Introduction
Cerebral infarction (CI) has the characteristics of
long recovery time and unsatisfactory prognosis. The disease
progresses gradually or stepwise, and is the main cause of human
disability and death (1). In recent
years, the number of people who died or became disabled from
cerebral infarction has shown an upward trend.
Clinical treatment of cerebral infarction often uses
thrombolysis technology. Reperfusion after thrombolysis is
beneficial to the recovery of neurons in reversible damage in the
ischemic area, leading to brain microvascular damage, blood-brain
barrier (BBB) injury, and angiogenic brain edema, aggravating brain
damage, which are also serious complications of thrombolysis, so
protecting or reducing BBB injury is of great significance for the
treatment of cerebral infarction. BBB is a special barrier between
the blood circulation and nerve tissue of the brain. Its function
is to maintain the homeostasis of the brain and regulate the
balance of material exchange in the brain. BBB injury is the main
cause of vasogenic cerebral edema and worsening of the disease in
ischemic stroke, maintenance and improvement of BBB damage are
important for the treatment of ischemic stroke and improvement of
prognosis (2–4). The nuclear transcription factor
NRF-2/hemeoxygenase 1 (NRF-2/HO-1) signaling pathway is the most
important cell defense mechanism, and studies have shown that
NRF-2/HO-1 signaling pathway activation can improve the integrity
and stability of the BBB, and is a key target for the treatment of
BBB in cerebral infarction (5,6).
Edaravone is a clinically used drug for the treatment and
prevention of ischemic stroke. It inhibits delayed neuronal death,
and inhibits the production of lipid peroxide, damage of vascular
endothelial cells and ischemic cerebral edema and improves
neurological function (7,8), there are reports that edaravone also
has effects in improving BBB, but the mechanism is still unclear
(9,10), and opinions vary. Niu et al
(11) considered that edaravone
inhibits the expression of occludin around the injured area of BBB,
and Tsuruoka et al (12) that
the expression of MMP-9 inhibited damaged brain tissue to protect
the function of BBB. However, the relationship between edaravone
and NRF-2/HO-1 signaling pathway has rarely been reported.
The aim of the study was to investigate the effect
of edaravone on the treatment of ischemic stroke with BBB injury
and the influence on NRF-2/HO-1 signaling pathway, in order to
provide a reference for the clinical treatment of ischemic stroke
with edaravone.
Materials and methods
Test animals
Eighty adult healthy male SD rats, with body weight
of 200–250 g, were provided by Hunan Weitong Lihua Experimental
Animal Co., Ltd., animal certificate number: SCXK (Hunan)
2009-0004. After 1 week of adaptive feeding, the experiment was
carried out. During the feeding period, the rats were given free
access to water, with constant temperature and humidity, and
artificial lighting for 12 h each day. All the operations strictly
abided by the ethical rules for experimental animals.
The study was approved by the Ethics Committee of
The Third Affiliated Hospital of Qiqihar Medical University
(Qiqihar, China).
Permanent cerebral artery occlusion
model and evaluation
A modified Longa method was used to make a permanent
middle cerebral artery occlusion model (cerebral infarction rats):
after abdominal anesthesia, rats were fixed on the operating table
in supine position, with preserved skin sterilized. Then 1 cm
median incision of the neck was taken to bluntly separate the neck,
and the right common carotid artery (CCA), external carotid artery
(ECA) and internal carotid artery (ICA)were separated, ECA trunk
was separated, ligated 2 mm from the CCA bifurcation and separated,
continuing to separate from the ICA to the base of the skull, the
ICA was exposed, CCA was temporarily clamped, a small opening was
cut in the ECA stump, the suture was inserted into the ICA through
the ECA stump, when slight resistance of the incoming suture was
felt, the end of the filament suture was ligated and cut, CCA blood
flow was restored, and wounds were sutured. After successful model
preparation, the rats were sacrificed at 24 h after surgery for
neurological function scores, and ≥2 were selected as study
subjects. Neurological function score: 0 point, no neurological
deficit; 1 point, the left forelimb of the rat was difficult to
fully extend; 2 points, left front limb flexion; 3 points, slightly
to the left when walking; 4 points, severe left side turn; 5
points, rats fell to the left while walking.
Grouping and administration
Eighty rats with cerebral infarction were randomly
divided into 4 groups: cerebral infarction model group, edaravone
low, medium and high dose groups. Healthy rats were taken from the
same batch as sham operation group, the rat model was established
in the same way as the ‘permanent middle cerebral artery occlusion
model’, but only the vascular was separated and the filament suture
was not inserted (n=20 per group). Rats in the cerebral infarction
model group and the control group were given normal saline by
intragastric administration. The edaravone low, medium and high
dose groups were intragastrically administered with 10, 15 and 20
mg/kg, respectively, for 14 days.
Indicator detection
Survival status
The survival status of all the groups of rats was
recorded, including activity, fur and diet.
Body mass and neurological function
scores
The body mass and neurological function scores of
all the groups of rats before and after administration were
recorded. The neurological function score was the same as
above.
Brain tissue water content
After treatment, 10 rats in each group were
sacrificed, and the brain tissue of the first half of the hematoma
center was taken, weighed and placed in an oven at about 100°C for
24 h and the dry weight was recorded. The water content of brain
tissue was calculated as follows: brain tissue water content (%) =
(fresh mass - dry mass)/fresh mass 100%.
Blood-brain barrier permeability
The Evans blue formamide (EB) method was used. After
treatment, the remaining 10 rats in each group were taken. After
general anesthesia, 2 ml/kg of 2% EB was injected through the tail
vein. The thoracic left ventricle was perfused with normal saline
at a pressure of 110 mmHg until the liquid flowing out of the right
atrium was colorless. The brain was taken by decapitation, and the
inner part of the hematoma was weighed and placed in formamide, 1
ml/100 g of brain tissue, and incubated at 60°C overnight. After
centrifugation at 300 × g and 4°C for 10 min with a radius of 8 cm,
the OD value of the supernatant was determined by UV751GD
ultraviolet spectrophotometer (Shanghai Precision Scientific
Instrument Co., Ltd.) at a wavelength of 632 nm, and the EB level
was calculated. The higher the EB, the higher the blood-brain
barrier permeability.
Western blot analysis of NRF-2 and
HO-1 protein expression
The total protein in remaining hematoma brain tissue
was extracted by RIPA buffer, and was detected by BCA method. Then,
10% SDS-PAGE was performed and transferred to a PVDF membrane and
30 µg of protein was loaded per lane. The cells were blocked with
5% skim milk powder at 4°C for 1 h. NRF-2 (1:250) and HO-1
(1:2,000) primary antibodies were added dropwise and incubated
overnight. The antibodies were incubated again and visualized with
horseradish enzyme-labeled secondary antibody (1:2,000) prior to
scanning by a Bio-Rad 2000 gel imaging system. Absorbance values
were analyzed using the Image-Pro Plus 6.0 Quantityone software.
NRF-2 (bs-1074R-1) and HO-1 (bs-0827R-1) were rabbit anti-human
antibodies and were from Shanghai Hengfei Biotechnology Co., Ltd.
β-actin (item no.: SC.1616, Santa Cruz, Biotechnology, Inc.) was
the internal reference.
Oxidative stress index
After treatment of rats in all the groups, blood was
taken from ocular venous plexus prior to euthanasia, and the serum
was obtained via centrifugation at 4,000 × g at 25°C for 5 min.
ELISA method was performed and the OD values of malonyldialdehyde
(MDA), superoxide dismutase (SOD) and glutathione (GSH) in serum
was read by MRX Revelation type microplate reader (Dynex) according
to the kit instructions, the expression levels were determined by
the standard curve of each index. MDA, SOD and GSH kits were
purchased from Nanjing Jiancheng Bioengineering Co., Ltd.
Statistical analysis
The data were processed by SPSS statistical software
package (SPSS, Inc.). Measurement data, such as NRF-2 and HO-1
protein relative expression, were expressed as mean standard
deviation (mean ± SD), and intra-group comparison was performed
using variance analysis. The SNQ-q test was used for pairwise
comparison between groups, α=0.05 for the test level. P<0.05 was
considered to indicate a statistically significant difference.
Results
Survival status of the five groups of
rats
The rats in the sham operation group were active,
the diet was normal, the hair color was regular, sensitive to
external stimuli, the activity of rats with cerebral infarction was
significantly reduced, the diet was significantly reduced, the hair
color of the rats was poor, and the spirit and aggression both were
poor, and the state of the edaravone rats was improved to a
different extent than that of cerebral infarction rats.
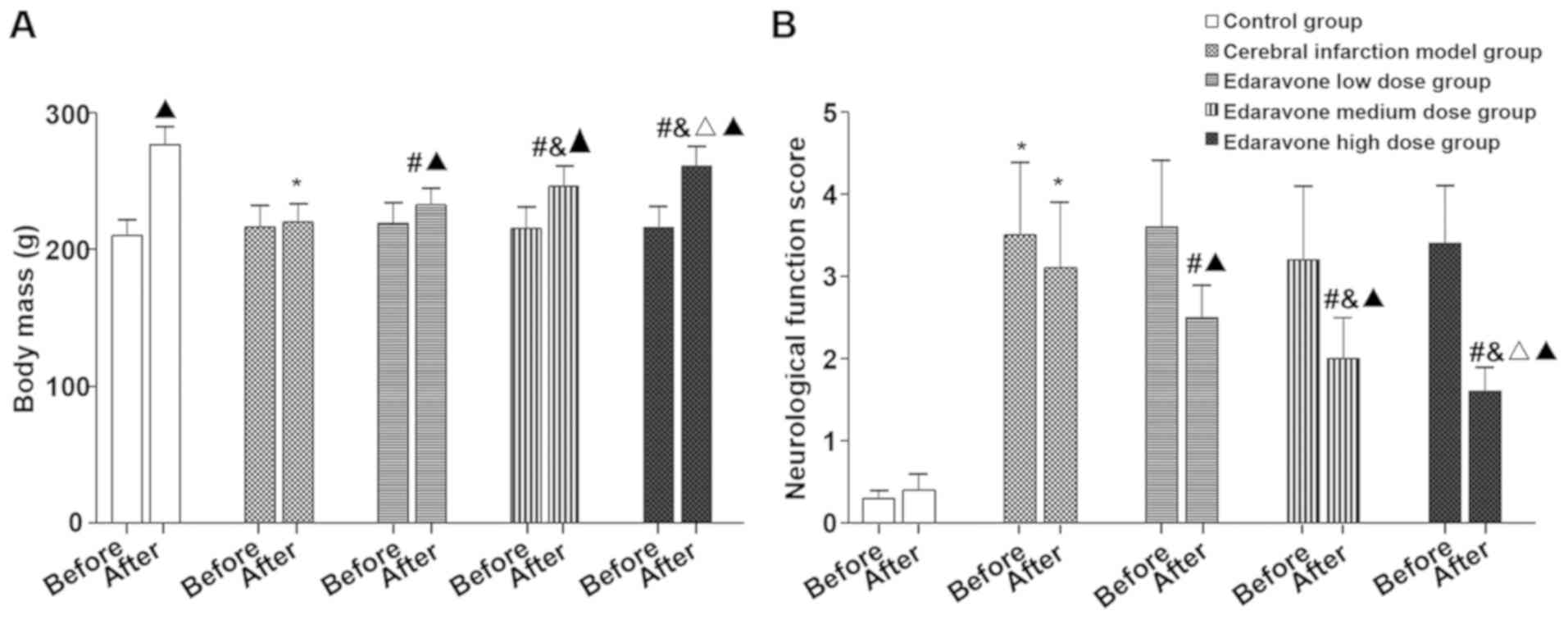
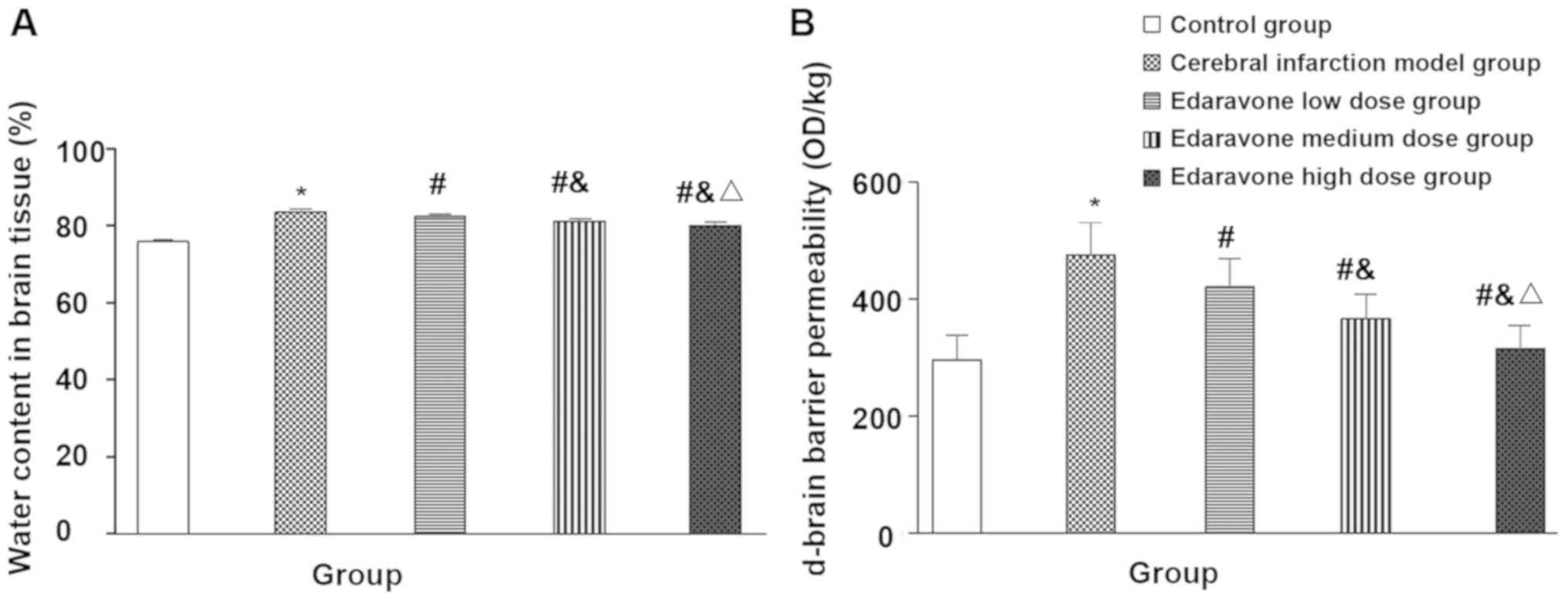
Comparison of the body weight,
neurological function score, brain tissue water content and
blood-brain barrier permeability of the five groups of rats
Compared with the control group, the body mass of
the cerebral infarction model group was significantly reduced after
treatment (t=13.937, P<0.001), neurological function score
(t=14.623, P<0.001), brain tissue water content (t=19.711,
P<0.001) and blood-brain barrier permeability (t=26.068,
P<0.001) were significantly increased. Compared with the
cerebral infarction model group, the body weight of the edaravone
group was significantly increased after treatment (F=31.122,
P<0.001), neurological function score (F=29.474, P<0.001),
brain tissue water content (F=60.185, P<0.001) and blood-brain
barrier permeability (F=22.986, P<0.001) were significantly
reduced, and showed a dose-dependent trend (Figs. 1 and 2).
Expression of NRF-2 and HO-1 in the
brain of five groups of rats
Compared with the control group, the expression of
NRF-2 (t=2.460, P=0.036) and HO-1 (t=2.683, P=0.025) in the brain
infarction model was significantly increased. Compared with the
cerebral infarction model group, the expression of NRF-2 (F=21.481,
P<0.001) and HO-1 (F=65.333, P<0.001) in the edaravone group
was significantly increased and in a dose-dependent manner
(Fig. 3).
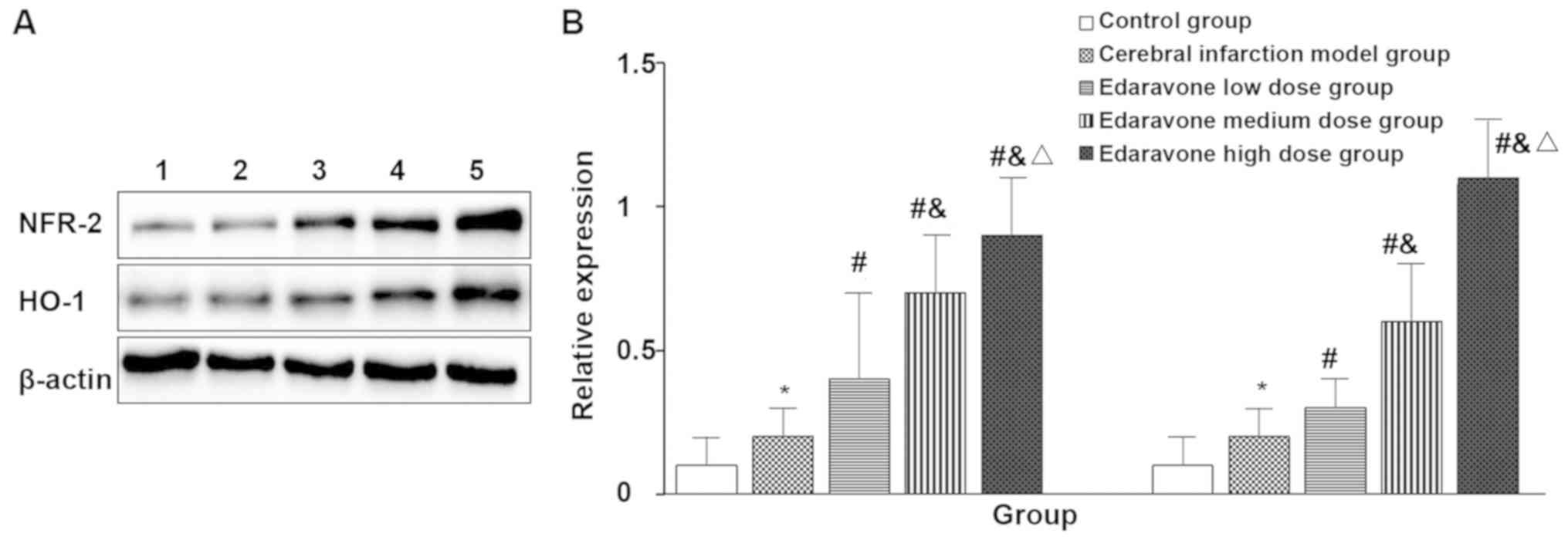 | Figure 3.Expression of NRF-2 and HO-1 in the
brains of five groups of rats (western blot, n=10). Lane 1, control
group; 2, cerebral infarction model group; 3, edaravone low dose
group; 4, edaravone medium dose group; 5, edaravone high dose
group. (A) Western blot result of NRF-2 and HO-1 protein. (B)
Quantitative analysis of NRF-2 and HO-1 protein. The brain
infarction model group compared with the control group, *P<0.05;
compared with the cerebral infarction model group,
#P<0.05; compared with the edaravone low dose group,
&P<0.05; compared with the edaravone medium dose
group, △P<0.05. |
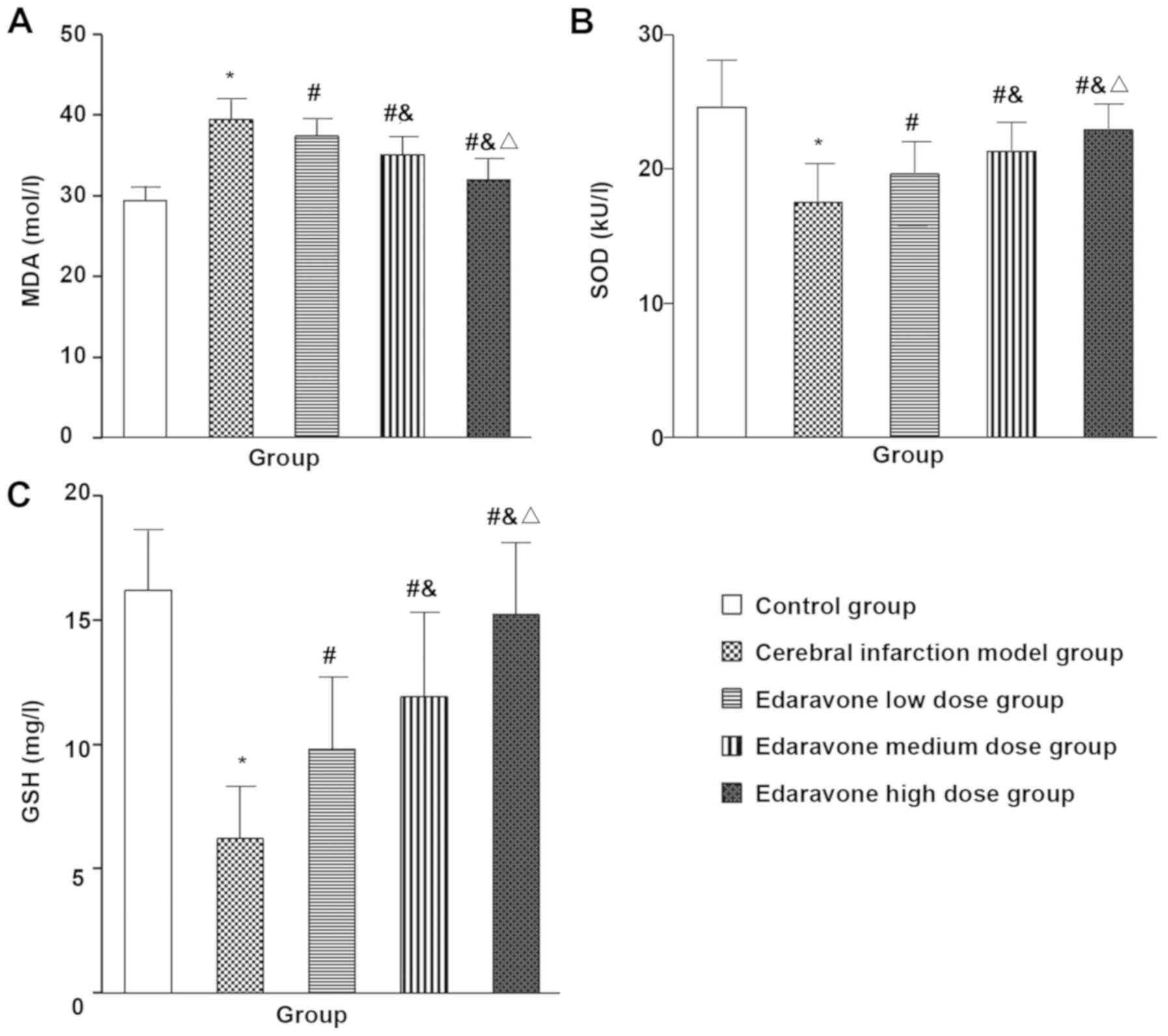
Comparison of oxidative stress
indicators in the peripheral blood of five groups of rats
Compared with the control group, the MDA (t=10.646,
P<0.001) in the cerebral infarction model group were
significantly increased, and the GSH (t=49.40, P=0.001) and SOD
(t=9.916, P<0.001) were significantly decreased, and compared
with the brain infarction model group, the MDA (F=19.051,
P<0.001) was significantly lower in the edaravone group, and the
GSH (F=9.667, P<0.001) and SOD (t=17.374, P<0.001) were
significantly increased, with a dose-dependence trend (Fig. 4).
Discussion
Cerebral edema after cerebral infarction is
vasogenic cerebral edema caused by impaired BBB, increased
permeability and plasma exudation, and the cells secrete a large
number of reactive oxygen species (ROS), which can participate in
oxidative stress and the release of cytotoxic substances, as shown
in the results of this study, the survival state of rats with
cerebral infarction decreased, the brain water content increased,
while the MDA of peripheral blood with oxidative stress were
significantly increased, and the antioxidant GSH and SOD were
significantly reduced.
The NRF2/HO-1 pathway is an important antioxidant
and cytotoxic defense mechanism in cells, which is of great
significance in stabilizing and protecting the integrity of the
blood-brain barrier. Under physiological conditions, the molecular
chaperone Kelch-like epichlorohydrin-related protein-1 (Keapl) is
coupled with NRF2, binds to actin, rapidly degraded by
ubiquitination, and has a short half-life. Under oxidative stress
conditions such as ischemia and hypoxia, NRF2 is decoupled from
Keap, and a large number of NRF2s are activated to enter the
nucleus and initiate NRF2 downstream target gene expression. As a
brain protective factor, NRF-2, through the binding of cell stress
signaling proteins, participates in the transcription process, and
then regulates the expression of various detoxification enzymes and
antioxidant enzymes downstream, which can regulate oxidative stress
after cerebral infarction (13).
HO-1 is an important downstream target gene of NRF2. HO-1 catalyzes
the degradation of heme, and the products of degradation have
anti-oxidative damage function. It has anti-inflammatory,
anti-apoptotic and anti-proliferative synergistic effects on HO-1.
It plays an important protective role in various diseases.
A large number of studies have shown that activation
of the NRF2/HO-1 pathway and induction of high expression of NRF2
and/or HO-1 may play an important role in anti-oxidation,
anti-apoptosis and protection of the blood-brain barrier (14). The results of this study showed that
compared with the control group, the expression of NRF2 and HO-1
protein in the brain of rats with cerebral infarction increased,
suggesting that the NRF2/HO-1 pathway is activated, which is the
endogenous and adaptive protection mechanism of the body to
initiate cerebral infarction, but this compensatory ability is
relatively limited, no drug intervention, no improvement in
neurological function scores in rats, suggesting that nerve
function may be lost, brain tissue damage may occur, with the
administration of edaravone and increased dose of edaravone, the
expression of NRF2 and HO-1 protein in the brain increase in a
dose-dependent manner, edaravone may promote the decoupling of NRF2
from the cytoplasmic chaperone Keap through the pathway of
inhibiting apoptosis, transport to the nucleus, initiate the
relative expression of the downstream gene HO-1 of NRF2, promote
the body to exert stronger anti-oxidative stress, improve the
integrity of BBB, and then protect the cranial nerves of infarcted
rats (15). This result is similar
to that reported in the literature. For example, studies of Liu
et al (16) have shown that
edaravone protects the hippocampal neurons of kainic acid-induced
epileptic rats by activating Nrf2/HO-1 channels; Zhang et al
(17) considered that edaravone
reduces neuronal apoptosis in the hippocampus by activating the
Nrf2/HO-1 signaling pathway, which in turn produces neuroprotection
in a mouse model of pneumococcal meningitis. Cechetti et al
(18) reported that edaravone
reduces oxidative stress induced by chronic cerebral hypoperfusion
injury and is associated with activation of Nrf2/HO-1 signaling
pathway.
There is insufficient research on this topic in
vivo, although the administration of edaravone in rats with
cerebral infarction reduces the permeability of blood-brain barrier
and brain edema, increases the expression of Nrf2 and HO-1 protein
in brain tissue, whether the upregulation of Nrf2 and HO-1 protein
expression is a direct or indirect effect of edaravone is not
clear, and further studies should be combined with ex vivo
analysis.
In summary, edaravone may exert a blood-brain
barrier protection by activating the NRF-2/HO-1 signaling
pathway.
Acknowledgements
Not applicable.
Funding
This study was supported by the Qiqihar Science and
Technology Plan Project (SFGG-201512).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL wrote the manuscript. JL and YJ were responsible
for the cerebral artery occlusion model construction. GZ and ZL
performed western blotting. SD was responsible for the data
analysis and interpretation. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Third Affiliated Hospital of Qiqihar Medical University
(Qiqihar, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nagai N, Yoshioka C, Ito Y, Funakami Y,
Nishikawa H and Kawabata A: Intravenous administration of
cilostazol nanoparticles ameliorates acute ischemic stroke in a
cerebral ischemia/reperfusion-induced injury model. Int J Mol Sci.
16:29329–29344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen S, Chen Z, Cui J, McCrary ML, Song H,
Mobashery S, Chang M and Gu Z: Early abrogation of gelatinase
activity extends the time window for tPA thrombolysis after embolic
focal cerebral ischemia in mice. eNeuro. 5(pii):
ENEURO.0391-17.2018. 2018.
|
|
3
|
Li M, Wen Y, Zhang R, Xie F, Zhang G and
Qin X: Adenoviral vector-induced silencing of RGMa attenuates
blood-brain barrier dysfunction in a rat model of MCAO/reperfusion.
Brain Res Bull. 142:54–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X, Kiss GK, Mellender SJ, Weiss HR and
Chi OZ: Activation of Akt by SC79 decreased cerebral infarct in
early cerebral ischemia-reperfusion despite increased BBB
disruption. Neurosci Lett. 681:78–82. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu C, Zhong L, Tian XL and Han YC:
Protective effects of 8-MOP on blood-brain barrier via the
Nrf-2/HO-1 pathway in mice model of cerebral infarction. Eur Rev
Med Pharmacol Sci. 22:4278–4287. 2018.PubMed/NCBI
|
|
6
|
Liu Z, Ma C, Shen J, Wang D, Hao J and Hu
Z: SDF-1/CXCR4 axis induces apoptosis of human degenerative nucleus
pulposus cells via the NF-κB pathway. Mol Med Rep. 14:783–789.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamada H, Kikuchi R, Nakamura A and
Miyazaki H: Severe reversible cerebral vasoconstriction syndrome
with large posterior cerebral infarction. J Stroke Cerebrovasc Dis.
27:3043–3045. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohta Y, Takamatsu K, Fukushima T, Ikegami
S, Takeda I, Ota T, Goto K and Abe K: Efficacy of the free radical
scavenger, edaravone, for motor palsy of acute lacunar infarction.
Intern Med. 48:593–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y and Zhao Y: Curative efficacy of
penehyclidine combined with edaravone on acute cerebral infarction
and their effects on serum TNF-α and NDS score in rats. Eur Rev Med
Pharmacol Sci. 22:223–228. 2018.PubMed/NCBI
|
|
10
|
Tóth AE, Walter FR, Bocsik A, Sántha P,
Veszelka S, Nagy L, Puskás LG, Couraud PO, Takata F, Dohgu S, et
al: Edaravone protects against methylglyoxal-induced barrier damage
in human brain endothelial cells. PLoS One. 9:e1001522014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niu F, Song XY, Hu JF, Zuo W, Kong LL,
Wang XF, Han N and Chen NH: IMM-H004, A new coumarin derivative,
improved focal cerebral ischemia via blood-brain barrier protection
in rats. J Stroke Cerebrovasc Dis. 26:2065–2073. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuruoka A, Atsumi C, Mizukami H, Imai T,
Hagiwara Y and Hasegawa Y: Effects of edaravone, a free radical
scavenger, on circulating levels of MMP-9 and hemorrhagic
transformation in patients with intravenous thrombolysis using
low-dose alteplase. J Stroke Cerebrovasc Dis. 23:2894–2899. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li C, Wang R, Hu C, Wang H, Ma Q, Chen S
and He Y: Pyridoxine exerts antioxidant effects in cell model of
Alzheimer's disease via the Nrf-2/HO-1 pathway. Cell Mol Biol.
64:119–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang W, Wang X, Zhang XS and Liang CZ:
Cryptotanshinone attenuates oxidative stress and inflammation
through the regulation of Nrf-2 and NF-κB in mice with unilateral
ureteral obstruction. Basic Clin Pharmacol Toxicol. 123:714–720.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Zhang X, Ma K, Zhang R, Hou P, Sun
B, Yuan S, Wang Z and Liu Z: Matrine alleviates early brain injury
after experimental subarachnoid hemorrhage in rats: Possible
involvement of PI3K/Akt-mediated NF-κB inhibition and
Keap1/Nrf2-dependent HO-1 inductionn. Cell Mol Biol
(Noisy-le-grand). 62:38–44. 2016.PubMed/NCBI
|
|
16
|
Liu Z, Yang C, Meng X, Li Z, Lv C and Cao
P: Neuroprotection of edaravone on the hippocampus of
kainate-induced epilepsy rats through Nrf2/HO-1 pathway. Neurochem
Int. 112:159–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang D, Xiao Y, Lv P, Teng Z, Dong Y, Qi
Q and Liu Z: Edaravone attenuates oxidative stress induced by
chronic cerebral hypoperfusion injury: Role of ERK/Nrf2/HO-1
signaling pathway. Neurol Res. 40:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cechetti F, Worm PV, Elsner VR, Bertoldi
K, Sanches E, Ben J, Siqueira IR and Netto CA: Forced treadmill
exercise prevents oxidative stress and memory deficits following
chronic cerebral hypoperfusion in the rat. Neurobiol Learn Mem.
97:90–96. 2012. View Article : Google Scholar : PubMed/NCBI
|