Introduction
Mast cells (MCs) are important effector cells and
immune regulatory cells in vivo (1). It originates from hematopoietic stem
cells in bone marrow, matures in the peripheral tissues,
distributes in the mucosa and connective tissue of whole body
(2,3). MCs were mainly divided into mucous
membranes and connective tissue MCs two subgroups according to the
distribution site and particles containing tryptase or chymase
(4). Many stimulus may activate the
MCs through the FcεRIdependent and non-dependent (such as c-Kit,
TLR) way. Activated MCs can produce three types of effector
molecules: The first is material stored in particles, such as 5-HT,
histamine, tryptase and chymase; the second category is the new
synthetic substances such as lipid metabolites, prostaglandins; the
third category is cytokines, such as IL-1, 3, TNF-α, VEGF and so on
(5–7). It is precisely because of the
generation of so many types of effector molecules, so MCs can
participate in a variety of biological processes of body. Now
studies show that MCs play a key role in the development of allergy
(8,9). Stem cell factor (SCF) (i.e. c-Kit
ligand), an important growth factor, has soluble and membrane-bound
two forms. SCF can be produced from both fibroblasts and
endothelial cells in vivo (10,11). MCs
characteristically express SCF receptor c-Kit. SCF is a potential
growth factor of MCs, in addition to affecting the development of
MCs, but also on its apoptosis, chemotaxis, adhesion, degranulation
and other biological characteristics (12,13). But
there are few studies on the effect of SCF signal on the production
of cytokines (especially Th2 type cytokines) in MCs. In the present
study, we investigated the effects of SCF on the production of
IL-13 and its mechanisms in mouse mast cell line P815 cells.
Materials and methods
Cell lines and experimental
reagents
Mouse mast cell line P815 cells purchased from
Shanghai Institute of life sciences, Chinese Academy of sciences.
DMEM (high glucose type) culture was purchased from Thermo Fisher
Company. Fetal bovine serum is product of Hangzhou Sijiqing
biological company. Fluorescein (PE-Cy5)-labeled CD117 (c-Kit)
antibody was purchased from eBioscience company. Recombinant mouse
SCF and U0126 was respectively purchased from PeproTech and Gene
Operation. JSI-124 and Curcumin are the product of Sigma company.
Wortmannin, NP-40 lysate, β-actin, p42/44 antibody, Phospho-p42/44
antibody, horseradish peroxidase labeled goat anti-mouse IgG
antibody, the BCA Protein Assay kit, nuclear proteins and
cytoplasmic protein extraction kit, H-89, PDTC, chemiluminescent
EMSA kit, biotin-labeled EMSA probes of CREB were purchased from
Beyotime Institute of Biotechnology (Haimen, China). Mouse
Interleukin 13 (IL-13) ELISA kit was purchased from Wuhan Huamei
Biological Engineering Co., Ltd. Trizol Reagent was purchased from
Invitrogen Corporation. TransScript First-Strand cDNA Synthesis
SuperMix and TransStart Top Green qPCR SuperMix are products of
Transgen Biotech company.
Cell culture
P815 cells were suspended in DMEM complete solution
(containing 4.0 mM L-glutamine, 4500 mg/l glucose, 10% fetal bovine
serum, 50,000 U/l gentamicin, 1 mmol/l sodium pyruvate), and
cultured in 37°C, 5% CO2 incubator. P815 cells were
seeded in 24-well plates and cells density is 5×105
cells/ml. After cultured in DMEM without serum for starvation 12 h,
the cells were treated with different concentrations of SCF for
different time.
Detection of c-kit receptor on P815
cell surface by flow cytometry
The cultured cells were washed 2 times with the
staining buffer solution. P815 cells were incubated with
PE-Cy5-c-Kit antibody (concentration based on the specification
instruction) in 100 µl reaction system at dark 4°C. After washed
two times with staining buffer, stained cells were fixed with 1%
paraformaldehyde (PFA), then were detected by flow cytometry.
Reverse transcription-quantitaive
polymerase chain reaction (RT-qPCR)
After P815 cells were washed two times with PBS,
their total RNA was extracted using Trizol reagent. cDNA was
reversed to synthesized in accordance with manufacturer's
instructions. PCR amplification was conducted in 25 µl reaction
system (including 2×TS Top Green qPCR SuperMix 12.5 µl, Passive
Reference Dye 0.5 µl, cDNA 1 µl, Forward Primer 0.5 µl, Reverse
Primer 0.5 µl, RNase-free water 10 µl). PCR reaction conditions
(two step method): 94°C 30s, 94°C 5s, 60°C 30s, a total of 40
cycles. IL-13 primer sequences are listed below: Upstream primer:
5′-GCAGCAGCTTGAGCACATT-3′, downstream primer:
5′-GGCATAGGCAGCAAACCA-3′. Gene expression was analyzed using
2−ΔΔCq method (14).
ELISA
To collect the culture supernatant of P815 cells
under various conditions, the concentration of IL-13 in supernatant
was detected by ELISA according to manufacturer's instructions.
Western blot analysis
Cytoplasmic protein was extracted from P815 cells of
control group and SCF stimulation group according to manufacturer's
instructions. After quantified by BCA method, the protein was
electrophoresed by SDS-PAGE (10% separating gel and 5% stacking
gel). After electrophoresis, NC film was used to transfer the
protein. The membrane was blocked 2 h with 5% BSA, and washed 30
min using TBST, then the membrane was incubated with primary
antibody or loading control antibody overnight at 4°C. After the
membrane was washed 30 min with TBST, then was incubated with
HRP-labeled second antibody 2 h. The positive signal was detected
by chemiluminescence method, and then the light density analysis
was carried out.
Electrophoretic mobility shift assay
(EMSA)
Cell nuclear proteins were collected from P815 cells
in the control group and the SCF stimulation group and
biotin-labeled CREB probe and nuclear protein binding reaction was
detected according to Beyotime kit instructions. Probe and protein
mixture was electrophoresis 45 min at 120V using 12% non-denaturing
PAGE gel. After electrophoresis, the protein was transferred to
nylon membrane using 380 mA constant current for 1 h and was
cross-linked 10 min using ultraviolet rays. Enhanced
chemiluminescence method was used for the detection of biotin
labeled CREB probes. CREB consensus oligo sequences are as
follows:
5′-AGAGATTGCCTGACGTCAGAGAGCTAG-3′,
3′-TCTCTAACGGACTGCAGTCTCTCGATC-5′.
Statistical analysis
The experimental data is indicated as mean ±
standard deviation, and one-way analysis of variance was used with
the Least Significant Difference post hoc test for the comparison
between groups. All analyses were carried out using SPSS 16.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of c-Kit receptor on the
surface of mast cell P815
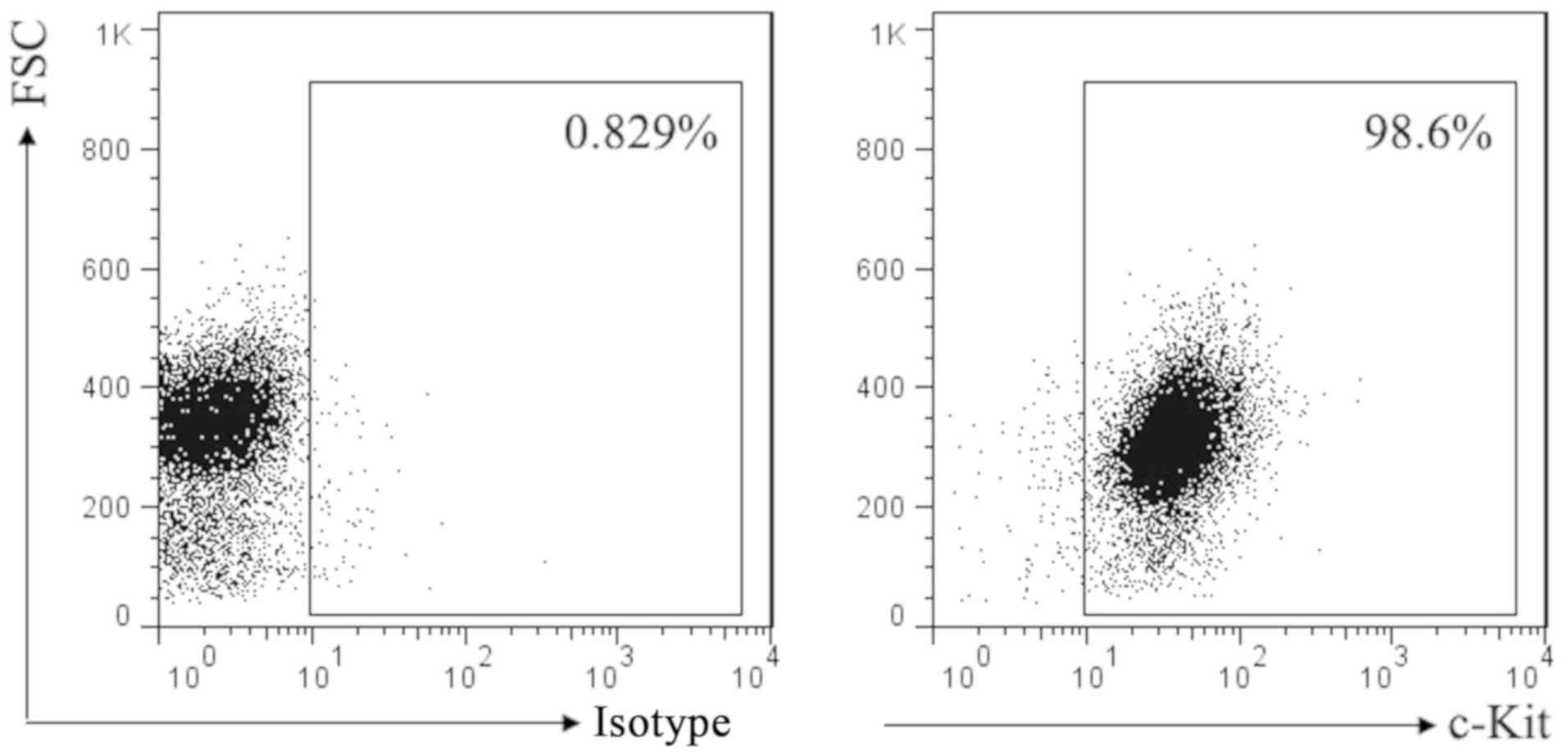
SCF corresponding receptor is c-Kit. To investigate
the effect of SCF signal on mast cell, we first used flow cytometry
to detect the expression of c-Kit in P815 cells. Result as shown in
Fig. 1, almost all of the P815 cells
membrane surface express c-Kit.
Effect of SCF on IL-13 production in
P815 cells
P815 cells were stimulated 6 h with
different concentrations of SCF
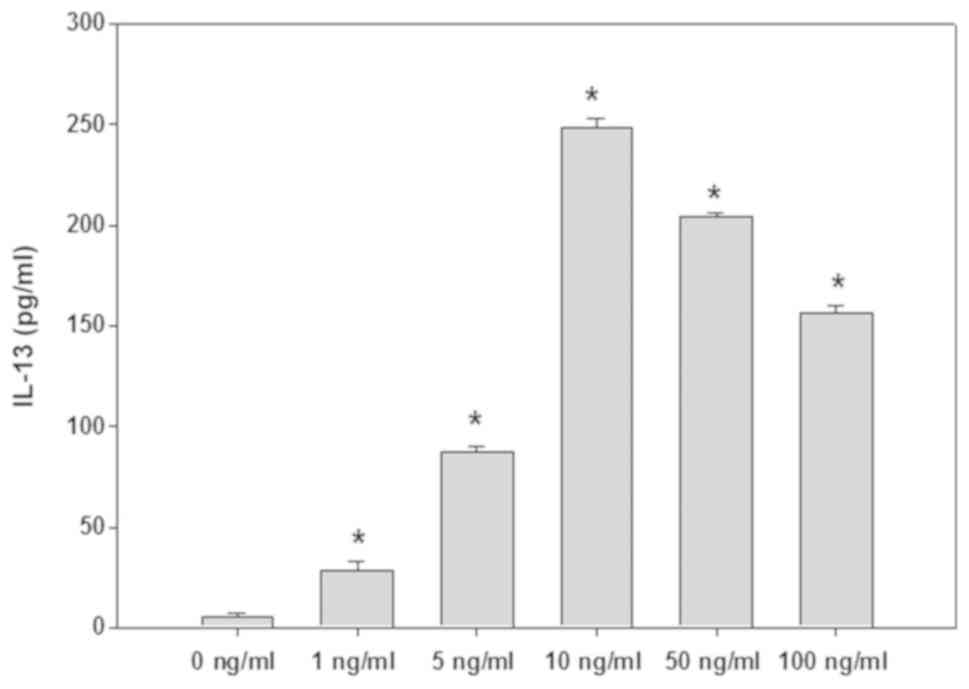
The supernatant in culture well was detected by
ELISA, and the results were shown in Fig. 2. SCF (1–100 ng/ml) can promote P815
cells to produce IL-13, among which the 10–50 ng/ml effect is the
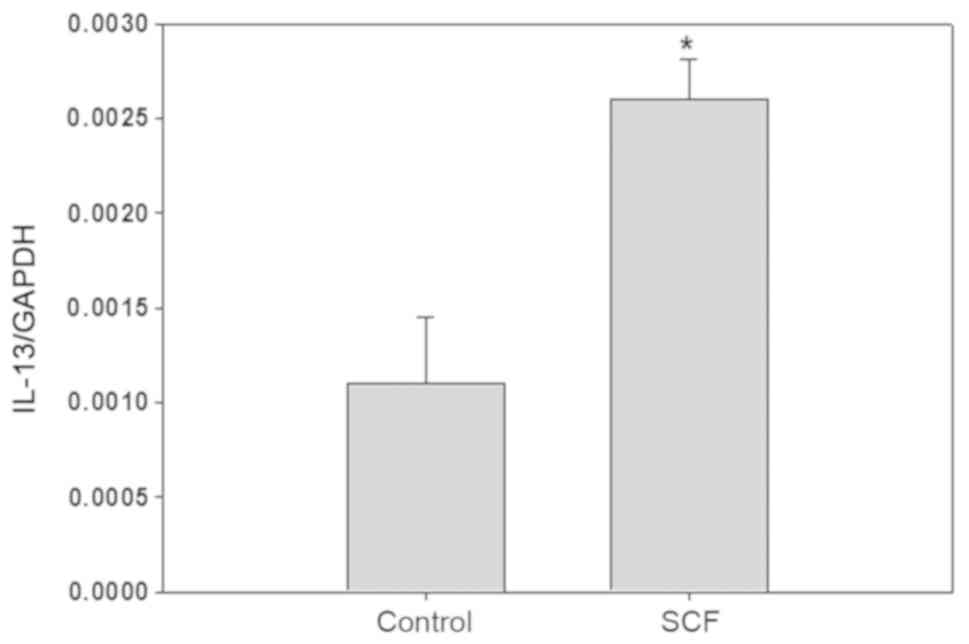
most obvious. After P815 cells were stimulated with SCF (50 ng/ml)
6 h, IL-13 gene expression in P815 cells is increased by about
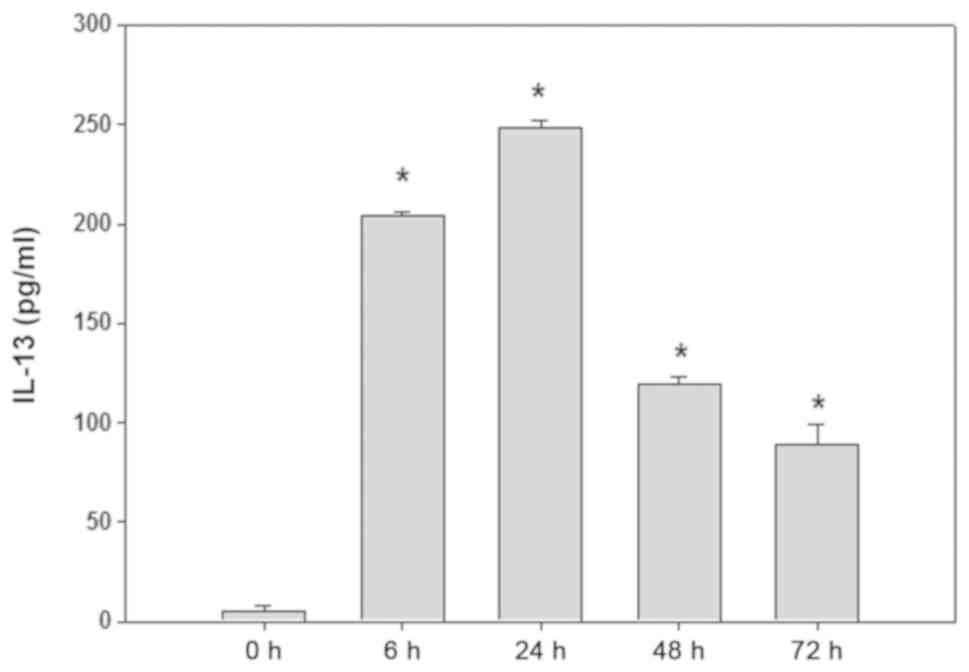
3-fold (Fig. 3). P815 cells were
stimulated with SCF (50 ng/ml) at different times, the content of
IL-13 in supernatant are shown in Fig.
4. Among them, the production of IL-13 reachs a higher level
when P815 cells were stimulated 6–24 h.
SCF promotes the production of IL-13
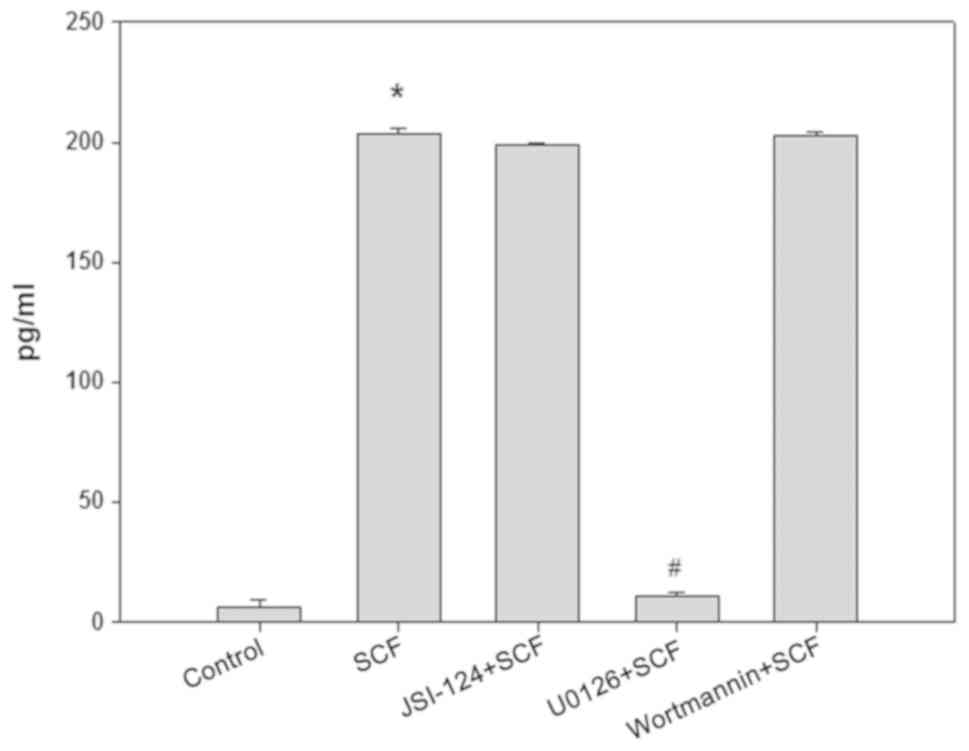
in MCs by MEK-ERK signaling pathway
After pretreated 30 min with MEK/ERK pathway
inhibitor U0126 (10 µM) or JAK/STAT3 pathway inhibitor JSI-124 (100
nM) or PI3K/Akt pathway inhibitor wortmannin (1 µM), P815 cells
were stimulated 6 h with SCF (50 ng/ml), then the content of IL-13
in the culture supernatant of P815 cells was detected by ELISA. The
results are shown in Fig. 5. U0126
completely blocked the effect of SCF on promotion IL-13 production
in P815 cells, but JSI-124 and Wortmannin had no effect on this
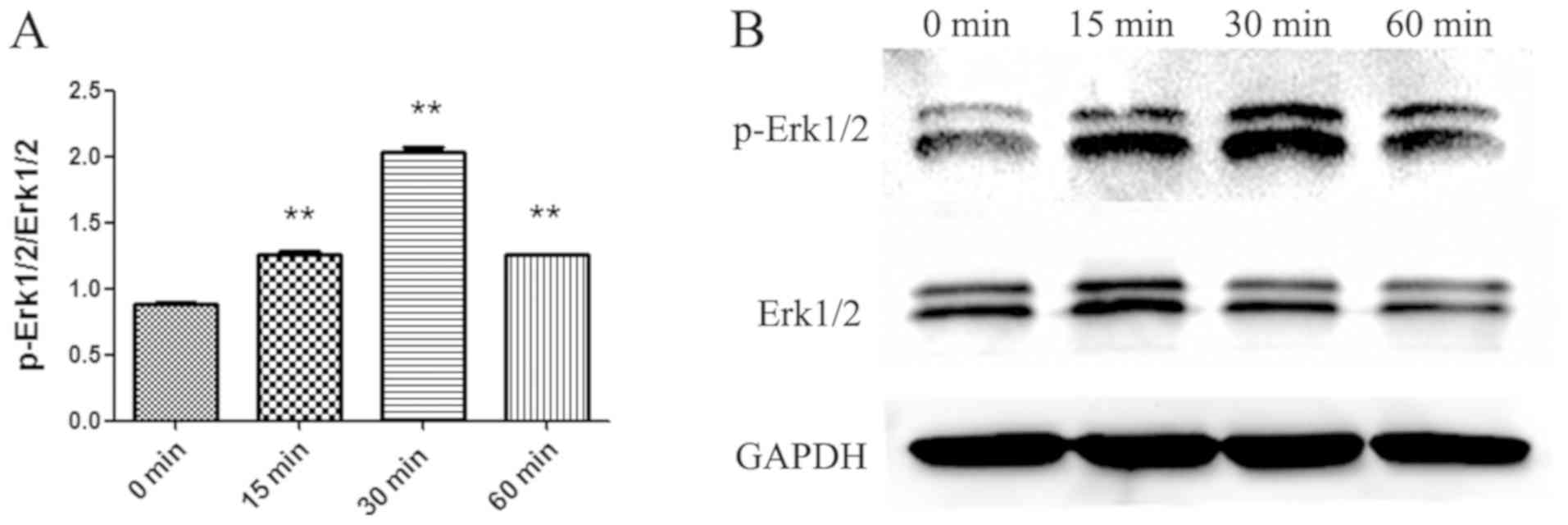
role of SCF. To demonstrate the activation of the MEK-ERK signaling
pathway, cytosolic proteins was extracted from P815 cells
stimulated with SCF (50 ng/ml) at different times and the
activation of Erk1/2 was detected by Western blot (Fig. 6). Erk1/2 phosphorylation was the
maximum in P815 cells stimulated 30 min with SCF. So it can be
proved that the SCF signal can promote the production of IL-13 by
activating the MEK-ERK signaling pathway in P815 cells.
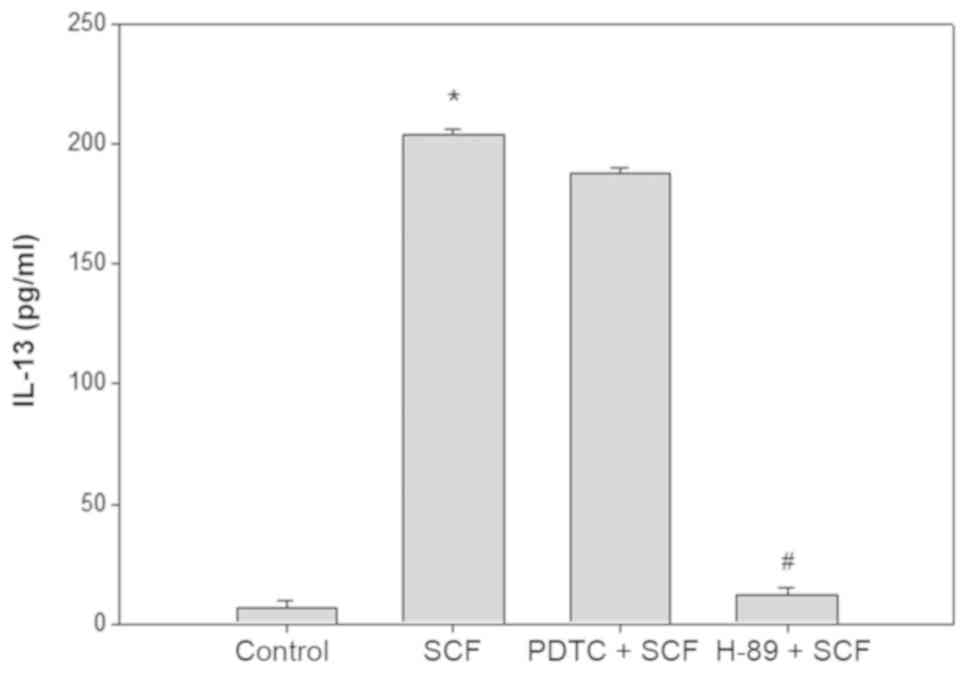
SCF activates CREB to promote the
production of IL-13 in P815 cells
To further clarify the role of the downstream factor
of MEK-ERK pathway in SCF promoting the IL-13 production in P815
cells. After pretreated 30 min with H-89 (20 µM, CREB blocker) or
PDTC (50 µM, NF-κB inhibitor), P815 cells were stimulated with SCF
(50 ng/ml) 6 h, and the content of IL-13 in supernatant was
detected by ELISA (Fig. 7). PDTC had
no effect on the SCF promoting P815 cells to produce IL-13, whereas
H-89 completely inhibited the effect of SCF.
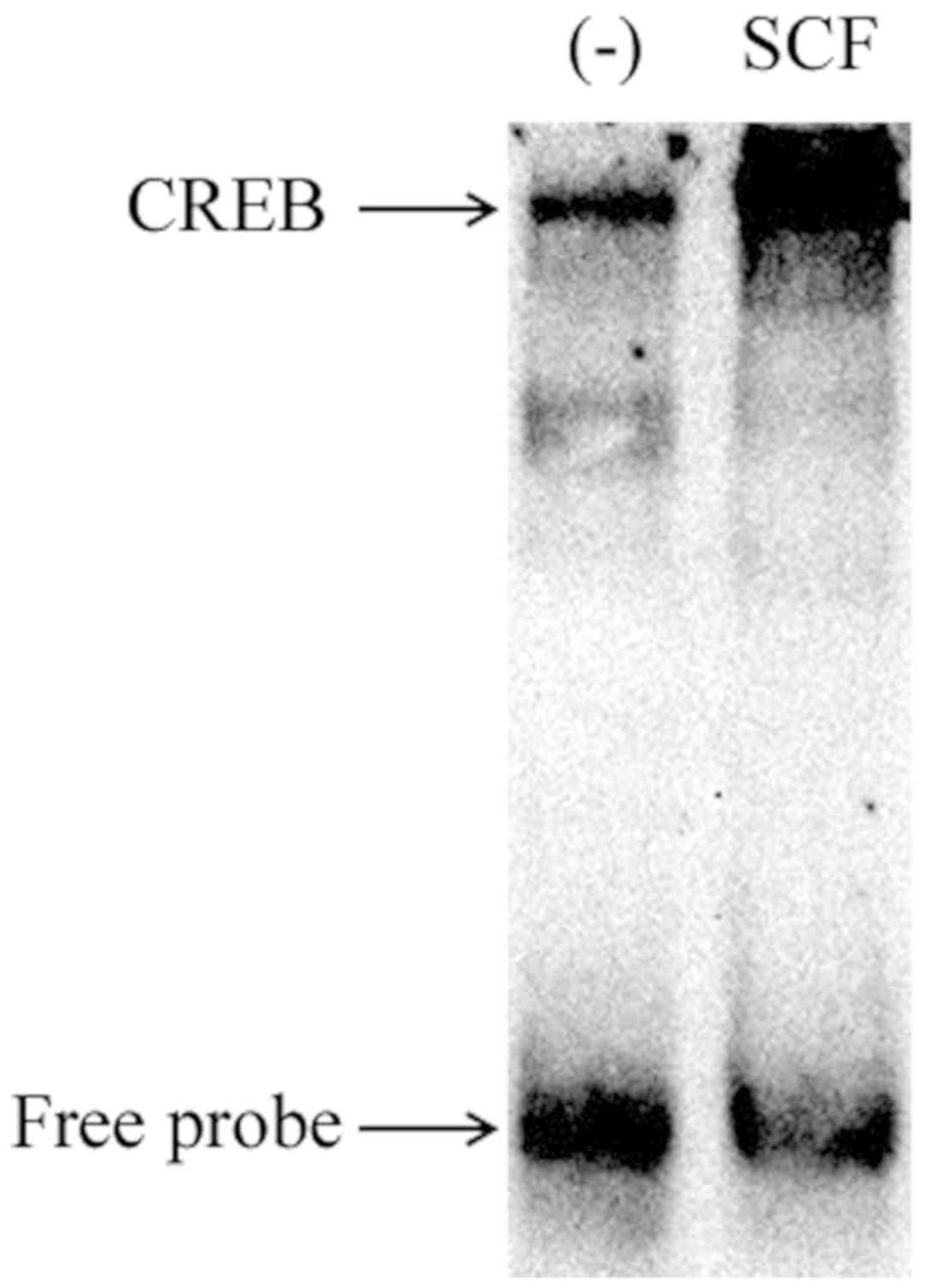
To demonstrate the activation of CREB, nuclear
proteins of P815 cells stimulated 1 h by SCF (50 ng/ml) was
extracted for EMSA. The results are shown as in Fig. 8. SCF signal induced significant CREB
activation in P815 cells. These suggest that SCF signaling can
regulate the production of IL-13 in P815 cells by activating
transcription factor CREB.
Discussion
In the present study we demonstrate that there is
the presence of c-kit expression on the P815 cells membrane
surface, and SCF signaling can induce MEK-ERK-CREB signaling
pathway activation in P815 cells, blocking the pathway inhibits the
effect of SCF promoting P815 cells to produce IL-13.
SCF, also known as c-Kit ligand, is an important
growth factor in the body, including soluble and membrane-bound two
forms. SCF is mainly produced by fibroblasts and endothelial cells
and it can promote the proliferation, migration, survival and
differentiation of hematopoietic precursor cells, melanocytes, germ
cells and other cells. Studies have indicated that SCF-c-Kit
signaling also plays an important role in the survival, growth,
adhesion and other biological activities of MCs (15,16).
Since MCs play a key role in allergic reactions, SCF-c-Kit
signaling is an important research target in the study of allergic
reactions. SCF start its effect by binding to c-Kit, the
combination of SCF led to c-Kit dimerization and open its protein
kinase activity. Activation of c-Kit activates multiple
intracellular signaling pathways, such as Src kinase, PI3K, PLC-γ,
MAPK and so on (11,17). SCF induced activation of Src family
is associated with the gene transcription and chemical chemotaxis
of MCs (18). The PI3K pathway
activation induced by SCF is associated with the development of MCs
(19). MacNeil's results showed that
SCF signal induced mast cell to produce IL-6 by P38MAPK and JNK
signaling (20). In this study, we
found that the SCF signal can activate the MEK-ERK-CREB signaling
pathway, and the activation of this pathway is related to the
production of IL-13 in MCs. Therefore, activation of each signal
pathway induced by SCF is associated with specific biological
functions.
Previous studies have suggested that the content of
SCF in sputum and alveolar lavage fluid of asthma patients was
increased. SCF was strongly correlated with IgE levels and the
state of lung function in patients with allergic and non-allergic
asthma patients (21,22). But the exact relationship between SCF
and asthma is still not very clear. Asthma is a chronic airway
inflammation, which involves a variety of cells and cytokines
(23). Epidemiological studies have
shown that there is an increasing trend in all parts of the world
(including China) about the morbidity and mortality of asthma
(24). MCs as the key effector cells
in asthma, hay fever and other allergic reaction has been known to
everyone, but more and more studies show the product of mast cells
after activation can regulate the adaptive immune response
intensity, duration and dynamics. Mast cells can also be used as
immune regulatory cells to play an important role in a variety of
biological processes (25). Once
activated, MCs can secrete lipid products, cytokines and chemokines
three types of chemical media. This study found that the production
of IL-13 was increased in mast cells stimulated by SCF signal. Li's
results showed that SCF signal induced mast cell to produce IL-13
through the early growth response factor-1 (26), which is consistent with our research
results.
IL-13, molecular weight of 12 KDa, is a pleiotropic
cytokine which regulates IgE synthesis, mucus hypersecretion,
subepithelial fibrosis, eosinophil tissue infiltration, chemokine
receptors (eg CCR5) expression, which is also closely linked with
airway inflammation and bronchial remodeling. Current studies
suggest that IL-13 plays a central role in the pathogenesis of
asthma (27,28). Thus, SCF signaling may play an
important role in the pathogenesis of asthma by affecting the
production of IL-13 in mast cells.
In conclusion, this study suggests that SCF
signaling can induce mast cells P815 to produce IL-13, and this
effect is related to the MEK-ERK-CREB signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Science
Foundation of China (grant no. 81273273), Anhui Provincial Natural
Science Foundation (grant no. 1708085MH218) and the Scientific
Research Innovation Team Project of Anhui Colleges and Universities
(grant no. 2016-40).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CS and SG designed the experiments. YW, HM, XT, YL,
HW and JH performed the experiments. QF, SG and CS analyzed the
data. CS wrote the manuscript. CS and SG revised the manuscript.
The manuscript has been read and approved by each author, and all
authors believe that the manuscript represents honest work.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Bengbu Medical College.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morita H, Saito H, Matsumoto K and Nakae
S: Regulatory roles of mast cells in immune responses. Semin
Immunopathol. 38:623–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang H, Li Y and Liu B: Transcriptional
regulation of mast cell and basophil lineage commitment. Semin
Immunopathol. 38:539–548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boeckxstaens G: Mast cells and
inflammatory bowel disease. Curr Opin Pharmacol. 25:45–49. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galli SJ, Nakae S and Tsai M: Mast cells
in the development of adaptive immune responses. Nat Immunol.
6:135–142. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gilfillan AM and Tkaczyk C: Integrated
signalling pathways for mast-cell activation. Nat Rev Immunol.
6:218–230. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arthur G and Bradding P: New developments
in mast cell biology: Clinical implications. Chest. 150:680–693.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cardamone C, Parente R, Feo GD and
Triggiani M: Mast cells as effector cells of innate immunity and
regulators of adaptive immunity. Immunol Lett. 178:10–14. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Savage JH, Courneya JP, Sterba PM,
Macglashan DW, Saini SS and Wood RA: Kinetics of mast cell,
basophil and oral food challenge responses in omalizumab-treated
adults with peanut allergy. J Allergy Clin Immunol.
130:1123–1129.e2. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andersson C, Tufvesson E, Diamant Z and
Bjermer L: Revisiting the role of the mast cell in asthma. Curr
Opin Pulm Med. 22:10–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reber L, Da Silva CA and Frossard N: Stem
cell factor and its receptor c-Kit as targets for inflammatory
diseases. Eur J Pharmacol. 533:327–340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lennartsson J and Rönnstrand L: Stem cell
factor receptor/c-Kit: From basic science to clinical implications.
Physiol Rev. 92:1619–1649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lorentz A and Bischoff SC: Regulation of
human intestinal mast cells by stem cell factor and IL-4. Immunol
Rev. 179:57–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Azzam N, Kondeti V, Duah E, Gombedza F,
Thodeti CK and Paruchuri S: Modulation of mast cell proliferative
and inflammatory responses by leukotriene d4 and stem cell factor
signaling interactions. J Cell Physiol. 230:595–602. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faber TW, Pullen NA, Fernando JF, Kolawole
EM, McLeod JJ, Taruselli M, Williams KL, Rivera KO, Barnstein BO,
Conrad DH and Ryan JJ: ADAM10 is required for SCF-induced mast cell
migration. Cell Immunol. 290:80–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smrž D, Bandara G, Beaven MA, Metcalfe DD
and Gilfillan AM: Prevention of F-actin assembly switches the
response to SCF from chemotaxis to degranulation in human mast
cells. Eur J Immunol. 43:1873–1882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stankov K, Popovic S and Mikov M: C-KIT
signaling in cancer treatment. Curr Pharm Des. 20:2849–2880. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Laughlin-Bunner B, Radosevic N, Taylor
ML, Shivakrupa, DeBerry C, Metcalfe DD, Zhou M, Lowell C and
Linnekin D: Lyn is required for normal stem cell factor-induced
proliferation and chemotaxis of primary hematopoietic cells. Blood.
98:343–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukao T, Yamada T, Tanabe M, Terauchi Y,
Ota T, Takayama T, Asano T, Takeuchi T, Kadowaki T, Hata Ji J and
Koyasu S: Selective loss of gastrointestinal mast cells and
impaired immunity in PI3K-deficient mice. Nat Immunol. 3:295–304.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
MacNeil AJ, Junkins RD, Wu Z and Lin TJ:
Stem cell factor induces AP-1-dependent mast cell IL-6 production
via MAPK kinase 3 activity. J Leukoc Biol. 95:903–915. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moaaz M, Abo El-Nazar S, Abd El-Rahman M
and Soliman E: Stem cell factor and interleukin-31 expression:
Association with IgE among Egyptian patients with atopic and
Nonatopic bronchial asthma. Immunol Invest. 45:87–106. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei Z, Liu G, Huang Q, Lv M, Zu R, Zhang
GM, Feng ZH and Huang B: SCF and IL-31 rather than IL-17 and BAFF
are potential indicators in patients with allergic asthma. Allergy.
63:327–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Samitas K, Delimpoura V, Zervas E and Gaga
M: Anti-IgE treatment, airway inflammation and remodelling in
severe allergic asthma: Current knowledge andfuture perspectives.
Eur Respir Rev. 24:594–601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anandan C, Nurmatov U, van Schayck OC and
Sheikh A: Is the prevalence of asthma declining? Systematic review
of epidemiological studies. Allergy. 65:152–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bulfone-Paus S and Bahri R: Mast cells as
regulators of T cell responses. Front Immunol. 6:3942015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li B, Berman J, Tang JT and Lin TJ: The
early growth response factor-1 is involved in stem cell factor
(SCF)-induced interleukin 13 production by mast cells, but is
dispensable for SCF-dependent mast cell growth. J Biol Chem.
282:22573–22581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitchell J, Dimov V and Townley RG: IL-13
and the IL-13 receptor as therapeutic targets for asthma and
allergic disease. Curr Opin Investig Drugs. 11:527–534.
2010.PubMed/NCBI
|
|
28
|
Corren J: Role of interleukin-13 in
asthma. Curr Allergy Asthma Rep. 13:415–420. 2013. View Article : Google Scholar : PubMed/NCBI
|