Introduction
Alzheimer's disease (AD) is a chronic age-associated
neurodegenerative disorder and the leading cause of dementia in
elderly individuals (1,2). AD is characterized by cognitive
dysfunction and memory loss (3). It
is estimated that the global prevalence of dementia has reached as
high as 24 million, which may double every 20 years, i.e. by 2040
(4). The pathogenic mechanisms of AD
include the increase of inflammation, death of neurons and atrophy
of the brain (5). The clinical
pathology of AD is aberrant amyloid protein deposition of amyloid β
(Aβ)42, as a result of abnormal amyloid precursor protein
processing. To date, the etiological mechanisms underlying the
neuropathological changes of AD remain to be fully elucidated and
diagnostic biomarkers for the early detection of AD remain limited
(6). Thus, it is of great
significance to identify more AD-associated genes and further
develop screening methods for these genes to improve the diagnosis
and therapy for AD.
MicroRNAs (miRNAs/miRs) are a class of small
non-coding RNAs with a length of 20–24 nucleotides (7). miRNAs regulate the degradation or
translational repression of their target mRNAs through binding to
their 3′-untranslated region (UTR) (8). Dysregulation of miRNA has been reported
to participate in multiple biological and pathological processes,
including AD. For instance, Kumar et al (9) reported that miR-455-3p was upregulated
in AD patients compared with that in healthy controls, suggesting
the potential role of miR-455-3p in the pathogenesis of AD. Hong
et al (10) indicated that
miR-125b, miR-9, miR-191-5p and miR-28-3p may be potential
biomarkers for AD, and their expression levels were validated in
SH-SY5Y cells and in a mouse model.
miR-133b is regarded as a specific miRNA of the
muscle, and it may be involved in myoblast differentiation and
certain myogenic diseases (11). Of
note, miR-133b has been indicated to have the ability to inhibit
the maturation and function of midbrain dopaminergic neurons in
primary rat midbrain cultures (12).
AD and Parkinson's disease (PD) all belong to neurodegenerative
diseases, which are characterized by neurodegenerative changes and
brain dysfunction (13).
Furthermore, multiple studies have proved the abnormal expression
of miR-133b in PD patients, indicating the crucial role of miR-133b
in this neurodegenerative disease (14–16).
However, to date, the expression and role of miR-133b in the
development of AD have remained to be fully determined.
Epidermal growth factor receptor (EGFR), also named
as ErbB-1 or HER1 in humans, belongs to the ErbB family. It is a
significant transmembrane receptor and has been reported to have
tyrosine kinase activity (17). EGFR
may be involved in various cellular processes, including cell
survival, differentiation, proliferation, migration and repair of
cell damage (18,19). Furthermore, the key ligands of the
EGFR protein, EGFs, has been indicated to regulate brain
development, and control the survival and function of nerve cells,
suggesting a potential role of EGFR in the development of AD
(20). Furthermore, in esophageal
squamous cell carcinoma (ESCC), miR-133b was proved to inhibit cell
cycle progression and bioinformatics analysis indicated that there
was a binding site of miR-133b on the 3′-UTR of EGFR (21). Thus, it may be hypothesized that EGFR
is a target gene of miR-133b in AD.
In the present study, the expression and diagnostic
value of miR-133b in patients with AD were assessed, and its
neuroprotective role in this disease was further explored.
Materials and methods
Study population
The protocol of the present study was reviewed and
approved by the Ethics Committee of Dongying People's Hospital
(Dongcheng, China). Written informed consent was obtained from each
participant.
A total of 105 patients with AD were recruited for
the present study, who were diagnosed according to the National
Institute on Aging-Alzheimer's Association criteria (1). All patients were aged between 60 and 85
years, and were admitted to Dongying People's Hospital between July
2014 and January 2017 (Dongcheng, China). Patients fulfilling the
following criteria were excluded from the study: i) Patients
receiving heparin therapy at the time of the blood collection, as
heparin may interfere with RNA isolation; ii) patients who had
other diseases/conditions, including acute myocardial infarction,
cancer, PD and multiple sclerosis (since these diseases may give
rise to expression differences of miRNA) (22). A total of 98 age- and gender-matched
healthy individuals were randomly selected as a control group when
receiving a routine physical examination. Each enrolled healthy
control had normal neurological function. The neurological function
of the controls was determined according to their clinical
features, laboratory examination results and Mini-Mental State
Examination (MMSE) scores. High blood pressure (HBP), diabetes
mellitus (DM), coronary atherosclerotic heart disease (CAHD) and
hyperlipidaemia (HLP) were diagnosed according to the current
diagnostic criteria (23–26). The MMSE score was determined for each
participant to assess cognitive and functional impairment (27). As presented in Table I, the AD group had a mean MMSE score
of 20.48±5.42, while the control group had a higher mean MMSE score
of 29.00±0.64.
 | Table I.Demographic and clinical
characteristics of the control and AD group. |
Table I.
Demographic and clinical
characteristics of the control and AD group.
| Variable | Control group
(n=98) | AD group
(n=105) | P-value |
|---|
| Age (years) | 75.38±7.11 | 76.46±5.87 | 0.242 |
| Gender (F/M) | 44/54 | 45/60 | 0.770 |
| MMSE scores | 29.00±0.64 | 20.48±5.42 | <0.001 |
| Other
diagnoses |
|
|
|
| HBP
(yes/no) | 42/56 | 49/56 | 0.585 |
| DM
(yes/no) | 48/50 | 52/53 | 0.938 |
| CAHD
(yes/no) | 46/52 | 49/56 | 0.969 |
| HLP
(yes/no) | 38/60 | 45/60 | 0.554 |
Sample collection
A total of 5 ml peripheral blood was collected from
each patient after fasting for 12 h. The serum was immediately
centrifuged at 3,000 × g for 10 min at room temperature, followed
by centrifugation at 12,000 × g for 5 min at 4°C. The separated
plasma was stored at −80°C until analysis. The serum samples were
examined by measurement of miR-133b expression ~two weeks from the
time-point of sample collection and the data were collected.
Cell culture and transfection
The human neuroblastoma cell line SH-SY5Y was
purchased from the American Type Culture Collection. It was
maintained in high-glucose Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 15% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and
penicillin/streptomycin (100 µg/ml; Keygen Biotechnology). The
cells were cultured in a humidified incubator with 5%
CO2 at 37°C. The medium was changed every day during
cell growth. When the cells reached 80–90% confluence, the cell
suspension was sub-cultured on a scale of 1:3. For the Aβ group,
cells were seeded into a 96-well plate at a density of
1×104 cells per well, and after culture for 24 h, medium
supplemented with Aβ25–35 (40 µM/l; Sigma-Aldrich; Merck KGaA) was
added and the cells were cultured for another 48 h (28). Prior to use, Aβ25–35 was dissolved in
sterile physiological saline and incubated at 37°C for one week to
allow for fibril formation. Medium containing an equal volume of
normal saline was used for the negative control. Each group was
independently repeated five times.
The miR-133b mimics, miR-133b inhibitor and the
corresponding negative controls of mimics and inhibitor (mimics NC
and inhibitor NC) were provided GenePharma. The sequences were as
follows: miR-133b mimics, 5′-UUUGGUCCCCUUCAACCAGCUA-3′; miR-133b
inhibitor, 5′-UAGCUGGUUGAAGGGGACCAAA-3′; mimics NC,
5′-UGUAGGGCCACUCAGUCAACUU-3′; inhibitor NC,
5′-AAUAUGGGCGAAAUGGGGCCAUC-3′. For the different transfection
groups, SH-SY5Y cells were seeded into a 96-well plate at the
density of 1×104 cells per well. After culture for 24 h,
cells were first transfected with 100 nM miR-133b mimics, miR-133b
inhibitor or corresponding mimics NC or inhibitor NC at room
temperature and then treated with Aβ25–35 for 48 h. The
transfection was performed using X-treme GENEHPDNA transfection
reagent (Roche) according to the manufacturer's protocol. At 48 h
post-transfection, the cells were harvested for the subsequent
experiments. The overexpression/knockdown efficiency was determined
by reverse transcription-quantitative (RT-q)PCR.
RNA extraction and RT-qPCR
The total RNA was extracted from the serum of all
enrolled participants using the miRVana miRNA Isolation Kit
(Ambion; Thermo Fisher Scientific, Inc.), while RNA was isolated
from SH-SY5Y cells of the Aβ25–35 group and control groups using
TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) based
on the manufacturer's protocol. RT reactions were performed using
the miScript Reverse Transcription Kit (Qiagen) with the following
reaction conditions: Incubation at 42°C for 60 min and at 85°C for
5 min. Real-time PCR was performed with the SYBR green I Master Mix
kit (Invitrogen; Thermo Fisher Scientific, Inc.) using the 7300
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with the following reaction conditions: Denaturation at 95°C
for 3 min and 45 PCR cycles (95°C for 15 sec, 60°C for 20 sec, 72°C
for 20 sec and 78°C for 20 sec). The 2−ΔΔCq method was
used to determine the relative expression of miR-133b and data were
normalized to U6 (29). The primer
sequences were as follows: miR-133b forward,
5′-GCGCTTTGGTCCCCTTC-3′ and reverse, 5′-CAGTGCAGGGTCCGAGGT-3′; U6
forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
MTT assay
The cell viability was measured using an MTT assay.
The stably transfected SH-SY5Y cells were seeded into a 96-well
plate at a density of 5×104 cells per well and cultured
for 24 h. The cells were then incubated with 20 µl MTT stock
solution (0.5 mg/ml; Sigma-Aldrich; Merck KGaA) and the supernatant
was removed after 4 h of further incubation at 37°C. Subsequently,
the precipitated formazan crystals were dissolved in a solution
containing 50% dimethyl sulfoxide (DMSO) (Sigma-Aldrich; Merck
KGaA). After 20 min, the absorbance was assessed at 490 nm using a
microplate reader (ELx800; Bio-Tek Instruments). The relative cell
viability was determined as the percentage of the control
group.
Apoptosis assay
An Annexin V-FITC Apoptosis Detection kit (Keygen
Biotechnology) was used for the assessment of cell apoptosis. Cells
of each group were collected and gently treated with EDTA-free
trypsin. Subsequently, all of the cells were centrifuged at 270 × g
for 5 min and washed twice with PBS. Subsequently, the cells were
re-suspended with the binding buffer and mixed with 5 µl Annexin
V-FITC and PI staining solution. After incubation at 2–8°C in the
dark for 10 min, the apoptotic rates were measured using a
FACSCalibur flow cytometer (BD Biosciences). CellQuest Pro 3.3
software (BD Biosciences) was applied for data analysis. The
apoptotic rates were determined as the sum of early and late
apoptotic rates, and experiments for each group were repeated in
three independent repetitions.
Luciferase reporter assay
The TargetScan analysis (version 7.1; www.targetscan.org) of the 3′-UTR of EGFR revealed a
putative binding site for miR-133b. To identify whether EGFR is a
target gene of miR-133b, the luciferase reporter gene assay was
performed. The 3′-UTR of the EGFR gene was amplified from human
genomic DNA using PCR and a mutation of the seed region in the
3′-UTR of EGFR mRNA was introduced using site-directed mutagenesis
with the megaprimer PCR method. The 3′-UTR of EGFR, the mutated PCR
fragment and the PmiR-RB-REPORT™ luciferase vector containing the
above fragment, were supplied by a service provider (Guangzhou
RiboBio Co. Ltd.). The dual-luciferase reporter assay was performed
as follows: Prior to transfection, SH-SY5Y cells were seeded into a
96-well plate at a density of 1×104 cells per well and
cultured for 24 h. Subsequently, SH-SY5Y cells were co-transfected
with 50 ng of either wild-type or mutant-type reporter vector and
200 ng miR-133b mimics or inhibitors and mimic NCs or inhibitor NCs
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). At 48 h post-transfection, the cells were harvested and
significant relative luciferase activity was measured using
Dual-Luciferase Reporter System (Promega Corp.) according to the
manufacturer's protocol. The luciferase activity was normalized to
that of the Renilla luciferase internal control.
Statistical analysis
SPSS version 18.0 software (SPSS Inc.) and GraphPad
Prism 5.0 software (GraphPad Software, Inc.) was applied for data
analysis. Demographic and clinical data were analyzed using an
independent-samples t-test or a chi-squared test. Differences
between two groups were analyzed by Student's t-test or a one-way
analysis of variance, followed by a Tukey's multiple-comparisons
test. The correlation between the MMSE score and miR-133b levels
was assessed by determining Spearman's correlation coefficient, as
the miR-133b levels in the patient and control groups did not
follow a normal distribution. Receiver operating characteristic
(ROC) curve analysis was applied to determine the specificity and
sensitivity of the miR-133b levels regarding the diagnosis of AD.
Values are expressed as the mean ± standard deviation. Each
experiment had at least three repetitions. P<0.05 was considered
to indicate statistical significance.
Results
Demographic and clinicopathological
characteristics of the study population
A total of 105 patients with AD were enrolled in the
present study (median age, 76.46±5.87 years; 45 females, 60 males).
A total of 98 healthy controls were also recruited (median age,
75.38±7.11 years; 44 females and 54 males). The demographic and
clinicopathological characteristics of the cohort are provided in
Table I. The AD and control groups
were matched regarding age and gender (P>0.05). The AD patients
had significantly lower MMSE score than the controls (P<0.05),
while the other parameters were not significantly different between
the AD group and the control group, including HBP, DM, CAHD and HLP
(P>0.05).
miR-133b expression levels in AD
patients and SH-SY5Y cells treated with Aβ25-35
The expression levels of miR-133b were determined
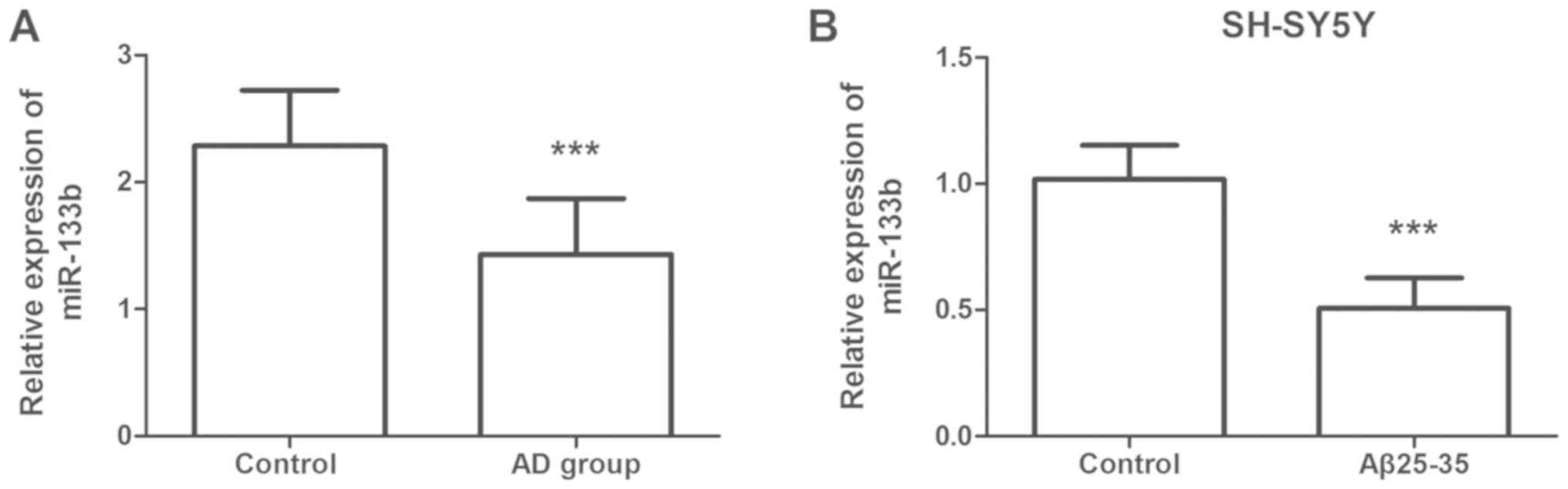
using RT-qPCR. As presented in Fig.
1A, the serum levels of miR-133b in the AD group were
significantly lower than those in the control group (P<0.001).
The miR-133b expression was also assessed in human SH-SY5Y cells.
It was noted that miR-133b was significantly downregulated in the
Aβ25–35 group compared with that in the control group (P<0.001;
Fig. 1B).
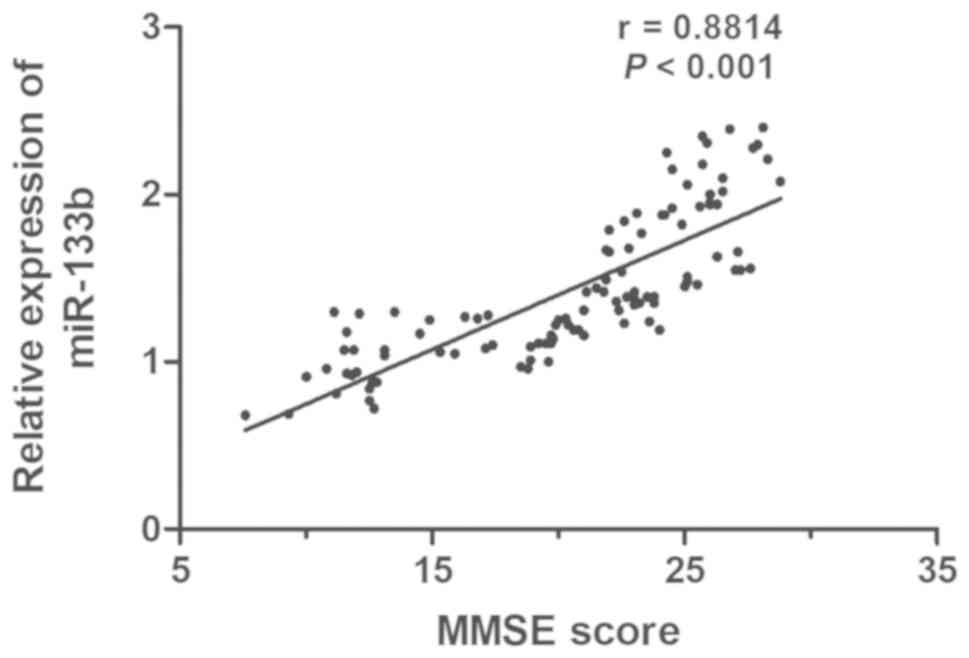
Correlation between the level of
miR-133b and MMSE score
MMSE is a common tool for dementia screening, as it
is able to fully, accurately and rapidly reflect the mental status
and the degree of cognitive impairment. To examine the association
between the serum level of miR-133b and the severity of AD, the
correlation between miR-133b expression levels and the MMSE score
in AD patients was determined. A positive correlation between the
level of miR-133b and MMSE score was identified (r=0.8814,
P<0.001; Fig. 2).
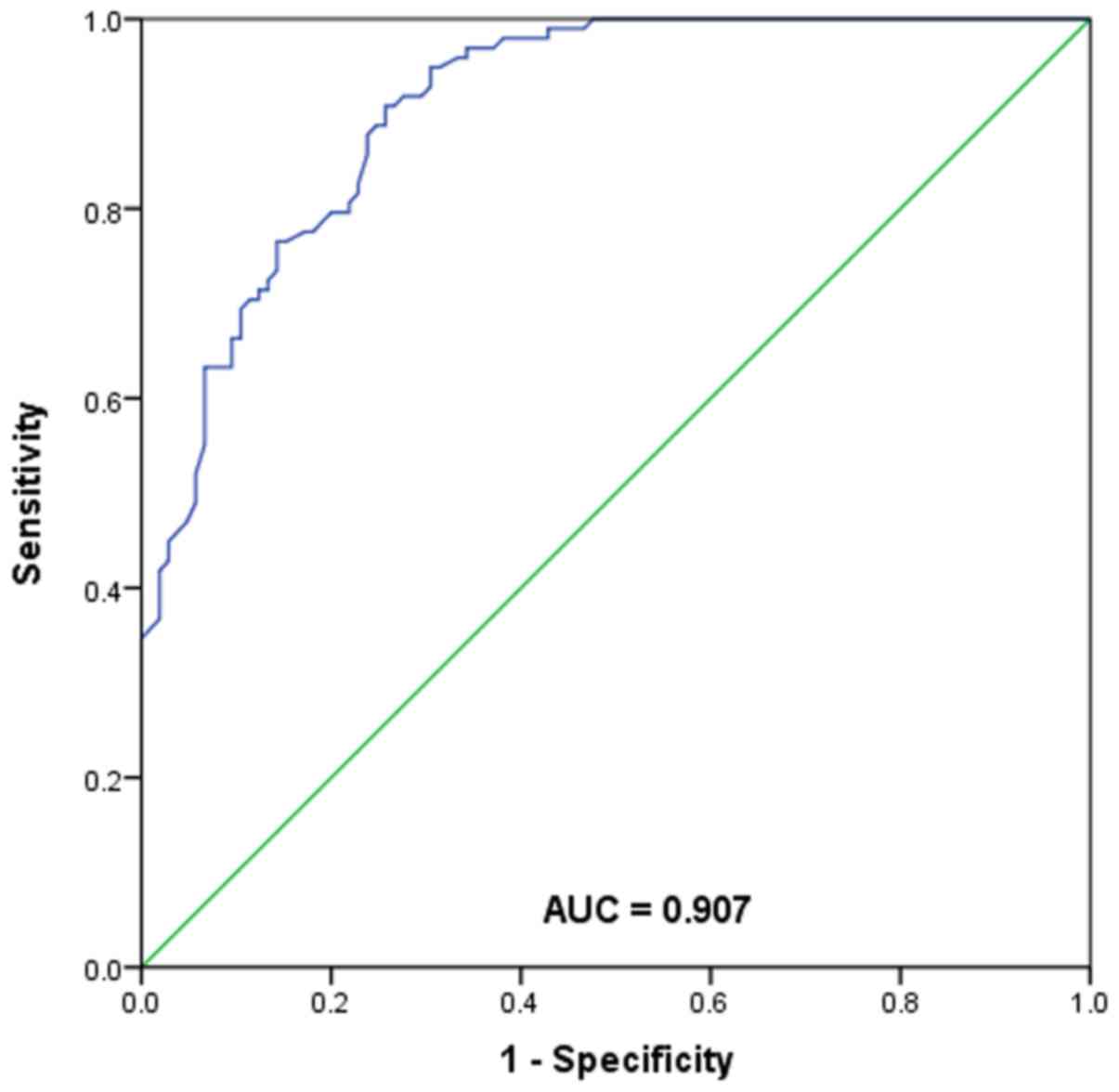
ROC analysis of the diagnostic value
of serum miR-133b for AD
The ROC curve is a graphical representation
reflecting the correlation between sensitivity and specificity of a
laboratory test. In the present study, a ROC curve was generated to
assess the diagnostic value of serum miR-133b for AD (Fig. 3). The area under the curve for
miR-133b was 0.907, with a sensitivity of 90.8% and specificity of
74.3% at the cutoff value of 1.70. The results suggested that
miR-133b may be a sensitive biomarker for differentiating AD
patients from healthy individuals.
Effects of miR-133b on Aβ25-35-induced
neurotoxicity in SH-SY5Y cells
The SH-SY5Y cells were first transfected with
miR-133b mimics, miR-133b inhibitor, mimics NC or inhibitor NC. To
explore the functional role of miR-133b in AD, an in vitro
model of AD was established by treating the transfected SH-SY5Y
cells with Aβ25–35 (Fig. 4). The
overexpression/knockdown efficiency was determined by RT-qPCR, and
the results demonstrated that transfection of miR-133b mimics led
to a marked increase in the expression levels of miR-133b, while
transfection of miR-133b inhibitor resulted in a significant
decrease in its expression, compared with the corresponding
negative controls (all P<0.001; Fig.
4A). It was noted that compared with the control group, the
viability of Aβ25-35-treated SH-SY5Y cells was significantly
reduced (P<0.001; Fig. 4B), while
cell apoptosis was significantly increased (P<0.001; Fig. 4C). As presented in Fig. 4B, transfection with miR-133b mimics
significantly attenuated the Aβ25-35-induced reduction in cell
viability, while transfection with miR-133b inhibitor markedly
promoted it to cause a further reduction in cell viability (all
P<0.01). In addition, transfection with miR-133b mimics
attenuated the Aβ25-35-induced cell apoptosis, while transfection
with miR-133b inhibitor further promoted the Aβ25-35-induced cell
apoptosis (all P<0.01; Fig.
4C).
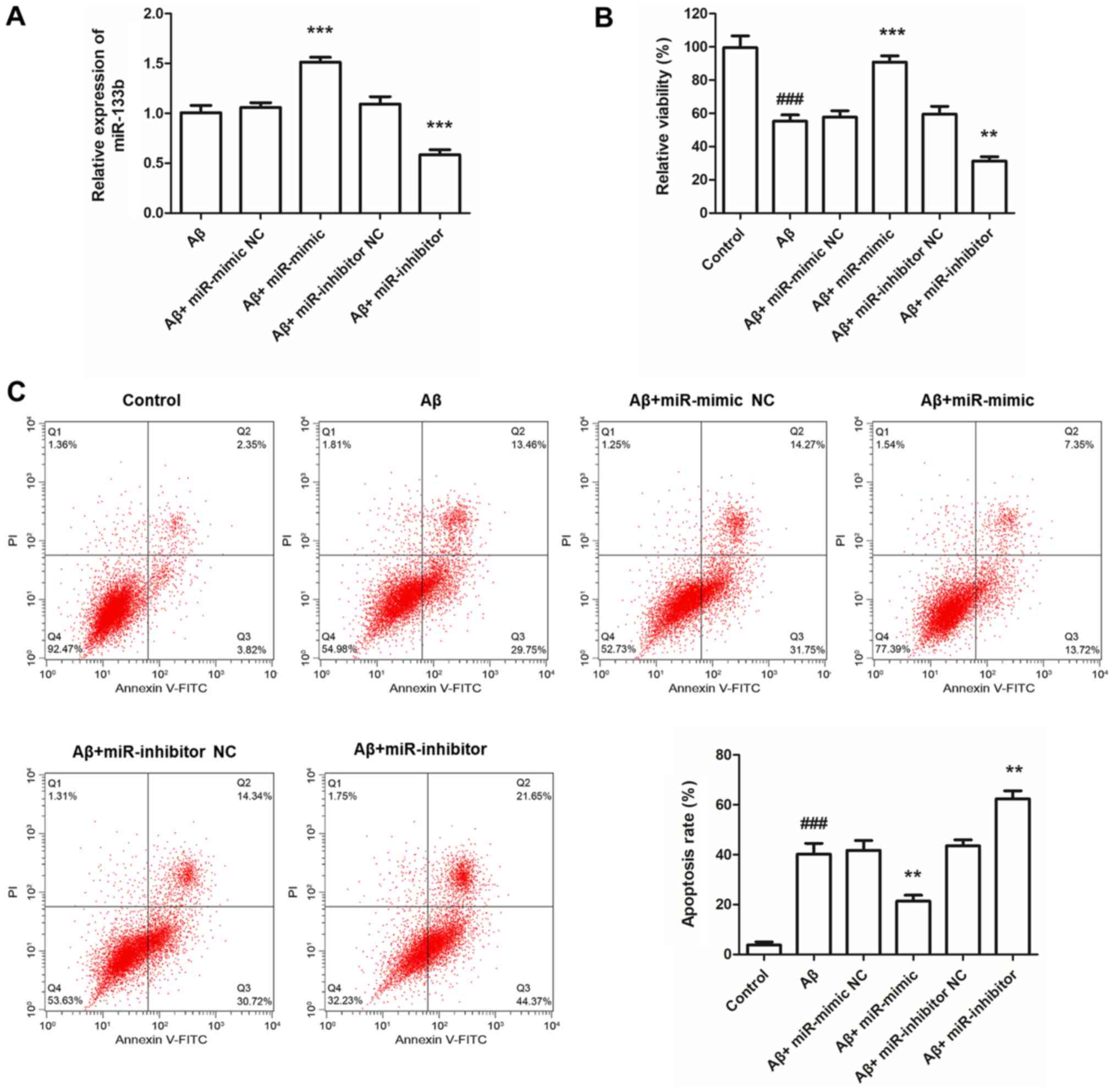 | Figure 4.Effects of miR-133b on
Aβ25-35-induced neurotoxicity in SH-SY5Y cells. (A) In SH-SY5Y
cells, the expression of miR-133b was significantly increased by
transfection with miR-133b mimics, but was decreased by miR-133b
inhibitor compared with that in the corresponding negative
controls. (B) Cell viability of SH-SY5Y cells detected via MTT
assay. (C) Flow cytometric analysis was performed to detect the
apoptotic rate of SH-SY5Y cells. Values are expressed as the mean ±
standard deviation (n=3). **P<0.01, ***P<0.001 vs. Aβ group;
###P<0.001 vs. control. Aβ group, SH-SY5Y cells
treated with Aβ25-35. miR, microRNA; FITC, fluorescein
isothiocyanate; PI, propidium iodide; Q, quadrant; NC, negative
control; Aβ, amyloid β. |
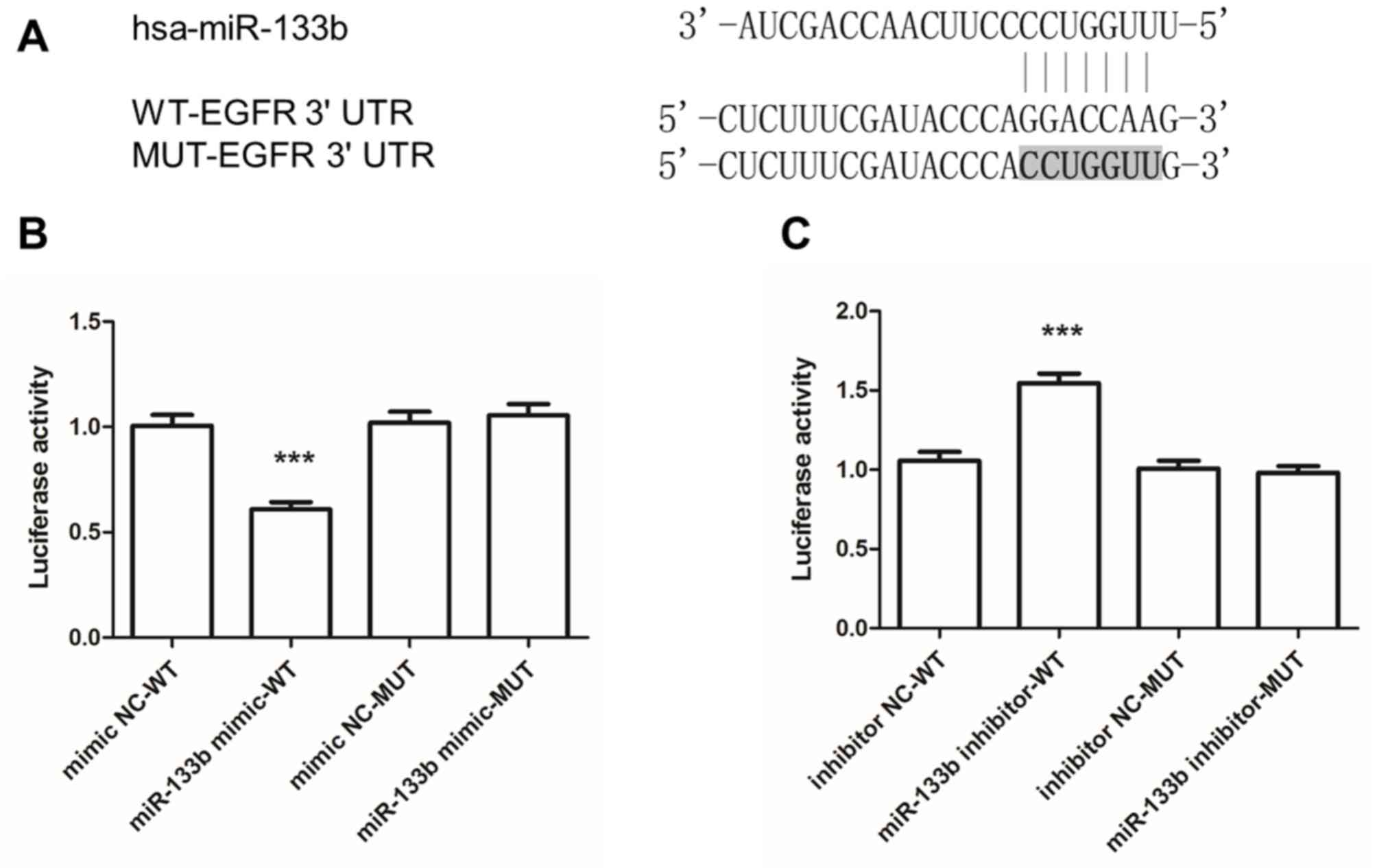
miR-133b directly targets the
conserved EGFR 3′-UTR sequence to regulate EGFR expression
TargetScan was utilized to identify target genes of
miR-133b, revealing that position 50–56 in the 3′-UTR of EGFR mRNA
contained a seven-nucleotide seed match with miR-133b (Fig. 5A). A luciferase reporter assay was
applied to confirm the predicted targeting interaction between
miR-133b and the 3′-UTR of EGFR. The results suggested that
co-transfection with miR-133b mimics attenuated the luciferase
activity of the vector driven by the wild-type 3′-UTR of EGFR,
while this effect was absent when the luciferase vector driven by
the mutant EGFR 3′-UTR was used (Fig.
5B). Conversely, inhibitor of miR-133b promoted the luciferase
activity of the vector containing the wild-type 3′-UTR of EGFR,
while the luciferase activity of the vector driven by the mutant
3′-UTR of EGFR was not affected (Fig.
5C).
Discussion
AD is considered to be the principal cognitive
disorder in humans. As an effect of the aging of the population,
the morbidity of AD has significantly increased in recent years. As
there is currently no known cure for AD and limited early
diagnostic biomarkers, it is of great importance to explore novel
strategies of early diagnosis and treatment for AD. In contrast to
cerebrospinal fluid collection, serum collection is relatively
simple and effective. Furthermore, with the development of
next-generation sequencing technology, the detection of serum
miRNAs is becoming increasingly convenient and accurate (30). A vast amount of studies have
demonstrated the significant role of aberrant miRNAs in the
progression of multiple human diseases (31).
It has been indicated that certain miRNAs are
involved in the development, differentiation and synaptic
plasticity of neurons; furthermore, their dysregulation may be
linked to various diseases, e.g. central nervous system (CNS)
diseases, including AD, multiple sclerosis and Huntington's disease
(32). To date, a number of miRNAs
have been reported to have crucial roles in neurogenesis and
neuronal maturation in the CNS (33). Significant differences have been
detected regarding miRNA expression levels between AD patients and
healthy individuals (34). miRNAs
represent a novel class of biomarkers for AD, which are
non-invasive and sensitive. miR-133b is general abnormally
expressed in various human cancer types, including bladder cancer,
breast cancer and glioma (35–37). It
has been reported that miR-133b is enriched in the midbrain, which
has a marked effect on the differentiation and degeneration of
midbrain dopaminergic neurons (12).
Furthermore, multiple studies have proved abnormal expression of
miR-133b in PD patients. All of this evidence supports the
potential role of miR-133b in neurodegenerative disorders.
In the present study, the serum level of miR-133b
was significantly downregulated in AD patients, which was
consistent with the results of previous studies on PD patients
(14,15). The expression of miR-133b was also
assessed in the human neuroblastoma cell line SH-SY5Y. The
formation of neurofibrillary tangles is regarded to be one of the
important pathological characteristics of AD, which may be caused
by the accumulation of Aβ25–35 (38). Thus, a cell model of AD was
established by treating SH-SY5Y cells with Aβ25-35, and the
concentration of 40 µm/l has been proved to be most efficient
(28). The present results
demonstrated a significant downregulation of miR-133b in SH-SY5Y
cells treated with Aβ25-35. Collectively, the present results
suggested that miR-133b may be a potential diagnostic biomarker for
AD. MMSE is a commonly used screening tool for providing an overall
assessment of cognitive impairment in the clinic, and the MMSE
score has important value in the diagnosis of mild cognitive
impairment that may develop into AD (39). In the present study, the serum levels
of miR-133b were proved to be positively correlated with the MMSE
scores of AD patients, indicating a significant correlation of
miR-133b with the severity of AD and serum miR-133b may therefore
be a useful clinical tool for predicting AD occurrence. In order to
assess the diagnostic sensitivity and specificity of serum miR-133b
for AD, ROC curve analysis was performed. The results suggested
that miR-133b is a sensitive biomarker for differentiating AD
patients from healthy individuals.
miR-133b is aberrantly expressed in various cancer
types, and it may be involved in tumor development via regulation
of tumor cell proliferation and apoptosis (40,41). The
functional role of miR-133b in AD was also explored in a cell model
of AD. The Aβ25-35-treated SH-SY5Y cells were transfected with
miR-133b mimics, miR-133b inhibitor, mimics NC or inhibitor NC. It
was observed that overexpression of miR-133b significantly
attenuated the Aβ25-35-induced inhibition of cell viability.
Furthermore, flow cytometric analysis demonstrated that
upregulation of miR-133b prevented Aβ25-35-induced cell apoptosis.
All of these results indicated that miR-133b may be involved in the
impairment of SH-SY5Y cells treated with Aβ25-35. Taken together,
it was concluded that miR-133b may have a neuroprotective role in
AD.
An important molecular interaction was determined
between miR-133b and EGFR in the present study. The TargetScan tool
predicted EGFR as a direct target gene of miR-133b. The 3′-UTR of
EGFR mRNA was identified to contain a miR-133b seven-nucleotide
seed match at position 50–56. The luciferase reporter assay further
confirmed that miR-133b targeted the 3′-UTR of EGFR, which was
consistent with the bioinformatics prediction.
EGFR, a member of the ErbB family, is located on
chromosome 7p12.1–12.3. It has been reported to be involved in cell
survival, differentiation, proliferation, migration and repair of
cell damage (42,43). Previous studies suggested that EGFR
is able to activate several signaling pathways, particularly the
mitogen-activated protein kinase/ERK and PI3K/AKT pathways
(44), which have been noted to have
crucial roles in neuronal growth, survival and plasticity (45). Furthermore, the important role of
these two major signaling pathways in AD has been widely
demonstrated (46,47). In addition, a key study has indicated
that miR-133b inhibits ESCC cells by targeting EGFR (21). All evidence suggests that miR-133b
may be involved in the development of AD via targeting EGFR.
However, the exact mechanisms require to be fully elucidated in
further studies.
In conclusion, the results of the present study
suggested that miR-133b may serve as a novel diagnostic biomarker
for AD, and it may exert its neuroprotective role in AD and targets
EGFR.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YY and QY conceived and designed the experiment. QY
and QZ performed all of the experiments. YY, QY and QZ wrote and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was designed under the review and
approval of the Ethics Committee of Dongying People's Hospital.
Written informed consent was collected from each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Balin BJ and Hudson AP: Etiology and
pathogenesis of late-onset Alzheimer's disease. Curr Allergy Asthma
Rep. 14:4172014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gu QF, Yu JZ, Wu H, Li YH, Liu CY, Feng L,
Zhang GX, Xiao BG and Ma CG: Therapeutic effect of Rho kinase
inhibitor FSD-C10 in a mouse model of Alzheimer's disease. Exp Ther
Med. 16:3929–3938. 2018.PubMed/NCBI
|
|
3
|
Alzheimer's Association National Plan
Milestone Workgroup, Fargo KN, Aisen P, Albert M, Au R, Corrada MM,
DeKosky S, Drachman D, Fillit H, Gitlin L, et al: 2014 report on
the milestones for the US national plan to address Alzheimer's
disease. Alzheimers Dement 10 (5 Suppl). S430–S452. 2014.
|
|
4
|
Mayeux R and Stern Y: Epidemiology of
Alzheimer disease. Cold Spring Harb Perspect Med. 2(pii):
a0062392012.PubMed/NCBI
|
|
5
|
Tan MS, Yu JT and Tan L: Bridging
integrator 1 (BIN1): Form, function, and Alzheimer's disease.
Trends Mol Med. 19:594–603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bekris LM, Yu CE, Bird TD and Tsuang DW:
Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol.
23:213–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paul S: Integration of miRNA and mRNA
expression data for understanding etiology of gynecologic cancers.
Methods Mol Biol 1912. 323–338. 2019. View Article : Google Scholar
|
|
8
|
Huang X, Liang M, Dittmar R and Wang L:
Extracellular microRNAs in urologic malignancies: chances and
challenges. Int J Mol Sci. 14:14785–14799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar S, Vijayan M and Reddy PH:
MicroRNA-455-3p as a potential peripheral biomarker for Alzheimer's
disease. Hum Mol Genet. 26:3808–3822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong H, Li Y and Su B: Identification of
circulating miR-125b as a potential biomarker of Alzheimer's
disease in APP/PS1 transgenic mouse. J Alzheimers Dis.
59:1449–1458. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tao J, Wu D, Xu B, Qian W, Li P, Lu Q, Yin
C and Zhang W: microRNA-133 inhibits cell proliferation, migration
and invasion in prostate cancer cells by targeting the epidermal
growth factor receptor. Oncol Rep. 27:1967–1975. 2012.PubMed/NCBI
|
|
12
|
Kim J, Inoue K, Ishii J, Vanti WB, Voronov
SV, Murchison E, Hannon G and Abeliovich A: A MicroRNA feedback
circuit in midbrain dopamine neurons. Science. 317:1220–1224. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goedert M: NEURODEGENERATION. Alzheimer's
and Parkinson's diseases: The prion concept in relation to
assembled Aβ, tau, and α-synuclein. Science. 349:12555552015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao N, Jin L, Fei G, Zheng Z and Zhong C:
Serum microRNA-133b is associated with low ceruloplasmin levels in
Parkinson's disease. Parkinsonism Relat Disord. 20:1177–1180. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Yang R, Hu BL, Lu P, Zhou LL, He
ZY, Wu HM and Zhu JH: Reduced circulating levels of miR-433 and
miR-133b are potential biomarkers for Parkinson's disease. Front
Cell Neurosci. 11:1702017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niu M, Xu R, Wang J, Hou B and Xie A:
MiR-133b ameliorates axon degeneration induced by MPP(+) via
targeting RhoA. Neuroscience. 325:39–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Igawa S, Ryuge S, Ichinoe M, Nakashima H,
Otani S, Nakahara Y, Fukui T, Sasaki J, Kubota M, Katagiri M, et
al: Impact of EGFR-tyrosine kinase inhibitors on postoperative
recurrent non-small-cell lung cancer harboring EGFR mutations.
Oncol Res Treat. 40:7–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka T, Kaida T, Yokoi K, Ishii S,
Nishizawa N, Kawamata H, Katoh H, Sato T, Nakamura T, Watanabe M
and Yamashita K: Critical relevance of genomic gains of
PRL-3/EGFR/c-myc pathway genes in liver metastasis of colorectal
cancer. Oncol Lett. 17:1257–1266. 2019.PubMed/NCBI
|
|
19
|
Guo N, Zhao Y, Zhang W, Li S and Yu J:
MicroRNA-133a downregulated EGFR expression in human non-small cell
lung cancer cells via AKT/ERK signaling. Oncol Lett. 16:6045–6050.
2018.PubMed/NCBI
|
|
20
|
Bruban J, Voloudakis G, Huang Q, Kajiwara
Y, Al Rahim M, Yoon Y, Shioi J, Gama Sosa MA, Shao Z,
Georgakopoulos A and Robakis NK: Presenilin 1 is necessary for
neuronal, but not glial, EGFR expression and neuroprotection via
γ-secretase-independent transcriptional mechanisms. FASEB J.
29:3702–3712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng W, Zhu JF, Liu JY, Li YL, Dong X,
Huang H and Shan L: miR-133b inhibits cell proliferation, migration
and invasion of esophageal squamous cell carcinoma by targeting
EGFR. Biomed Pharmacother. 111:476–484. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lugli G, Cohen AM, Bennett DA, Shah RC,
Fields CJ, Hernandez AG and Smalheiser NR: Plasma exosomal miRNAs
in persons with and without Alzheimer disease: Altered expression
and prospects for biomarkers. PLoS One. 10:e01392332015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whitworth JA; World Health Organization
and International Society of Hypertension Writing Group, : 2003
World Health Organization (WHO)/International Society of
Hypertension (ISH) statement on management of hypertension. J
Hypertens. 21:1983–1992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Puavilai G, Chanprasertyotin S and
Sriphrapradaeng A: Diagnostic criteria for diabetes mellitus and
other categories of glucose intolerance: 1997 criteria by the
expert committee on the diagnosis and classification of diabetes
mellitus (ADA), 1998 WHO consultation criteria, and 1985 WHO
criteria. World Health Organization. Diabetes Res Clin Pract.
44:21–26. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang B, Qiu Q, Yin L, Yao Y, Wang C and
Wu X: Measurement of retinal function with
flash-electroretinography in Chinese patients with hyperlipidemia.
Graefes Arch Clin Exp Ophthalmol. 252:1385–1392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Zhao X, Li L, Yao H, Jiang Y and
Zhang J: Effects of Combination of ezetimibe and rosuvastatin on
coronary artery plaque in patients with coronary heart disease.
Heart Lung Circ. 25:459–465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Jia J and Yang Z: Mini-mental state
examination in elderly chinese: A population-based normative study.
J Alzheimers Dis. 53:487–496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu L, Zhang R, Yuan Q, Gao Y, Yang MQ,
Zhang C, Huang J, Sun Y, Yang W, Yang JY, et al: The emerging role
of microRNA-4487/6845-3p in Alzheimer's disease pathologies is
induced by Aβ25–35 triggered in SH-SY5Y cell. BMC Syst Biol. 12
(Suppl 7):S1192018. View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amodio N, Stamato MA, Gullà AM, Morelli E,
Romeo E, Raimondi L, Pitari MR, Ferrandino I, Misso G, Caraglia M,
et al: Therapeutic targeting of miR-29b/HDAC4 epigenetic loop in
multiple myeloma. Mol Cancer Ther. 15:1364–1375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tian Y, Yan M, Zheng J, Li R, Lin J, Xu A,
Liang Y, Zheng R and Yuan Y: miR-483-5p decreases the
radiosensitivity of nasopharyngeal carcinoma cells by targeting
DAPK1. Lab Invest. 99:602–611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maniati MS, Maniati M, Yousefi T,
Ahmadi-Ahangar A and Tehrani SS: New insights into the role of
microRNAs and long noncoding RNAs in most common neurodegenerative
diseases. J Cell Biochem. 120:8908–8918. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Díaz NF, Cruz-Reséndiz MS, Flores-Herrera
H, García-López G and Molina-Hernández A: MicroRNAs in central
nervous system development. Rev Neurosci. 25:675–686.
2014.PubMed/NCBI
|
|
34
|
Maldonado-Lasuncion I, Atienza M,
Sanchez-Espinosa MP and Cantero JL: Aging-related changes in
cognition and cortical integrity are associated with serum
expression of candidate MicroRNAs for Alzheimer disease. Cereb
Cortex. Dec 22–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi Z, Kadeer A, Wang M, Wen B, Li M,
Huang J, Gao Y, Liu E, Liu D, Jia D and Liang C: The deregulation
of miR-133b is associated with poor prognosis in bladder cancer.
Pathol Res Pract. 215:354–357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang QY, Zhou CX, Zhan MN, Tang J, Wang
CL, Ma CN, He M, Chen GQ, He JR and Zhao Q: MiR-133b targets Sox9
to control pathogenesis and metastasis of breast cancer. Cell Death
Dis. 9:7522018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Q, Fan X, Xu B, Pang Q and Teng L:
miR-133b acts as a tumor suppressor and negatively regulates EMP2
in glioma. Neoplasma. 65:494–504. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thaker AA, Weinberg BD, Dillon WP, Hess
CP, Cabral HJ, Fleischman DA, Leurgans SE, Bennett DA, Hyman BT,
Albert MS, et al: Entorhinal cortex: Antemortem cortical thickness
and postmortem neurofibrillary tangles and amyloid pathology. AJNR
Am J Neuroradiol. 38:961–965. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arevalo-Rodriguez I, Smailagic N, Roqué I
Figuls M, Ciapponi A, Sanchez-Perez E, Giannakou A, Pedraza OL,
Bonfill Cosp X and Cullum S: Mini-mental state examination (MMSE)
for the detection of Alzheimer's disease and other dementias in
people with mild cognitive impairment (MCI). Cochrane Database Syst
Rev. CD0107832015.PubMed/NCBI
|
|
40
|
Li H, Xiang Z, Liu Y, Xu B and Tang J:
MicroRNA-133b inhibits proliferation, cellular migration, and
invasion via targeting LASP1 in hepatocarcinoma cells. Oncol Res.
25:1269–1282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhen Y, Liu J, Huang Y, Wang Y, Li W and
Wu J: miR-133b inhibits cell growth, migration, and invasion by
targeting MMP9 in non-small cell lung cancer. Oncol Res.
25:1109–1116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Appert-Collin A, Hubert P, Crémel G and
Bennasroune A: Role of ErbB receptors in cancer cell migration and
invasion. Front Pharmacol. 6:2832015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim SB, Ly P, Kaisani A, Zhang L, Wright
WE and Shay JW: Mitigation of radiation-induced damage by targeting
EGFR in noncancerous human epithelial cells. Radiat Res.
180:259–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guillot F, Kemppainen S, Lavasseur G,
Miettinen PO, Laroche S, Tanila H and Davis S: Brain-specific basal
and novelty-induced alternations in PI3K-Akt and MAPK/ERK signaling
in a middle-aged AβPP/PS1 mouse model of Alzheimer's disease. J
Alzheimers Dis. 51:1157–1173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Feng MG, Liu CF, Chen L, Feng WB, Liu M,
Hai H and Lu JM: MiR-21 attenuates apoptosis-triggered by amyloid-β
via modulating PDCD4/PI3K/AKT/GSK-3β pathway in SH-SY5Y cells.
Biomed Pharmacother. 101:1003–1007. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zang G, Fang L, Chen L and Wang C:
Ameliorative effect of nicergoline on cognitive function through
the PI3K/AKT signaling pathway in mouse models of Alzheimer's
disease. Mol Med Rep. 17:7293–7300. 2018.PubMed/NCBI
|