Introduction
Bone remodeling, an essential and strictly regulated
process for maintaining the mass and the strength of skeletal
tissue in adults, is known to be initiated by osteoclastic bone
resorption, followed by osteoblastic bone formation (1). In bone formation stage, the
Wnt/β-catenin signaling pathway, called the canonical Wnt signaling
pathway, plays a crucial role in the expression of target genes,
including Runx2, a pivotal element of bone formation (2). Wnts form a complex by binding to
Frizzled receptors and low-density lipoprotein receptor-related
protein 5 or 6 (LRP5/6), which leads to inactivation via the
phosphorylation of glycogen synthase kinase-3β (GSK-3β), resulting
in the stabilization of β-catenin and promoting its translocation
into the nucleus (3). Nuclear
β-catenin up-regulates the transcription of target genes, including
Runx2 (2). It
Constitutively-activated β-catenin is known to potently induce the
up-regulation of Runx2, resulting in osteogenesis (2). Thus, the canonical Wnt signaling
pathway is an essential positive regulator of osteogenesis in
mammals.
Regarding osteoblast functions, we previously
reported that Wnt3a upregulates the synthesis of vascular
endothelial growth factor stimulated by fibroblast growth factor-2
(FGF-2), transforming growth factor-β (TGF-β) and prostaglandin
F2α (PGF2α) but downregulates the
interleukin-6 (IL-6) synthesis stimulated by tumor necrosis
factor-α (TNF-α) in osteoblast-like MC3T3-E1
cells (4–7).
Osteocalcin is a highly differentiated osteoblast
secretary phenotype and the most abundant non-collagenous protein
(8). Osteocalcin is
post-translationally modified by vitamin K-dependent
γ-carboxylation, which is known to be a bone Gla-protein (8). An increase in bone formation is
reportedly observed in osteocalcin-deficient mice without impaired
bone resorption (9). Thus,
osteoblast-producing osteocalcin is considered to play a pivotal
role in the regulation of bone formation. In addition,
uncarboxylated osteocalcin reportedly regulates energy metabolism
by stimulating the secretion of insulin from β cells of pancreatic
islets and up-regulating the insulin sensitivity of target organs
(10). Thus, bone may function as an
endocrine organ through osteocalcin release.
Thyroid hormone plays an essential role as a
regulator of bone metabolism as well as whole-body metabolism. An
excess of thyroid hormone, namely hyperthyroidism, is well known to
boost the bone metabolic turnover and increase the ratio of bone
resorption to bone formation, leading to osteoporosis and
subsequently increased risk of bone fracture (11). The bone mineral density actually
decreases in patients with hyperthyroidism (12). Thyroid hormone receptor is a member
of the nuclear receptor superfamily (13), and its actions are chiefly mediated
through its binding to specific nuclear receptors, with the complex
of receptor and hormone complex activating the transcription of
corresponding genes (14). In
osteoblasts, thyroid hormone induces alkaline phosphatase and
modulates cell proliferation (15).
We previously showed that triiodothyronine (T3) elicits
the expression of osteocalcin via p38 mitogen-activated protein
(MAP) kinase at least in part, and the adenylyl cyclase-cAMP system
regulates the osteocalcin expression via the suppression of p38 MAP
kinase activation in osteoblast-like MC3T3-E1 cells (16,17).
However, the exact mechanism behind thyroid hormone-induced
expression of osteocalcin including a role of Wnt signaling remains
to be elucidated.
In the present study, we investigated the effect of
Wnt3a on the T3-stimulated osteocalcin expression in
osteoblast-like MC3T3-E1 cells.
Materials and methods
Materials
T3 and SB216763 were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Wnt3a was purchased from
R&D Systems, Inc. (Minneapolis, MN, USA). The mouse osteocalcin
enzyme-linked immunosorbent assay (ELISA) kit was purchased from
Alfa Aesar, Thermo Fisher Scientific (Heysham, Lancashire, UK).
Antibodies against phospho-specific β-catenin, β-catenin,
phospho-specific p38 MAP kinase and p38 MAP kinase were purchased
from Cell Signaling Technology, Inc., (Biverly, MA, USA).
Antibodies against GAPDH were purchased from Santa Cruz
Biotechnology, Inc., (Santa Cruz, CA, USA). Other materials and
chemicals were purchased from commercial sources. T3 was
dissolved in 0.1 M NaOH, and SB216763 was dissolved in DMSO. The
maximum concentration of NaOH or SB216763 was 10 µM or 0.1%,
respectively, which did not affect the assay for osteocalcin,
reverse transcription-polymerase chain reaction (RT-PCR) or western
blotting.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells derived from
newborn mouse calvaria (18) were
maintained as previously described (19). In brief, the cells were cultured in
α-minimum essential medium (α-MEM) containing
10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere of
5% CO2/95% air. The cells were seeded into 35-mm
diameter dishes (5×104 cells/dish) or 90-mm diameter
dishes (2×105 cells/dish) in α-MEM containing 10% FBS.
After 5 days, the medium was exchanged for α-MEM
containing 0.3% FBS. The cells were used for experiments following
a 48 h incubation period at 37°C.
Assay for osteocalcin
The cultured cells were stimulated by 10 nM of
T3 or vehicle in 1 ml of α-MEM containing
0.3% FBS for the indicated periods. When indicated, the cells were
pretreated with various dose of Wnt3a or SB216763 for 60 min. The
conditioned medium was collected at the end of incubation, and the
osteocalcin concentration in the medium was then measured using the
mouse osteocalcin ELISA kit according to the manufacturer's
protocol.
RT-quantitative PCR (RT-qPCR)
The cultured cells were pretreated with 30 ng/ml of
Wnt3a, 10 µM of SB216763 or vehicle for 60 min, and then stimulated
by 10 nM of T3 or vehicle in α-MEM containing
0.3% FBS for 48 h. Total RNA was isolated and reverse transcribed
into complementary DNA using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and Omniscript Reverse Transcriptase kit
(Qiagen Inc., Valencia, CA, USA), respectively. RT-qPCR was
performed in capillaries using a LightCycler system with the
LightCycler FastStart DNA Master SYBR Green I (Roche
Diagnostics, Basel, Switzerland). Sense and antisense primers were
synthesized based on the reports of Zhang et al (20) for mouse osteocalcin and Simpson et
al for mouse GAPDH (21). The
amplified products were determined using a melting curve analysis
according to the system protocol. The Bglap2 mRNA levels were
normalized to those of GAPDH mRNA.
Luciferase reporter assay
A reporter plasmid, pDR4 (thyroid hormone
response element)-Luc was purchased from Stratagene (La Jolla,
CA, USA). The cultured cells were pretreated with 30 ng/ml of
Wnt3a, 10 µM of SB216763 or vehicle at 6 h after transfection with
the pDR4-Luc reporter plasmid (1 µg/dish) using UniFector
transfection reagent (B-Bridge International, Mountain View, CA,
USA). After pretreatment (60 min) with Wnt3a, the cells were
stimulated by 10 nM of T3 or vehicle for 48 h. Samples
were lysed using passive lysis buffer (Promega Corp., Madison, WI,
USA) and collected with a cell scraper. The luciferase activity of
the cell lysates was measured using a dual luciferase reporter
assay system (Promega Corp., Madison, WI, USA) according to the
manufacture's protocol. The cells were cotransfected with pRL-CMV
(Renilla luciferase; 0.1 µg/dish) as an internal standard to
normalize transfection efficiency.
Western blot analysis
Western blotting was performed as previously
described (22). In brief, the
cultured cells were treated with various doses of SB216763 for 120
min or pretreated with various doses of Wnt3a for 60 min. The
Wnt3a-pretreated cells were then stimulated by 10 nM of
T3 in α-MEM containing 0.3% FBS for 2 h. The
cells were washed twice with phosphate-buffered saline and then
lysed, homogenized and sonicated in a lysis buffer containing 62.5
mM Tris-HCL, pH6.8, 3% sodium dodecyl sulfate (SDS), 50 mM
dithiothreitol, and 10% glycerol. SDS-polyacrylamide gel
electrophoresis (PAGE) was performed according to Laemmli (23) on 10% polyacrylamide gel. The protein
was fractionated and transferred onto an Immun-Blot PVDF membrane
(Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5%
fat-free dry milk in Tris-buffered saline-Tween (TBS-T; 20 mM
Tris-HCl, pH 7.6, 137 mM NaCl, 0.1% Tween 20) for 1 h before
incubation with primary antibodies. Phospho-specific β-catenin
antibodies, β-catenin antibodies, phospho-specific p38 MAP kinase
antibodies, p38 MAP kinase antibodies and GAPDH antibodies were
used as primary antibodies, and peroxidase-labeled antibodies
raised in goat against rabbit IgG (KPL, Inc., Gaitherburg, MD, USA)
were used as secondary antibodies. The primary and secondary
antibodies were diluted at 1:1,000 with 5% fat-free dry milk in
TBS-T. The peroxidase activity on the PVDF sheet was visualized on
X-ray film by an ECL Western blotting detection system.
Statistical analysis
The data were analyzed by an analysis of variance
followed by Bonferroni's method for multiple comparisons between
pairs, and P<0.05 was considered to indicate a statistically
significant difference. All data are presented as the mean ±
standard error of the mean of triplicate determinations from three
independent cell preparations.
Results
Effects of Wnt3a and SB216763 on the
T3-stimulated osteocalcin release in MC3T3-E1 cells
In our previous study (16), we showed that
T3-stimulated osteocalcin release could be detected
after 48 to 96 h of stimulation in osteoblast-like MC3T3-E1 cells.
We therefore first examined the effect of Wnt3a on the release of
osteocalcin stimulated by T3 in these cells. Regarding
the working concentrations of Wnt3a, we previously reported that
Wnt3a at a dose of 10 to 30 ng/ml caused a remarkable amplification
in the VEGF synthesis induced by FGF-2, TGF-β and PGF2α
in osteoblast-like MC3T3-E1 cells (4–6).
Wnt3a by itself did not affect the basal levels of
osteocalcin, but significantly reduced the release of osteocalcin
stimulated by T3 (Fig.
1). The suppressive effect of Wnt3a on the
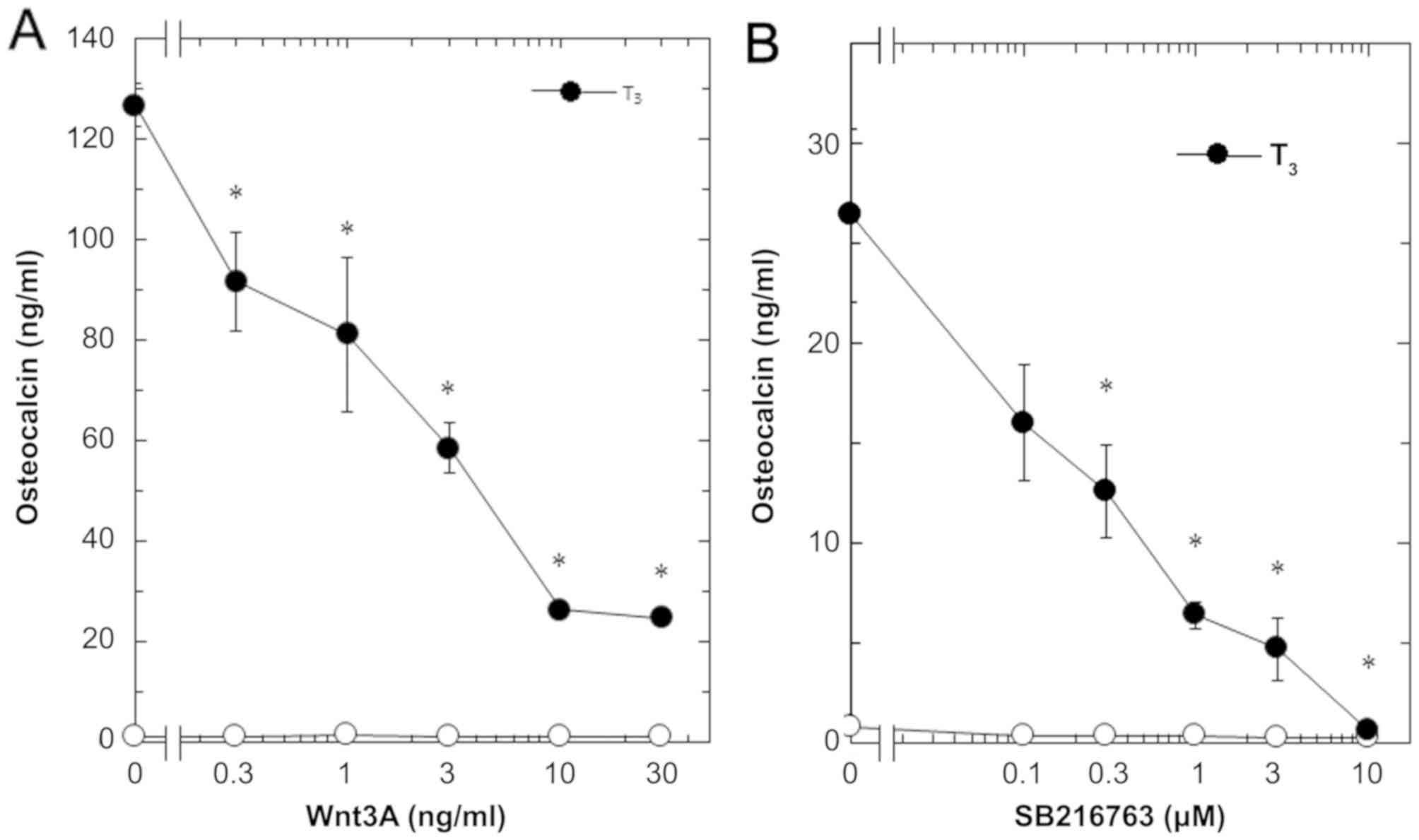
T3-stimulated osteocalcin release was dose-dependent in
between 0.3 and 30 ng/ml (Fig. 2A).
The maximum inhibitory effect of Wnt3a observed at 30 ng/ml was
approximately 80% in the T3-effect of osteocalcin
release in these cells. The effects of Wnt3a at doses of 10 and 30
ng/ml on the T3-induced release of osteocalcin were
similar. It is unlikely that we would have been able to obtain a
further inhibitory effect of Wnt3a at a dose higher than 30
ng/ml.
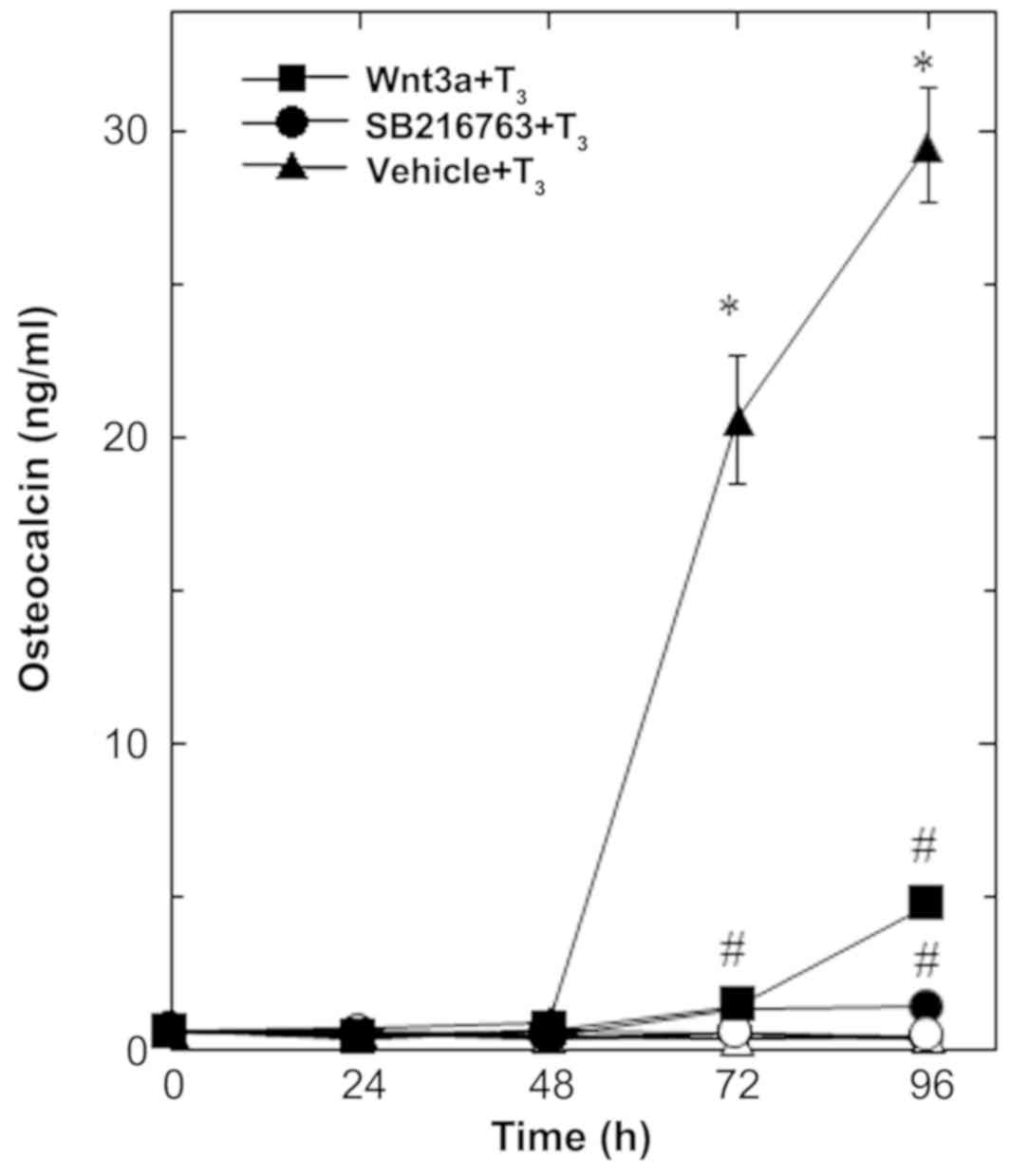 | Figure 1.Effects of Wnt3a on the
T3-stimulated osteocalcin release in MC3T3-E1 cells. The
cultured cells were pretreated with 30 ng/ml of Wnt3a (■,□), 10 µM
of SB216763 (•, ◦) or vehicle (▲,∆) for 60 min, and subsequently
stimulated with 10 nM of T3 (■,•,▲) or vehicle (□,◦,∆)
for the indicated periods. Osteocalcin concentrations in the
culture medium were determined by an ELISA. Each value represents
the mean ± SEM of triplicate determinations from three independent
cell preparations. Each line binding the closed boxes (■) and the
closed circles (•) are the experimental groups indicating Wnt3a or
SB216763, respectively, relative to the control line (T3
alone) binding the closed triangles (▲) *P<0.05 vs. the value of
the control; #P<0.05 vs. the value of T3
alone. T3, triiodothyronine. |
Wnt3a stimulation has been established to induce the
inactivation of GSK-3β, resulting in the stabilization of β-catenin
and the promotion of its translocation into the nucleus, called the
canonical Wnt pathway (3). To
elucidate the effect of GSK-3β inhibition, we examined the effect
of SB216763, a selective inhibitor of GSK-3β (24), on the T3-stimulated
osteocalcin release in osteoblast-like MC3T3-E1 cells. As for the
working concentrations of SB216763, we previously reported that
SB216763 at a dose of 10 µM remarkably enhanced the VEGF synthesis
stimulated by FGF-2, TGF-β and PGF2α in these cells
(4–6). SB216763, which by itself hardly
affected the release of osteocalcin, significantly inhibited the
T3-stimulated release of osteocalcin as well as Wnt
(Fig. 1). The inhibitory effect of
SB216763 was dose-dependent between 0.1 µM and 10 µM (Fig. 2B). SB216763 at 10 µM almost
completely inhibited the release of osteocalcin stimulated by
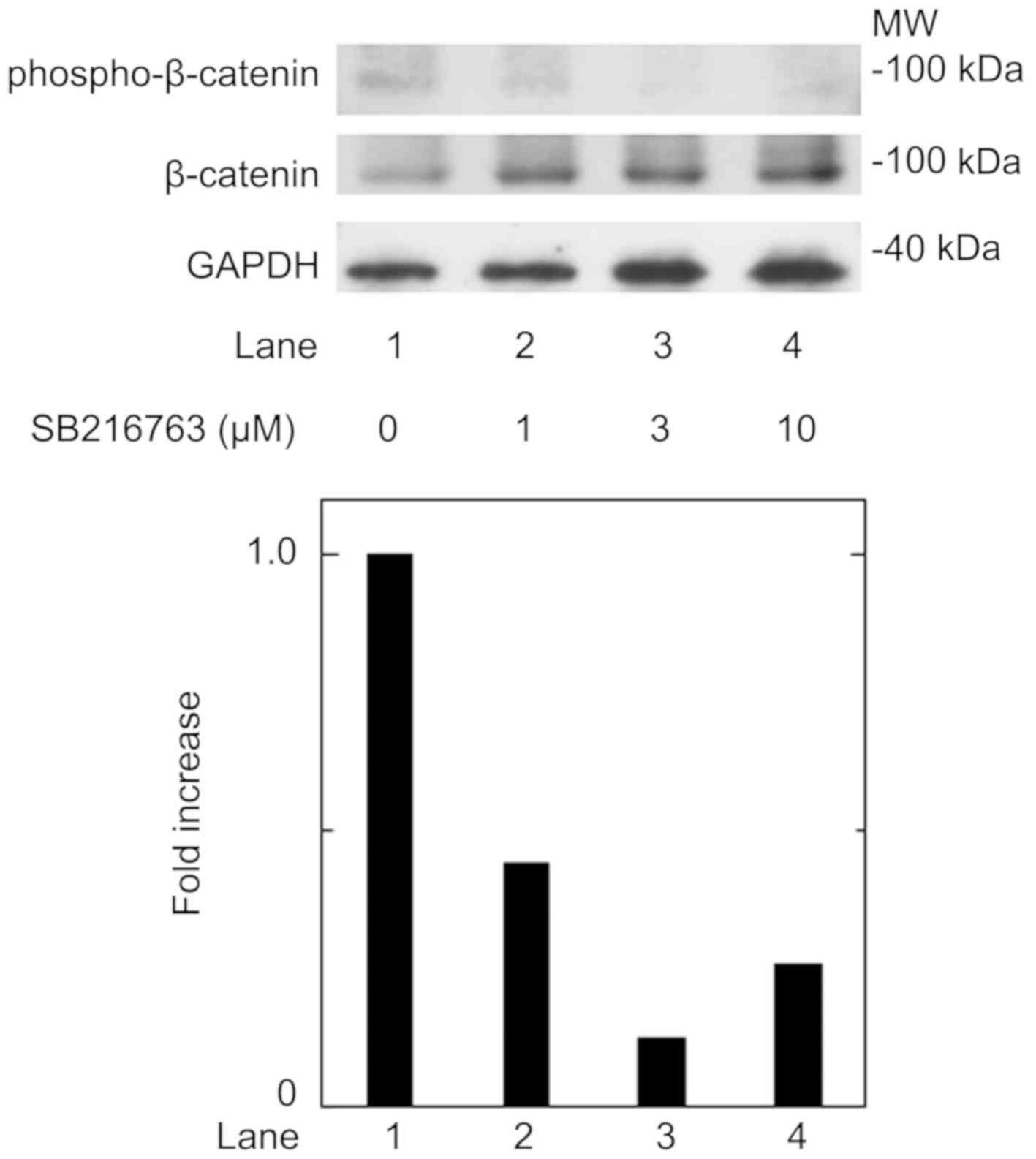
T3 in these cells. We confirmed that SB216763 truly
reduced the phosphorylation of β-catenin, the functional substrate
of GSK-3β (3), in a dose-dependent
manner between 1 and 10 µM (Fig. 3).
We also confirmed that neither Wnt3a nor SB216763 affected the cell
numbers of MC3T3-E1 cells with or without T3 up to 96 h
(data not shown).
Effects of Wnt3a and SB216763 on the
T3-induced expression of Bglap2 mRNA in MC3T3-E1
cells
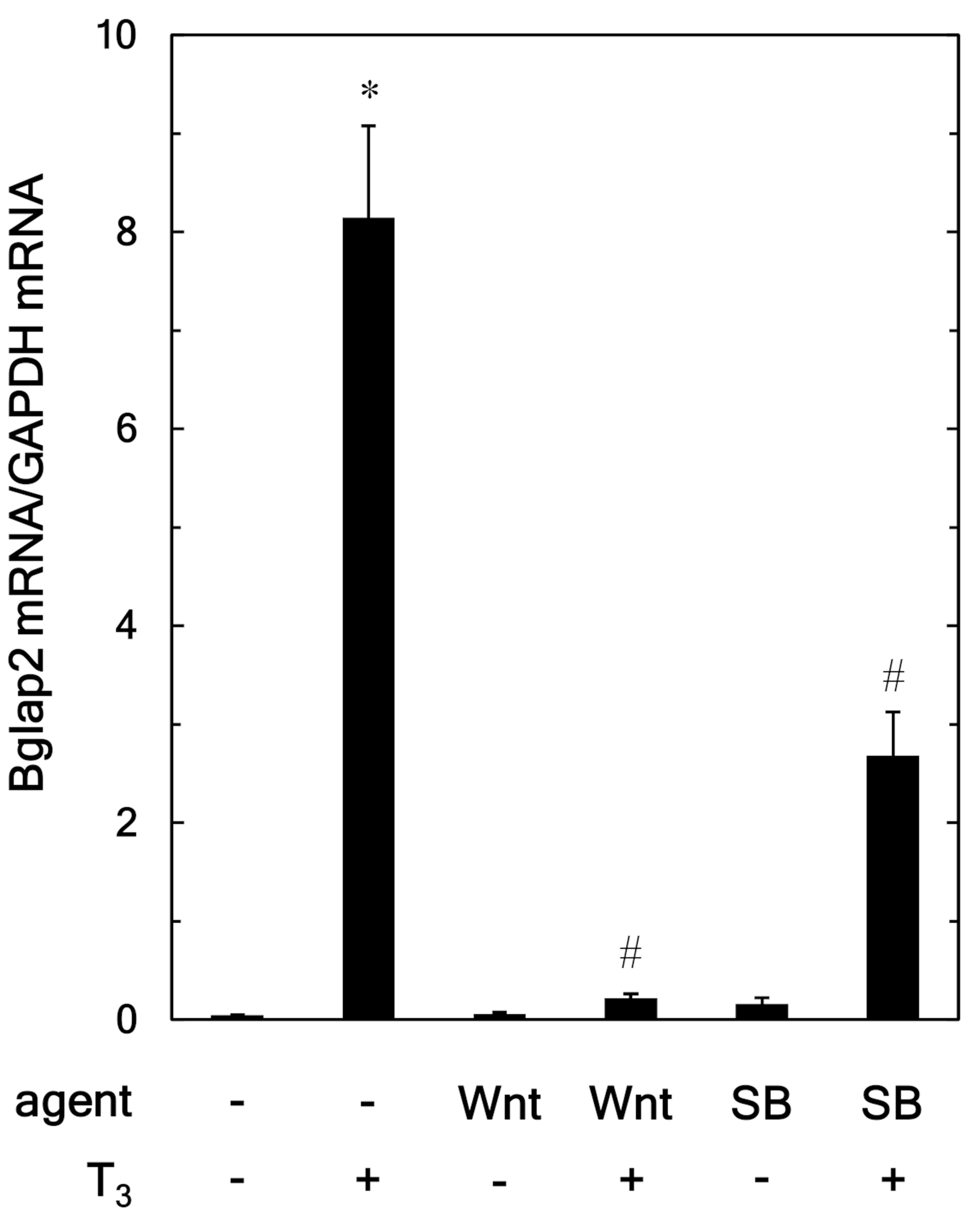
To elucidate whether the suppressive effect of Wnt3a
on the osteocalcin release stimulated by T3 was elicited
through transcriptional events, we examined the effect of Wnt3a on
the Bglap2 mRNA expression induced by T3, using RT-qPCR.
Wnt3A significantly attenuated the expression of Bglap2 mRNA
induced by T3, whereas, Wnt3a by itself had little
effect on the expression levels (Fig.
4). SB216763, which alone had little effect on the levels of
Bglap2 mRNA, also suppressed the T3-stimulated
upregulation of Bglap2 mRNA expression (Fig. 4).
Effects of Wnt3a and SB216763 on the
T3-stimulated transactivation activity of the thyroid
hormone-responsive element in MC3T3-E1 cells
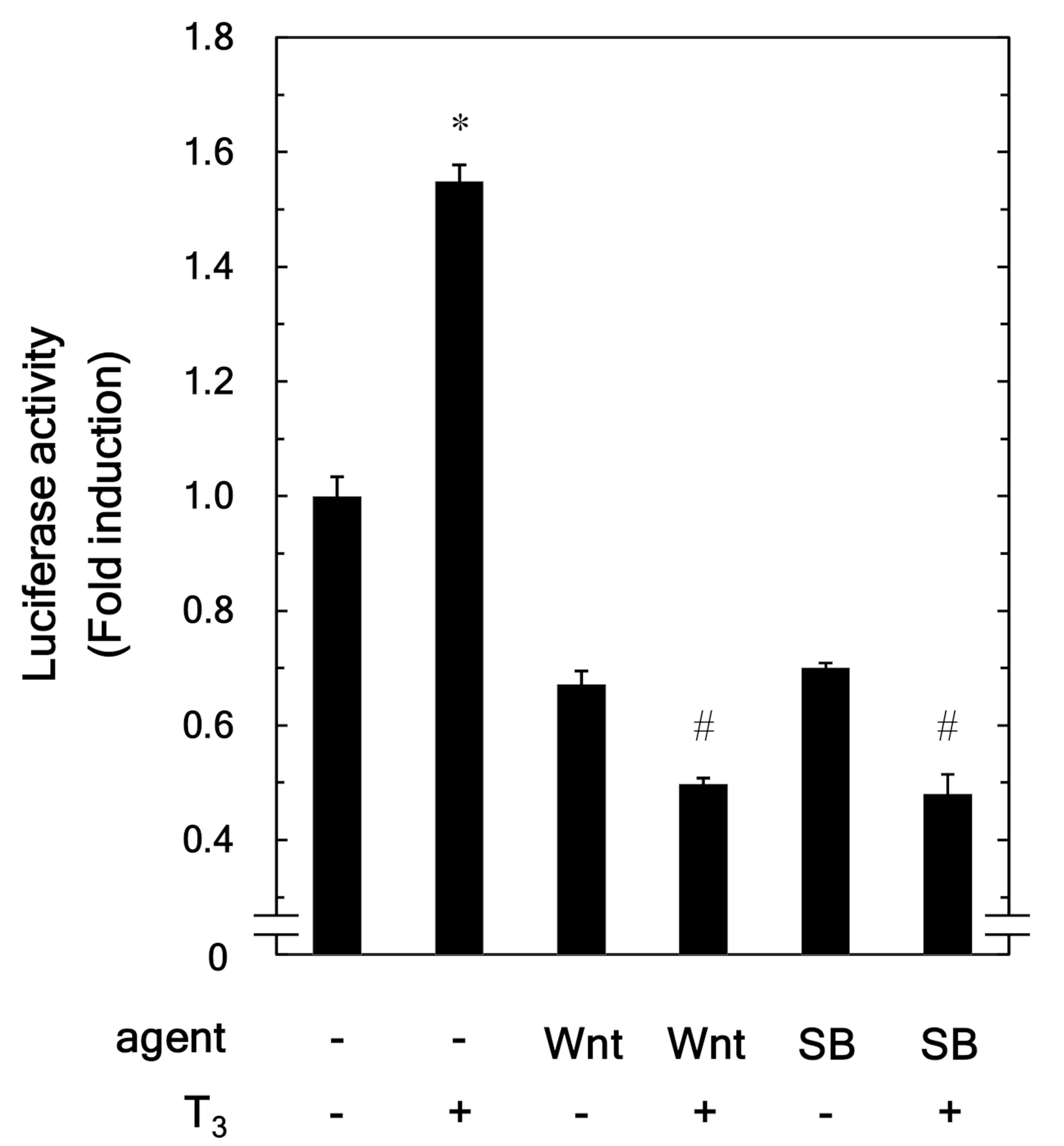
Using a luciferase reporter assay, we further
examined the effect of Wnt3a on the T3-stimulated
transactivation of thyroid hormone response element in
osteoblast-like MC3T3-E1 cells. Wnt3a significantly reduced the
T3-upregulated transactivation activity (Fig. 5). In addition, SB216763 markedly
suppressed the transactivation activity stimulated by T3
(Fig. 5).
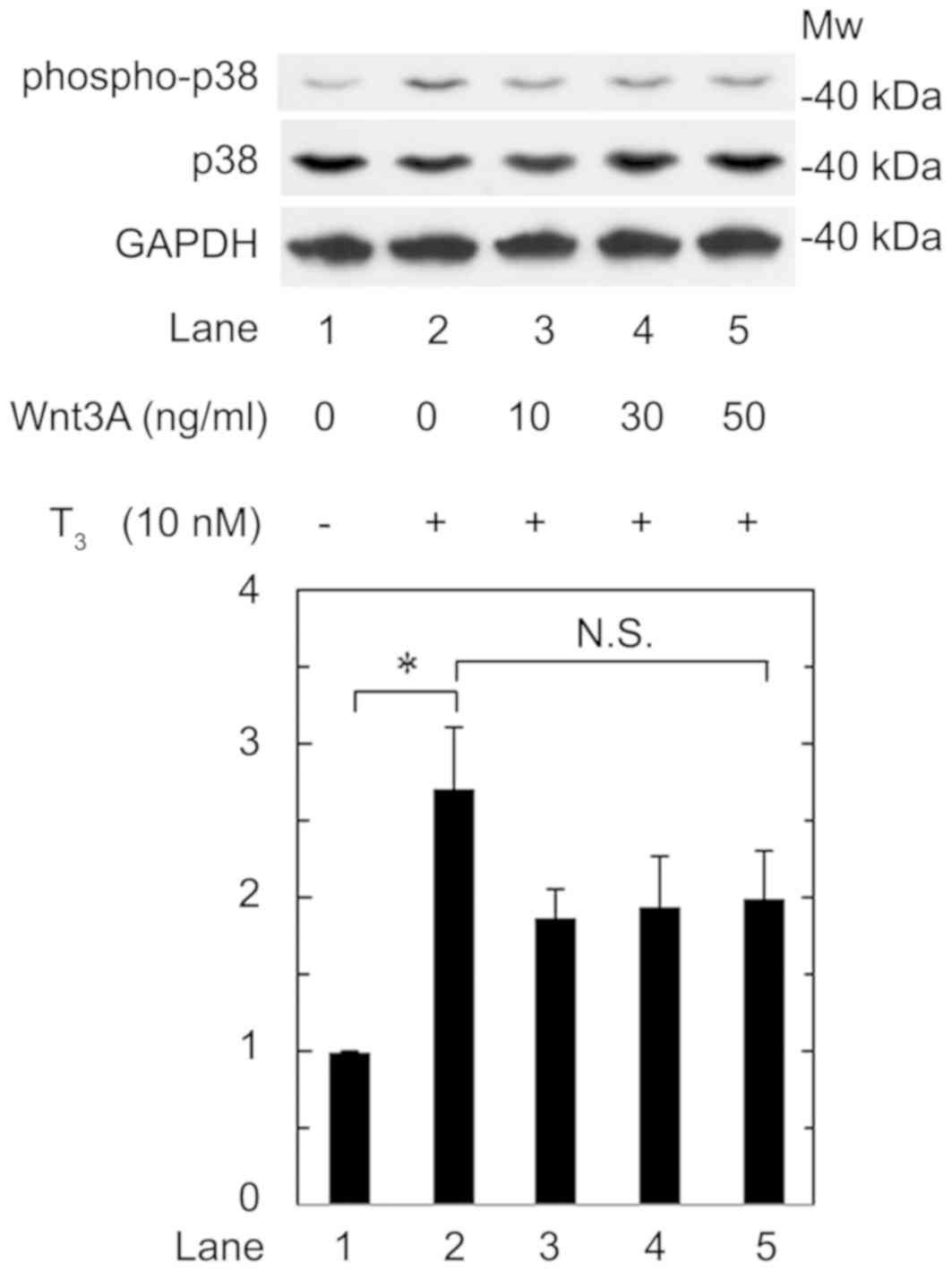
Effect of Wnt3a on the
T3-stimulated phosphorylation of p38 MAP kinase in
MC3T3-E1 cells
We previously reported that p38 MAP kinase is
involved in the T3-stiumulated osteocalcin expression in
osteoblast-like MC3T3-E1 cells (16). Therefore, we further investigated the
effect of Wnt3a on the phosphorylation of p38 MAP kinase stimulated
by T3 in these cells. We confirmed that T3
significantly upregulated p38 MAP kinase phosphorylation (Fig. 6, lane 1 vs. lane 2). However, Wnt3a
hardly affected the phosphorylation at a dose up to 50 ng/ml
(Fig. 6, lane 2 vs. lane 5). Wnt3a
at a dose up to 50 ng/ml had little effect on the phosphorylation
of p38 MAP kinase induced by T3. Wnt3a likely had little
effect on the phosphorylation at a dose up to 50 ng/ml, which was
sufficient to suppress the release of osteocalcin induced by
T3.
Discussion
In the present study, we clearly demonstrated that
Wnt3a reduced the osteocalcin release stimulated by T3
in osteoblast-like MC3T3-E1 cells. We also showed that Wnt3a
suppressed the Bglap2 mRNA expression induced by T3 in
these cells. These results suggest that Wnt3a suppressed the gene
transcription of osteocalcin stimulated by T3 or
potentiate the degradation of Bglap2 mRNA in osteoblasts.
Thyroid hormone is established to belong nuclear
receptor superfamily, and its biological functions are mediated
through the activation of the transcription of target genes,
including osteocalcin, via the binding of hormone and specific
nuclear receptor (13). Thus, using
a luciferase reporter assay, we examined the effect of Wnt3a on the
transactivation activity of thyroid hormone response element
stimulated by T3 and found that Wnt3a truly reduced the
T3-stimulated transactivation activity in these cells.
Therefore, it is probable that Wnt3a downregulates the osteocalcin
expression stimulated by T3, and the effect is exerted
through gene transcription in osteoblast-like MC3T3-E1 cells. To
our knowledge, this is the first report to clearly demonstrate the
suppressive effect of Wnt3a on the osteocalcin expression in
osteoblast-like cells.
Wnts is well known to comprise a complex of Fizzled
receptors and LRP5/6, resulting in the inactivation of GSK-3β and
the accumulation of β-catenin, which translocates into the nucleus
and upregulates the transcription of target genes, in a process
known as the Wnt canonical pathway (2,3). To
clarify the suppressive effect of Wnt3a on the osteocalcin
expression stimulated by T3, we examined the effects of
SB216763, a selective inhibitor of GSK-3β (20), on the osteocalcin release and the
Bglap2 mRNA expression stimulated by T3 in
osteoblast-like MC3T3-E1 cells. We found that SB216763 actually
mimicked the suppressive effects of Wnt3a on both the osteocalcin
release and the Bglap2 mRNA expression levels stimulated by
T3 in these cells. We confirmed that SB216763 truly
reduced the phosphorylation of β-catenin. It is most likely that
the effects of SB216763 presented here resulted from the inhibition
of GSK-3β activity in these cells. SB216763 appears to exert a
small inhibitory effect on the up-regulation of Bglap2
(osteocalcin) mRNA expression induced by T3 stimulation
compared with Wnt3a, while suppressing the T3-stimulated
transactivation activity of thyroid hormone response element,
similar to Wnt3a. The mRNA degradation might be upregulated in
Wnt3a-treated cells.
In addition, we confirmed that SB216763 as well as
Wnt3a truly inhibited the T3-stimulated transactivation
activity of the thyroid hormone response element in osteoblast-like
MC3T3-E1 cells. It is likely that the inactivation of GSK-3β and,
in turn, the activation of the Wnt canonical pathway caused the
downregulation of the osteocalcin expression stimulated by
T3 in these cells. Therefore, it is most likely that
Wnt3a downregulates the T3-induced osteocalcin
expression through the canonical pathway in osteoblast-like cells.
Treatment of Wnt3a or SB216763 alone evidently reduced the
transactivation activity of the thyroid hormone response element.
It is likely that the upregulation of β-catenin resulting from the
inhibition of GSK-3β causes deterioration of the transactivation
activity of the thyroid hormone response element without
T3 in osteoblast-like MC3T3-E1 cells.
Regarding the signaling mechanism of T3
in osteoblasts, we previously showed that p38 MAP kinase is
involved, at least in part, in the T3-stimulated
osteocalcin expression in osteoblast-like MC3T3-E1 cells (16). We therefore additionally examined the
effect of Wnt3a on the phosphorylation of p38 MAP kinase induced by
T3; however, this was hardly affected by Wnt3a in these
cells. It is unlikely that the suppressive effect of Wnt3a on the
osteocalcin expression stimulated by T3 is mediated
through the regulation of p38 MAP kinase in osteoblasts. Taking our
present findings into account as a whole, it is most likely that
Wnt3a downregulates the T3-induced osteocalcin
expression through the canonical pathway without affecting p38 MAP
kinase in osteoblast-like cells.
The Wnt/β-catenin signaling pathway is known to play
a role in osteogenesis (2). We
previously reported that Wnt3a upregulates the syntheses of
vascular endothelial growth factor stimulated by FGF-2, TGF-β and
PGF2α, but downregulates the IL-6 synthesis stimulated
by TNF-α in osteoblast-like MC3T3-E1 cells (4–7). These
findings prompted us to speculate that Wnt3a exerts pleiotropic
effects on the osteoblastic functions, leading to the promotion of
osteogenesis. However, it is widely accepted that osteocalcin, a
mature osteoblast phenotype, is specifically produced by these
cells and placed in the bone matrix during the process of bone
formation (3). The Gla residues in
osteocalcin are essential for its function, and Gla-carboxylated
osteocalcin becomes attached to hydroxyapatite with a high affinity
and thereby supports calcification (3). In addition, the bone mass and strengty
in osteocalcin-deficient mice have been reported to be more highly
expressed than in wild-type mice, namely, osteocalcin has been
reported to play a regulatory role in bone formation (4). T3 definitely plays a pivotal
role in physiological bone metabolism. Therefore, our present
findings showing the downregulation by Wnt3a of
T3-induced osteocalcin in osteoblast-like cells may
suggest that Wnt3a promotes osteogenesis through relief of the
regulation of bone formation by osteocalcin, at least in part.
These findings might also support a novel therapeutic strategy for
bone metabolic diseases, such as osteoporosis and distress of bone
fracture repair, by modulating the Wnt/β-catenin signaling pathway.
Further investigations will be required to clarify the exact roles
of Wnt3a in bone metabolism.
In conclusion, our present findings strongly suggest
that Wnt3a downregulates T3-induced osteocalcin
expression through canonical pathways in osteoblasts without
affecting p38 MAP kinase.
Acknowledgements
The authors would like to thank Mrs Yumiko Kurokawa
(Department of Pharmacology, Gifu University Graduate School of
Medicine) for her skillful technical assistance.
Funding
The present study was supported in part by
Grant-in-Aid for Scientific Research (grant nos. 19591042 and
26462289) from the Ministry of Education, Science, Sports and
Culture of Japan, the Research Funding for Longevity Sciences
(grant no. 28-9, 29-12) from National Center for Geriatrics and
Gerontology (Obu, Japan).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KF, TO, OK and HT conceived and designed the
experiments. KF, TK, GS, WK and RM-N performed the experiments. KF,
TK, GS, RM-N, OK and HT analyzed the data. KF, TO, OK and HT wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Komori T: Signaling networks in
RUNX2-dependent bone development. J Cell Biochem. 112:750–755.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moon RT, Bowerman B, Boutros M and
Perrimon N: The promise and perils of Wnt signaling through
beta-catenin. Science. 296:1644–1646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tokuda H, Adachi S, Matsushima-Nishiwaki
R, Kato K, Natsume H, Otsuka T and Kozawa O: Enhancement of basic
fibroblast growth factor-stimulated VEGF synthesis by Wnt3a in
osteoblasts. Int J Mol Med. 27:859–864. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Natsume H, Tokuda H, Matsushima-Nishiwaki
R, Kato K, Yamakawa K, Otsuka T and Kozawa O: Wnt3a upregulates
transforming growth factor-β-stimulated VEGF synthesis in
osteoblasts. Cell Biochem Funct. 29:373–377. 2011. View Article : Google Scholar
|
|
6
|
Kondo A, Tokuda H, Mizutani J,
Matsushima-Nishiwaki R, Kozawa O and Otsuka T: Wnt3a upregulates
prostaglandin F2α-stimulated vascular endothelial growth factor
synthesis in osteoblasts. Mol Med Rep. 6:421–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Natsume H, Tokuda H, Adachi S,
Matsushima-Nishiwaki R, Kato K, Minamitani C, Otsuka T and Kozawa
O: Wnt3a regulates tumor necrosis factor-α-stimulated interleukin-6
release in osteoblasts. Mol Cell Endocrinol. 331:66–72. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hauschka PV, Lian JB, Cole DE and Gundberg
CM: Osteocalcin and matrix Gla protein: Vitamin K-dependent
proteins in bone. Physiol Rev. 69:990–1047. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ducy P, Desbois C, Boyce B, Pinero G,
Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, et
al: Increased bone formation in osteocalcin-deficient mice. Nature.
382:448–452. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karsenty G and Ferron M: The contribution
of bone to whole-organism physiology. Nature. 481:314–320. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gogakos AI, Duncan Bassett JH and Williams
GR: Thyroid and bone. Arch Biochem Biophys. 503:129–136. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vestergaard P and Mosekilde L:
Hyperthyroidism, bone mineral, and fracture risk-a meta-analysis.
Thyroid. 13:585–593. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng SY, Leonard JL and Davis PJ:
Molecular aspects of thyroid hormone actions. Endocr Rev.
31:139–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mullur R, Liu YY and Brent GA: Thyroid
hormone regulation of metabolism. Physiol Rev. 94:355–382. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kasono K, Sato K, Han DC, Fujii Y,
Tsushima T and Shizume K: Stimulation of alkaline phosphatase
activity by thyroid hormone in mouse osteoblast-like cells
(MC3T3-E1): A possible mechanism of hyperalkaline phosphatasia in
hyperthyroidism. Bone Miner. 4:355–363. 1988.PubMed/NCBI
|
|
16
|
Ishisaki A, Tokuda H, Yoshida M, Hirade K,
Kunieda K, Hatakeyama D, Shibata T and Kozawa O: Activation of p38
mitogen-activated protein kinase mediates thyroid
hormone-stimulated osteocalcin synthesis in osteoblasts. Mol Cell
Endocrinol. 214:189–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanno Y, Ishisaki A, Yoshida M, Nakajima
K, Tokuda H, Numata O and Kozawa O: Adenylyl cyclase-cAMP system
inhibits thyroid hormone-stimulated osteocalcin synthesis in
osteoblasts. Mol Cell Endocrinol. 229:75–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2 in
osteoblast-like cells. Exp Cell Res. 198:130–134. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Yang N and Shi XM: Regulation of
mesenchymal stem cell osteogenic differentiation by
glucocorticoid-induced leucine zipper (GILZ). J Biol Chem.
283:4723–4729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simpson DA, Feeney S, Boyle C and Stitt
AW: Retinal VEGF mRNA measured by SYBR green I fluorescence: A
versatile approach to quantitative PCR. Mol Vis. 6:178–183.
2000.PubMed/NCBI
|
|
22
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and alpha B-crystallin by cyclic AMP in C6 rat glioma cells.
J Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coghlan MP, Culbert AA, Cross DA, Corcoran
SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee
Cox L, et al: Selective small molecule inhibitors of glycogen
synthase kinase-3 modulate glycogen metabolism and gene
transcription. Chem Biol. 7:793–803. 2000. View Article : Google Scholar : PubMed/NCBI
|