Introduction
Macular degeneration represents the main cause of
severe visual impairment in patients over 60 years of age in
industrialized countries (1). The
prevalence of macular degeneration in people of 40–49 years is
2.1%, rising dramatically to 35% in the population aged over 80
years (2).
The severe deterioration of central vision occurs
mainly in the exudative form of age-related macular degeneration
(AMD) (3), as a result of the
formation of pathological choroidal neovessels - choroidal
neovascular membranes (CNVM) that induce structural and functional
changes in the macular region (4).
Exudative AMD dominates the etiologic spectrum of
conditions involving CNVM; therefore, our study focused on the
current therapeutic options for the choroidal neovascular membranes
in the wet form of AMD.
In advanced stages of AMD, CNVM can cause
irreversible loss of central vision to the level of ‘legal
blindness’. This ‘blindness’, though not complete (there is a
remaining peripheral visual field), reduces the individual's
adaptation to the environment, making it impossible to read, drive,
recognize facial features of people around them, thus altering the
quality of life and autonomy of those affected (4).
Until 2006, the methods of treatment for neovascular
choroidal pathology differed from the prescription of
prophylactically antioxidant medication, with no effect on the
existing membranes, to the ‘heroic’ surgery that tried to extract
the membranes under the retina and possibly ‘relocate’ the retina,
by turning it on healthy pigment epithelium areas, with sometimes
ambiguous effects. At this point, because of a new class of drugs,
namely vascular endothelial growth factor blockers (anti-VEGF), we
have entered a new therapeutic era in the management of these
diseases. Thirteen years after having started the intravitreal
anti-VEGF treatment in Romania, we are reviewing the steps, the
results and the changes in our daily practice.
Materials and methods
The study was performed based on a number of cases
diagnosed with exudative AMD, the inclusion criteria being the
presence of choroidal neovascularization in the context of AMD,
evidenced clinically and on OCT images. Exclusion criteria
consisted in the absence of clinical and angiofluorographic
exudation features and in the presence of excessive fibrous
components. The epidemiological strategy was an analytical,
interventional case series without a control group, with a
prospective component, which was the introduction of new
therapeutic methods (intravitreal injection of bevacizumab) in a
pathology (an exudative type of age-related macular degeneration)
where treatment solutions were mostly ineffective. The functional
and anatomical effectiveness of this treatment was followed-up over
short (1–3 months) and medium (6–18 months) periods.
In October 2006, we started to perform the first
intravitreal bevacizumab injections. At the beginning of this
therapy, its legal aspect was uncertain (‘off-label’ use of
bevacizumab drug - outside the therapeutic indications that were
safe) (5). The medium and long-term
results were unknown; there was no available data regarding
possible systemic side effects of the substance. Regarding the
‘off-label’ status of this medication, we followed the American
model, elaborating a well-informed consent, based on the content
used overseas. This study adhered to the tenets of the Declaration
of Helsinki and was approved by the Ethics Committee of our
hospital, ‘Prof. Dr. Agrippa Ionescu’ Clinical Emergency Hospital.
Written informed consent was obtained from all patients before the
examinations and surgeries were performed. As we obtained favorable
results in patients with low VA, we began to use intravitreal
bevacizumab in patients with the same disease (neovascular AMD),
but with better visual acuity (VA) (best VA for initiating
treatment was 20/40-0.5). When included in the treatment group,
each patient was evaluated for the best corrected visual acuity
(BCVA) with Snellen chart and they underwent a complete eye
examination, which included measurement of intraocular pressure,
refractometry and biomicroscopy of the anterior and posterior pole.
Most times, especially at the beginning of the study, the main
element that allowed the patient to have access to the treatment
algorithm was slit lamp examination with Volk Centralis Direct
contact lens. Where clinical macular edema, exudative elements
and/or subretinal haemorrhage were found, we used bevacizumab
without imaging confirmation. In cases where the fibrovascular
component was predominant or the subfoveal changes did not show
clinical signs of exudation, we performed FA and/or OCT in order to
specify the diagnosis. Central retinal thickness was measured in
patients who performed OCT with ‘5-mm raster line scans’ for
patients examined with ZeissStratus model and ‘6-mm raster line
scans’ for the investigations with Topcon 3D 1000. Fluoresceine
angiographies were performed mainly in patients from the first part
of the study group because OCT examination was less accessible at
that time. Afterwards, FA was used only as a method to determine
the leakage site (extravasation of fluid) at certain period of
times after the end of therapy, when there was clinical doubt or VA
tended to change.
All patients had previously received antioxidant
medication administered orally. The therapeutic protocol consisted
of intravitreal administration of bevacizumab 1.25 mg/0.05 ml every
four weeks in three sessions for each of the eyes of the patients
in the study. Each eye was considered a separate entity. Increasing
the number of injections (to 4 or 5 injections) over the standard,
a protocol was performed to cases which showed clinical and
functional improvement after 3 months of treatment. However,
relapses followed at intervals varying between at least 3 months
and at most 12 months. Patients were clinically reviewed by retinal
imagistic after 1 month and 3 months. Some patients continued to be
monitored at 6, 12 and 18 months. This was the first study
performed in Romania regarding the treatment of wet AMD using
bevacizumab. We intend to show our first results from the beginning
of the anti-VEGF era to the international ophthalmologic community,
in order to demonstrate that our experience, which started in 2006,
is consistent with all the data from the literature and our
approach, which was not influenced by other authors, can be
superimposed on the standard protocols nowadays.
Patients
There were 376 intravitreal injections to 117 eyes
[67 left eyes (57.2%) and 50 right eyes (42.8%)] from 96 patients
[59 males (61.45%) and 37 females (38.55%)], starting October 2006.
A total of 21 patients had both eyes treated. Patients were
classified into three age groups: 55–65 years (average age 62.4
years, 18 patients), 65–75 years (average age 71.7, 37 patients)
and over 75 (average age 82.3 years, 41 patients). The average age
was 72.1 years.
Treatment protocol
The three ‘standard’ injections were administered to
all patients. Among the 117 treated eyes, 13 received four
injections and six eyes received five injections, in addition to
standard injections. The criteria by which treatment protocol was
supplemented will be discussed further. The structure of the
injected study group was as follows: 96 patients: 21 treated in
both eyes (OU), 42 eyes, 126 injections; 75 treated in one/single
eye (SE), 225 injections. Total number of injections performed, 376
(average number of injections performed/eyes, 3.2).
Follow-up
The patients were functionally monitored, being
evaluated with the Snellen chart for central visual acuity (VA). We
have opted to express it as decimal VA, because it is simply and
easily understood by the patient and it is easier to convert it in
the minimum visual resolution angle logarithm (logMAR), a
universally accepted mathematical unit in statistical
calculations.
The follow-up of retinal structural changes involved
performing either optical coherence tomography, which allowed the
assessment of macular thickness variations (i.e. the degree of
macular edema resolution) or fluorescein angiographies, which
verified the degree of activity of choroidal neovascular membranes
according to the presence or absence of the extravasation/diffusion
fluid (leakage).
Central visual acuity
Central visual acuity was measured at baseline and
then after one month, 2 months and 3 months. These data were
obtained for all treated eyes (100% of cases). For some patients,
VA follow-up could also be done after 6 months, 12 months and 18
months. Tailure to follow-up the medium controls (6–12/18 months)
of patients was determined both by a lower compliance and by old
age associated with other systemic diseases, which reduced the
addressability to the ophthalmologist.
We identified eight groups of patients according to
the initial value of VA (assessed with the best optical
correction): Group A: Initial VA 0.01 (0.5/50), 26 eyes; Group B:
Initial VA 0.02 (1/50), 43 eyes; Group C: Initial VA 0.04 (2/50),
26 eyes; Group D: Initial VA 0.06 (3/50), 8 eyes; Group E: Initial
VA 0.1 (5/50), 7 eyes; Group F: Initial VA 0.2, 4 eyes; Group G:
Initial VA 0.3, 1 eye; Group H: Initial VA 0.5, 2 eyes.
The number of patients available for follow-up was:
76 eyes from 63 patients after 6 months, 39 eyes from 28 patients
after 9 months, 31 eyes from 21 patients after 12 months and 23
eyes from 19 patients after 18 months.
From the initial study groups, only some patients
remained to be followed-up. Thirteen eyes received four injections
and six eyes received five injections. Reinjections were performed
in cases where a favorable initial response had been found after
the first three injections of the therapeutic drug, but followed by
a functional relapse (reduction in VA) doubled by exudative
phenomena (neovascular membranes' activity marker). Therefore, it
was decided to resume therapy in those cases, this time according
to the circumstances and adapting, that is injecting bevacizumab
not following a scheme, but observing the clinical and paraclinical
evolution of the patient - the actual PRN (pro re nata) scheme.
VA measurement involves a subjective component, the
patient's response, but in controlled and standardized conditions
(Snellen chart, standard lighting conditions). In some patients, we
did not detect favorable changes of central visual acuity. However,
many patients with unchanged VA reported an improvement in vision.
We could not quantify this aspect; therefore, we were unable to
integrate it into a statistical analysis. We noted the main
favorable elements reported by the patients 3 months after
injection (Table I). Among the 117
eyes treated, after 3 months 40 of them (34%) presented no VA
changes.
 | Table I.Correlation of the study group
patients without change in VA with subjective elements changes. |
Table I.
Correlation of the study group
patients without change in VA with subjective elements changes.
| Group | Perception of
color | Delimitation of the
contours | The decrease of
central ‘darkness’ | Reduction of image
distortion | No. of patients |
|---|
| A (0.01) | 2 | 1 | 5 | 2 | 11 |
| B (0.02) | 4 | 3 | 5 | 2 | 16 |
| C (0.04) | 5 | 3 | 4 | 4 | 8 |
| D (0.06) | 1 | 0 | 1 | 1 | 2 |
| E (0.1) | 1 | 1 | 1 | 1 | 2 |
| F (0.2) | 1 | 0 | 0 | 0 | 1 |
| G (0.3) | 0 | 0 | 0 | 0 | 0 |
| H (0.5) | 0 | 0 | 0 | 0 | 0 |
| Total | 14 | 8 | 16 | 10 | 40 |
Central retinal thickness (foveal
thickness)
Central thickness changes were determined by optical
coherence tomography (OCT). Among the 96 patients (117 eyes), 42
patients (43%) (51 eyes) (43%) could perform imaging investigation
such as angiofluorography and/or OCT. Among these, 51 eyes could be
monitored by OCT at baseline and after 3 months from the first
injection (Table II).
 | Table II.Structure of the remaining group of
patients that was imagistically followed-up on mid-term. |
Table II.
Structure of the remaining group of
patients that was imagistically followed-up on mid-term.
| Period | Eyes | OCT performed |
|---|
| 6 months | 76 | 9 |
| 9 months | 39 | 11 |
| 12 months | 29 | 9 |
| 18 months | 23 | 3 |
Among the patients remaining for follow-up after the
required period of 3 months, only some of them underwent imaging
investigation. Actually, OCT and/or AFG were performed only in
patients who had been reinjected, several of whom were monitored by
OCT 3 months after reinjection.
Activity signs of choroidal
neovascularization
The existence and/or persistence of signs of
choroidal neovascular membrane activity, expressed by ‘leakage’ -
extravasation and diffusion of fluid, were followed-up by
fluorescein angiography. A minority of patients performed FA - in
cases of diagnostic doubt, or those at baseline when the
tomographic examination was not available. Numerical data do not
provide a coherent statistical overview of the treated group
evolution and, therefore, it will not be used.
Statistical analysis
The statistical analyses have been performed
starting with 2009. All data were analyzed using the Statistical
Package for Social Sciences software (version 16.0, SPSS, Inc.) and
shown as mean ± standard deviation. Graphs were plotted by
Microsoft Excel 2008 (Microsoft Corporation). Means and standard
deviations were calculated for all continuous variables. Frequency
distribution and percentages were determined for categorical
variables. Comparisons between post-operative and pre-operative
values, for visual acuity (VA) and foveal thickness (FT) were
carried out using the paired sample t-test. Concordance tests
(Kolmogorov-Smirnov) have been performed for each data vector in
order to determine their normality. For a 0.05 confidence interval,
the data have been tested as normally distributed, which allowed
the use of parametric methods (for quantitative data).
Non-parametric tests have been performed for bivariate categorical
data. Differences in the distributions between groups were analyzed
using the independent t-test. Differences in the distributions of
categorical variables between groups were analyzed using the
Chi-squared test. In order to analyze the correlations, both
Pearson correlation parametric tests and non-parametric Spearman
and Kendall were used. Regression linear curves have been
represented to determine the functional dependencies between some
characteristics. Their adaptation over the represented point clouds
has been appreciated by coefficients of determination. All tests
have been performed bilaterally. Two significance thresholds were
considered in order to test the statistical significance, α=0,05
and α=0,01, respectively. The statistical significance was set for
P-values lower than the significance threshold. The graphic data
processing included percentage, comparison diagrams, boxplots and
regression functional plots.
Results
The differentiated approach of the
study according to the purpose
The personal study had proposed two major directions
of experimental argumentation and statistical validation of the
results: a) Follow-up of the evolution of individual VA (visual
acuity) parameter in patients treated with bevacizumab intravitreal
both in the unprocessed rough groups and in the representative
groups, statistically adapted to random the loss of subjects. b)
Follow-up of the VA parameter evolution in correlation with the
foveal thickness (FT) parameter.
Although the groups of patients observed are unitary
in achieving both objectives, for the second one it must be taken
into account that a significant number of patients abandoned the
study. For this reason, we have split the results of the
statistical analysis according to the two objectives proposed.
Univariate results follow-up (referring only to VA) will be
distinguished from a bivariate approach where the visual acuity
(VA) is correlated with foveal thickness (FT).
The analysis of VA parameter pursued
independent batches of raw patients
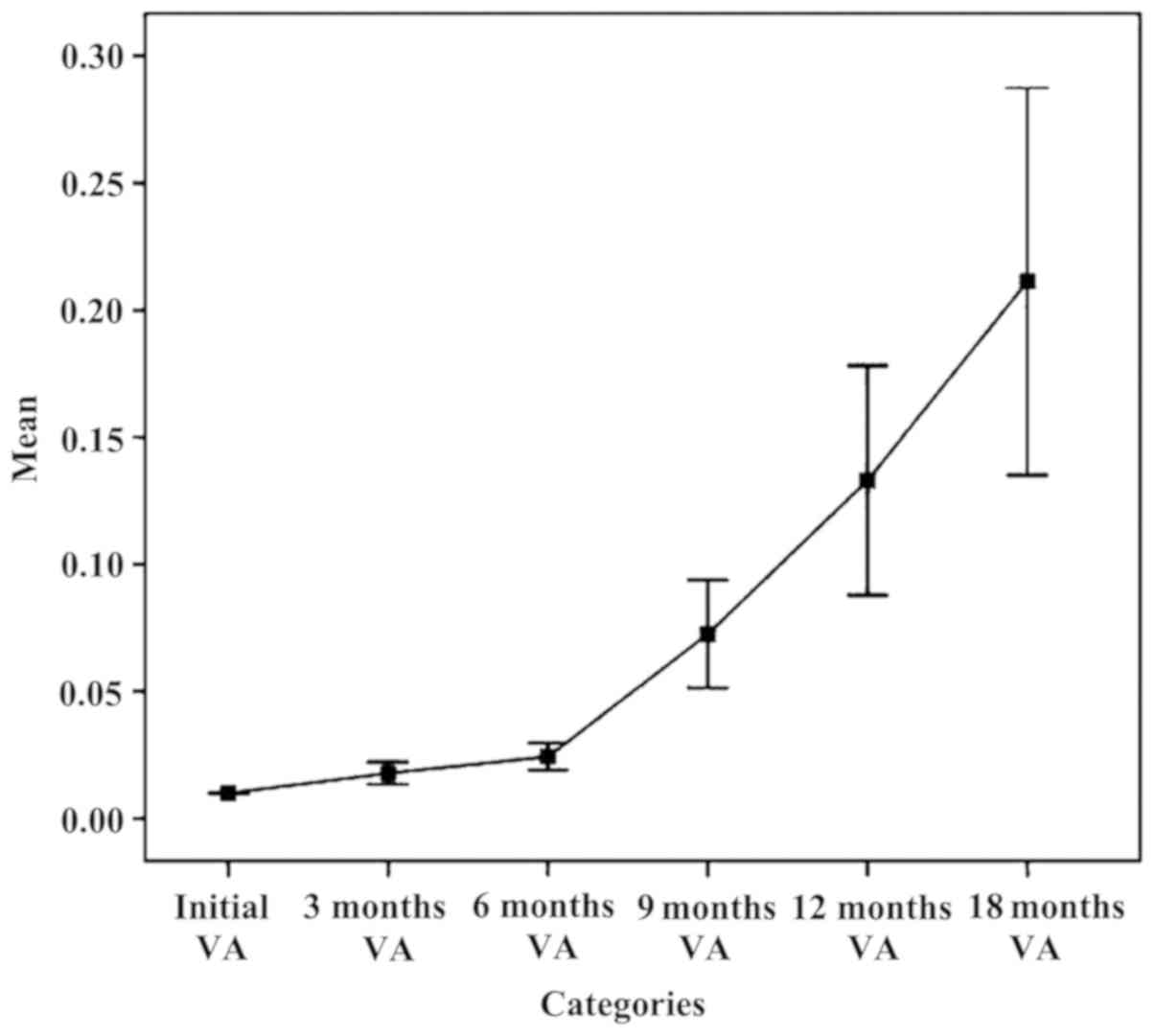
The chart in Fig. 1
established an upward trend in mean parameter AV on intervals
during follow-up. It looks favorable and functional, but
statistically insignificant, due to alteration in time of lots
followed and the remaining, not representative study subjects.
Increasing the average parameter ranges VA 6 months - 9 months - 12
months - 18 months is an unreal situation. Practically, we planned
and realized an improvement in visual acuity within 3 months and
even within 6 months, maintaining relatively constant limits for
longer periods of time (9, 12 or even 18 months), but failed, at
least for now, to achieve both significant growth and progress over
time. It is a gross analysis with rough results. For this reason,
we cannot make quantitative judgments of value on the results of
intravitreal bevacizumab therapy relying on such data, but we can
make qualitative ones.
The analysis of VA parameter pursued
independently on the lots processed and statistically adjusted by
patients (Fig. 2)
Preoperative visual acuity in the initial batch
ranged from 0.01 to 0.5. In the initial batch, mean visual acuity
was 0.04 and after 3 months it was 0.12. Visual acuity improved in
the assessment of 3 months in 77 eyes (66%), subjectively
quantified by the patient or objectively by physician. In 40 eyes
(34%) there was no change in visual acuity. There was no case of
decreased visual acuity postoperatively. In order to validate the
statistical data, processing, adaptation and conformity tests were
applied to patients' groups. The overall conclusion regarding
visual acuity independent parameter (VA) is that it highlights a
clear increasing trend of the parameter VA from the initial
interval to 3 months, with a flat profile (maintaining a constant
level) in the 3-, 6-, 9- and 12-month intervals.
The analysis of VA parameter pursued
in conjunction with foveal thickness (FT)
Analysis of the VA time intervals highlighting the
environmental functional effect and analysis of the average
thickness of the fovea on time intervals to highlight the effect of
structural environment (Table III,
Figs. 3 and 4).
 | Table III.Average VA time intervals on the
effect of functional environment and average thickness of the fovea
per time intervals with medium structural effect. |
Table III.
Average VA time intervals on the
effect of functional environment and average thickness of the fovea
per time intervals with medium structural effect.
| Time | Average VA | Change in VA during
treatment from the previous stage | Average FT | FT variation during
treatment from the initial stage |
|---|
| Initial | 0.071 |
| 450.941 |
|
| 1 month | 0.021 |
0.7 |
|
|
| 2 months | 0.023 | −0.11 |
|
|
| 3 months | 0.204 |
0.13 | 309.137 | −0.31 |
| 6 months | 0.074 | −0.72 | 431.556 |
0.4 |
| 9 months | 0.208 | −0.64 | 331.222 | −0.23 |
| 12 months | 0.065 |
0.69 | 400.500 |
0.21 |
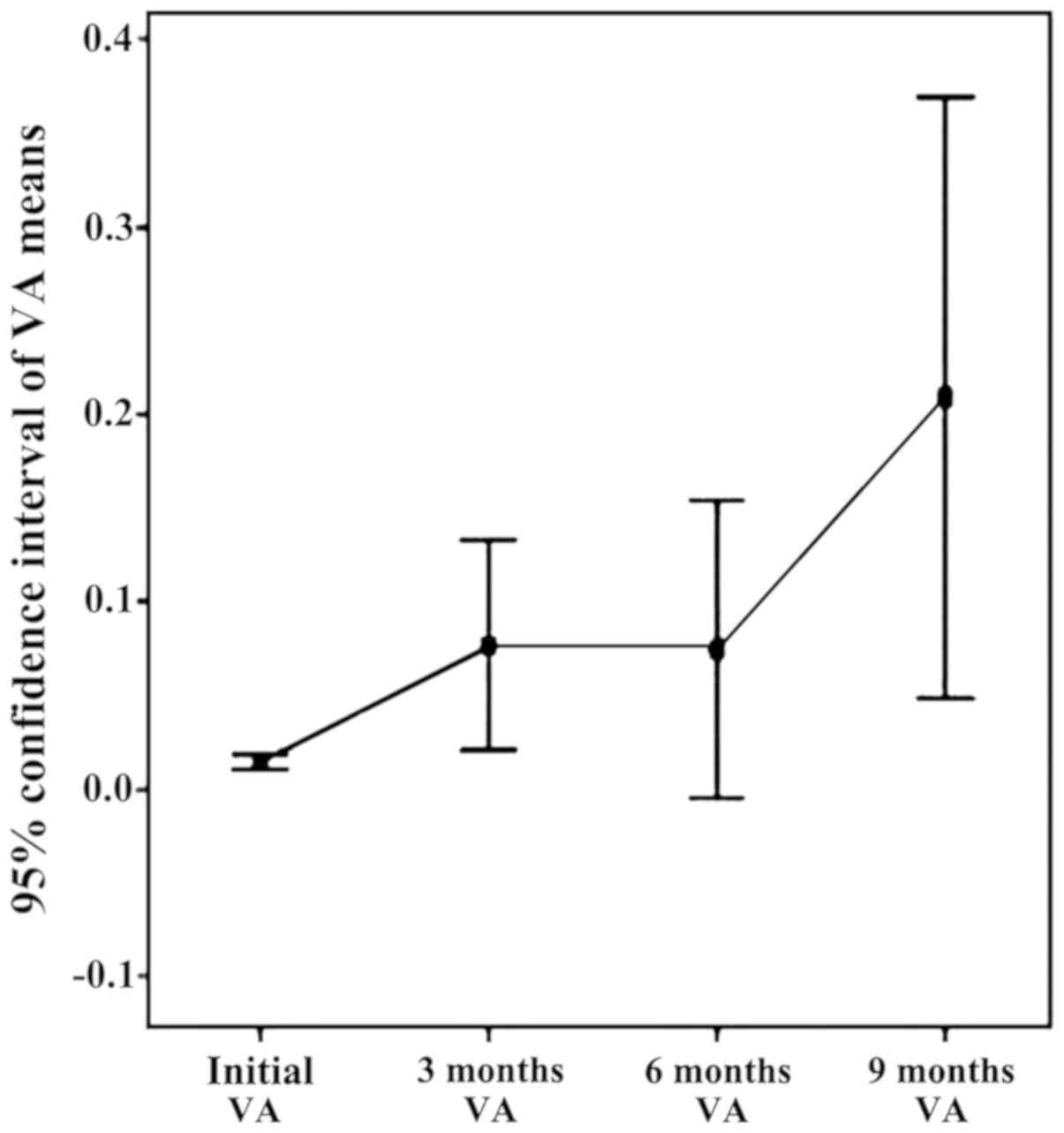
There is an ascending trend of visual acuity in
baseline period, 9 months, due to the effect of bevacizumab
injections (Fig. 3). Note the two
slopes upward initially 3 months (by applying the standard
protocol) and 6–9 months (by re-injection performed at 6 months).
The appearance slightly decreasing on the 3–6 month interval is due
to the reactivation of neovascular membranes.
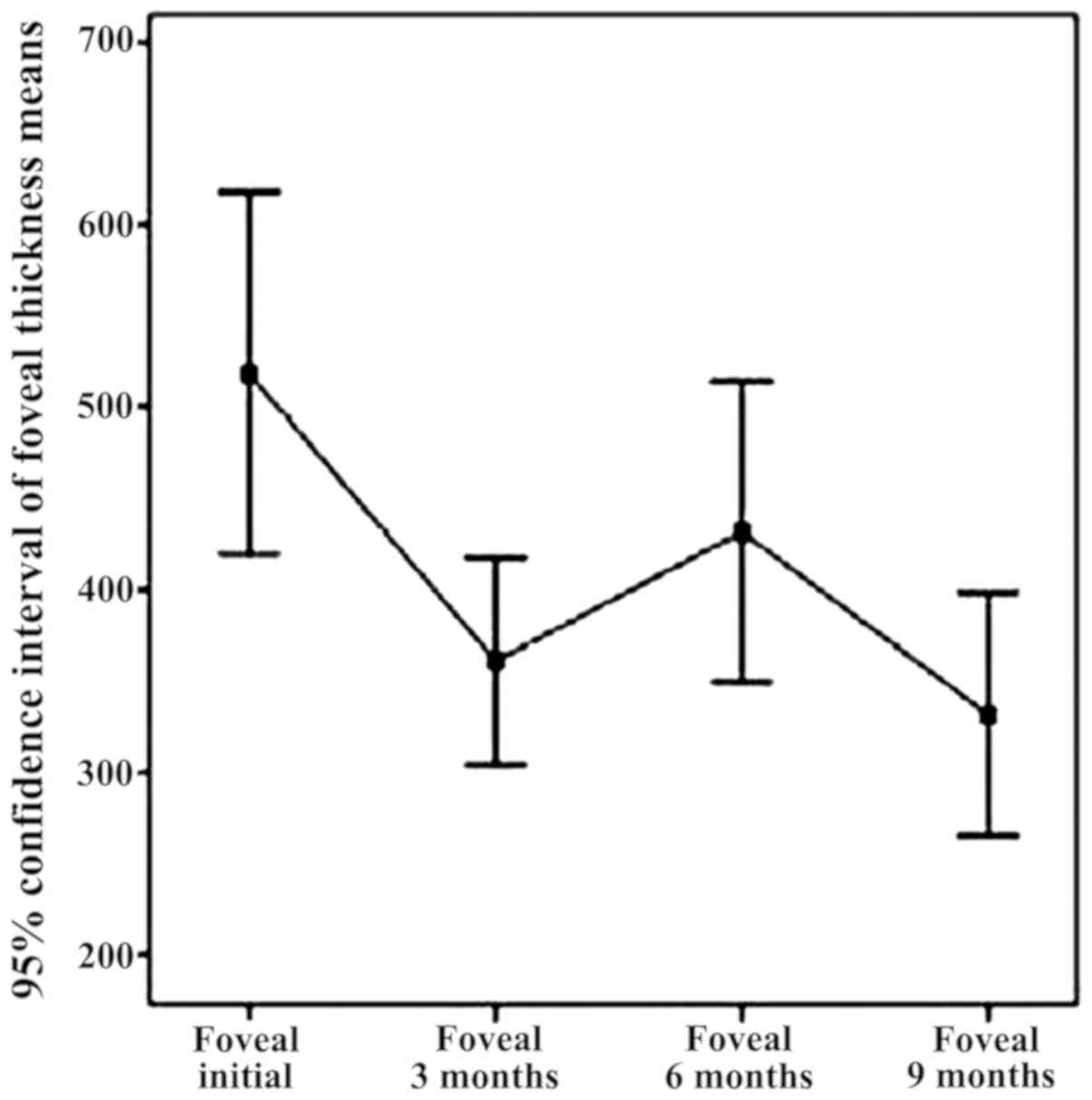
In Fig. 4, a decrease
of central retinal thickness in the baseline range of 9 months
under the effect of intravitreal bevacizumab injection can be
noted.
Fovea thickness (central retina), the initial batch,
determined by OCT, ranged from 266 microns to 720 microns, with an
average of 450.94 (±104.5) microns. Fovea thickness was
significantly reduced following treatment with intravitreal
bevacizumab, 3 months recorded an average of 309.13 (±66.57)
microns. The overall conclusion regarding fovea thickness
independent parameter refers to the downward trend in the range of
parameter FT from initial to 3 months. In addition, it refers to an
increase in central retinal thickness (6 months), explained by
recurrent exudative phenomena, again significantly reduced FT (9
months) through augmentation of antiangiogenic effect by
re-injection of 6 months, as well as a relatively stable appearance
of reference parameter within 9–12 months, item also followed and
expected from the treatment.
The statistical analysis of the two parameters
followed (visual acuity and foveal thickness) was statistically
validated in an initial period of 9 months, the data gathered at 12
months and 18 months being inconsistent. The conclusion of this
correlation is that for the initial time of 9 months, VA has an
upward trend, while presenting a downward trend for FT in the
pursued group, identifying a solid negative correlation between two
variables for all periods included (initially 3 months, 3–6 months
and 6–9 months).
The statistical analysis of the two followed
parameters (visual acuity and foveal thickness) also showed that
there were patients with choroidal neovascular membrane recurrence
(VA reduction and FT increase) at varying intervals from baseline
(6, 9 or 12 months), for which re-injection option has led to
significant improvement or stabilization of both structural and
functional parameters.
Discussion
Considerations on intravitreal
bevacizumab use complications
Within the studied patient groups, we did not have
systemic complications that could be associated with bevacizumab,
such as cardiac or cerebral ischemia. There were, however, minor
and local complications related to the technique of injection:
subconjunctival hemorrhage, 31 cases of the 376 injections (8.2%),
corneal erosion, 2 cases (0.5%), post-injection ocular surface
discomfort such as: burning, stinging, mild pain at the injection
site, itching, 11 cases (2.9%). There were no serious local
complications, such as retinal tears, rhegmatogenous retinal
detachment, endophthalmitis, increased intraocular pressure,
cataract, retinal pigment epithelium rupture or systemic
complications like cardiac or cerebral thromboembolic
accidents.
We have reviewed the results of prestigious research
groups regarding pharmacokinetics, the effects on the blood-ocular
barrier, electrophysiological effects, CNV histology, the general
conclusion being that bevacizumab is not toxic at the currently
used dose and can be used safely in patients with neovascular AMD
(6–11).
Arguments and counter-arguments
related to the personal study
In the series of cases diagnosed with choroidal
neovascularization secondary to AMD and treated according to a
personal algorithm with bevacizumab intravitreal injection, in the
non-comparative retrospective analysis, there was a functional
improvement from a quantitative and qualitative point of view
(improvement of visual acuity, color perception optimizing,
widening visual field, better appreciation of the spatial
elements); in addition, good anatomical results (central retinal
thickness reduction, reduction or disappearance of ‘leakage’) were
also noted. The retrospective analysis of personal cases treated
and followed was favorable to the use of intravitreal bevacizumab
in short-term and medium treatment for choroidal neovascular
membranes associated with AMD.
The study had several weaknesses: first of all, the
design of the study - retrospective, non-randomized,
non-controlled, interventional type on a number of cases - only
allowed assessment of effectiveness treatment under subjective
conditions chosen by the authors on a group of patients in whom
they identified a common element, namely choroidal
neovascularization associated with AMD. The absence of
randomization and control group does not allow making proper
interpretation of the epidemiological value. The ‘selection’ of
cases based on clinical criteria (significant exudation, minimal
fibrosis) allowed to obtain good results, situated somewhat above
the average of other studies that used randomization and control
elements. Another element inducing limits to the study is the
presence of short-term follow-up data (3 months), reducing the
database to track the medium term (9–12 months), but this
represents a subjective aspect and it is difficult to control by
the physician. In order to make valid judgments on the medium and
long-term, the study group should not impair over time. The tests
applied are of pair type (same subject is tested based on the same
clinical parameters intervals and different conditions) always
watching the number of subjects who remained in the study and
therefore would not affect the representativeness and statistical
significance of the results. For this reason, statistical analysis
stopped at the 12 months interval, virtually ignoring the values
recorded at 18 months.
Considerations regarding the use of
intravitreal bevacizumab in treating neovascular AMD
Preclinical studies have suggested that full-length
antibodies could not penetrate the retina and are therefore
ineffective in the treatment of subretinal pathology, in this case
choroidal neovascularization associated with AMD (12). The publication of clinical cases that
showed an impressive resolution of the macula intraretinal fluid in
Exudative AMD or Central Venous Occlusions have raised doubts over
the previous term (13,14). Subsequently, several studies have
shown that bevacizumab molecule can penetrate the retina in the
intravitreal administration (6,15). A
series of cases were also published showing favorable anatomic and
functional effect of bevacizumab therapy in various ocular
conditions associated with neovascular disease (16–20). The
first clinical results showed a promising efficiency profile for
Avastin in neovascular AMD (21,22).
There seems to be significant difference in terms of responsiveness
to anti-VEGF between different types of choroidal neovascular
membranes in classic or occult AMD, myopia, or juxtafoveal
telangiectasis and angioid streaks (21,23–31).
The intravitreal injection rate of adverse effects
for bevacizumab did not exceed that observed in control groups from
trials with ophthalmology accepted anti-VEGF (ranibizumab). There
was no more than 0.21% incidence of any adverse effect on an
average follow-up period of 3.5 months.
In cconclusion, the increased prevalence of wet AMD,
along with the severity of functional visual impairment have led to
significant research concentration on all issues identified in
correlation with the background condition. In the last thirteen
years of ophthalmology, there was a huge explosion belonging both
to the anti-angiogenic agents in the usual therapeutic arsenal and
to the optical coherence tomography as a standard tool of
diagnosis. The introduction of intravitreal bevacizumab in Romania
has also led to a fantastic increase in number of OCT equipment,
from four units in 2006 to more than two hundred. The huge
potential of anti-VEGF medication is revealed more and more while
allowing the development of new therapeutic principles and new
classes of drugs. Although treatment with anti-VEGF first addressed
choroidal neovascular membranes from AMD, the range of therapeutic
indications of this new class of substances was expanded
considerably to other pathologies (16–18,32–38).
To the best of our knowledge, this is the first
study in Romania that assessed, analyzed and correlated clinical
and statistically functional (improvement of visual acuity) and
structural (central retinal thickness reduction) results of
intravitreal bevacizumab treatment in patients with neovascular
AMD, while comparing them with results of baseline studies in
literature on the same topic.
We have been using bevacizumab in our daily practice
for thirteen years and we have decided to publish our results from
the beginning of our own experience (2006) as a tribute to the
remarkable results of this efficient, safe and accessible drug for
wet AMD patients. By using anti-VEGF agents in our clinical
approach, we could improve and prolong visual function for many
patients who did not have any viable option till antiangiogenics
was born. Bevacizumab is an affordable treatment when other, more
expensive anti-VEGF agents, are not available. Soon, there will
probably be new options at our disposal, aiming to overcome macular
atrophy and also subretinal fibrosis, items which bevacizumab could
not address. As physicians, our goal is to offer the best medical
solution to our patients; thus, we must continuously prepare to
fight against ‘unresolved’ issues.
Acknowledgements
Professional editing, linguistic and technical
assistance performed by Individual Service Provider Irina Radu,
certified translator in Medicine and Pharmacy.
Funding
Not applicable.
Availability of data and materials
All data and materials supporting the results of the
present study are available in the article.
Authors' contributions
HTS contributed to the conception and design of the
study, the acquisition, analysis and interpretation of data of the
study. Also contributed in drafting the work and revising it
critically for important intellectual content. SS contributed to
the design of the study and in drafting the study and revising it
critically for important intellectual content. BT and MD
contributed to the acquisition, analysis and interpretation of data
of the study and in the drafting the work. FB contributed to the
conception and design of the study and in revising the work
critically for important intellectual content. All authors read and
approved the final version of the manuscript and agreed to be
accountable for all aspects of the study in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
This study adhered to the tenets of the Declaration
of Helsinki and was approved by the Ethics Committee of our
hospital, ‘Prof. Dr. Agrippa Ionescu’ Clinical Emergency Hospital.
Written informed consent was obtained from all the patients before
the examinations and surgeries were performed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Klein R, Klein BE, Jensen SC and Meuer SM:
The five-year incidence and progression of age-related maculopathy:
The Beaver Dam Eye Study. Ophthalmology. 104:7–21. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Eye Institute, . Statistics and
Data. Prevalence of Blindness Data. Data Tables [online]. 2007,
https://nei.nih.gov/eyedata/pbd_tables
|
|
3
|
Fine SL, Berger JW, Maguire MG and Ho AC:
Age-related macular degeneration. N Engl J Med. 342:483–492. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ciulla TA, Harris A and Martin BJ: Ocular
perfusion and age-related macular degeneration. Acta Ophthalmol
Scand. 79:108–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moshfeghi AA, Rosenfeld PJ, Puliafito CA,
Michels S, Marcus EN, Lenchus JD and Venkatraman AS: Systemic
bevacizumab (Avastin) therapy for neovascular age-related macular
degeneration: Twenty-four-week results of an uncontrolled
open-label clinical study. Ophthalmology. 113:2002.e1–2002.e12.
2006. View Article : Google Scholar
|
|
6
|
Rosenfeld PJ, Fung AE and Puliafito CA:
Optical coherence tomography findings after an intravitreal
injection of bevacizumab (avastin) for macular edema from central
retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 36:336–339.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bakri SJ, Cameron JD, McCannel CA, Pulido
JS and Marler RJ: Absence of histologic retinal toxicity of
intravitreal bevacizumab in a rabbit model. Am J Ophthalmol.
142:162–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manzano RP, Peyman GA, Khan P and Kivilcim
M: Testing intravitreal toxicity of bevacizumab (Avastin). Retina.
26:257–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiss C, Michels S, Prager F, Weigert G,
Geitzenauer W and Schmidt-Erfurth U: Anterior chamber inflammatory
activity in patients with neovascular age-related macular
degeneration treated with intravitreal bevacizumab (Avastin).
Retina. 26:877–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spitzer MS, Wallenfels-Thilo B, Sierra A,
Yoeruek E, Peters S, Henke-Fahle S, Bartz-Schmidt KU and Szurman P;
Tuebingen Bevacizumab Study Group, : Antiproliferative and
cytotoxic properties of bevacizumab on different ocular cells. Br J
Ophthalmol. 90:1316–1321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ziemssen F, Warga M, Neuhann IM, Leitritz
M, Biester S, Grisanti S and Bartz-Schmidt KU: Does intravitreal
injection of bevacizumab have an effect on the blood-aqueus barrier
function? Br J Ophthalmol. 90:9222006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mangione CM, Lee PP, Gutierrez PR,
Spritzer K, Berry S and Hays RD; National Eye Institute Visual
Function Questionnaire Field Test Investigators, : Development of
the 25-item National Eye Institute Visual Function Questionnaire.
Arch Ophthalmol. 119:1050–1058. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mordenti J, Cuthbertson RA, Ferrara N,
Thomsen K, Berleau L, Licko V, Allen PC, Valverde CR, meng YG, Fei
DT, et al: Comparisons of the intraocular tissue distribution,
pharmacokinetics, and safety of 125I-labeled full-length
and Fab antibodies in rhesus monkeys following intravitreal
administration. Toxicol Pathol. 27:536–544. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosenfeld PJ, Moshfeghi AA and Puliafito
CA: Optical coherence tomography findings after an intravitreal
injection of bevacizumab (avastin) for neovascular age-related
macular degeneration. Ophthalmic Surg Lasers Imaging. 36:331–335.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shahar J, Avery RL, Heilweil G, Barak A,
Zemel E, Lewis GP, Johnson PT, Fisher SK, Perlman I and Loewenstein
A: Electrophysiologic and retinal penetration studies following
intravitreal injection of bevacizumab (Avastin). Retina.
26:262–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spaide RF, Laud K, Fine HF, Klancnik JM
Jr, Meyerle CB, Yannuzzi LA, Sorenson J, Slakter J, Fisher YL and
Cooney MJ: Intravitreal bevacizumab treatment of choroidal
neovascularization secondary to age-related macular degeneration.
Retina. 26:383–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heiduschka P, Fietz H, Hofmeister S,
Schultheiss S, Mack AF, Peters S, Ziemssen F, Niggemann B, Julien
S, Bartz-Schmidt KU, et al Tübingen Bevacizumab Study Group, :
Penetration of bevacizumab through the retina after intravitreal
injection in the monkey. Invest Ophthalmol Vis Sci. 48:2814–2823.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iturralde D, Spaide RF, Meyerle CB,
Klancnik JM, Yannuzzi LA, Fisher YL, Sorenson J, Slakter JS, Freund
KB, Cooney M, et al: Intravitreal bevacizumab (Avastin) treatment
of macular edema in central retinal vein occlusion: A short-term
study. Retina. 26:279–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spaide RF and Fisher YL: Intravitreal
bevacizumab (Avastin) treatment of proliferative diabetic
retinopathy complicated by vitreous hemorrhage. Retina. 26:275–278.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Avery RL: Regression of retinal and iris
neovascularization after intravitreal bevacizumab (Avastin)
treatment. Retina. 26:352–354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Avery RL, Pearlman J, Pieramici DJ, Rabena
MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R and Patel A:
Intravitreal bevacizumab (Avastin) in the treatment of
proliferative diabetic retinopathy. Ophthalmology.
113:1695.e1–1695.e15. 2006. View Article : Google Scholar
|
|
22
|
Aisenbrey S, Ziemssen F, Völker M,
Gelisken F, Szurman P, Jaissle G, Grisanti S and Bartz-Schmidt KU:
Intravitreal bevacizumab (Avastin) for occult choroidal
neovascularization in age-related macular degeneration. Graefes
Arch Clin Exp Ophthalmol. 245:941–948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avery RL, Pieramici DJ, Rabena MD,
Castellarin AA, Nasir MA and Giust MJ: Intravitreal bevacizumab
(Avastin) for neovascular age-related macular degeneration.
Ophthalmology. 113:363–372.e5. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoganathan P, Deramo VA, Lai JC, Tibrewala
RK and Fastenberg DM: Visual improvement following intravitreal
bevacizumab (Avastin) in exudative age-related macular
degeneration. Retina. 26:994–998. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakaguchi H, Ikuno Y, Gomi F, Kamei M,
Sawa M, Tsujikawa M, Oshima Y, Kusaka S and Tano Y: Intravitreal
injection of bevacizumab for choroidal neovascularisation
associated with pathological myopia. Br J Ophthalmol. 91:161–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laud K, Spaide RF, Freund KB, Slakter J
and Klancnik JM Jr: Treatment of choroidal neovascularization in
pathologic myopia with intravitreal bevacizumab. Retina.
26:960–963. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tewari A, Dhalla MS and Apte RS:
Intravitreal bevacizumab for treatment of choroidal
neovascularization in pathologic myopia. Retina. 26:1093–1094.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teixeira A, Moraes N, Farah ME and Bonomo
PP: Choroidal neovascularization treated with intravitreal
injection of bevacizumab (Avastin) in angioid streaks. Acta
Ophthalmol Scand. 84:835–836. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lommatzsch A, Spital G, Trieschmann M and
Pauleikhoff D: Intraocular application of bevacizumab for the
treatment of choroidal neovascularization secondary to angioid
streaks. Ophthalmologe. 104:325–328. 2007.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jorge R, Costa RA, Calucci D and Scott IU:
Intravitreal bevacizumab (Avastin) associated with the regression
of subretinal neovascularization in idiopathic juxtafoveolar
retinal telangiectasis. Graefes Arch Clin Exp Ophthalmol.
245:1045–1048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Munteanu M and Rosca C: Repositioning and
follow-up of intralenticular dexamethasone implant. J Cataract
Refract Surg. 39:1271–1274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jianu DC, Jianu SN, Dan TF, Motoc AGM and
Poenaru M: Pulsatile tinnitus caused by a dilated left
petrosquamosal sinus. Rom J Morphol Embryol. 57:319–322.
2016.PubMed/NCBI
|
|
33
|
Stanca HT, Suvac E, Munteanu M, Jianu DC,
Motoc AGM, Roşca GC and Boruga O: Giant cell arteritis with
arteritic anterior ischemic optic neuropathy. Rom J Morphol
Embryol. 58:281–285. 2017.PubMed/NCBI
|
|
34
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomarkers Med. 9:513–528. 2015. View Article : Google Scholar
|
|
35
|
Solomon I, Voiculescu VM, Caruntu C, Lupu
M, Popa A, Ilie MA, Albulescu R, Caruntu A, Tanase C, Constantin C,
et al: Neuroendocrine factors and head and neck squamous cell
carcinoma: An affair to remember. Dis Markers. 2018:97878312018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ion A, Popa IM, Papagheorghe LML,
Lisievici C, Lupu M, Voiculescu V, Caruntu C and Boda D: Proteomic
approaches to biomarker discovery in cutaneous T-cell lymphoma. Dis
Markers. 2016:96024722016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non-melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in vivo
reflectance confocal microscopy. Exp Ther Med. 15:1241–1246.
2018.PubMed/NCBI
|