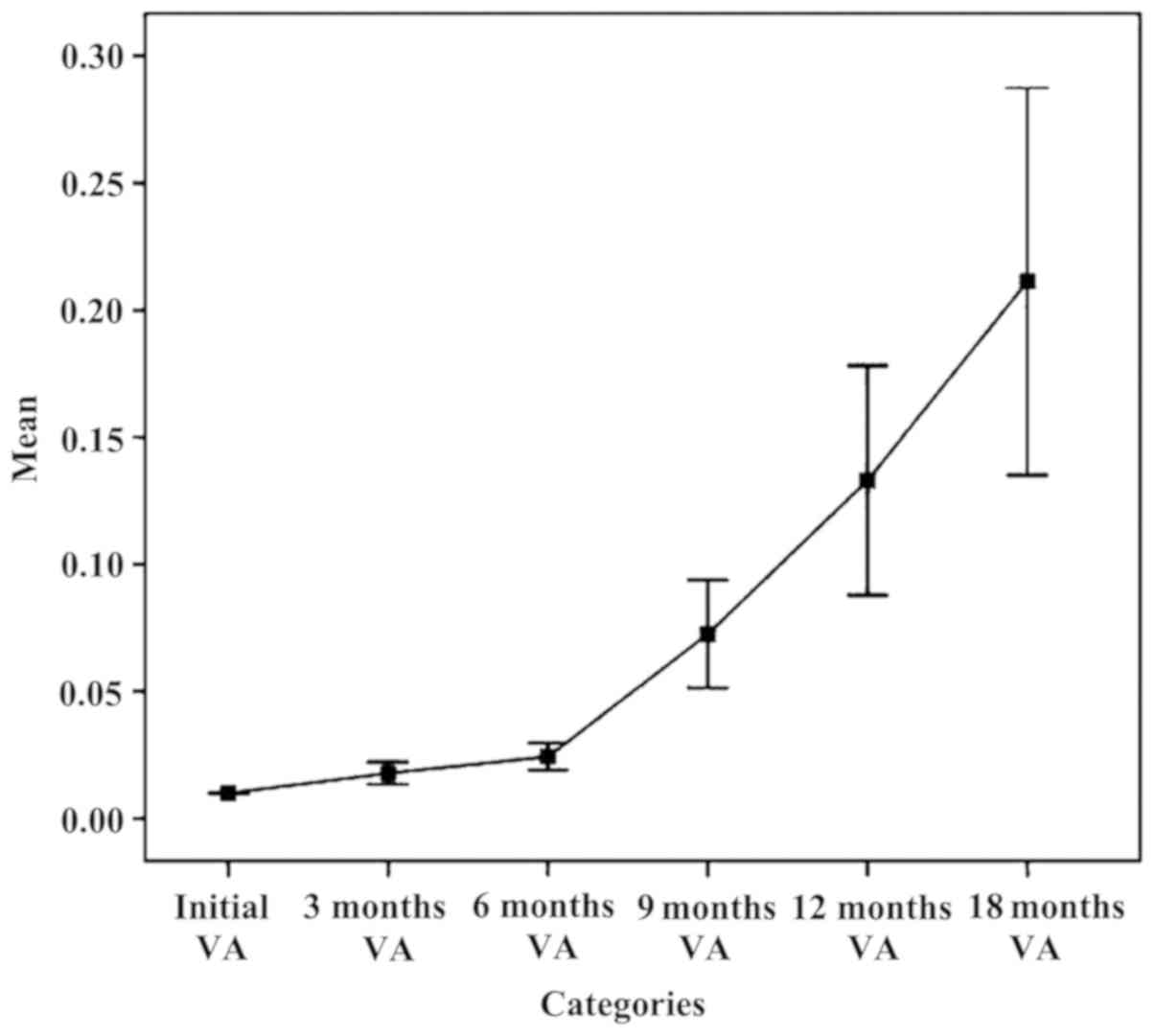
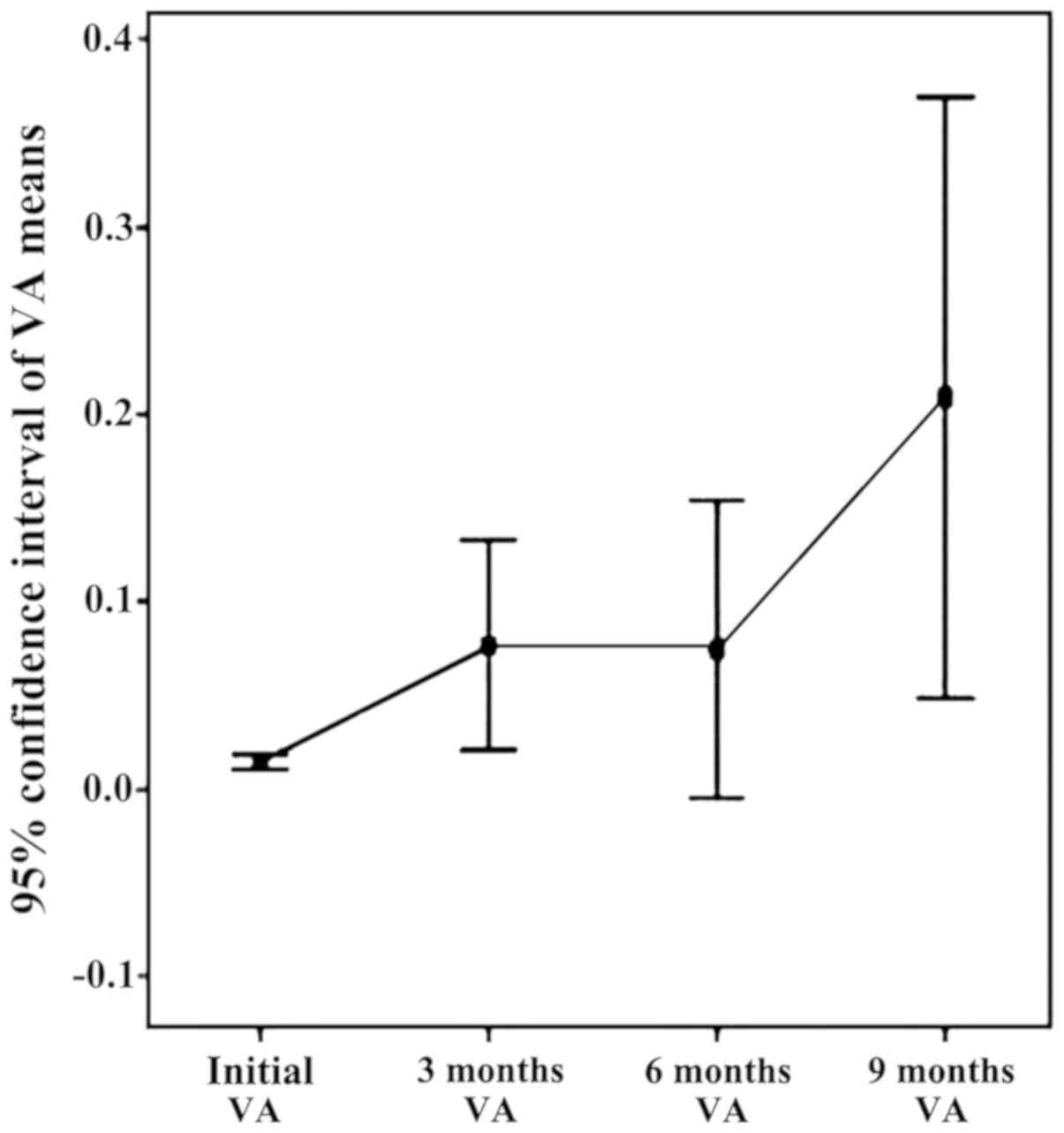
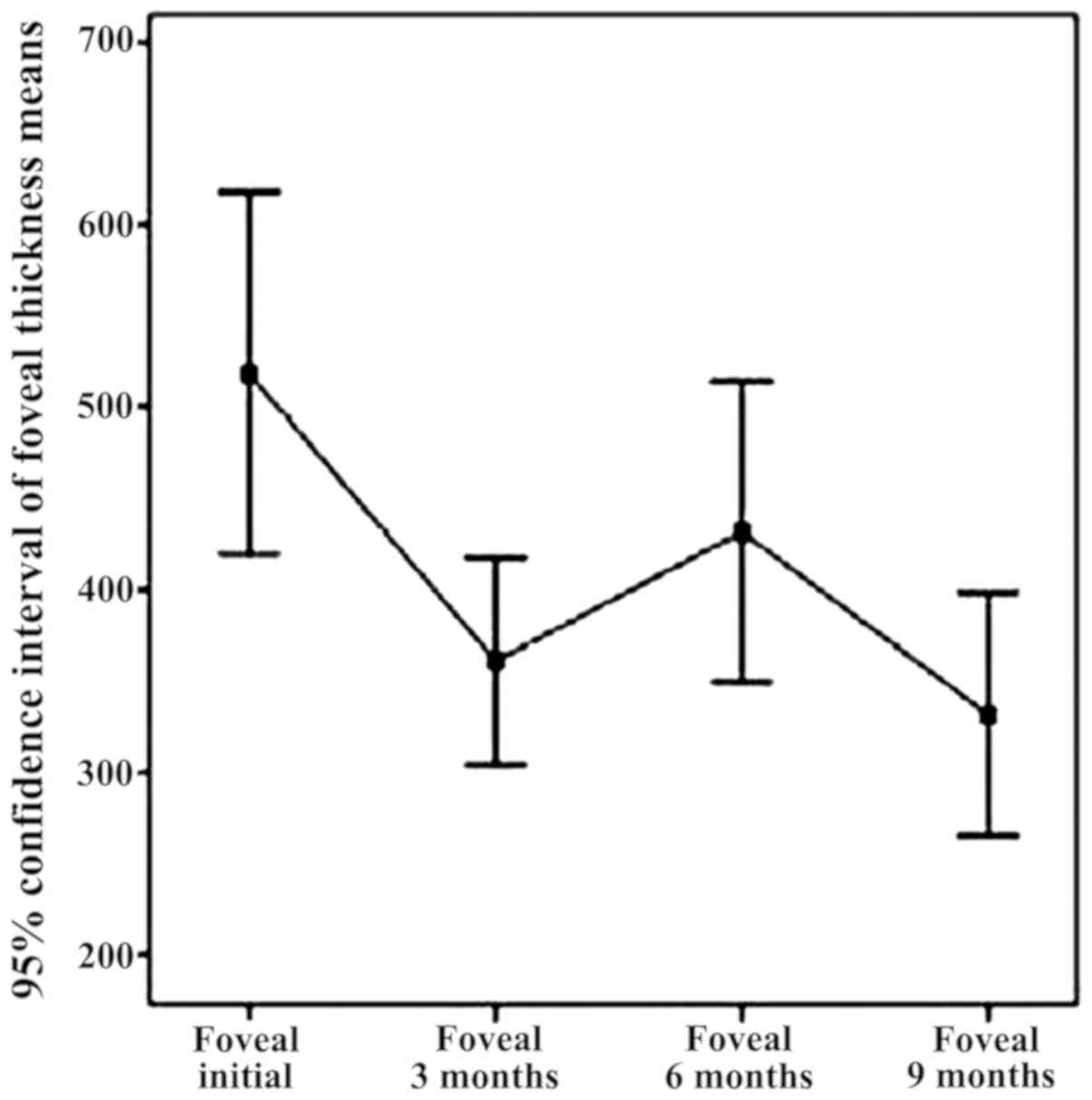
|
1
|
Klein R, Klein BE, Jensen SC and Meuer SM:
The five-year incidence and progression of age-related maculopathy:
The Beaver Dam Eye Study. Ophthalmology. 104:7–21. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Eye Institute, . Statistics and
Data. Prevalence of Blindness Data. Data Tables [online]. 2007,
https://nei.nih.gov/eyedata/pbd_tables
|
|
3
|
Fine SL, Berger JW, Maguire MG and Ho AC:
Age-related macular degeneration. N Engl J Med. 342:483–492. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ciulla TA, Harris A and Martin BJ: Ocular
perfusion and age-related macular degeneration. Acta Ophthalmol
Scand. 79:108–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moshfeghi AA, Rosenfeld PJ, Puliafito CA,
Michels S, Marcus EN, Lenchus JD and Venkatraman AS: Systemic
bevacizumab (Avastin) therapy for neovascular age-related macular
degeneration: Twenty-four-week results of an uncontrolled
open-label clinical study. Ophthalmology. 113:2002.e1–2002.e12.
2006. View Article : Google Scholar
|
|
6
|
Rosenfeld PJ, Fung AE and Puliafito CA:
Optical coherence tomography findings after an intravitreal
injection of bevacizumab (avastin) for macular edema from central
retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 36:336–339.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bakri SJ, Cameron JD, McCannel CA, Pulido
JS and Marler RJ: Absence of histologic retinal toxicity of
intravitreal bevacizumab in a rabbit model. Am J Ophthalmol.
142:162–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manzano RP, Peyman GA, Khan P and Kivilcim
M: Testing intravitreal toxicity of bevacizumab (Avastin). Retina.
26:257–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiss C, Michels S, Prager F, Weigert G,
Geitzenauer W and Schmidt-Erfurth U: Anterior chamber inflammatory
activity in patients with neovascular age-related macular
degeneration treated with intravitreal bevacizumab (Avastin).
Retina. 26:877–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spitzer MS, Wallenfels-Thilo B, Sierra A,
Yoeruek E, Peters S, Henke-Fahle S, Bartz-Schmidt KU and Szurman P;
Tuebingen Bevacizumab Study Group, : Antiproliferative and
cytotoxic properties of bevacizumab on different ocular cells. Br J
Ophthalmol. 90:1316–1321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ziemssen F, Warga M, Neuhann IM, Leitritz
M, Biester S, Grisanti S and Bartz-Schmidt KU: Does intravitreal
injection of bevacizumab have an effect on the blood-aqueus barrier
function? Br J Ophthalmol. 90:9222006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mangione CM, Lee PP, Gutierrez PR,
Spritzer K, Berry S and Hays RD; National Eye Institute Visual
Function Questionnaire Field Test Investigators, : Development of
the 25-item National Eye Institute Visual Function Questionnaire.
Arch Ophthalmol. 119:1050–1058. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mordenti J, Cuthbertson RA, Ferrara N,
Thomsen K, Berleau L, Licko V, Allen PC, Valverde CR, meng YG, Fei
DT, et al: Comparisons of the intraocular tissue distribution,
pharmacokinetics, and safety of 125I-labeled full-length
and Fab antibodies in rhesus monkeys following intravitreal
administration. Toxicol Pathol. 27:536–544. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosenfeld PJ, Moshfeghi AA and Puliafito
CA: Optical coherence tomography findings after an intravitreal
injection of bevacizumab (avastin) for neovascular age-related
macular degeneration. Ophthalmic Surg Lasers Imaging. 36:331–335.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shahar J, Avery RL, Heilweil G, Barak A,
Zemel E, Lewis GP, Johnson PT, Fisher SK, Perlman I and Loewenstein
A: Electrophysiologic and retinal penetration studies following
intravitreal injection of bevacizumab (Avastin). Retina.
26:262–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spaide RF, Laud K, Fine HF, Klancnik JM
Jr, Meyerle CB, Yannuzzi LA, Sorenson J, Slakter J, Fisher YL and
Cooney MJ: Intravitreal bevacizumab treatment of choroidal
neovascularization secondary to age-related macular degeneration.
Retina. 26:383–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heiduschka P, Fietz H, Hofmeister S,
Schultheiss S, Mack AF, Peters S, Ziemssen F, Niggemann B, Julien
S, Bartz-Schmidt KU, et al Tübingen Bevacizumab Study Group, :
Penetration of bevacizumab through the retina after intravitreal
injection in the monkey. Invest Ophthalmol Vis Sci. 48:2814–2823.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iturralde D, Spaide RF, Meyerle CB,
Klancnik JM, Yannuzzi LA, Fisher YL, Sorenson J, Slakter JS, Freund
KB, Cooney M, et al: Intravitreal bevacizumab (Avastin) treatment
of macular edema in central retinal vein occlusion: A short-term
study. Retina. 26:279–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spaide RF and Fisher YL: Intravitreal
bevacizumab (Avastin) treatment of proliferative diabetic
retinopathy complicated by vitreous hemorrhage. Retina. 26:275–278.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Avery RL: Regression of retinal and iris
neovascularization after intravitreal bevacizumab (Avastin)
treatment. Retina. 26:352–354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Avery RL, Pearlman J, Pieramici DJ, Rabena
MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R and Patel A:
Intravitreal bevacizumab (Avastin) in the treatment of
proliferative diabetic retinopathy. Ophthalmology.
113:1695.e1–1695.e15. 2006. View Article : Google Scholar
|
|
22
|
Aisenbrey S, Ziemssen F, Völker M,
Gelisken F, Szurman P, Jaissle G, Grisanti S and Bartz-Schmidt KU:
Intravitreal bevacizumab (Avastin) for occult choroidal
neovascularization in age-related macular degeneration. Graefes
Arch Clin Exp Ophthalmol. 245:941–948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avery RL, Pieramici DJ, Rabena MD,
Castellarin AA, Nasir MA and Giust MJ: Intravitreal bevacizumab
(Avastin) for neovascular age-related macular degeneration.
Ophthalmology. 113:363–372.e5. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoganathan P, Deramo VA, Lai JC, Tibrewala
RK and Fastenberg DM: Visual improvement following intravitreal
bevacizumab (Avastin) in exudative age-related macular
degeneration. Retina. 26:994–998. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakaguchi H, Ikuno Y, Gomi F, Kamei M,
Sawa M, Tsujikawa M, Oshima Y, Kusaka S and Tano Y: Intravitreal
injection of bevacizumab for choroidal neovascularisation
associated with pathological myopia. Br J Ophthalmol. 91:161–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laud K, Spaide RF, Freund KB, Slakter J
and Klancnik JM Jr: Treatment of choroidal neovascularization in
pathologic myopia with intravitreal bevacizumab. Retina.
26:960–963. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tewari A, Dhalla MS and Apte RS:
Intravitreal bevacizumab for treatment of choroidal
neovascularization in pathologic myopia. Retina. 26:1093–1094.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teixeira A, Moraes N, Farah ME and Bonomo
PP: Choroidal neovascularization treated with intravitreal
injection of bevacizumab (Avastin) in angioid streaks. Acta
Ophthalmol Scand. 84:835–836. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lommatzsch A, Spital G, Trieschmann M and
Pauleikhoff D: Intraocular application of bevacizumab for the
treatment of choroidal neovascularization secondary to angioid
streaks. Ophthalmologe. 104:325–328. 2007.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jorge R, Costa RA, Calucci D and Scott IU:
Intravitreal bevacizumab (Avastin) associated with the regression
of subretinal neovascularization in idiopathic juxtafoveolar
retinal telangiectasis. Graefes Arch Clin Exp Ophthalmol.
245:1045–1048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Munteanu M and Rosca C: Repositioning and
follow-up of intralenticular dexamethasone implant. J Cataract
Refract Surg. 39:1271–1274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jianu DC, Jianu SN, Dan TF, Motoc AGM and
Poenaru M: Pulsatile tinnitus caused by a dilated left
petrosquamosal sinus. Rom J Morphol Embryol. 57:319–322.
2016.PubMed/NCBI
|
|
33
|
Stanca HT, Suvac E, Munteanu M, Jianu DC,
Motoc AGM, Roşca GC and Boruga O: Giant cell arteritis with
arteritic anterior ischemic optic neuropathy. Rom J Morphol
Embryol. 58:281–285. 2017.PubMed/NCBI
|
|
34
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomarkers Med. 9:513–528. 2015. View Article : Google Scholar
|
|
35
|
Solomon I, Voiculescu VM, Caruntu C, Lupu
M, Popa A, Ilie MA, Albulescu R, Caruntu A, Tanase C, Constantin C,
et al: Neuroendocrine factors and head and neck squamous cell
carcinoma: An affair to remember. Dis Markers. 2018:97878312018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ion A, Popa IM, Papagheorghe LML,
Lisievici C, Lupu M, Voiculescu V, Caruntu C and Boda D: Proteomic
approaches to biomarker discovery in cutaneous T-cell lymphoma. Dis
Markers. 2016:96024722016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non-melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in vivo
reflectance confocal microscopy. Exp Ther Med. 15:1241–1246.
2018.PubMed/NCBI
|