Introduction
Acute diarrhea is a common intestinal disease in
children, and also one of the diseases with high incidence in
childhood, ranking second in pediatric diseases in China (1). The clinical manifestations of acute
diarrhea in children before the confirmed etiology is the increase
of defecation frequency and the change of trait (2). Diet regulation, oral rehydration salt
(ORS) and other methods are the routine treatment for controlling
the development of the disease (3),
which may lead to intestinal dysbacteriosis due to its prolonged
effective time of stopping diarrhea. A study (4) has shown that infantile diarrhea not
only cause acid-base imbalance in the intestine, but also affect
the respiratory system of the children.
Saccharomyces boulardii powder is a
biological antidiarrheal agent (5).
The study of Micklefield (6) showed
that Saccharomyces boulardii had a good preventive effect on
antibiotic-associated diarrhea, acute infection, irritable bowel
syndrome and nonspecific diarrhea. Its main active ingredient is
lyophilized and viable Saccharomyces boulardii (7), and it is the first choice of drugs in
the treatment of infantile diarrhea (8). Saccharomyces boulardii powder
has nutritive effect on intestinal mucosa (9) and antitoxin effect on microorganism
(10), it is effective in treating
diarrhea in children and diarrhea caused by intestinal flora
disorders and can also prevent and treat diarrhea caused by
antibiotics (11). Saccharomyces
boulardii does not degrade its activity due to gastrointestinal
fluids, sulfanilamides or antimicrobial agents and has natural
tolerance to antibiotics (12).
Studies of O'Mahony et al (13) have shown that in animal experiments,
Saccharomyces boulardii has antimicrobial activity
(including albicans Saccharomyces) in vivo and in
vitro. Bifidobacterium triple viable powder is a compound
preparation (14), the main
components are bifidobacterium, lactobacillus and
enterococcus viable organism (15).
After oral administration, it can directly act on the body to
supplement normal human bacteria, adjust the balance of intestinal
flora (16), inhibit the growth of
pathogenic bacteria, promote the digestive function of the body,
synthesize vitamins needed for normal metabolism and growth
(17), prevent the fixed value of
pathogenic bacteria on intestinal mucosa and improve the local
immunity of intestinal mucosa (18).
Moreover, it has small adverse reactions and high application value
in clinic. T lymphocyte subsets are important indexes for the
detection of cellular immune function, and are of great
significance for the diagnosis and observation of therapeutic
effects of some diseases (19).
CD4+ is an important immune cell in the human immune
system, which is mainly responsible for assisting T cells and
directing the body against microorganisms. CD8+ cells
are inhibitory lymphocytes with the main function of killing the
target cells directly. Whether infantile diarrhea affects cellular
immune function is also worth clarification. Therefore, its
treatment has drawn the attention of the medical field.
The therapeutic effect of Saccharomyces
boulardii combined with bifidobacterium and its effect
on cellular immune function in children with acute diarrhea were
analyzed in this study.
Patients and methods
General information
In total, 116 cases of children with acute diarrhea
admitted to Xuzhou Children's Hospital (Xuzhou, China) from March
2015 to March 2017 were collected, 59 children treated with
Saccharomyces boulardii were the control group and 57
children treated with Saccharomyces boulardii combined with
bifidobacterium were the experimental group. In the control
group, there were 28 males and 31 females, aged 1–11 years, with an
average age of 5.2 years. In the experimental group, there were 30
males and 27 females, aged 4–12 years, with an average age of 6.8
years.
This study was approved by the Εthics Committee of
Xuzhou Children's Hospital. The signed informed consents were
obtained from the patients or the guardians.
Inclusion criteria: Patients with no
contraindications, with treatment for more than 3 days and patinets
with positive bacterial culture.
Exclusion criteria: Patients with antibiotics before
treatment, with severe malnutrition, with digestive system disease,
congenital heart disease, respiratory disease and patients with
moderate dehydration, hypovolemic shock or other critical
disease.
Administration methods
The control group was treated with Saccharomyces
boulardii, 0.25 g Saccharomyces boulardii powder
(Laboratories Biocodex; SFDA approval no. H20046379) was given
orally, children over 3 years old were given 0.25 g each time, once
every 12 h, children under 3 years old were given 0.25 g each time,
once per day. The experimental group was treated with 0.1 g
bifidobacterium triple viable powder (Shanghai Shangmiao
Xinyi Pharmaceutical Co., Ltd.; SFDA approval no. S10970105) on the
basis of the control group, children aged 0–1 years old were given
0.05 g each time, 3 times per day, children aged 1–5 years were
given 0.1 g each time, 3 times per day, children over 6 years old
were given 0.2 g each time, 3 times per day. The course of
treatment in the two groups was one week.
Observation indicators and judgement criteria. The
judgment criteria for therapeutic effect (20) are shown in Table I.
 | Table I.Criteria for therapeutic effect of
diarrhea. |
Table I.
Criteria for therapeutic effect of
diarrhea.
| Therapeutic
effect | Medication
duration | Stool
character | Stool
frequency | Clinical
symptoms |
|---|
| Apparent | 24-48 h | Return to
normal | ≤ Twice daily | Complete
disappearance |
| Effective | 48-72 h | Improvement and
water reduction | ≤ Twice daily | Basic
disappearance |
| Non-effective | 72 h | No obvious
improvement | > Twice
daily | Exacerbation of
illness |
Standard for the detection of cellular immunological
indexes. The cellular immune indexes CD3+,
CD4+, CD8+ in Xuzhou Children's Hospital at
the time of visit and one month after treatment were detected by
flow cytometry, and the changes of CD4+/CD8+
were measured and calculated.
Main observation indicators and secondary
observation indicators of infantile diarrhea. Main observation
indicators: Stool frequency in different time periods, mean
antidiarrheal time, mean antipyretic time and length of stay in the
two groups. Fasting venous blood samples were collected before and
after treatment. The ratio of Th1 and Th2 in peripheral blood was
detected by flow cytometry, and the ratio of Th1/Th2 was
calculated. Cellular immunological indexes were examined before and
one month after treatment in both groups. Secondary observation
indicators: clinical efficacy (apparent, effective,
non-effective).
Statistical analysis
The data were analyzed by SPSS19.0 (SPSS, Inc.). The
enumeration data were expressed as n (%). The comparison method was
Chi-square test. t-test was used for comparison between the
experimental and the control group. P<0.05 was statistically
significant.
Results
Comparison of basic data between the
two groups
There was no significant difference in sex, age,
height, weight, fever, vomiting, abdominal pain, fecal routine,
crying and loss of appetite between the two groups (P>0.05)
(Table II).
 | Table II.Basic data of 116 children with acute
diarrhea [n (%)]. |
Table II.
Basic data of 116 children with acute
diarrhea [n (%)].
| Basic data | Control group
(n=59) | Experimental group
(n=57) | χ2
value | P-value |
|---|
| Sex |
|
Male | 28 (47.46) | 30 (52.63) | 0310 | 0.577 |
|
Female | 31 (52.54) | 27 (47.37) |
|
|
| Age (years) |
|
|
|
|
|
<6 | 33 (55.93) | 31 (54.39) | 0.028 | 0.867 |
| ≥6 | 26 (44.07) | 26 (45.61) |
|
|
| Weight (kg) |
|
|
|
|
|
<15 | 34 (57.63) | 38 (66.67) | 1.006 | 0.316 |
|
≥15 | 25 (42.37) | 19 (33.33) |
|
|
| Height (m) |
|
|
|
|
|
<1 | 36 (61.02) | 34 (59.65) | 0.023 | 0.880 |
| ≥1 | 23 (38.98) | 23 (40.35) |
|
|
| Fever |
|
|
|
|
|
Yes | 48 (81.36) | 45 (78.95) | 0.106 | 0.745 |
| No | 11 (18.64) | 12 (21.05) |
|
|
| Vomiting |
|
|
|
|
|
Yes | 45 (76.27) | 49 (85.96) | 1.773 | 0.183 |
| No | 14 (23.73) | 8
(14.04) |
|
|
| Abdominal pain |
|
|
|
|
|
Yes | 47 (79.66) | 47 (82.46) | 0.147 | 0.701 |
| No | 12 (20.34) | 10 (17.54) |
|
|
| Fecal routine |
|
|
|
|
|
Normal | 49 (83.05) | 51 (89.47) | 1.006 | 0.316 |
|
Abnormal | 10 (16.95) | 6
(10.53) |
|
|
| Crying |
|
|
|
|
|
Yes | 47 (79.66) | 49 (85.96) | 0.807 | 0.369 |
| No | 12 (20.34) | 8
(14.04) |
|
|
| Loss of
appetite |
|
|
|
|
|
Yes | 48 (81.36) | 48 (84.21) | 0.166 | 0.684 |
| No | 11 (18.64) | 9
(15.79) |
|
|
Comparison of clinical efficacy
The cure rate (73.68%) and the total effective rate
(87.72%) in the experimental group were significantly higher than
those in the control group (47.46 and 71.19%) and there was a
statistical difference between the two groups (P<0.05) (Table III).
 | Table III.Comparison of clinical efficacy
between the two groups [n (%)]. |
Table III.
Comparison of clinical efficacy
between the two groups [n (%)].
| Groups | Cure (%) | Effective (%) | Non-effective
(%) | Total effective
rate (%) |
|---|
| Control (n=59) | 28 (47.46) | 14 (23.73) | 17 (28.81) | 42 (71.19) |
| Experimental
(n=57) | 42 (73.68) | 8
(14.04) | 7
(12.28) | 50 (87.72) |
| χ2
value | 8.333 | 1.773 | 4.829 | 4.829 |
| P-value | 0.004 | 0.183 | 0.030 | 0.030 |
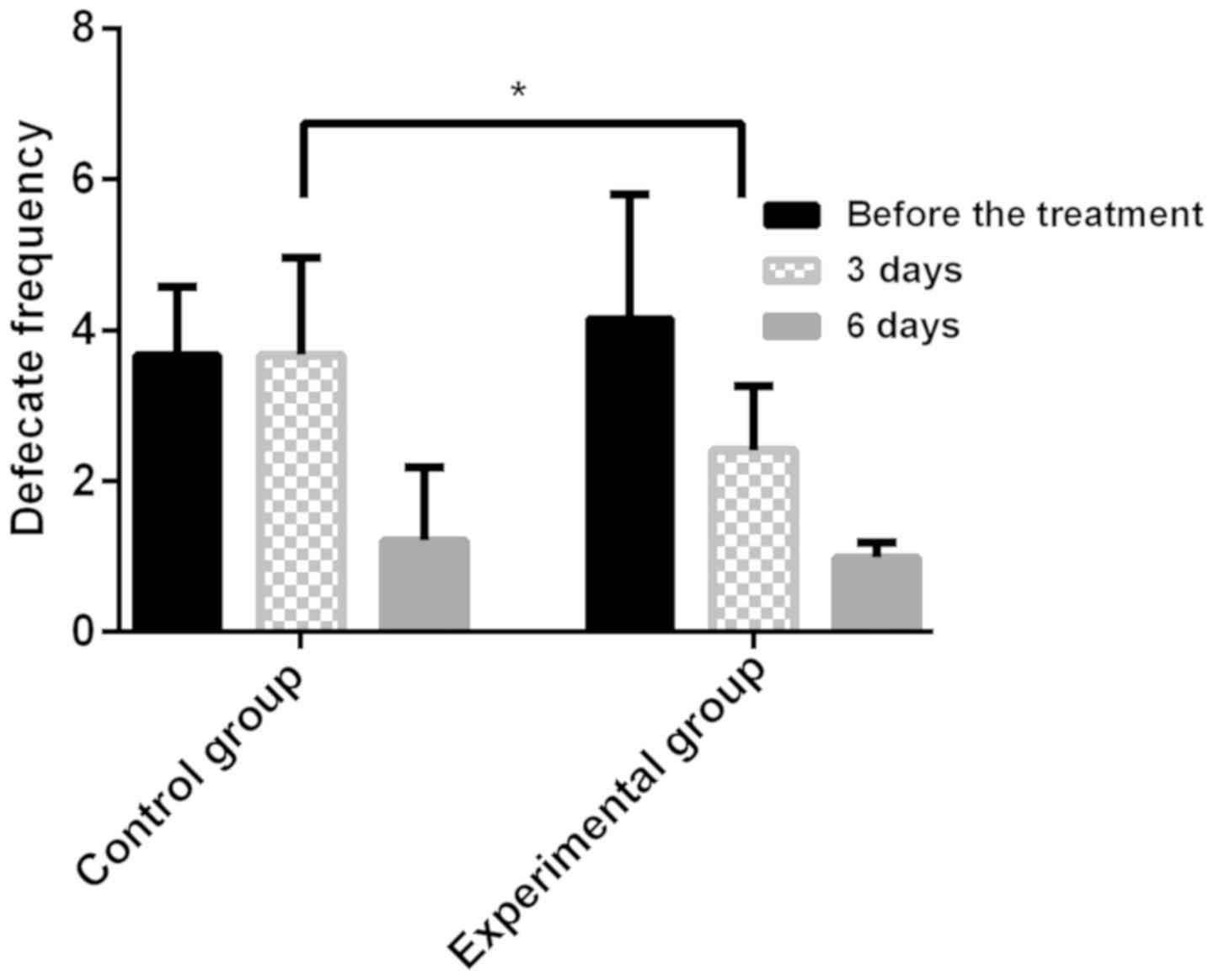
Stool frequency in different time period in the two
groups. There was no significant difference in the average stool
frequency before treatment between the experimental group and the
control group (P>0.05), the stool frequency in the two groups
was significantly decreased after treatment, and the experimental
group was significantly lower than the control group 3 days after
treatment (P<0.05). There was no significant difference in
average stool frequency between the two groups after 6 days of
treatment (P>0.05). The stool frequency in different time
periods in the two groups are shown in Fig. 1.
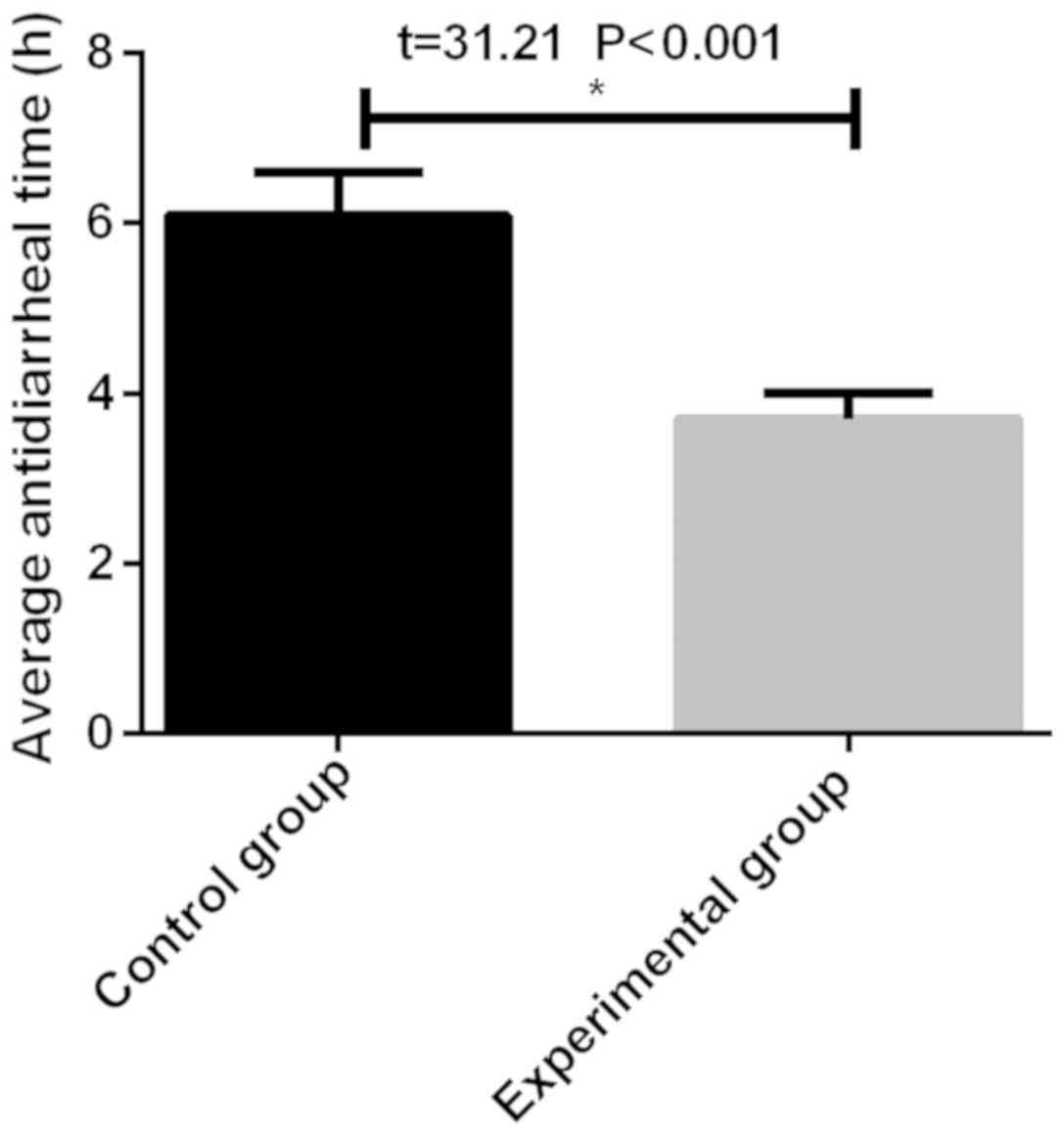
Comparison of mean antidiarrheal time,
mean antipyretic time and mean length of stay between the two
groups
The mean antidiarrheal time in the experimental
group (3.7±0.3 h) was significantly shorter than that in the
control group (6.1±0.5 h), and there was a statistical difference
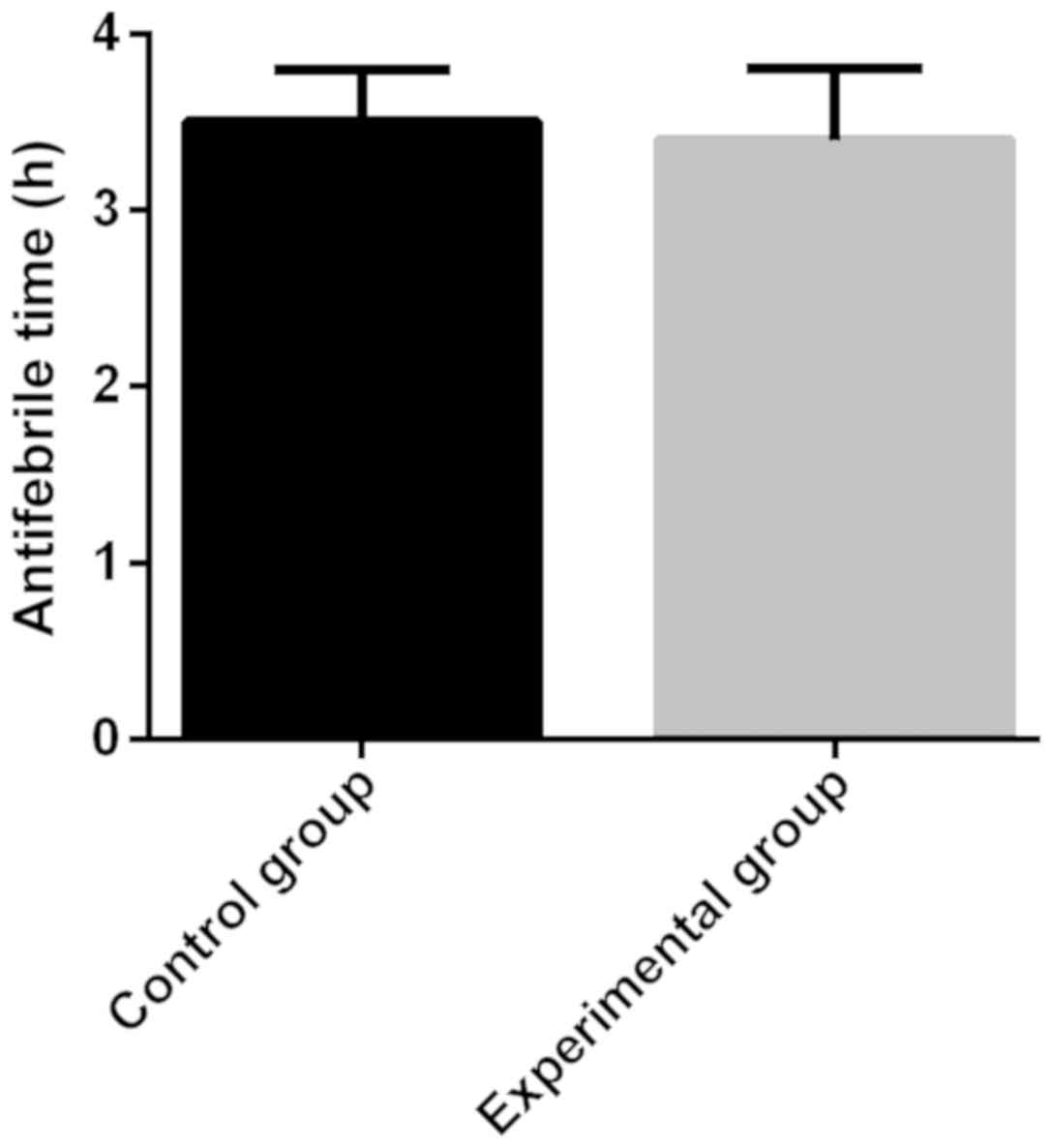
(P<0.05) (Fig. 2). The
antipyretic time in the control group (3.5±0.3 h) was longer than
that in the experimental group (3.4±0.4 h), and there was no
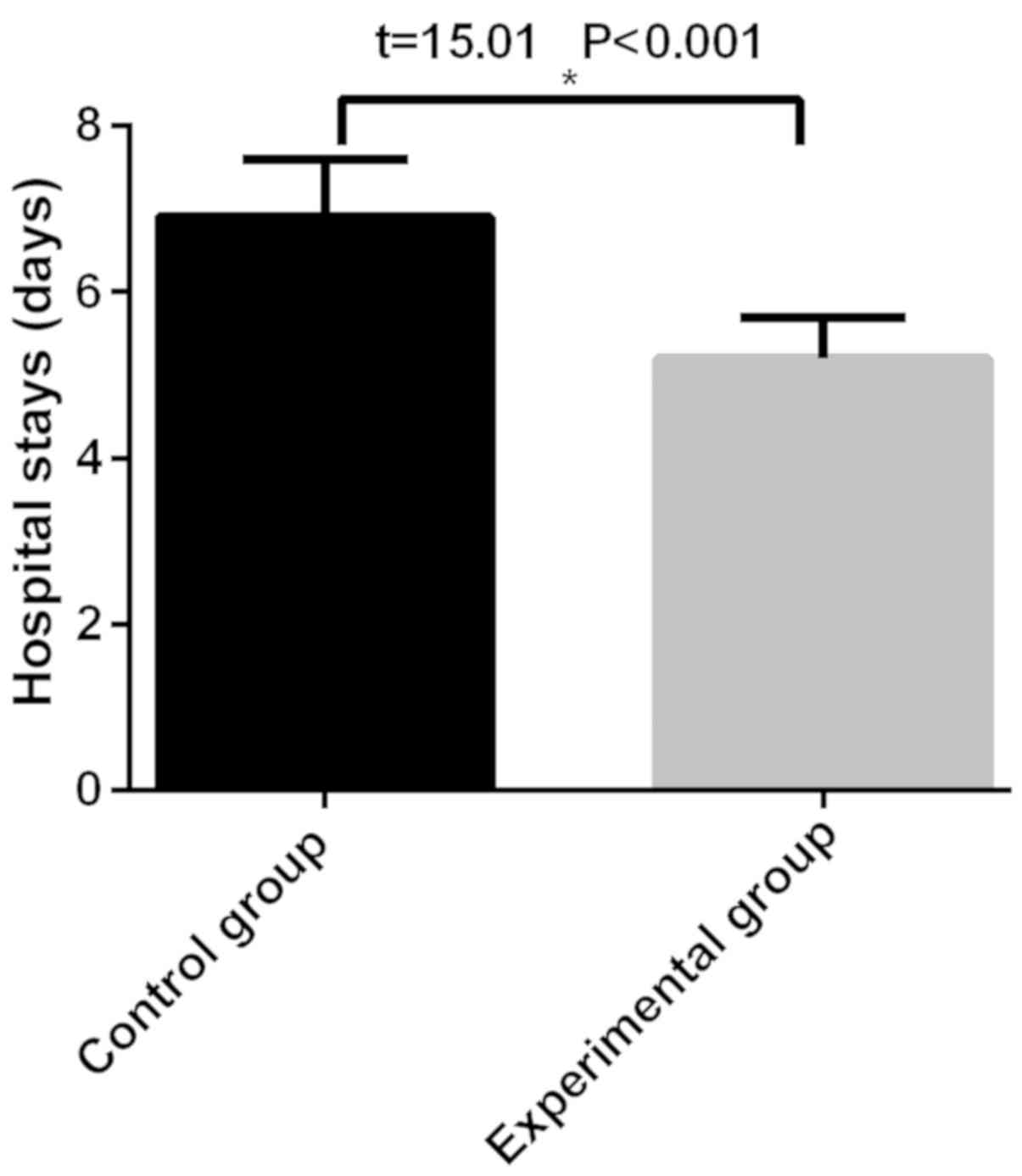
statistical significance (P>0.05) (Fig. 3). The length of stay in the control
group (6.9±0.7 h) was significantly longer than that in the
experimental group (5.2±0.5 h), with a statistical significance
(P<0.05) (Fig. 4).
Comparison of the results of cellular
immunologic indexes before treatment and one month after treatment
between the two groups
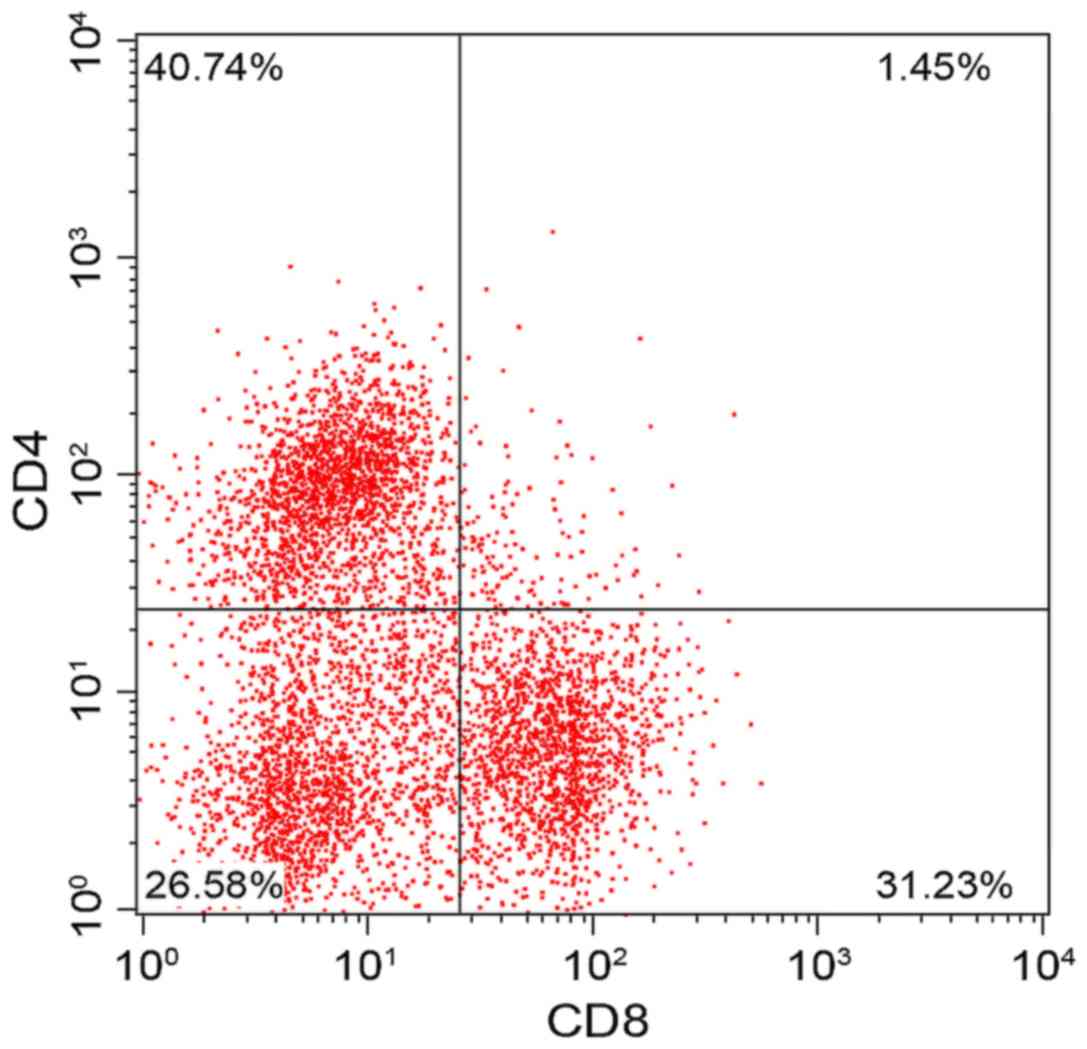
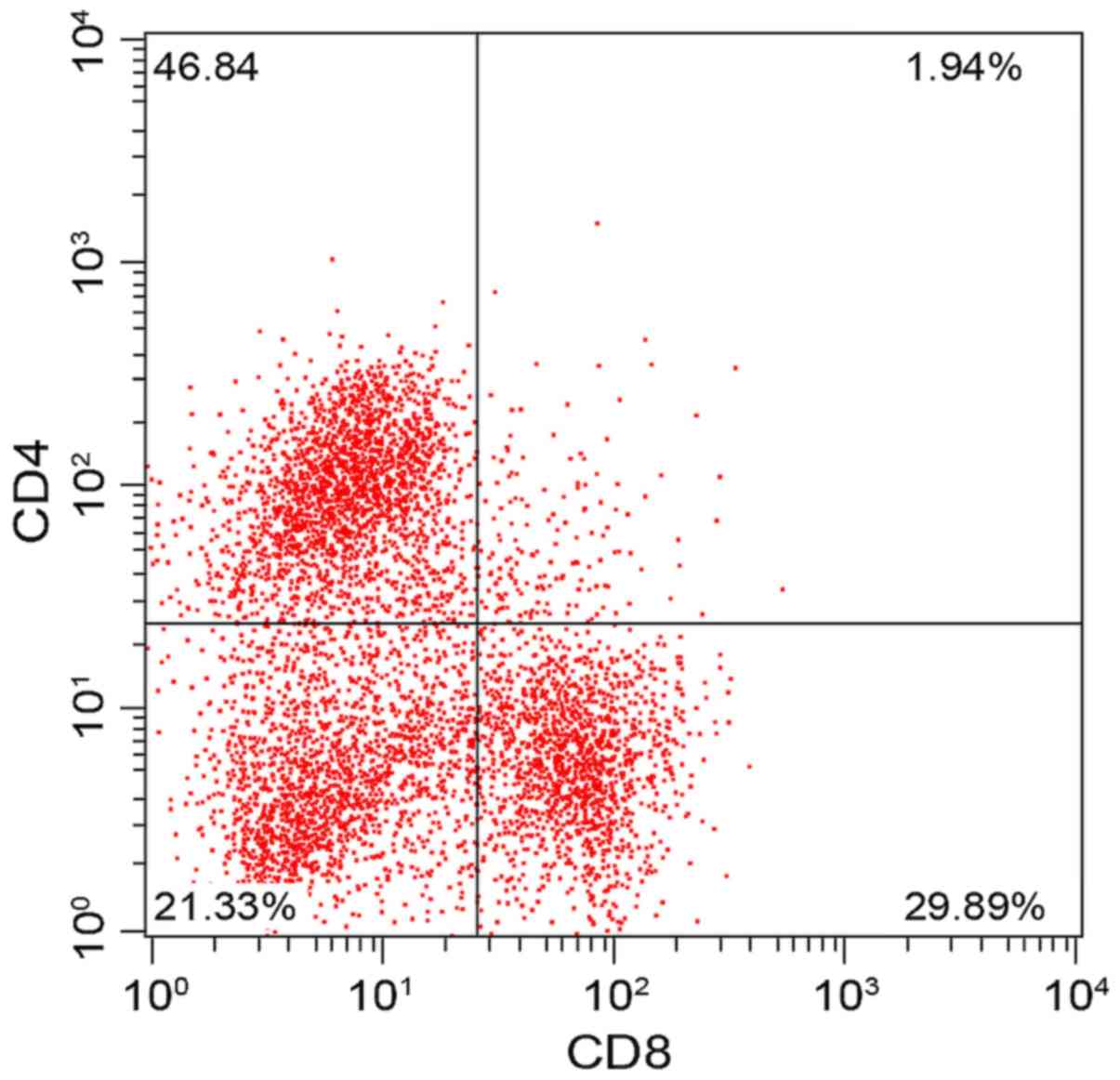
There was no significant difference in CD3+, CD4+,
CD8+ and CD4+/CD8+ between the two groups before treatment
(P>0.05). The results of review conducted one month after
treatment showed that CD3+, CD4+ and CD4+/CD8+ increased
significantly in the experimental group while CD8+ decreased
significantly (P<0.05), which indicated that the cellular immune
function of the children in the experimental group was
significantly improved. Compared with those before treatment, CD3+,
CD4+ and CD4+/CD8+ increased slightly in the control group while
CD8+ decreased slightly, and the difference was statistically
significant (P<0.05) (Figs. 5,
6 and Table IV).
 | Table IV.Comparison of the results of cellular
immunologic indexes before and after treatment between the two
groups. |
Table IV.
Comparison of the results of cellular
immunologic indexes before and after treatment between the two
groups.
| Groups | Time | CD3+
(%) | CD4+
(%) | CD8+
(%) | CD4/CD8+
(%) |
|---|
| Control | Before
treatment | 57.28±1.54 | 37.24±7.12 | 28.52±1.68 | 1.19±0.57 |
| (n=59) | One month after
treatment |
61.84±2.95a |
40.74±6.31a |
31.23±1.50a |
1.45±0.61a |
| Experimental | Before
treatment | 62.54±3.54 | 41.23±6.87 | 32.14±1.94 | 1.59±0.65 |
| (n=57) | One month after
treatment |
65.48±2.51a,b |
46.84±4.54a,b |
29.89±2.14a,b |
1.94±0.51a,b |
| t value | 1.172 | 1.250 | 1.159 | 0.964 |
|
| P-value | 0.362 | 0.338 | 0.366 | 0.437 |
|
Comparison of T lymphocyte levels
between the two groups
After treatment, the ratio of Th1 and Th2 in the two
groups decreased significantly compared with before treatment, with
a statistical significance (P<0.05), and the experimental group
was significantly lower than the control group, with a statistical
significance (P<0.05). After treatment, Th1/Th2 ratio was
significantly higher than that before treatment, with a statistical
significance (P<0.05), and the experimental group was
significantly higher than the control group, with a statistical
significance (P<0.05) (Table
V).
 | Table V.Comparison of T lymphocyte levels
between the two groups. |
Table V.
Comparison of T lymphocyte levels
between the two groups.
|
| Th1 (%) | Th2 (%) | Th1/Th2 |
|---|
|
|
|
|
|
|---|
| Groups | Before
treatment | After
treatment | Before
treatment | After
treatment | Before
treatment | After
treatment |
|---|
| Experimental
(n=57) | 19.37±2.47 |
11.72±2.01a | 28.37±3.26 |
13.47±3.17a | 0.63±0.12 |
0.89±0.13a |
| Control (n=59) | 19.28±2.51 |
15.63±2.27a | 28.38±3.12 |
19.37±3.38a | 0.61±0.16 |
0.81±0.11a |
| t value | 0.195 |
9.809 | 0.017 |
9.690 | 0.760 | 0.005 |
| P-value | 0.846 | <0.001 | 0.987 | <0.001 | 0.449 | 3.582 |
Discussion
As a common disease in children with a high
incidence in children at 6 months to 2 years of age (21), the main clinical symptoms of
infantile diarrhea are thin stool, a small amount of gas and
sometimes mucus discharging, increased defecation and often
accompanied by copracrasia and shortness of defecation and other
symptoms (22). Attention should be
paid to the disease once it is contracted, the exacerbation can
cause severe dehydration, leading to shock and even be
life-threatening. Its influencing factors are complex, mainly
divided into infectious and non-infectious categories (23). Infectious diarrhea is caused by
pathogenic organisms entering the digestive tract with contaminated
food or drinking water (24). The
ability of pathogenic organisms to cause intestinal infection also
determines the strength of their defense and the size of infected
pathogenic organisms (25). Most of
the cases are viral infectious diarrhea in clinic because
children's immune system is not mature, the immune function is not
perfect and the pathogen can easily invade (26). Infectious diarrhea is mainly caused
by improper diet. For example, improper composition and food
intolerance can cause malfunction of digestion, making food
difficult to digest and absorb, thus leading to food stagnation in
the upper small intestine and decreased acidity of the intestinal
cavity, which is more favorable for bacteria reproduction and
upward migration in the lower part of the small intestine (27).
Results in this study showed that the cure rate
(73.68%) and the total effective rate (87.72%) in the experimental
group were significantly higher than those in the control group
(47.46 and 71.19%), and there was statistical difference between
the two groups (P<0.05), which suggested that therapeutic effect
of Saccharomyces boulardii combined with
bifidobacterium on infantile diarrhea is better than that of
single use of Saccharomyces boulardii. It is inferred that
when the Saccharomyces boulardii and bifidobacterium
triple therapy are used together, the Saccharomyces
boulardii may proliferate rapidly in the intestine, decompose
peroxide and consume oxygen to produce anaerobic environment, which
is more beneficial to the growth of bifidobacterium.
Bifidobacterium triple viable powder can directly supplement human
normal flora, regulate intestinal balance and eliminate harmful
bacteria. Bifidobacterium can also promote intestinal absorption,
reduce the absorption and production of toxic substances, and
maintain ecological balance. Therefore, the combined use of the two
drugs may be compatible with each other. The results of Martins
et al (28) showed that the
combined use of Saccharomyces boulardii and
bifidobacterium triple therapy was more effective than that
of Bifidobacterium triple therapy alone, which gives support to our
results. However, the data analysis of this experiment was more
comprehensive, and the experimental results were more convincing.
Then we analyzed the mean antipyretic time, mean antidiarrheal
time, and the length of stay. The results showed that there was no
difference in the mean antipyretic time, and the mean antidiarrheal
time, and length of stay in the experimental group was
significantly shorter than that in the control group. These results
indicated that Saccharomyces boulardii combined with
bifidobacterium could shorten the mean antidiarrheal time
and length of stay. In the study of El-Soud et al (29), a randomized controlled trial showed
that probiotics of bifidobacterium significantly decreased
frequency, duration of antidiarrheal and length of stay. The
results of the detection of cellular immunological indexes before
and after treatment and the level of T lymphocyte showed that there
was no significant difference in CD3+, CD4+,
CD8+ and CD4+/CD8+ between the two
groups before treatment. The results of review conducted one month
after treatment showed that CD3+, CD4+ and
CD4+/CD8+ increased significantly in the
experimental group, while CD8+ decreased significantly.
After treatment, the ratio of Th1 and Th2 in the two groups
decreased significantly compared with before treatment, and the
experimental group was significantly lower than the control group.
After treatment, Th1/Th2 ratio was significantly higher than that
before treatment and the experimental group was significantly
higher than the control group. Thus, our study indicated that the
cellular immune function of the children in the experimental group
was significantly improved. The results suggest that infantile
diarrhea is closely related to cellular immunity, so attention
should not be paid only to clinical efficacy, but also to the
prevention and treatment of cellular immune dysfunction in the
course of treatment, so as to achieve better clinical effect. The
observation of intestinal immunology found that the distribution of
memory T cells and macrophages changed obviously. After early
infantile treatment, macrophages were concentrated in the local
intestine to activate the natural immunity. In the study of Akatsu
et al (30), the elderly were
studied. The results showed that long-term bifidobacterium
intake had potential in increasing the number of
bifidobacterium cells in intestinal microflora and
regulating the immune function of the elderly.
There are some defects in this study. All the
children with acute diarrhea included in the study were treated,
and untreated children were not included. The cellular
immunological indicators and T lymphocyte levels were not observed
in the untreated patients. The number of patients included is
small.
In conclusion, treatment of acute diarrhea in
children with Saccharomyces boulardii combined with
bifidobacterium can effectively improve the clinical
efficacy, shorten antidiarrheal time and hospital stay, and promote
the improvement of immune function in children.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW recorded and analyzed observation indicators. GW
and DF collected, analyzed and interpreted general information of
patients. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Εthics Committee of
Xuzhou Children's Hospital (Xuzhou, China). The signed informed
consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang SX, Zhou YM, Xu W, Tian LG, Chen JX,
Chen SH, Dang ZS, Gu WP, Yin JW, Serrano E, et al: Impact of
co-infections with enteric pathogens on children suffering from
acute diarrhea in southwest China. Infect Dis Poverty. 5:642016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Drossman DA: Functional gastrointestinal
disorders: History, Pathophysiology, Clinical Features and Rome IV.
Gastroenterology. Feb 19–2016.(Epub ahead of print). doi:
10.1053/j.gastro.2016.02.032. View Article : Google Scholar
|
|
3
|
Guandalini S: Probiotics for prevention
and treatment of diarrhea. J Clin Gastroenterol. 45
(Suppl):S149–S153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Jia CS, Shi J, Wang JL, Li XF,
Zhang XP, Sun YH and Qin L: Meta analysis of effectiveness of
acupoint application therapy for infantile diarrhea. Zhen Ci Yan
Jiu. 38:319–323. 2013.(In Chinese). PubMed/NCBI
|
|
5
|
Riaz M, Alam S, Malik A and Ali SM:
Efficacy and safety of Saccharomyces boulardii in acute
childhood diarrhea: A double blind randomised controlled trial.
Indian J Pediatr. 79:478–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Micklefield G: Saccharomyces boulardii in
the treatment and prevention of antibiotic-associated diarrhea. MMW
Fortschr Med. 156 (Suppl 1):18–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Czerucka D and Rampal P: Experimental
effects of Saccharomyces boulardii on diarrheal pathogens.
Microbes Infect. 4:733–739. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szajewska H, Skórka A, Ruszczyński M and
Gieruszczak-Białek D: Meta-analysis: Lactobacillus GG for treating
acute diarrhoea in children. Aliment Pharmacol Ther. 25:871–881.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murzyn A, Krasowska A, Augustyniak D,
Majkowska-Skrobek G, Łukaszewicz M and Dziadkowiec D: The effect of
Saccharomyces boulardii on Candida albicans-infected
human intestinal cell lines Caco-2 and Intestin 407. FEMS Microbiol
Lett. 310:17–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moré MI and Swidsinski A: Saccharomyces
boulardii CNCM I-745 supports regeneration of the intestinal
microbiota after diarrheic dysbiosis - a review. Clin Exp
Gastroenterol. 8:237–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McFarland LV: Can Saccharomyces
boulardii prevent antibiotic-associated diarrhea in children?
Nat Clin Pract Gastroenterol Hepatol. 2:262–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qamar A, Aboudola S, Warny M, Michetti P,
Pothoulakis C, LaMont JT and Kelly CP: Saccharomyces
boulardii stimulates intestinal immunoglobulin A immune
response to Clostridium difficile toxin A in mice. Infect
Immun. 69:2762–2765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Mahony L, McCarthy J, Kelly P, Hurley G,
Luo F, Chen K, O'Sullivan GC, Kiely B, Collins JK, Shanahan F, et
al: Lactobacillus and bifidobacterium in irritable
bowel syndrome: Symptom responses and relationship to cytokine
profiles. Gastroenterology. 128:541–551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang ZH, Gao QY and Fang JY: Meta-analysis
of the efficacy and safety of Lactobacillus-containing and
Bifidobacterium-containing probiotic compound preparation in
Helicobacter pylori eradication therapy. J Clin
Gastroenterol. 47:25–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meile L, Le Blay G and Thierry A: Safety
assessment of dairy microorganisms: Propionibacterium and
Bifidobacterium. Int J Food Microbiol. 126:316–320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Shimizu T, Hosaka A, Kaneko N,
Ohtsuka Y and Yamashiro Y: Effects of bifidobacterium breve
supplementation on intestinal flora of low birth weight infants.
Pediatr Int. 46:509–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin HZ, Fan XB, Hang XM, Li KB and Yang H:
Analysis of the probiotic Bifidobacterium and Lactobacillus
community in child intestinal flora. Wei Sheng Wu Xue Bao.
45:567–570. 2005.(In Chinese). PubMed/NCBI
|
|
18
|
Grönlund MM, Gueimonde M, Laitinen K,
Kociubinski G, Grönroos T, Salminen S and Isolauri E: Maternal
breast-milk and intestinal bifidobacteria guide the compositional
development of the Bifidobacterium microbiota in infants at
risk of allergic disease. Clin Exp Allergy. 37:1764–1772. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reinherz EL and Schlossman SF: Current
concepts in immunology: Regulation of the immune response - inducer
and suppressor T-lymphocyte subsets in human beings. N Engl J Med.
303:370–373. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fischbach W, Andresen V, Eberlin M, Mueck
T and Layer P: A comprehensive comparison of the efficacy and
tolerability of racecadotril with other treatments of acute
diarrhea in adults. Front Med (Lausanne). 3:442016.PubMed/NCBI
|
|
21
|
Bern C, Martines J, de Zoysa I and Glass
RI: The magnitude of the global problem of diarrhoeal disease: A
ten-year update. Bull World Health Organ. 70:705–714.
1992.PubMed/NCBI
|
|
22
|
Benninga MA, Faure C, Hyman PE, St James
Roberts I, Schechter NL and Nurko S: Childhood functional
gastrointestinal disorders: Neonate/toddler. Gastroenterology.
150:1443–1455. 2016. View Article : Google Scholar
|
|
23
|
Turin CG and Ochoa TJ: The role of
maternal breast milk in preventing infantile diarrhea in the
developing world. Curr Trop Med Rep. 1:97–105. 2014.PubMed/NCBI
|
|
24
|
Shane AL, Mody RK, Crump JA, Tarr PI,
Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, et
al: Infectious Diseases Society of America Clinical Practice
Guidelines for the diagnosis and management of infectious diarrhea.
Clin Infect Dis. 65:e45–e80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maeda I, Siddiki MS, Nozawa-Takeda T,
Tsukahara N, Tani Y, Naito T and Sugita S: Population abundance of
potentially pathogenic organisms in intestinal microbiome of jungle
crow (Corvus macrorhynchos) shown with 16S rRNA gene-based
microbial community analysis. BioMed Res Int. 2013:4389562013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hunter P: Co-infection: when whole can be
greater than the sum: The complex reaction to co-infection of
different pathogens can generate variable symptoms. EMBO Rep.
19:e466012018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mushtaq I, Cheema HA and Malik HS, Waheed
N, Hashmi MA and Malik HS: Causes of chronic non-infectious
diarrhoea in infants less than 6 months of age: Rarely recognized
entities. J Ayub Med Coll Abbottabad. 29:78–82. 2017.PubMed/NCBI
|
|
28
|
Martins FS, Silva AA, Vieira AT, Barbosa
FH, Arantes RM, Teixeira MM and Nicoli JR: Comparative study of
Bifidobacterium animalis, Escherichia coli, Lactobacillus
casei and Saccharomyces boulardii probiotic properties.
Arch Microbiol. 191:623–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El-Soud NH, Said RN, Mosallam DS, Barakat
NA and Sabry MA: Bifidobacterium lactis in treatment of
children with acute diarrhea. A randomized double blind controlled
trial. Open Access Maced J Med Sci. 3:403–407. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Akatsu H, Iwabuchi N, Xiao JZ, Matsuyama
Z, Kurihara R, Okuda K, Yamamoto T and Maruyama M: Clinical effects
of probiotic Bifidobacterium longum BB536 on immune function
and intestinal microbiota in elderly patients receiving enteral
tube feeding. JPEN J Parenter Enteral Nutr. 37:631–640. 2013.
View Article : Google Scholar : PubMed/NCBI
|