfort degree increases with the
increase of the number. The effective PCIA press times and the
effect of analgesic agents were observed and recorded for all
patients within 20 h after operation; the incidence of adverse
reactions during analgesia was observed and recorded, including
cough, respiratory depression (breathing <8 times/min),
drowsiness and pruritus.
Follow-up
The subjects in this group were followed up using
ward round and other follow-up methods, and the analgesic methods
and prognosis of patients were observed, and they were followed up
for a maximum of 60 days. Analysis of the clinical analgesic effect
of different doses of remifentanil combined with nalbuphine on
postoperative gastric cancer patients was performed.
Statistical analysis
The data obtained from the records were
statistically processed using the SPSS 20.0 statistical package
(IBM Corp., Armonk, NY, USA). Measurement data were expressed in
mean ± standard deviation (mean ± SD), and the comparison between
two groups was tested by Student's t-test. Repeated measures
analysis of variance was used for the comparison of different time
points within the group. LSD test was the post hoc test. The
enumeration data was expressed in percentage [n/(%)], and the
comparison between the groups was tested by Chi-square test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Analysis of the basic clinical data of
the two groups
The subjects included in this study were 100 gastric
cancer patients who were divided into two groups, with 50 cases in
each group. The t-test and Chi-square test were used for
statistical analysis of the clinical data of the two groups. The
results showed that there were no statistically significant
differences between the two groups in main vital signs, proportion
of male and female and medical history (P>0.05) (Table I). The two groups of patients were
comparable. The proportion of patients who had a history of
drinking and irregular diet, and the proportion of male patients
were high patients.
 | Table I.Basic clinical data [mean ± SD or
n/(%)]. |
Table I.
Basic clinical data [mean ± SD or
n/(%)].
| Items | Group A | Group B | t/χ2
value | P-value |
|---|
| Number | 50 | 50 |
|
|
| Sex |
|
| 0.208 | 0.648 |
| Male | 38 (76) | 36 (72) |
|
|
|
Female | 12 (24) | 14 (28) |
|
|
| Age distribution
(years) | 50.12±5.6 | 52.43±6.3 | 1.930 | 0.057 |
| Systolic pressure
before operation (mmHg) | 137.02±6.10 | 134.69±5.89 | 1.943 | 0.055 |
| Diastolic pressure
before operation (mmHg) | 81.34±9.36 | 79.6±8.66 | 0.937 | 0.351 |
| Heart rate before
operation (times/min) | 76.51±10.35 | 77.27±9.52 | 0.382 | 0.703 |
| Breathing before
operation (times/min) | 17.84±1.22 | 18.12±1.64 | 0.969 | 0.335 |
| Operation time
(min) | 217.83±5.25 | 219.42±4.93 | 1.561 | 0.122 |
| History of
drinking | 32 (64.00) | 33 (66.00) | 0.044 | 0.834 |
| History of irregular
diet | 39 (78.00) | 39 (78.00) | 0.000 | 1.000 |
| Tumor size |
|
| 0.049 | 0.826 |
| <4
cm | 35 (70.00) | 36 (72.00) |
|
|
| ≥4
cm | 15 (30.00) | 14 (28.00) |
|
|
| Degree of tumor
differentiation |
|
| 0.220 | 0.896 |
| High
differentiation | 2 (4.00) | 3 (6.00) |
|
|
| Middle
differentiation | 26 (52.00) | 25 (50.00) |
|
|
| Poor
differentiation | 22 (44.00) | 22 (44.00) |
|
|
| Tumor
infiltration |
|
| 0.539 | 0.970 |
|
T1 | 14 (28.00) | 13 (26.00) |
|
|
|
T2 | 12 (24.00) | 12 (24.00) |
|
|
|
T3 | 19 (38.00) | 20 (40.00) |
|
|
|
T4a | 3 (6.00) | 4 (8.00) |
|
|
|
T4b | 2 (4.00) | 1 (2.00) |
|
|
| Number of lymph
node metastases |
|
| 1.450 | 0.996 |
|
N0 | 21 (42.00) | 18 (36.00) |
|
|
|
N1 | 10 (20.00) | 12 (24.00) |
|
|
|
N2 | 11 (22.00) | 13 (26.00) |
|
|
|
N3 | 8 (16.00) | 7 (14.00) |
|
|
| Distant
metastasis |
|
| 0.502 | 0.919 |
|
M0 | 47 (94.00) | 48 (96.00) |
|
|
|
M1 | 3 (6.00) | 2 (4.00) |
|
|
Patients' VAS pain condition and BCS
comfort scores
The VAS scores of patients were observed and
recorded at 2, 6, 12, 24 and 48 h after operation. The VAS scores
of patients were higher in group A than those in group B at each
time point (P<0.05). Patients' comfort degree (BCS scores) was
lower in group A than that in group B at each time point
(P<0.05)(Table II).
 | Table II.Comparison of VAS pain scores and BCS
comfort scores between the two groups of patients at 2, 6, 12, 24
and 48 h (mean ± SD, scores). |
Table II.
Comparison of VAS pain scores and BCS
comfort scores between the two groups of patients at 2, 6, 12, 24
and 48 h (mean ± SD, scores).
|
| VAS scores |
|
| BCS scores |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Time (h) | Group A | Group B | t value | P-value | Group A | Group B | t value | P-value |
|---|
| 2 | 5.08±0.75 | 3.02±0.25 | 18.430 | <0.001 |
1.52±0.12a | 2.85±0.12 | 55.420 | <0.001 |
| 6 |
4.51±0.54a | 3.05±0.15 | 18.420 | <0.001 |
1.66±0.08a | 3.05±0.16 | 54.940 | <0.001 |
| 12 |
3.95±0.45a | 2.68±0.13 | 19.170 | <0.001 |
1.85±0.08a | 2.95±0.23 | 31.940 | <0.001 |
| 24 |
3.71±0.24a | 2.45±0.14 | 31.090 | <0.001 |
1.95±0.13a | 2.98±0.45 | 15.500 | <0.001 |
| 48 |
3.61±0.34a | 2.45±0.14 | 22.310 | <0.001 |
1.86±0.16a | 2.96±0.45 | 16.290 | <0.001 |
| F value | 77.110 | 153.200 |
|
| 108.900 | 2.596 |
|
|
| P-value | <0.001 | <0.001 |
|
| <0.001 | 0.037 |
|
|
PCIA press times and adverse reactions
of patients
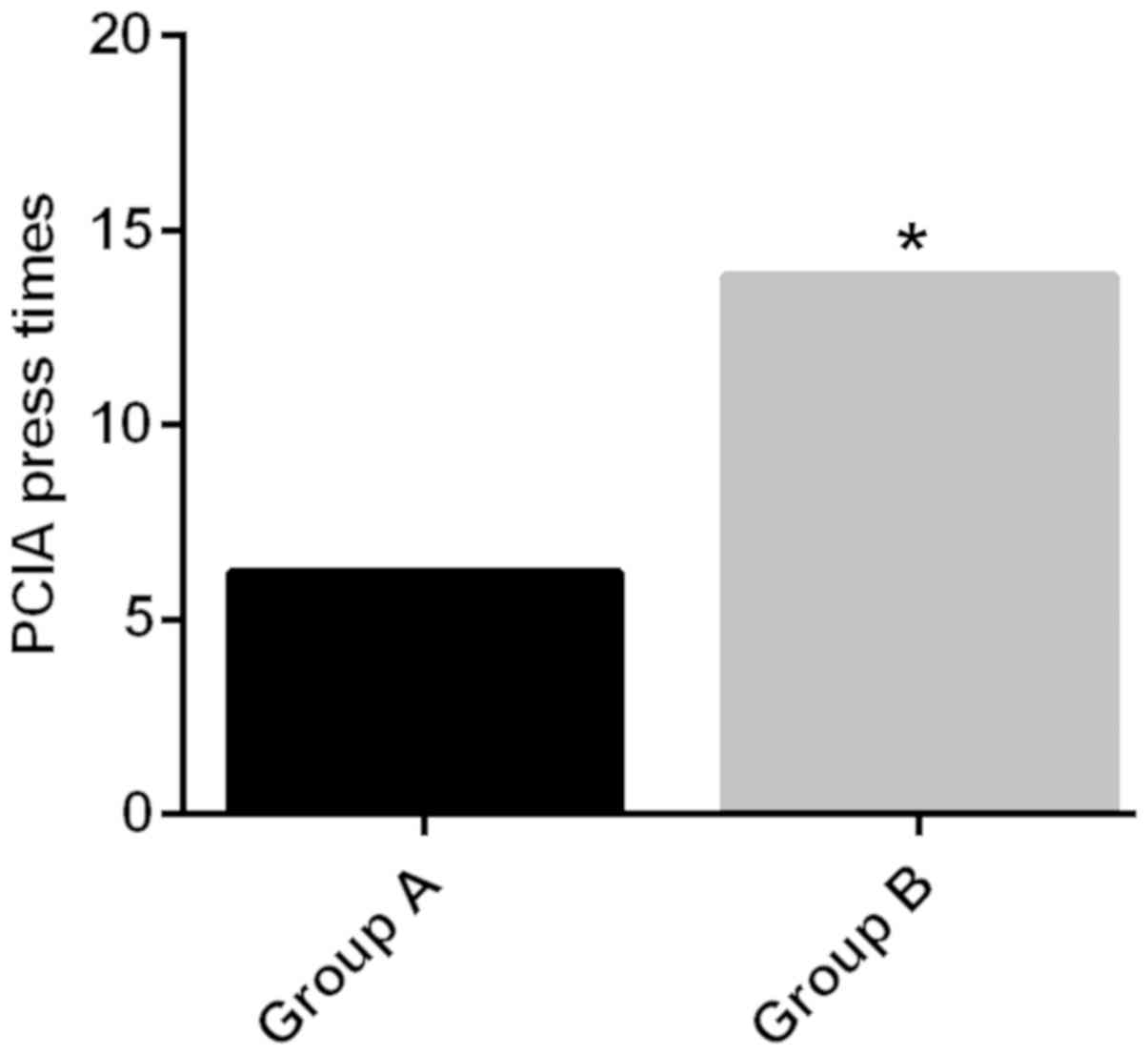
After observation and record, it was found that
within 20 h after operation, the effective PCIA press times in
group A (6.2±1.5 times) were lower than those in group B (13.8±2.5
times), and the difference was statistically significant
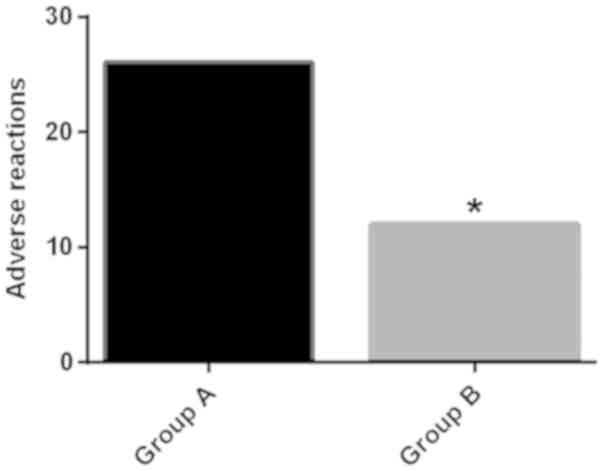
(P<0.05) (Fig. 1); the incidence
of adverse reactions such as nausea, vomiting and respiratory
depression (breathing <8 times/min) in group A of patients was
52.00%, higher than 24.00% of patients in group B (P<0.05). The
difference was not statistically significant in epigastric
discomfort (P>0.05) (Table III
and Fig. 2).
 | Table III.Comparison of adverse reactions
between the two groups [n (%)]. |
Table III.
Comparison of adverse reactions
between the two groups [n (%)].
| Items | Group A | Group B | χ2
value | P-value |
|---|
| n | 50 | 50 |
|
|
| Epigastric
discomfort | 15 (30.00) | 6
(12.00) | 0.012 | 0.911 |
| Nausea,
vomiting | 11 (22.00) | 3 (6.00) | 5.316 | 0.021 |
| Cough | 10 (20.00) | 2 (4.00) | 6.061 | 0.014 |
| Respiratory
depression | 17 (34.00) | 5
(10.00) | 8.392 | 0.004 |
| Drowsiness | 12 (24.00) | 4 (8.00) | 4.762 | 0.029 |
| Pruritus | 7
(14.00) | 1 (2.00) | 4.891 | 0.027 |
| Total | 26 (52.00) | 12 (24.00) | 8.319 | 0.004 |
Discussion
Radical gastrectomy is an open surgery that severely
damages the immune system. Under the influence of minimally
invasive techniques, both the surgical method and the wound area
have been optimized. However, it is still a hot topic how to
effectively relieve or eliminate acute pain of the patient caused
by surgical trauma and minimize the incidence of side effects.
Scientific statistics have proven that effective postoperative
analgesia can accelerate postoperative recovery (19). First, it can effectively improve
postoperative sleep quality of the patient. Moreover, it can reduce
postoperative pain and encourage cough and expectoration. Finally,
the complications caused by surgical trauma have also been
improved. Rose and Kam (20) found
that postoperative complications are mainly caused by the
inhibition of the immune system, its mechanism of action is
generally that the pituitary is excessively activated caused by the
stimulation of postoperative excessive pain, thus releasing a large
number of hormones that inhibit the immune system. In this study,
remifentanil combined with nalbuphine was used for the
postoperative stabilization of radical gastrectomy, and the effect
of its dose on clinical analgesia was investigated.
Compared with conventional analgesic methods, PCIA
can be administered by patients themselves to meet individual
analgesic needs, and the titration of doses is more accurate,
avoiding obvious fluctuations in blood drug concentration, thus
achieving the greatest analgesic effect in the shortest time
(21). The convenient administration
of PCIA also makes the dosage individualized, and greatly reduces
the workload of medical staff (22).
Epidural anesthesia cannot ensure the anesthesia effect of patients
because the dosage cannot be completely individualized. In the
occurrence of certain emergencies such as insufficient depth of
anesthesia, it is passive for the patient's anesthesia treatment,
and when the anesthesia level is lower, it has a greater impact on
blood pressure and other hemodynamic factors (23,24).
Nerve block is also a common analgesic method, but the technical
requirements for the operator are higher, the cost of anesthesia is
more expensive, and when the operator is not experienced enough, a
small error can cause nerve stimulation symptoms, even serious
complications (25). As a result, in
this study, we used PCIA to relieve postoperative pain.
The dose in analgesic regime A was 0.2 mg/kg of
nalbuphine plus 0.2 µg/kg of remifentanil and in the analgesic
regime B it was 0.3 mg/kg of nalbuphine plus 0.1 µg/kg of
remifentanil. The results of the study were as follows: The VAS
scores in group A were higher than those in group B; the BCS scores
in group A were lower than those in group B, and the difference was
statistically significant (P<0.05), indicating that the
postoperative discomfort and pain value in group A were overall
higher than those in group B, that is, analgesic regime B was
better. At the same time, related research (7) has also shown that the analgesic effect,
drug efficacy duration and drug resistance to extensive surgical
trauma have been improved when remifentanil at a dose of 0.1
µg/(kg·min) is used, which is consistent with this study. From a
study on the pharmacological aspect of remifentanil (26), it was found that inhibitory G protein
and excitatory G protein were conjugated to this type of drug at
the time of analgesic effect, which increased body's sensitivity to
pain. Therefore, the high-content remifentanil in group A also
triggered more acute pain at the same time as high-efficiency
analgesia, resulting in the comfort in group A being lower than
that in group B, and the pain higher than that in group B.
This study also found that postoperative PCIA press
times in group A of patients (6.2±1.5 times) were lower than those
in group B (13.8±2.5 times) (P<0.05), and the incidence of
adverse reactions such as nausea, vomiting and respiratory
depression (breathing <8 times/min) in group A of patients was
52.00%, higher than 24.00% in group B (P<0.05). It further
verifies this conclusion, indicating that high-content remifentanil
has extended its drug resistance time, but it also increases the
incidence frequency of adverse reactions. Compared to other studies
of remifentanil combined with non-nalbuphine (27–29),
under the same conditions as recording patient postoperative
analgesia and the same setting of analgesic pump at the same time
point, in this study, group B had lower VAS, higher BCS, and
superior analgesic effect. It was proposed to be due to the optimal
addition of remifentanil combined with nalbuphine in pharmacology
(30). In terms of receptors,
nalbuphine belongs to the κ-receptor but remifentanil belongs to
the µ-receptor, and different receptors reduce receptor
competition. In addition, nalbuphine contains antagonistic part of
the µ-receptor, the excitement of which will cause adverse
reactions, and the addition of remifentanil promotes the
elimination of analgesic drugs. Therefore, the combination of
remifentanil and nalbuphine optimizes the analgesic and
anti-adverse reaction effects.
Due to limitations such as small experimental sample
size and limited experimental conditions, this study can only
preliminarily determine that the analgesic scheme of 0.3 mg/kg of
nalbuphine plus 0.1 of µg/kg remifentanil is safer and more
effective than that of 0.2 mg/kg of nalbuphine plus 0.2 µg/kg of
remifentanil.
The intravenous analgesia scheme of 0.1 µg/kg of
remifentanil plus 0.3 mg/kg of nalbuphine were superior to other
schemes in analgesic effect, comfort and times of pressing, which
reduces the incidence of adverse reactions. Low dose remifentanil
combined with nalbuphine may have a higher security, and is worth
further exploring.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ conceived and designed the study. RZ collected
and analyzed the data. YZ and RZ performed the experiments. ND was
responsible for analgesia and follow-up. YZ, RZ and ND wrote the
manuscript and revised it critically. All the authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Τhis study was approved by the Ethics Committee of
the Xiangyang No. 1 People's Hospital (Xiangyang, China). Patients
who participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R,
Dandona L, et al: Global, regional, and national cancer incidence,
mortality, years of life lost, years lived with disability, and
disability-adjusted life-years for 32 cancer groups, 1990 to 2015:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 3:524–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao H, Xiao Y, Quan H, Liu W, Pan S and
Ouyang Y: Intra-abdominal infection after radical gastrectomy for
gastric cancer: Incidence, pathogens, risk factors and outcomes.
Int J Surg. 48:195–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bencivenga M, Verlato G, Han DS, Marrelli
D, Roviello F, Yang HK and de Manzoni G; Italian Research Group for
Gastric Cancer (GIRCG), : Validation of two prognostic models for
recurrence and survival after radical gastrectomy for gastric
cancer. Br J Surg. 104:1235–1243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stessel B, Theunissen M, Marcus MA,
Joosten EA, van Kuijk SMJ, Fiddelers AAA, Peters ML, Hoofwijk DMN,
Buhre WFFA and Gramke HF: Prevalence and predictors of patient
nonadherence to pharmacological acute pain therapy at home after
day surgery: A prospective cohort study. Pain Pract. 18:194–204.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daccache G, Caspersen E, Pegoix M,
Monthé-Sagan K, Berger L, Fletcher D and Hanouz JL: A targeted
remifentanil administration protocol based on the analgesia
nociception index during vascular surgery. Anaesth Crit Care Pain
Med. 36:229–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eagleman SL, Drover CM, Drover DR,
Ouellette NT and MacIver MB: Remifentanil and nitrous oxide
anesthesia produces a unique pattern of EEG activity during loss
and recovery of response. Front Hum Neurosci. 12:1732018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maguire DR and France C: Impact of
delta-9-tetrahydrocannabinol on the reinforcing effects of
remifentanil in rhesus monkeys responding under a food/drug choice
procedure. FASEB J. 31 (Suppl 1):lb5912017.
|
|
10
|
Eleveld DJ, Proost JH, Vereecke H, Absalom
AR, Olofsen E, Vuyk J and Struys MMRF: An allometric model of
remifentanil pharmacokinetics and pharmacodynamics. Anesthesiology.
126:1005–1018. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cornelissen JC, Obeng S, Rice KC, Zhang Y,
Negus SS and Banks ML: Application of receptor theory to the design
and use of fixed-proportion mu-opioid agonist and antagonist
mixtures in rhesus monkeys. J Pharmacol Exp Ther. 365:37–47. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Günther T, Dasgupta P, Mann A, Miess E,
Kliewer A, Fritzwanker S, Steinborn R and Schulz S: Targeting
multiple opioid receptors - improved analgesics with reduced side
effects. Br J Pharmacol. 175:2857–2868. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Apfelbaum JL, Horlocker TT, Agarkar M,
Connis RT, Hebl JR, Nickinovich DG, Palmer CM, Rathmell JP,
Rosenquist RW and Wu CL; American Society of Anesthesiologists
Committee on Standards, Practice Parameters, : Practice guidelines
for the prevention, detection, and management of respiratory
depression associated with neuraxialn opioid administration: An
updated report by the American Society of Anesthesiologists Task
Force on Neuraxial Opioids and the American Society of Regional
Anesthesia and Pain Medicine. Obstet Anesthes Dig. 37:112017.
View Article : Google Scholar
|
|
14
|
Cao X, Yumul R, Elvir Lazo OL, Friedman J,
Durra O, Zhang X and White PF: A novel visual facial anxiety scale
for assessing preoperative anxiety. PLoS One. 12:e01712332017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Price DD, McGrath PA, Rafii A and
Buckingham B: The validation of visual analogue scales as ratio
scale measures for chronic and experimental pain. Pain. 17:45–56.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao ZY, Jia Z, Xie YH, Zhang LL, Zhang HS,
Wu WQ, Zhang CK and Gan LJ: Analgesic effect of dezocine in
different doses on elderly patients undergoing abdominal operation
under general anesthesia and its influence on stress response to
postoperative tracheal extubation. Eur Rev Med Pharmacol Sci.
21:5223–5229. 2017.PubMed/NCBI
|
|
17
|
Cho JS, Lee MH, Kim SI, Park S, Park HS,
Oh E, Lee JH and Koo BN: The Effects of perioperative anesthesia
and analgesia on immune function in patients undergoing breast
cancer resection: A prospective randomized study. Int J Med Sci.
14:970–976. 2018. View Article : Google Scholar
|
|
18
|
Sun HL, Dong YC, Wang CQ, Qian YN and Wang
ZY: Effects of postoperative analgesia with the combination of
tramadol and lornoxicam on serum inflammatory cytokines in patients
with gastric cancer. Int J Clin Pharmacol Ther. 52:1023–1029. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McEvoy MD, Scott MJ, Gordon DB, Grant SA,
Thacker JKM, Wu CL, Gan TJ, Mythen MG, Shaw AD and Miller TE;
Perioperative Quality Initiative (POQI) I Workgroup, : American
Society for Enhanced Recovery (ASER) and Perioperative Quality
Initiative (POQI) joint consensus statement on optimal analgesia
within an enhanced recovery pathway for colorectal surgery: Part
1-from the preoperative period to PACU. Perioper Med (Lond).
6:82017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rose MA and Kam PC: Gabapentin:
Pharmacology and its use in pain management. Anaesthesia.
57:451–462. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Viscusi ER, Grond S, Ding L, Danesi H,
Jones JB and Sinatra RS: A comparison of opioid-related adverse
events with fentanyl iontophoretic transdermal system versus
morphine intravenous patient-controlled analgesia in acute
postoperative pain. Pain Manag. 6:19–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bijur PE, Mills AM, Chang AK, White D,
Restivo A, Persaud S, Schechter CB, Gallagher EJ and Birnbaum AJ:
Comparative effectiveness of patient-controlled analgesia for
treating acute pain in the emergency department. Ann Emerg Med.
70:809–818.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loscovich A, Briskin A, Fadeev A,
Grisaru-Granovsky S and Halpern S: Emergency cesarean section in a
patient with Fontan circulation using an indwelling epidural
catheter. J Clin Anesth. 18:631–634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamada K, Abe Y, Satoh S, Yanagibashi Y,
Hyakumachi T and Masuda T: Large increase in blood pressure after
extubation and high body mass index elevate the risk of spinal
epidural hematoma after spinal surgery. Spine. 40:1046–1052. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Britt T, Sturm R, Ricardi R and Labond V:
Comparative evaluation of continuous intercostal nerve block or
epidural analgesia on the rate of respiratory complications,
intensive care unit, and hospital stay following traumatic rib
fractures: A retrospective review. Local Reg Anesth. 8:79–84.
2015.PubMed/NCBI
|
|
26
|
Sprenger C, Eichler IC, Eichler L, Zöllner
C and Büchel C: Altered signaling in the descending pain-modulatory
system after short-term infusion of the µ-opioid agonist
remifentanil. J Neurosci. 38:2454–2470. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ovari A, Bicker I, Machmueller S, Schuldt
T, Sauer M, Soltesz S, Noeldge-Schomburg G, Mlynski R and Mencke T:
Sevoflurane at 1.0 MAC together with remifentanil and propofol
produces clinically acceptable intubation conditions at the vocal
cords: A prospective randomized study. J Int Med Res. 45:1098–1108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang HY, Ting CK, Liou JY, Chen KH, Tsou
MY and Chang WK: A previously published propofol-remifentanil
response surface model does not predict patient response well in
video-assisted thoracic surgery. Medicine (Baltimore).
96:e68952017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Hoogd S, Ahlers SJGM, van Dongen EP,
van de Garde EMW, Daeter EJ, Dahan A, Tibboel D and Knibbe CAJ:
Randomized controlled trial on the influence of intraoperative
remifentanil versus fentanyl on acute and chronic pain after
cardiac surgery. Pain Pract. 18:443–451. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trescot AM, Datta S, Lee M and Hansen H:
Opioid pharmacology. Pain Physician. 11:133–153. 2008.PubMed/NCBI
|