Introduction
Pancreatic cancer is a common malignancy with a poor
prognosis. According to predictions by the American Cancer Society,
56,770 new pancreatic carcinoma diagnoses will be made in 2019 and
as such, will become the fourth-highest cause of mortality in the
US (with rates as high as 80%), irrespective of sex (1). It is also estimated that pancreatic
cancer will surpass liver cancer in becoming the second-highest
cause of cancer-associated deaths by 2030 (2). Due to the clinically vague nature of
early stage disease, the majority of pancreatic cancer diagnoses
are only possible after metastasis at more advanced stages
(3). Due to pancreatic cancer
exhibiting high chemotherapy and radiation therapy resistance,
surgical resection is the only effective therapeutic option
currently available (3). Novel
strategies for treating pancreatic cancer are therefore urgently
required.
Enhancer of zeste homolog 2 (EZH2) is the
enzymatically active subunit of polycomb repressive complex 2,
which catalyzes the trimethylation of lysine 27 on histone 3
(H3K27) by transferring a methyl group from S-adenosyl-L-methionine
(SAM), leading to the transcriptional silencing of target genes
(4,5). Although the underlying molecular
mechanism of EZH2 oncogenic activity is unclear, EZH2 has been
revealed to serve a key role in the progression of different types
of cancer, including prostate cancer, breast cancer, endometrial
cancer, melanoma and hematological malignancies (6–14). A
previous study demonstrated that there was a high expression of
EZH2 in pancreatic cancer cells and tissues, which was associated
with proliferation, invasion and chemoresistance (15). EZH2 overexpression may also be a
promising prognostic marker for patients with pancreatic cancer
after surgery (16).
GSK343 serves as a SAM-competitive inhibitor,
exerting an enzymatically repressive function on EZH2 (17). This is distinct from
3-deazaneplanocin A (DZNep), which inhibits the activity of
S-adenosyl-L-homocystenine hydeolase (SAH) (18). Numerous studies have reported an
elevated expression of EZH2 in various types of cancer, which is
associated with a poor prognosis and metastasis (19–23). The
inhibition of EZH2 has been regarded as a novel therapeutic target
for various carcinomas (24–28).
The current study aimed to elucidate the effects of
GSK343 on pancreatic cancer cells to determine a potential future
putative therapeutic strategy. The results of the present study
revealed that GSK343 may be a potential anti-cancer drug and may
exert an autophagy-inducing effect.
Materials and methods
Materials
GSK343 (cat. no. S7164; Selleck Chemicals) was
dissolved in 5 mmol/l DMSO. Primary antibodies against GAPDH (cat.
no. 60004-1-Ig; ProteinTech Group, Inc.), microtubule-associated
protein 1 light chain 3 beta (LC3B; cat. no. L7543; Sigma-Aldrich;
Merck KGaA), AKT (cat. no. 1085; Abcam), phospho-AKT (cat. no.
2118; Abcam), autophagy-related protein 5 (ATG5; cat. no. 12994;
Cell Signaling Technology, Inc.), mTOR (cat. no. 2983; Cell
Signaling Technology, Inc.) and phospho-mTOR (cat. no. 5536; Cell
Signaling Technology, Inc.). Horseradish peroxidase-conjugated
anti-rabbit secondary antibodies (cat. no. A00098; GenScript) or
anti-mouse (cat. no. A00160; GenScript) and fluorochrome-conjugated
anti-rabbit secondary antibodies (cat. no. E031220; EarthOx Life
Sciences) were used at the recommended concentrations outlined by
the manufacturer's protocol.
Cell culture
A primary cancer cell was randomly selected (PANC-1,
derived from pancreatic ductal epithelial cells) and a metastatic
cancer cell line (AsPC-1, ascites metastasis) was used in the
present study to clarify the function of GSK343 (all, American Type
Culture Collection). The two pancreatic cell lines were cultured in
RPMI 1640 medium (Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Sigma-Aldrich; Merck KGaA) and incubated at 37°C with 5%
CO2.
MTT assay
AsPC-1 and PANC-1 Cells were plated into 96-well
plates (3×103 cells/well) 24 h (37°C; 5% CO2)
prior to treatment with various concentrations (0, 2.5, 5.0, 7.5,
10.0, 12.5, 15, 17.5, 20 and 25 µmol/l) of GSK343 for an additional
48 h at 37°C with 5% CO2. A total of 5 mg/ml of MTT
(Beijing Solarbio Science & Technology Co., Ltd.) was added
into each well for an additional 4 h at 37°C with 5%
CO2. The purple MTT formazan precipitate was dissolved
in 200 µl DMSO and the absorption value was measured using a
spectrophotometer at 570 nm. Curves were fitted and analyzed with
GraphPad software version 6.0 to calculate the IC50 value (GraphPad
Software, Inc.).
Cell counting kit-8 (CCK-8) assay
AsPC-1 and PANC-1 cells were seeded at a density of
3×103 cells per well into 96-well plates 24 h (37°C; 5%
CO2) prior to treatment with the IC50 concentration of
GSK343 (12.71 µmol/l for AsPC-1, 12.04 µmol/l for PANC-1) at 37°C
with 5% CO2 for 0, 24, 48 and 72 h. A total of 10 µl
CCK-8 (Dojindo Molecular Technologies, Inc.) was subsequently added
to each well for an additional 2 h at 37°C with 5% CO2,
and optical density (OD450) was measured using a
multi-well plate reader.
EdU viability assay
A Cell-Light™ EdU Apollo567 In Vitro Imaging
kit (Guangzhou RiboBio Co., Ltd.) was used to evaluate the effect
of GSK343, according to the manufacturer's protocol. Following
treatment with IC50 concentration of GSK343 (12.71 µmol/l for
AsPC-1; 12.04 µmol/l for PANC-1) and 20 µmol/l of GSK343 for 48 h,
the proportion of AsPC-1 and PANC-1 cells that incorporated EdU,
was observed using a fluorescence microscope (Olympus IX71; Olympus
Corporation; magnification, ×200).
Flow cytometry analysis
For the analysis of apoptosis, AsPC-1 and PANC-1
cells were seeded at a density of 2×105 cells per well
in six-well plates. Cells of NC group were treated with 8 µl DMSO
per well. After treatment with IC50 concentration of GSK343 (12.71
µmol/l for AsPC-1; 12.04 µmol/l for PANC-1) and 20 µmol/l of
GSK343, cells were harvested and double stained with 5 µl Annexin
V-FITC and 5 µl PI (Annexin V, FITC Apoptosis Detection kit; cat.
no. AD10; Dojindo Molecular Technologies, Inc.) in the dark. For
the assessment of cell cycle distribution, cells were collected and
fixed in 75% ethanol overnight at 4°C after treatment with GSK343
for 48 h. Upon completion of treatment, cells were washed with PBS
and resuspended in 500 µl of staining solution (Cell Cycle Staining
kit; cat. no. CCS012; Hangzhou Multi Sciences Biotech Co., Ltd.) at
37°C for 30 min. Finally, apoptosis and cell cycle distribution
were detected using flow cytometry (CYTOMICS FC 500; Beckman
Coulter, Inc.). Flowjo software 7.6.1 (FlowJo LLC) and Modifit LT
software 3.1 (Verity Software House, Inc.) were used to analyze the
data of apoptosis and cell cycle.
Immunofluorescence microscopy
After treatment with IC50 concentration of GSK343
(12.71 µmol/l for AsPC-1; 12.04 µmol/l for PANC-1) for 48 h, AsPC-1
and PANC-1 cells were fixed in 100% methanol for 10 min at −20°C,
washed with PBST, blocked using 10% FBS at 37°C for 1 h and
subsequently incubated with rabbit polyclonal antibody against
LC3B, which includes LCBI and LC3BII (1:100) overnight at 4°C.
Fluorochrome-conjugated anti-rabbit IgG (1:100) was added to slides
and incubated at 37°C for 1 h, after which DAPI counterstaining was
performed at 37°C for 20 min and observations were made using a
fluorescence microscope (Olympus IX71; magnification, ×400).
Western blot analysis
Western blotting was performed as previously
described (29). AsPC-1 and PANC-1
cells were harvested and dissolved in a 100 µl mixture of
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Inc.) and complete protease inhibitor cocktail
(Roche Diagnostics) for 30 min so that total protein could be
extracted. Protein concentration was then measured using a BCA
protein assay kit (cat. no. 23250; Thermo Fisher Scientific, Inc.).
Equal quantities of protein (20 µg) were subsequently separated on
10% NuPAGE Bis-Tris gels (Invitrogen; Thermo Fisher Scientific,
Inc.) for electrophoresis and transferred to 0.45 µm PVDF membranes
for 60 min. Membranes were then blocked using 5% non-fat milk at
37°C for 1 h and incubated with primary antibodies against GAPDH,
LC3B, AKT, phospho-AKT, ATG5, mTOR and phospho-mTOR at 1:1,000
overnight at 4°C. Membranes were then incubated with horseradish
peroxidase-conjugated anti-rabbit or anti-mouse secondary
antibodies at 1:2,000 for 1 h at room temperature and protein bands
were detected using enhanced chemiluminescence (cat. no. G2014;
Wuhan Servicebio Technology Co., Ltd.). Band intensity of LC3B-I
and LC3B-II was quantified using Image Lab software 5.2.1 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data were analyzed using SPSS software 24 (IBM
Corp.) and figures were created using GraphPad software 6.0
(GraphPad Software, Inc.). The results were expressed as the mean ±
standard deviation from three independent experiments. A one-way
ANOVA was performed followed by a Tukey HSD post-hoc test to make
comparisons between multiple groups. Comparisons between two groups
were analyzed using a Student's t-test. P<0.05 was considered to
indicate a statistically significant result.
Results
GSK343 inhibits cell viability and
suppresses cell proliferation
GSK343, an inhibitor of EZH2, has been regarded to
be a therapeutic target for various types of cancer. In the present
study, the effect of GSK343 on the viability of pancreatic cancer
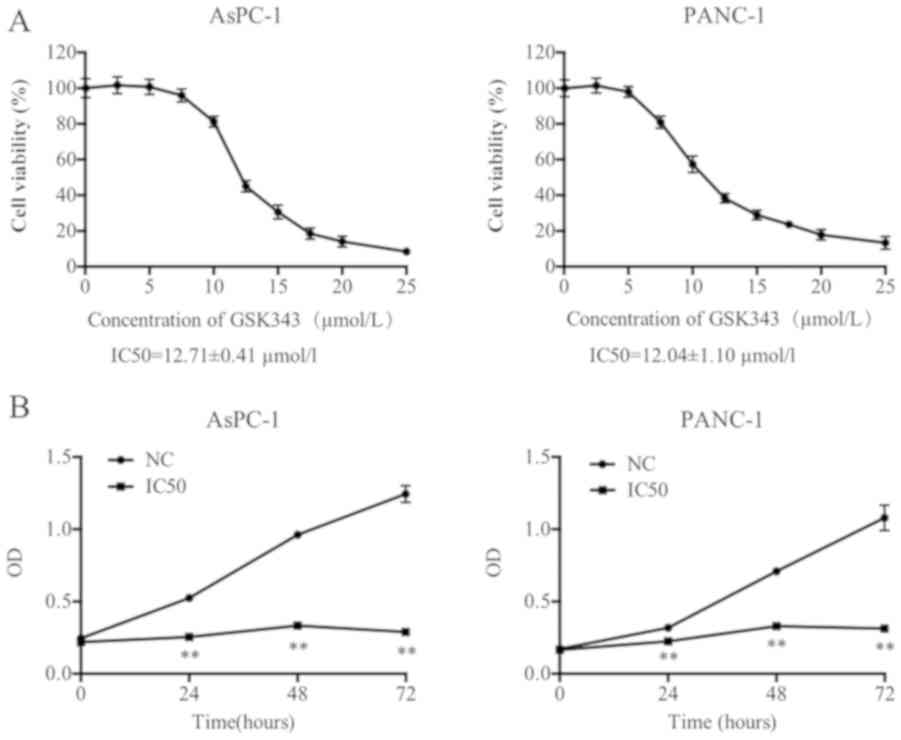
cells (AsPC-1 and PANC-1) was evaluated using an MTT assay. As
presented in Fig. 1A, the viability
of each pancreatic cancer cell line markedly decreased with
increasing GSK343 concentration. The IC50 values of AsPC-1 and
PANC-1 (12.71±0.41 and 12.04±1.10 µmol/l) revealed that neither
pancreatic cell line exhibited a statistically significant
difference between values.
The IC50 was selected as a suitable concentration
for pancreatic cancer cell treatment and viability after incubation
with GSK343 for 24, 48 and 72 h using a CCK-8 assay. As presented
in Fig. 1B, compared with NC, GSK343
(IC50) significantly reduced the OD values from 24 to 72 h. The
difference values were gradually increased, which revealed that
GSK343 may inhibit cell viability in AsPC-1 and PANC-1 cells in a
time-dependent manner.
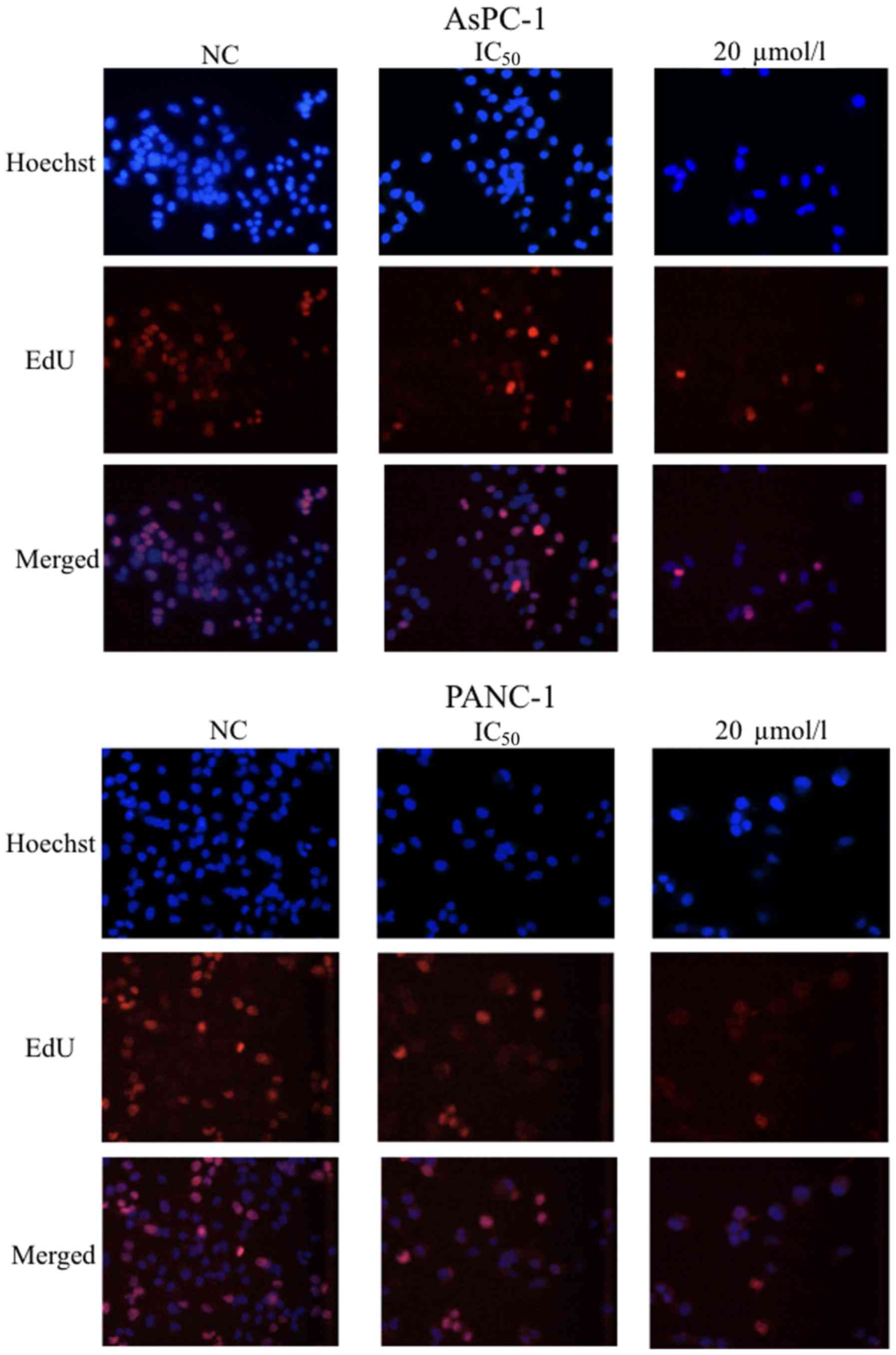
To demonstrate the effects of GSK343 on cell
proliferation, an EdU assay was performed using AsPC-1 and PANC-1
cells after treatment with the IC50 for the respective cell lines
and 20 µmol/l of GSK343 for 48 h. The percentage of EdU-positive
cancer cells (in AsPC-1 and PANC-1 lines) decreased markedly after
treatment with GSK343 (Fig. 2).
Therefore, GSK343 suppressed the proliferation of pancreatic cancer
cells in a dose-dependent manner.
GSK343 induces cell apoptosis and G1
arrest
To determine the underlying mechanisms that result
in the loss of cell viability and the suppression of cell
proliferation, apoptosis and cell cycle distribution were assessed
using flow cytometry. AsPC-1 and PANC-1 were treated with different
concentrations of GSK343 (0 µmol/l; IC50 for the respective cell
lines; 20 µmol/l).
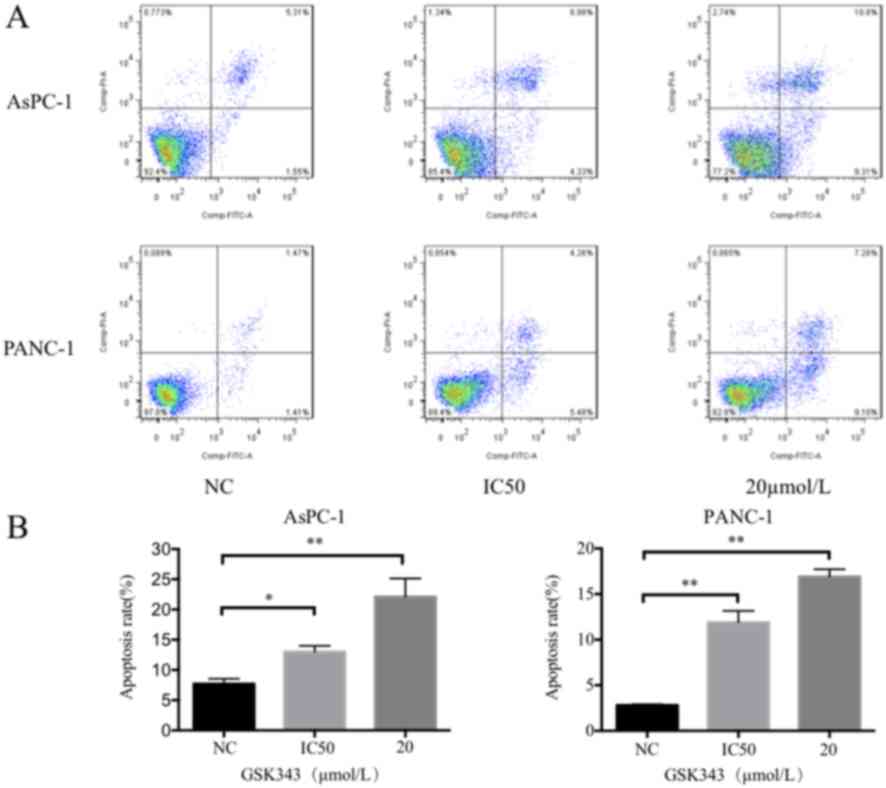
As presented in Fig.
3, the Annexin V-FITC/PI assay results reveal that GSK343
treatment induces significant cell apoptosis compared with the
untreated pancreatic cancer cells. One representative result was
selected, which is presented in Fig.
3A. In AsPC-1 cells, the IC50 dose and 20 µmol/l of GSK343
increased the percentage of apoptotic cells from 6.86 to 13.31 and
20.11%, respectively. The percentage of PANC-1 apoptotic cells
after treatment with GSK343, the IC50 concentration and 20 µmol/l
increased from 2.88 to 9.74 and 17.85%, respectively.
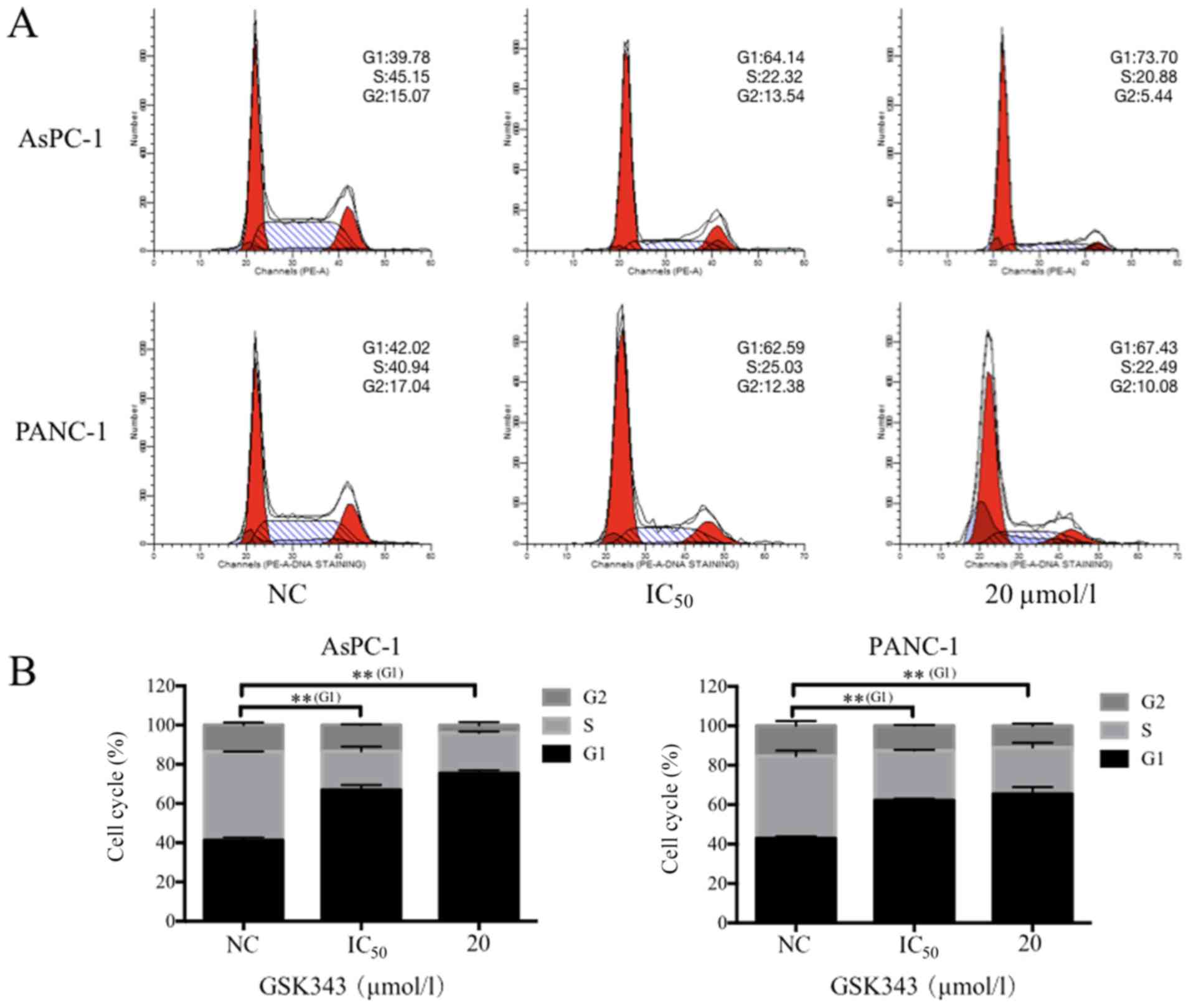
In Fig. 4, following
GSK343 treatment, an increase in the percentage of cells in
G1-phase and a reduction in S-phase cells was observed in AsPC-1
and PANC-1 cells. The IC50 of GSK343 increased the percentage of
AsPC-1 and PANC-1 cells in G1-phase from 39.78 to 64.14% and 42.02
to 62.59% (Fig. 4A), respectively.
GSK343 (IC50) also reduced the percentage of AsPC-1 and PANC-1
cells in S-phase from 45.15 to 22.32% and 40.94 to 25.03% (Fig. 4A). A concentration of 20 µmol/l of
GSK343 decreased the percentage of AsPC-1 in the G2-phase from
15.07 to 5.44% (Fig. 4A). In
Fig. 4B, compared with the NC, the
IC50 concentration and 20 µmol/l group exhibited significantly
increased percentages of G1-phase cells. However, 20 µmol/l of
GSK343 did not lead to an increase of pancreatic cancer cells in
the G1 phase compared with the IC50 concentration.
GSK343 induces autophagy in pancreatic
cancer cells
Autophagy is a process in which cytoplasmic proteins
and organelles are delivered to the lysosome for digestion. Various
studies have reported the induction of autophagy by GSK343 in
osteosarcoma cells, as well as in breast, liver and colorectal
cancer (30–32). Autophagy induction was analyzed using
immunofluorescence for microtubule-associated protein 1 light chain
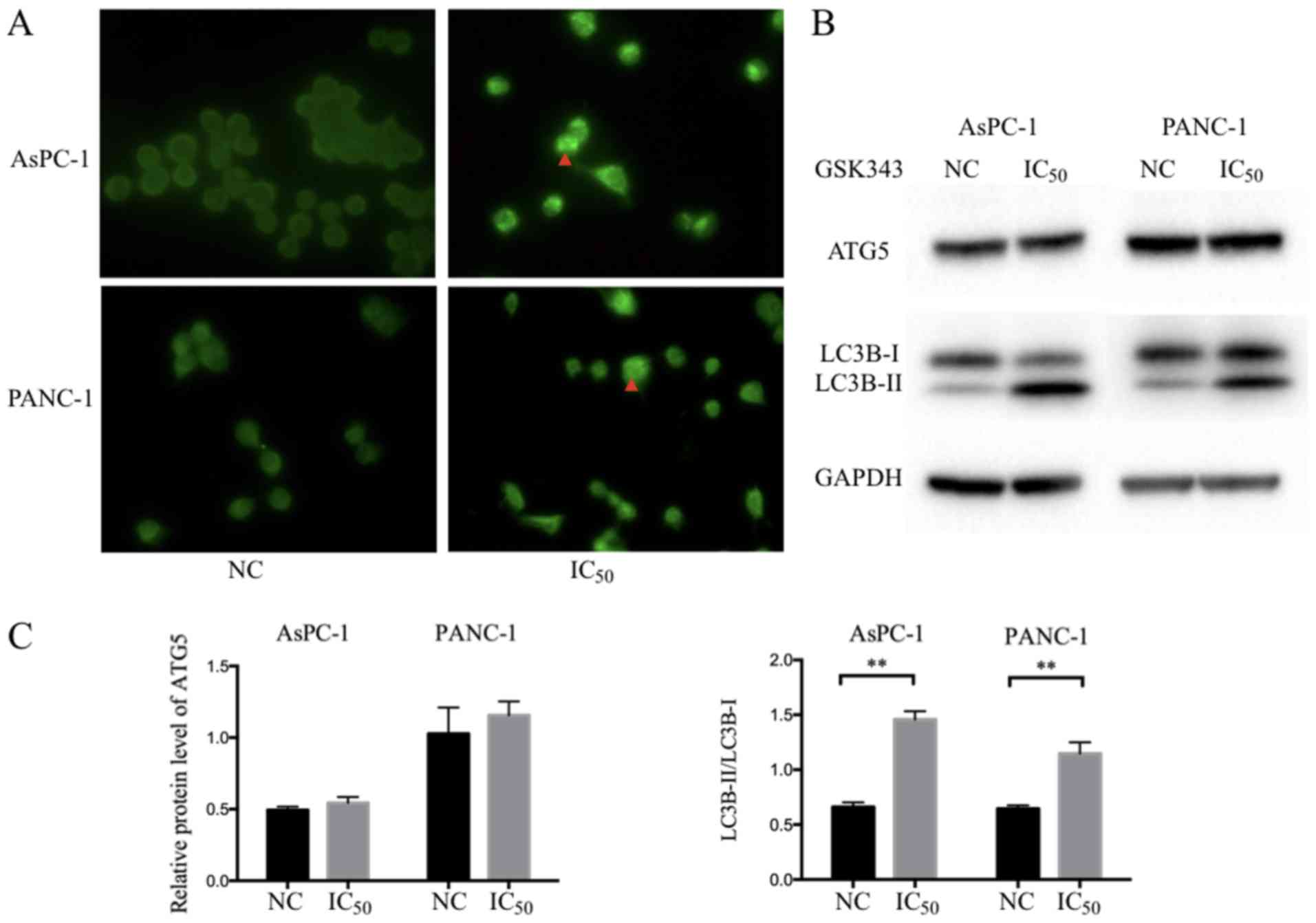
3 (LC3) positive punctate dots in GSK343 treatments. As presented
in Fig. 5A, LC3B punctate dots per
cell (indicated by the red arrow) were markedly increased after
GSK343 (IC50) treatment.
LC3 has been considered to be a crucial component in
the elongation and closure of autophagosome membranes. Accompanied
with this process, LC3 is converted from LC3-I to LC3-II (33). Therefore, autophagy was assessed
using the LC3-II/LC3-I ratio (34).
In Fig. 5B, following GSK343 (IC50)
treatment, a reduction of LC3B-I in AsPC-1 cell was exhibited, but
no significant change was observed in PANC-1 cells. However,
LC3B-II was markedly increased in both cell types. Western blot
analysis revealed that GSK343 (IC50) treatment significantly
increased the ratio of LC3B-II/LC3B-I in AsPC-1 and PANC-1 cells
(Fig. 5C).
ATG5, which can form the Atg12-Atg5-Atg16L complex,
is also the crucial component of the ubiquitin-like conjugation
system and promotes autophagosome formation (35). ATG5 did not reveal any significant
change after treatment with GSK343 (IC50) in AsPC-1 or PANC-1 cell
lines (Fig. 5B).
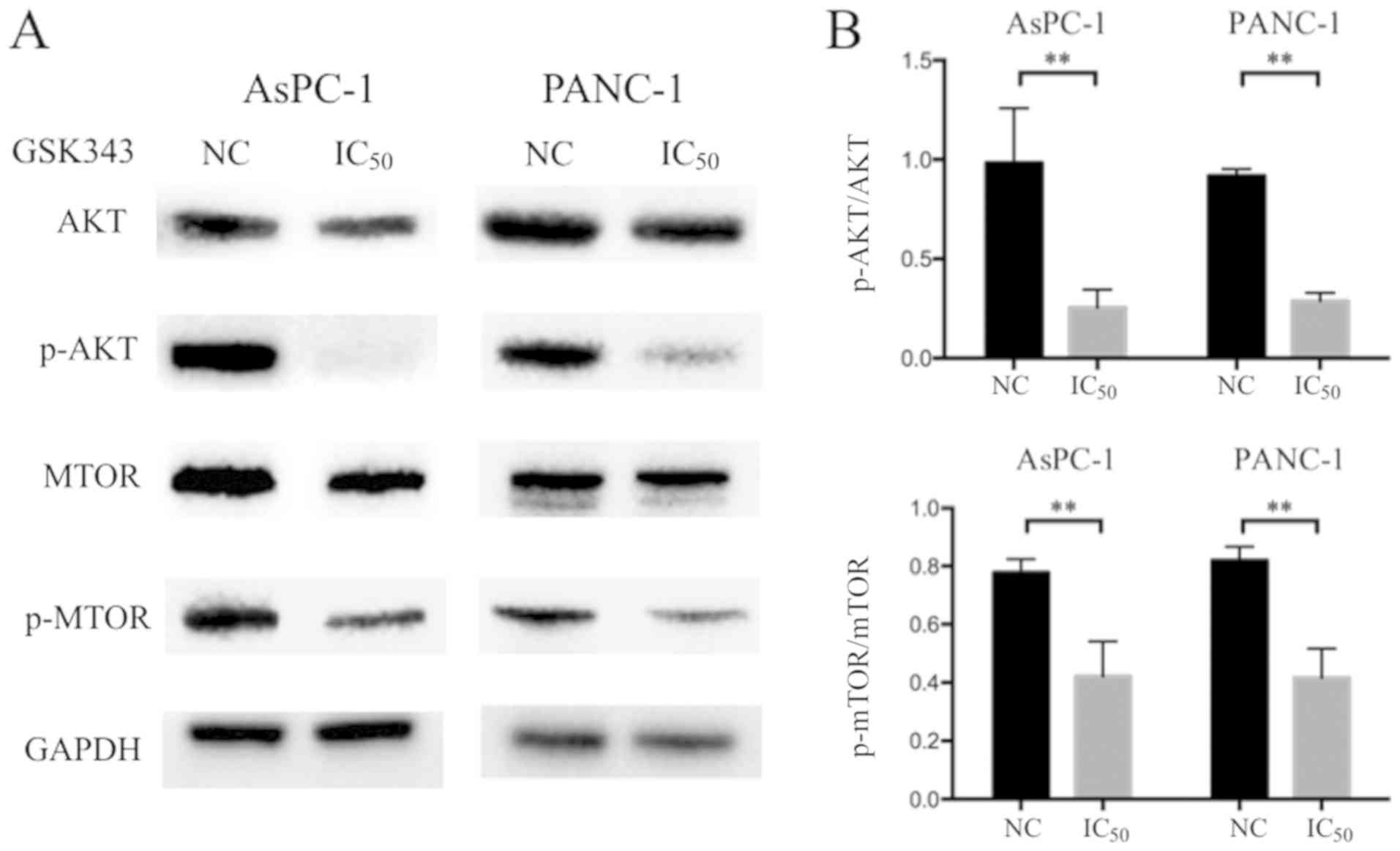
GSK343 inhibits the AKT/mTOR signaling
pathway
The AKT/mTOR signaling pathway is reported to be
activated in a variety of different types of cancer and negatively
regulates autophagy (36). To
identify the underlying signaling mechanism responsible for
GSK343-induced autophagy, the role of the AKT/mTOR signaling
pathway was investigated using western blot analysis. As presented
in Fig. 6A and B, a marked reduction
was observed in AKT and p-AKT results revealed a significant
decrease in pancreatic cancer cells. Similarly, although the total
mTOR level was variable in treated cells, p-mTOR was significantly
reduced after GSK343 (IC50) treatment. The results indicate that
GSK343 may downregulate the AKT/mTOR-mediated signaling
pathway.
Discussion
EZH2 has been demonstrated to be highly expressed in
malignant tumors, serving a crucial role in the progression of
breast, bladder and liver malignancies (19–21).
Therefore, inhibitors of EZH2 are of therapeutic interest in cancer
research. Distinct from DZNep, a classic EZH2 inhibitor that
decreases SAH, GSK343 suppresses EZH2 by competitively binding to a
methyl donor, SAM (17,18). In the present study, a significant
dose and time-dependent reduction in the cell viability of
pancreatic cancer cells by GSK343 was demonstrated. GSK343 also
suppressed cell proliferation, induced cell apoptosis and blocked
cell cycle progression at the G1-phase. These results are
consistent with observations in a variety of different types of
cancer and may help to identify a novel therapeutic approach for
pancreatic cancer.
Autophagy is an important cellular process that
digests endogenous proteins or organelles via lysosomal degradation
and is divided into macroautophagy, microautophagy and
chaperone-mediated autophagy (37).
Several studies have established that autophagy serves a crucial
role in cell development and in the maintenance of homeostasis
(37,38). While autophagy can recycle damaged
and/or dysfunctional cellular components (organelles and
macromolecules) to provide raw materials for cell reconstruction
and regeneration, the excessive upregulation of autophagy may
result in autophagic cell death, also called type II programmed
cell death (38,39). mTOR, a highly evolutionarily
conserved protein kinase, serves a key role in regulating autophagy
and may be associated with epigenetic regulatory mechanisms
(40,41). In the present study, the GSK343
induced autophagy of pancreatic cancer cells was observed using
immunofluorescence microscopy and western blot analysis. Although
GSK343 induces autophagy in pancreatic cancer, as a direct and
selective inhibitor of EZH2, whether EZH2 serves a role in
GSK343-induced autophagy is controversial. A previous study
demonstrated that EZH2 inhibited autophagy by epigenetically
repressing the mTOR signaling pathway (42). However, a previous report also
demonstrated that DZNep could not induce autophagy in breast, lung
and hepatocellular carcinoma cells, and that the suppression of
EZH2 expression was unnecessary for GSK343 to induce autophagy
(30). The knockdown of EZH2 by
small interfering RNA cannot induce autophagy sufficiently and its
overexpression is unable to reduce autophagy, which is induced by
EZH2 inhibitors in human colorectal cancer cells (31). GSK343-induced autophagy may be
associated with the non-canonical pathway-mediated transcriptional
upregulation of LC3B (31). These
contrasting results require further investigation into the
signaling mechanisms which govern the GSK343 induction of autophagy
in a variety different types of cancer.
The AKT/mTOR signaling pathway is activated in
different types of cancer and is a critical cellular pathway.
Upstream AKT kinase can repress the Tuberous Sclerosis complex
(TSC) 1/2 by phosphorylating TSC2 to promote mTOR activity, which
regulates cell proliferation, differentiation, apoptosis and
metabolism, and inhibits autophagy (40). In the current study, the mechanism of
GSK343-induced autophagy in pancreatic cancer cells was
investigated. Western blot analysis revealed a reduction in p-AKT
and p-mTOR after GSK343 treatment. These results indicated that
GSK343-induced autophagy involved the AKT/mTOR-mediated signaling
pathway in pancreatic cancer cells. However, the role of GSK343 in
the inhibition of the AKT pathway is unclear. A previous study has
revealed that increasing EZH2 can influence H3K27me3 and DNA
methylation across the promoter to silence phosphatase and tensin
homolog deleted on chromosome 10, which dephosphorylates
phosphatidylinositol 3,4,5-triphosphate to inactivate AKT and mTOR
complex 1 (43). Studies have also
revealed that EZH2 inhibitors may induce endoplasmic reticulum
stress, which negatively regulates the AKT/TSC/mTOR pathway to
enhance autophagy (31,44). Further experiments should be
performed to support the mechanism of GSK343-induced autophagy in
pancreatic cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81572307 and
81773096) and the Major Project of Medical and Health Technology
Development Program in Zhejiang Province (grant no. 7211902).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HX and LZ designed the present study. HX conducted
the experiments, analyzed the data and drafted the manuscript. XQ,
YY and JZ performed cell culture and flow cytometry analysis. XZ
performed immunofluorescence microscopy. WG and MA conducted the
western blot analysis. WW conceived the current study, and drafted
and revised the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Margueron R and Reinberg D: The Polycomb
complex PRC2 and its mark in life. Nature. 469:343–349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Croce L and Helin K: Transcriptional
regulation by Polycomb group proteins. Nat Struct Mol Biol.
20:1147–1155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bracken AP, Pasini D, Capra M, Prosperini
E, Colli E and Helin K: EZH2 is downstream of the pRB-E2F pathway,
essential for proliferation and amplified in cancer. EMBO J.
22:5323–5335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sauvageau M and Sauvageau G: Polycomb
group proteins: Multi-faceted regulators of somatic stem cells and
cancer. Cell Stem Cell. 7:299–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Croonquist PA and Van Ness B: The polycomb
group protein enhancer of zeste homolog 2 (EZH 2) is an oncogene
that influences myeloma cell growth and the mutant ras phenotype.
Oncogene. 24:6269–6280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kleer CG, Cao Q, Varambally S, Shen R, Ota
I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:11606–11611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chien YC, Liu LC, Ye HY, Wu JY and Yu YL:
EZH2 promotes migration and invasion of triple-negative breast
cancer cells via regulating TIMP2-MMP-2/-9 pathway. Am J Cancer
Res. 8:422–434. 2018.PubMed/NCBI
|
|
12
|
Bachmann IM, Halvorsen OJ, Collett K,
Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP and
Akslen LA: EZH2 expression is associated with high proliferation
rate and aggressive tumor subgroups in cutaneous melanoma and
cancers of the endometrium, prostate, and breast. J Clin Oncol.
24:268–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zingg D, Debbache J, Peña-Hernández R,
Antunes AT, Schaefer SM, Cheng PF, Zimmerli D, Haeusel J, Calçada
RR, Tuncer E, et al: EZH2-mediated primary cilium deconstruction
drives metastatic melanoma formation. Cancer Cell. 34:69–84.e14.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakagawa M and Kitabayashi I: Oncogenic
roles of enhancer of zeste homolog 1/2 in hematological
malignancies. Cancer Sci. 109:2342–2348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ougolkov AV, Bilim VN and Billadeau DD:
Regulation of pancreatic tumor cell proliferation and
chemoresistance by the histone methyltransferase enhancer of zeste
homologue 2. Clin Cancer Res. 14:6790–6796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maftouh M, Avan A, Funel N, Paolicchi E,
Vasile E, Pacetti P, Vaccaro V, Faviana P, Campani D, Caponi S, et
al: A polymorphism in the promoter is associated with EZH2
expression but not with outcome in advanced pancreatic cancer
patients. Pharmacogenomics. 15:609–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Verma SK, Tian X, LaFrance LV, Duquenne C,
Suarez DP, Newlander KA, Romeril SP, Burgess JL, Grant SW, Brackley
JA, et al: Identification of potent, selective, cell-active
inhibitors of the histone lysine methyltransferase EZH2. ACS Med
Chem Lett. 3:1091–1096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Glazer RI, Hartman KD, Knode MC, Richard
MM, Chiang PK, Tseng CK and Marquez VE: 3-Deazaneplanocin: A new
and potent inhibitor of S-adenosylhomocysteine hydrolase and its
effects on human promyelocytic leukemia cell line HL-60. Biochem
Biophys Res Commun. 135:688–694. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raaphorst FM, Meijer CJ, Fieret E,
Blokzijl T, Mommers E, Buerger H, Packeisen J, Sewalt RA, Otte AP
and van Diest PJ: Poorly differentiated breast carcinoma is
associated with increased expression of the human polycomb group
EZH2 gene. Neoplasia. 5:481–488. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raman JD, Mongan NP, Tickoo SK, Boorjian
SA, Scherr DS and Gudas LJ: Increased expression of the polycomb
group gene, EZH2, in transitional cell carcinoma of the bladder.
Clin Cancer Res. 11:8570–8576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sudo T, Utsunomiya T, Mimori K, Nagahara
H, Ogawa K, Inoue H, Wakiyama S, Fujita H, Shirouzu K and Mori M:
Clinicopathological significance of EZH2 mRNA expression in
patients with hepatocellular carcinoma. Br J Cancer. 92:1754–1758.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Xie D, Yin Li W, Man Cheung C, Yao
H, Chan CY, Chan CY, Xu FP, Liu YH, Sung JJ and Kung HF: RNAi
targeting EZH2 inhibits tumor growth and liver metastasis of
pancreatic cancer in vivo. Cancer Lett. 297:109–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsukawa Y, Semba S, Kato H, Ito A,
Yanagihara K and Yokozaki H: Expression of the enhancer of zeste
homolog 2 is correlated with poor prognosis in human gastric
cancer. Cancer Sci. 97:484–491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan J, Yang X, Zhuang L, Jiang X, Chen W,
Lee PL, Karuturi RK, Tan PB, Liu ET and Yu Q: Pharmacologic
disruption of Polycomb-repressive complex 2-mediated gene
repression selectively induces apoptosis in cancer cells. Genes
Dev. 21:1050–1063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirukawa A, Smith HW, Zuo D, Dufour CR,
Savage P, Bertos N, Johnson RM, Bui T, Bourque G, Basik M, et al:
Targeting EZH2 reactivates a breast cancer subtype-specific
anti-metastatic transcriptional program. Nat Commun. 9:25472018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Ai Z, Chen J, Teng Y and Zhu J:
Enhancer of zeste homolog 2 blockade by RNA interference is
implicated with inhibited proliferation, invasion and promoted
apoptosis in endometrial carcinoma. Oncol Lett. 15:9429–9435.
2018.PubMed/NCBI
|
|
27
|
Jones BA, Varambally S and Arend RC:
Histone methyltransferase EZH2: A therapeutic target for ovarian
cancer. Mol Cancer Ther. 17:591–602. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mohammad F, Weissmann S, Leblanc B, Pandey
DP, Højfeldt JW, Comet I, Zheng C, Johansen JV, Rapin N, Porse BT,
et al: EZH2 is a potential therapeutic target for H3K27M-mutant
pediatric gliomas. Nat Med. 23:483–492. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Xie Q, Zhou X, Yao J, Zhu X, Huang
P, Zhang L, Wei J, Xie H, Zhou L and Zheng S: Mitofusin-2 triggers
mitochondria Ca2+ influx from the endoplasmic reticulum to induce
apoptosis in hepatocellular carcinoma cells. Cancer Lett.
358:47–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu TP, Lo HL, Wei LS, Hsiao HH and Yang
PM: S-Adenosyl-L-methionine-competitive inhibitors of the histone
methyltransferase EZH2 induce autophagy and enhance drug
sensitivity in cancer cells. Anticancer Drugs. 26:139–147. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsieh YY, Lo HL and Yang PM: EZH2
inhibitors transcriptionally upregulate cytotoxic autophagy and
cytoprotective unfolded protein response in human colorectal cancer
cells. Am J Cancer Res. 6:1661–1680. 2016.PubMed/NCBI
|
|
32
|
Xiong X, Zhang J, Liang W, Cao W, Qin S,
Dai L, Ye D and Liu Z: Fuse-binding protein 1 is a target of the
EZH2 inhibitor GSK343, in osteosarcoma cells. Int J Oncol.
49:623–628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mizushima N, Noda T, Yoshimori T, Tanaka
Y, Ishii T, George MD, Klionsky DJ, Ohsumi M and Ohsumi Y: A
protein conjugation system essential for autophagy. Nature.
395:395–398. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao GS, Gao ZR, Zhang Q, Tang XF, Lv YF,
Zhang ZS, Zhang Y, Tan QL, Peng DB, Jiang DM and Guo QN: TSSC3
promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR
pathway to suppress tumorigenesis and metastasis in osteosarcoma
and predicts a favorable prognosis. J Exp Clin Cancer Res.
37:1882018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mizushima N: A brief history of autophagy
from cell biology to physiology and disease. Nat Cell Biol.
20:521–527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Berry DL and Baehrecke EH: Autophagy
functions in programmed cell death. Autophagy. 4:359–360. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei FZ, Cao Z, Wang X, Wang H, Cai MY, Li
T, Hattori N, Wang D, Du Y, Song B, et al: Epigenetic regulation of
autophagy by the methyltransferase EZH2 through an MTOR-dependent
pathway. Autophagy. 11:2309–2322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jarome TJ, Perez GA, Hauser RM, Hatch KM
and Lubin FD: EZH2 methyltransferase activity controls pten
expression and mTOR signaling during fear memory reconsolidation. J
Neurosci. 38:7635–7648. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qin L, Wang Z, Tao L and Wang Y: ER stress
negatively regulates AKT/TSC/mTOR pathway to enhance autophagy.
Autophagy. 6:239–247. 2010. View Article : Google Scholar : PubMed/NCBI
|