Introduction
A real-time cell-monitoring analysis (RTCA) system
was previously developed for continuous monitoring of adherent cell
cultures (1). This label-free and
non-invasive method is based on measurement of the electrical
impedance (cell index, CI) between interdigitated regions on the
base of tissue culture plates. The CI measurement provides
quantitative information about the biological status of adherent
cells. Actually, the meaning of CI is the number of survival cell
on the surface of E-plate. These data include the cell number,
viability, and morphology as a real-time profile (2–4). RTCA
system is known to early detection device of cell reaction as a
dynamic phenotype against some reagents (5).
In our previous study, imatinib cytotoxicity in oral
squamous cell carcinoma (OSCC) was assessed using the WST-8
(5-[2,4-Bis(sodiooxysulfonyl)phenyl]- 3-(2- methoxy- 4-
nitrophenyl)- 2-(4-nitrophenyl)-2H- tetrazole- 3- ium) assay as an
endpoint measurement (6), and then
later with an RTCA system (7).
Endpoint measurements by MTT (3-[4,5- dimethylthiazol-
2-yl]-2,5-diphenyltetrazolium bromide) and WST-8 assays are
commonly used to evaluate cytotoxicity. However, such assays are
limited by variations in the effects of different anticancer agents
on different cell lines. Furthermore, IC50 values
calculated by in vitro endpoint assays tend to be higher
than the effective concentrations in vivo (8,9).
In our previous study, the IC50 values
measured by the RTCA system were lower than those measured by the
WST-8 assay, suggesting that the RTCA system can sensitively
evaluate cytotoxicity and the influence of imatinib on cell
adhesion. However, it is unclear whether evaluation of the
IC50 values of other anticancer agents using the RTCA
system would be useful because there are differences in cytotoxic
reactions between molecular targeted drugs such as imatinib and
anticancer agents over time.
In this study, we need to select the some type of
cell lines in order to determine the difference of cell reaction
profile against anticancer reagents in real-time using RTCA system.
Non-invasive SQUU-A cell line and invasive SQUU-B cell line which
were established from local recurrent tongue cancer tumors in a
single patient, were selected because we were engaged in research
on metastasis of SQUU-B cell line using SQUU-A cell line and SQUU-B
cell line (10–12). SAS cell line were established from
poorly differentiated human squamous cell carcinoma of the tongue
(13). NA cell line was established
as a fibronectin-producing cell line (14). Furthermore, it has been reported that
the cytotoxicity of anti-cancer reagents in OSCC has been evaluated
in SQUU-A cell line and SQUU-B cell line (15), SAS cell line (16), NA cell line (17) using various conventional methods.
That is why, we selected four these cell lines with each
characteristic feature in the present study, which were also used
in cytotoxic assay in previous study.
In this study, we focused on a new RTCA device
developed for real-time measurement and evaluated the
IC50 values as a scale to assess the cytotoxicity of
four anticancer agents towards in four OSCC cell lines. This
allowed us to obtain information about the variations observed
between the different anticancer reagents and cell lines in
real-time.
The aim of the present study was to obtain
IC50 profiles from immediately after addition of
anticancer agents using an RTCA system. This study demonstrated the
advantage of evaluating the cytotoxicity of anticancer agents using
an RTCA system compared with an endpoint assay.
Materials and methods
Reagents and materials
5-Fluorouracil (5-FU) was diluted to 100 mM in
dimethylsulfoxide (DMSO; Sigma-Aldrich Inc., St. Louis, MO, USA).
Doxifluridine and carboplatin were diluted to 100 and 50 mM,
respectively, in distilled water. Docetaxel was diluted to 10 mM in
ethanol. All anticancer reagents were sourced from Wako Pure
Chemical Industries, Ltd., (Osaka, Japan) and stored at −20°C.
Cell culture
Human OSCC cell lines SQUU-A, SQUU-B, SAS, and NA
were derived from human tongue samples. SQUU-A and SQUU-B, were
kindly provided by Morifuji-Wilson M (Kumamoto University,
Kumamoto, Japan), were established from local recurrent tongue
cancer tumors (18). SAS (13,19,20) was
purchased from the Riken BRC Cell Bank (Tsukuba, Japan). NA
(14) was kindly provided by Dr.
Jun-ichi Iwata (Kyushu University; Fukuoka, Japan). All cell lines
were maintained in Dulbecco's modified Eagle's medium (DMEM;
Nacalai Tesque, Inc., Kyoto, Japan) containing 10% fetal calf serum
(Biowest, Nuaille, France) at 37°C in a humidified atmosphere with
5% CO2.
Measurement of OSCC cell proliferation
by the RTCA cytotoxicity assay
CI was acquired by the iCELLigence system (ACEA
Biosciences, Inc., San Diego, CA, USA) as the RTCA system. All
monitoring was performed at 37°C with regulated CO2
content (5%). E-plates (culture plates for the iCELLigence system)
containing 200 µl culture medium per well were equilibrated to
37°C, and CI was set to zero under these conditions. Cells
(2×104 cells/well unless specified otherwise) were added
in 560 µl culture medium Anticancer agents were added at 24 h after
seeding the cells. The CI was monitored in real-time for 96 h after
cell seeding. The IC50 values were calculated by RTCA
Data Analysis Software version 1.0 (ACEA Biosciences, Inc.).
Eight points concentration of four anticancer
reagents were set based on Cmax values in previous study
(21–25). Furthermore, time points were set
during 72 h including 24 h and 48 h, which were general method to
evaluate cytotoxicity in end-point assay.
Curve fitting of the IC50
data
We used the following sigmoidal dose-response
formula to calculate the IC50 values: Y=Low CI + (High
CI-Low CI)/{1+10 ^ (Log IC50-X)}, where ‘Low CI’
represents the minimum CI values, ‘High CI’ represents the maximum
cell index values, Y is the cell index, and X is the log of
concentration (M).
Measurement of OSCC cell proliferation
by the WST-8 assay
After the initial seeding and culture of OSCC cells,
the culture medium was removed and replaced with anticancer
agent-containing medium. After 24, 48, and 72 h of incubation, 20
µl WST-8 dye (Cell Counting Kit-8; Dojindo Corporation, Tokyo,
Japan) was added to each well. After 3 h, the plates were read at
450 nm/655 nm. The cell survival rate was calculated using the
formula below (7). IC50
values were calculated by linear approximation regression of the
percentage survival versus the drug concentration.
Cell survival rate (%)=(a-c)/(b-c) ×100
(a=absorbance at each concentration of the anticancer reagent,
b=absorbance at 0 µM of the anticancer reagent, and c=absorbance of
the blank).
Statistical analysis
All data are shown as the mean ± standard deviation
(SD) of three independent experiments. Correlations between
IC50 values obtained using the RTCA system and WST-8
assay were evaluated for statistical significance by the Spearman
test. Two-tailed values of P<0.05 were considered as
significant.
Results
Effect of the anticancer agent
concentration on OSCC cell proliferation using the RTCA system
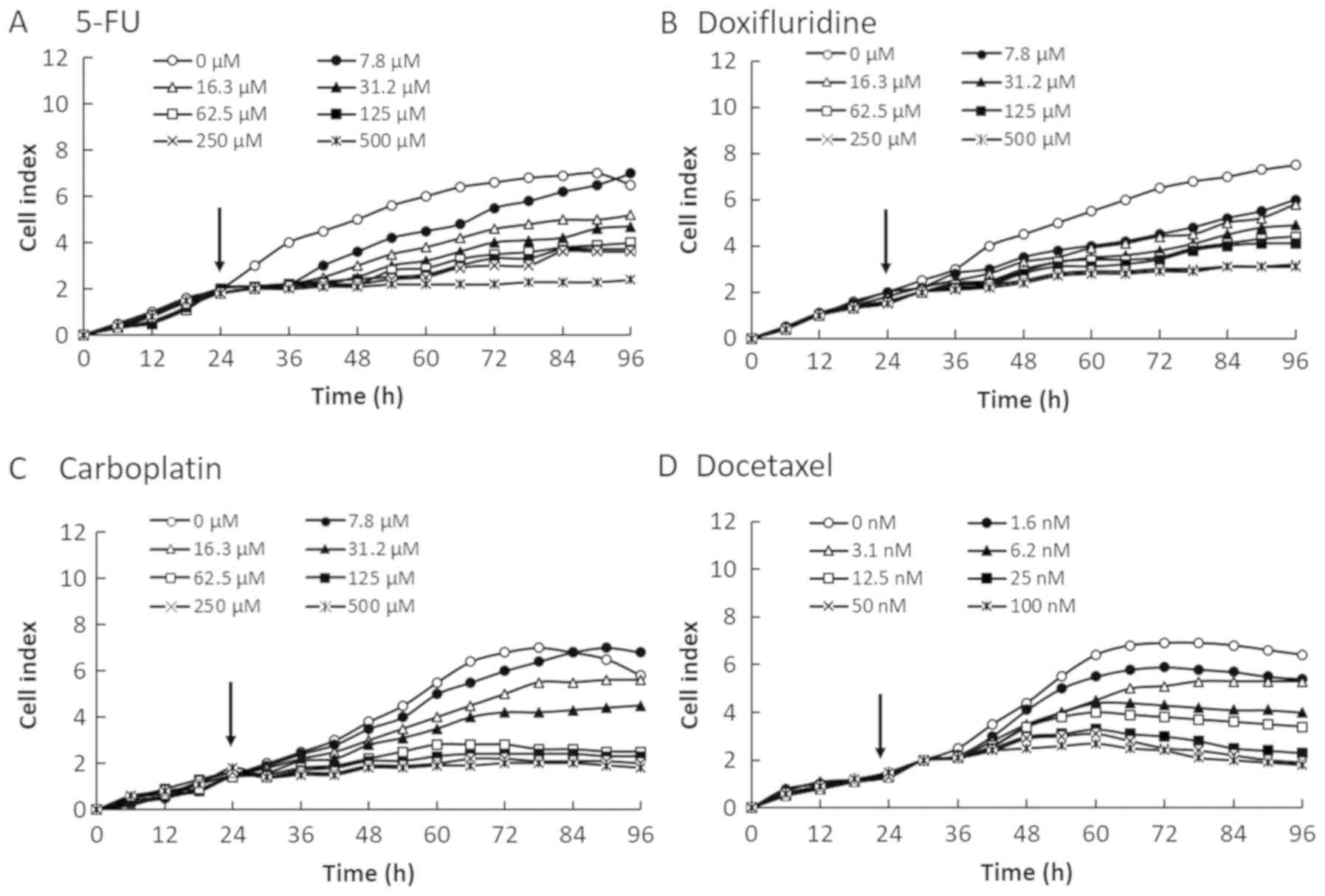
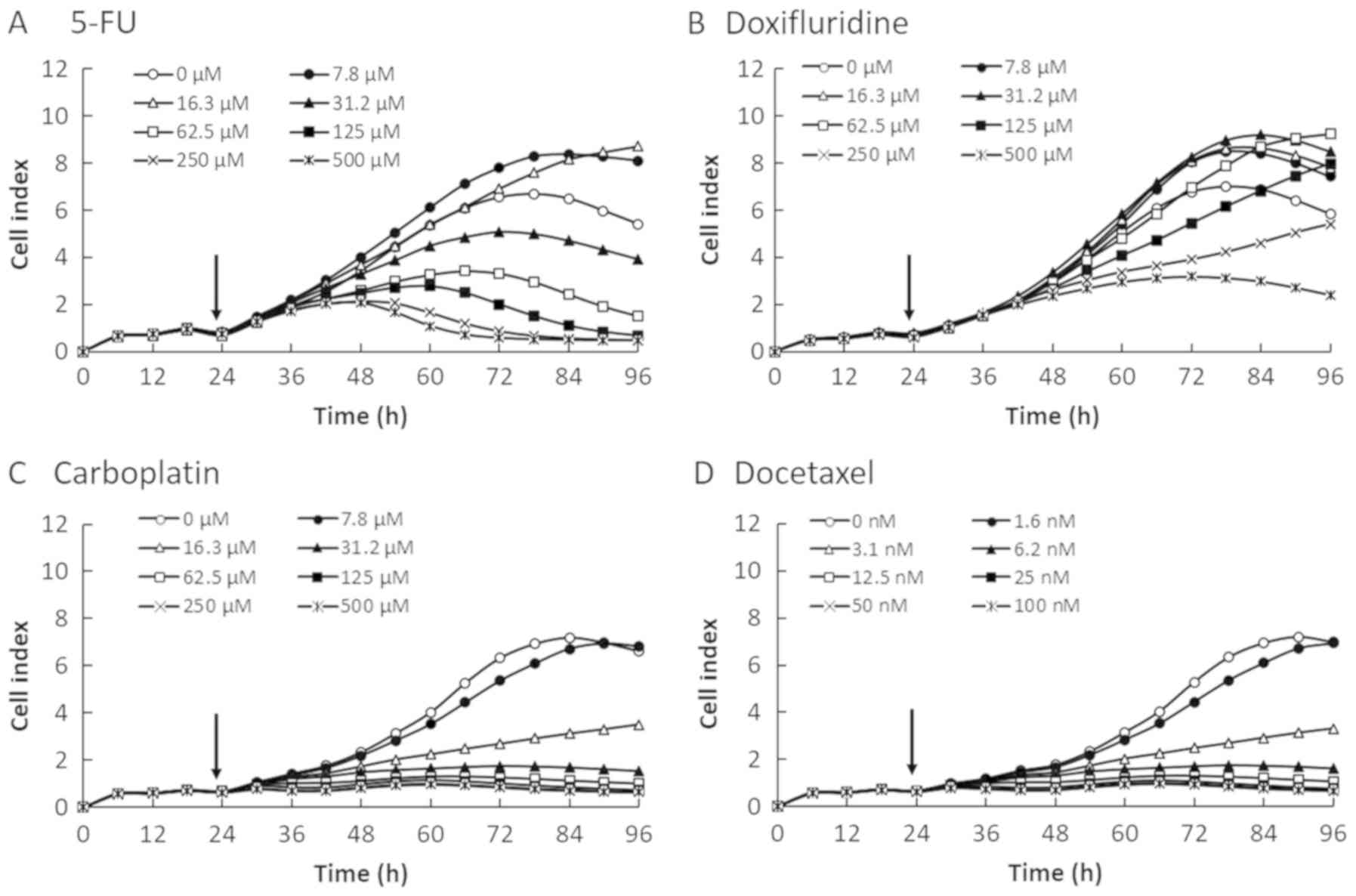
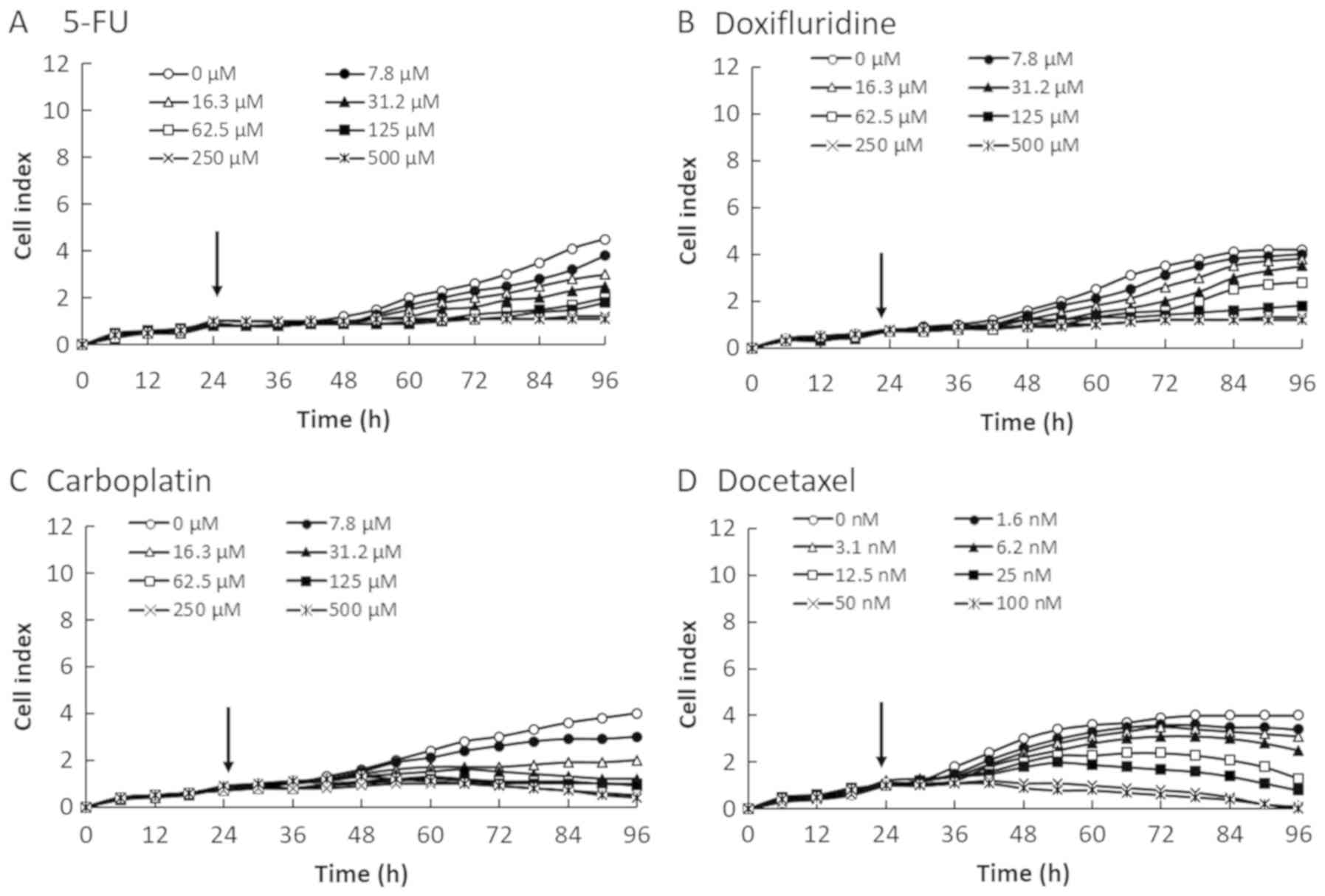
We evaluated the cytotoxicity of four anticancer
reagents (5-FU, doxifluridine, carboplatin, and docetaxel) in four
OSCC cell lines by monitoring the CI values for 96 h after the
cells were seeded at 2×104 cells/well on E-plates
(Figs. 1–4). The CI values were decreased in a
dose-dependent manner in all four OSCC cell lines. Therefore, the
reduction in CI values correlated with the decrease in cell number.
As shown in Fig. 2, the CI value for
invasive SQUU-B cell line was lower than those of other OSCC cells.
As shown in Fig. 3, CI profile
obtained for SAS cell line showed delayed increase after 48 h. As
shown in Fig. 4, the rate of
proliferation and max CI value in NA cell line were higher than one
of other cell lines.
Real-time measurement of the
IC50 profiles of anticancer agents in OSCC cells using
the RTCA system
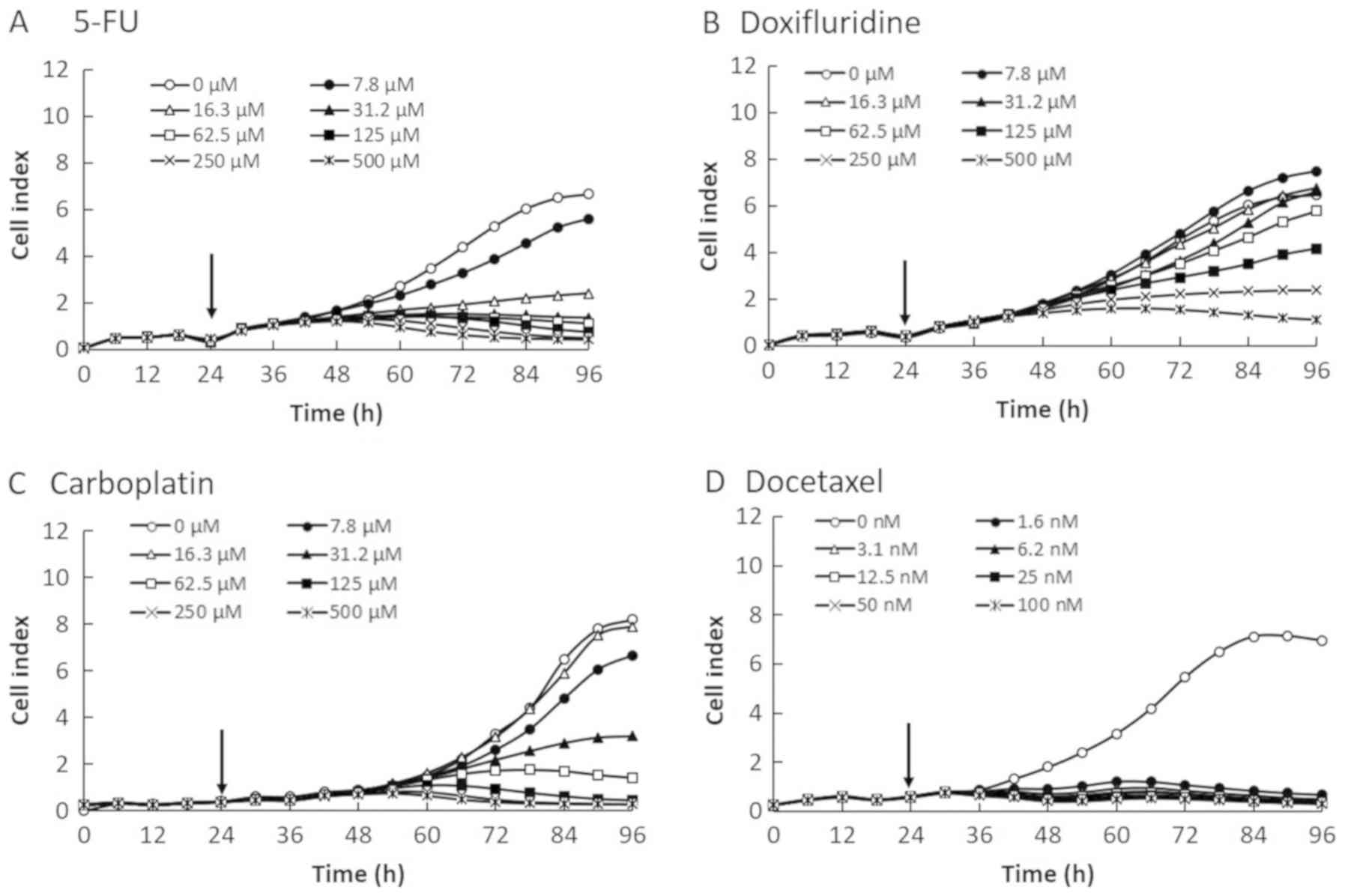
The IC50 profiles of the four anticancer
reagents in the four OSCC cell lines were determined by the RTCA
system and calculated by the commercial software provided with the
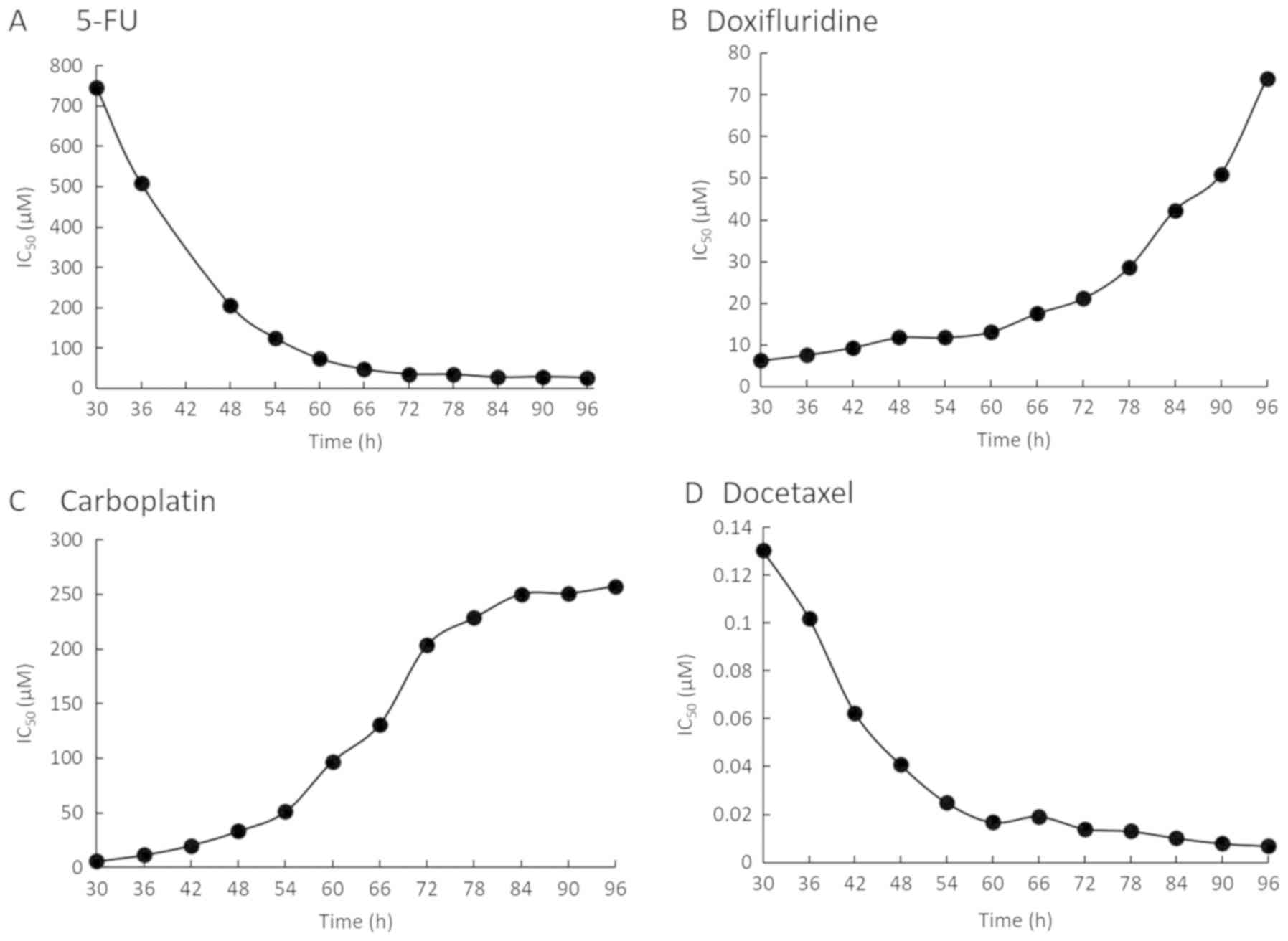
instrument (a subset of the SQUU-B data are shown in Fig. 5). The IC50 values were
plotted for 72 h after the addition of anticancer agents. The
IC50 values at 24, 48, and 72 h for all four cell lines
are summarized in Tables I–IV. As shown in Fig. 5, there was time lag in the cytotoxic
reactions of 5-FU (Fig. 5A) and
docetaxel (Fig. 5D), and recovery of
cell proliferation was observed at about 24 h after treatments.
However, cytotoxic reactions were observed immediately after
treatment with anticancer agents such as doxifluridine (Fig. 5B) and carboplatin (Fig. 5C).
 | Table I.IC50 values of 5-FU
determined with the RTCA system after 48, 72, and 96 h incubation
in OSCC cells. |
Table I.
IC50 values of 5-FU
determined with the RTCA system after 48, 72, and 96 h incubation
in OSCC cells.
|
| 48 h | 72 h | 96 h |
|---|
|
|
|
|
|
|---|
| Cell line | IC50
(µM) | r2 | IC50
(µM) | r2 | IC50
(µM) | r2 |
|---|
| SQUU-A | 48.2 | 0.99 | 3.3 | 0.99 | 8 | 0.99 |
| SQUU-B | 372 | 0.56 | 46.8 | 0.91 | 27.5 | 0.97 |
| NA | 3 | 0.91 | 3 | 0.94 | 7 | 0.9 |
| SAS | 28 | 0.99 | 36 | 1 | 18 | 0.92 |
 | Table IV.IC50 values of docetaxel
determined with the RTCA system after 48, 72 and 96 h incubation in
OSCC cells. |
Table IV.
IC50 values of docetaxel
determined with the RTCA system after 48, 72 and 96 h incubation in
OSCC cells.
|
| 48 h | 72 h | 96 h |
|---|
|
|
|
|
|
|---|
| Docetaxel | IC50
(nM) | r2 | IC50
(nM) | r2 | IC50
(nM) | r2 |
|---|
| SQUU-A | 27.3 | 0.96 | 5.4 | 0.96 | 3.1 | 0.95 |
| SQUU-B | 58.1 | 0.99 | 16 | 0.98 | 6.9 | 0.93 |
| NA | 1 | 0.99 | 0.9 | 0.98 | 0.8 | 0.99 |
| SAS | 3 | 0.99 | 2 | 0.92 | 2 | 0.92 |
Dose-response curves in SQUU-B cell line using RTCA
system were shown in supplemental materials (Figs. S1–S4) in order to show how to conversion the
data of Fig. 2 into Fig. 5. While, cell viability curves of
SQUU-B using WST-8 assay were also shown in supplemental materials
(Figs. S5–S8). R2 values at 72 h after
addition anticancer reagents in WST-8 assay were low in four
anticancer reagents compared with one of 24 h and 48 h. These
results showed that it is desirable that end-point assay be
performed at 24 h or 48 h to be used as a general protocol. In this
study, the cell viability curves of WST-8 assay were shown in
supplemental materials because there were no novelty in how to
calculation of IC50 values using WST-8 assay.
Correlations between real-time
measurements of IC50 values using the RTCA system and
endpoint measurements of IC50 values using the WST-8
assay in OSCC cells
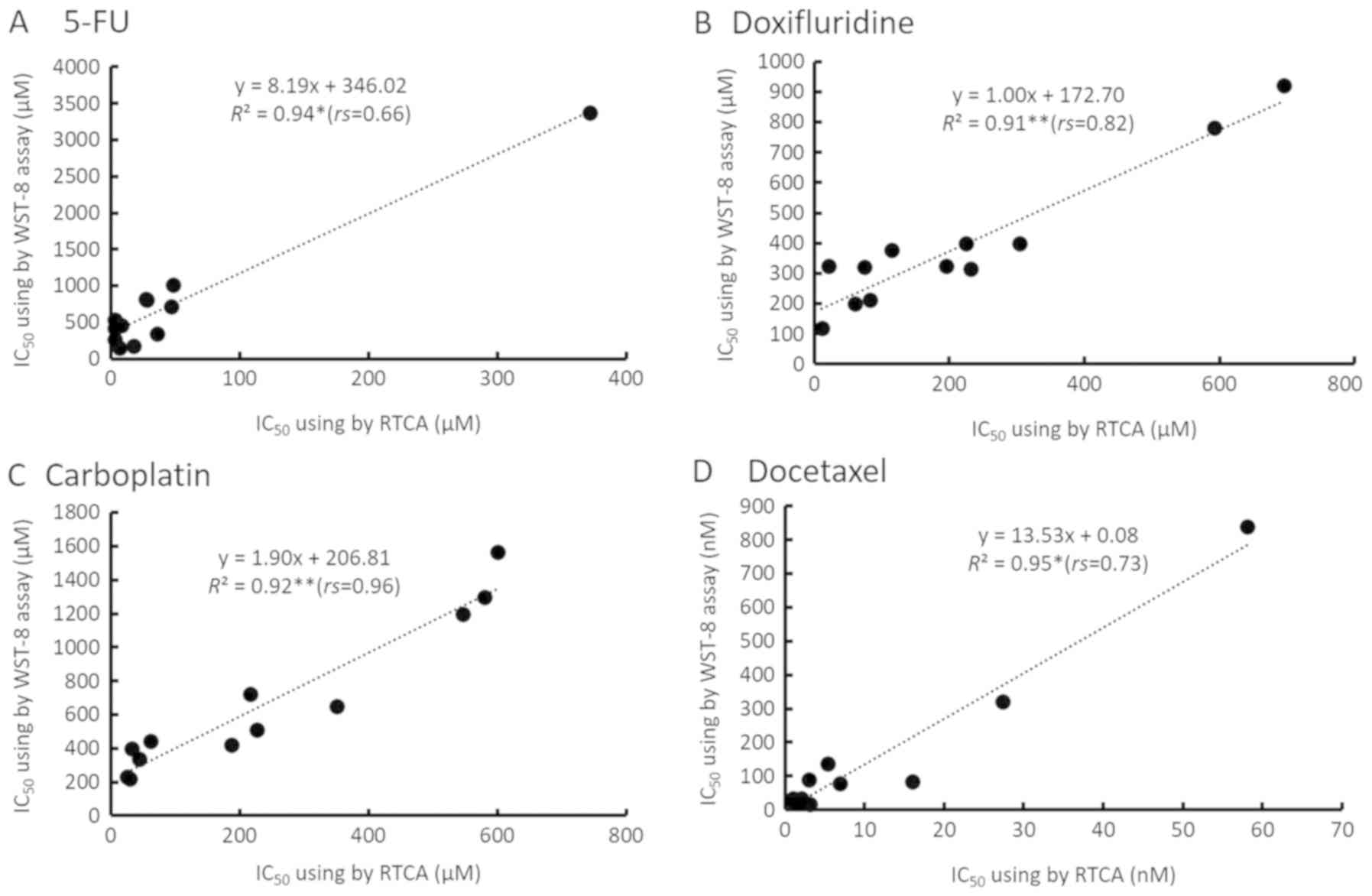
As shown in Fig. 6,
IC50 values at 24, 48, and 72 h using two methods were
plotted. The horizontal axis shows real-time IC50 values
measured using the RTCA system, while the longitudinal axis shows
endpoint IC50 values measured using the WST-8 assay. A
positive correlation was observed between the two types of assay
method to measure the IC50 for each anticancer agent.
The results of 5-FU, doxifluridine, carboplatin, and docetaxcel
were y=8.19x+346.02 (R2=0.94,
rs=0.66, *P<0.05), y=1.00x+172.70
(R2=0.91, rs=0.82, **P<0.01),
y=1.90x+206.81 (R2=0.92,
rs=0.96, **P<0.01), and
y=13.53x+0.08 (R2=0.95,
rs=0.73, *P<0.05), respectively. The real-time
IC50 values tended to be lower than the corresponding
endpoint IC50 values.
Discussion
CI values calculated by the RTCA are also
representing the cell status (3). As
shown in Figs. 1–5, SD values of CI values were too small to
see. For example, all SD values in Fig.
1 were under 0.05. The reason for low CI values of SQUU-B was
suggested that the adhesion protein E-cadherin plays an essential
role in metastasis, with reduced levels of E-cadherin promoting
cell migration and cell invasion (26). The reason for high max CI values of
NA was suggested that it has been reported that fibronectin
accelerated cell proliferation and adhesion due to the feature of
NA cell line as a fibronectin producing cell line (6). Thus, cell reactions of each cell lines
against four anticancer reagents were variable. We could not find
the causal relationship between IC50 values and the
feature of cell lines in this study. However, it is important to
detect a cell reaction in real-time as a CI profile to evaluate the
cytotoxicity of anticancer reagent when considering pharmaceutical
application to human.
The IC50 profile of SQUU-B cell line was
described in Fig. 5 as a
representative IC50 profile because there were no
differences in IC50 profile pattern in same cell line in
case of same reagent, though we calculated IC50 values
in all cell line using four anticancer reagents. We found that the
IC50 profiles varied for each anticancer agent and in
each OSCC cell line. As shown in Fig.
5, 5-FU and docetaxel required more than 24 h (48 h in the
figure) to start exerting a cytotoxic effect on the OSCC cells,
whereas the IC50 values had recovered from about 24 h
(48 h in the figure) after the addition of doxifluridine and
carboplatin. Thus, we suggested that the differences of real-time
IC50 profiles was caused by anticancer reagent. Such
observations would not have been possible without real-time
measurement using the RTCA. Real-time monitoring of the
IC50 values also revealed that these values changed
markedly over time. There is the possibility of not detecting
changes using conventional methods, because only one time point of
the IC50 after treatment with the anticancer agent is
evaluated by an endpoint assay.
The RTCA system is unlike traditional endpoint
assays be because the measurement of impedance is non-invasive and
can provide high quality, quantitative data on cytotoxicity in a
continuous manner. As shown in Fig.
6, there were significant positive correlations between
IC50 values obtained with the RTCA system and WST-8
assay, even though there were some differences in absolute values
of CI such as low CI values obtained for SQUU-B cell line. These
results suggested that even a low CI obtained for some cell lines
would be able to evaluate the cytotoxicity high sensitivity in RTCA
system. While, the real-time IC50 values were lower than
endpoint IC50 values when the real-time IC50
values obtained with the RTCA system were compared with the
endpoint IC50 values obtained with the WST-8 assay.
These results suggest that the RTCA system can be used to
sensitively evaluate the effect of anticancer agents on cell
proliferation and adhesion.
In RTCA system, adherent cell act as an insulator on
the surface of the electrode and change the ionic medium of the
electrode solution, increasing the impedance (27). Thus, CI is function of the cell
number and ratio of cells at different time intervals. CI=0 when
there is no cell adhesion (5). CI
changes described by RTCA system are reflected dynamic phenotype of
cell. These dynamic monitoring of cell-drug interaction enables us
to obtain a better understanding of temporal effects in
vitro, especially immediately after treatment with drug
(28). The kinetic feature of the
cells offers insightful information that cannot be acquired from a
conventional single end-point assay such as WST-8 assay. While,
results of WST-8 assay is reflected metabolic reaction of survival
cell (29). Toxicity using WST-8
assay might be underestimated if cell metabolic reaction were
remained though dynamic cell reaction was occurred. We suggested
that these differences of principal in two methods were induced a
huge differences of IC50 value up to 8 times between two
methods as shown in Fig. 6A. Our
previous study reported that IC50 of imatinib in SQUU-A
cells calculated by RTCA system and WST-8 assay were 4 µM and 60
µM, respectively (sensitivity of 15 times) (6,7). Other
research groups reported that IC50 of cisplatin in HK-2
cells calculated by RTCA system and WST-8 assay were 0.76 µM and 25
µM, respectively (sensitivity of 33 times) (30,31).
These previous data supported our data to show validity.
It is important to obtain measurements from
immediately after treatment with anticancer agents to assess both
the side effects of the agents and its desired effects. However,
there are few reports of useful methods that correlate in
vitro data with human in vivo data. We considered that
real-time measurement using the RTCA system would benefit
evaluation of the side effects of anticancer agents in normal
cells.
Previously reported Cmax values of 5-FU,
doxifluridine, carboplatin, and docetaxel are 60 (21), 40–800 (22), 40 (23), and 2 µM (24,25),
respectively, when used for intravenous treatment in humans. Our
data correlated with the human data because the Cmax
values were included in the range of the experimental doses used in
this study.
RTCA systems are the new principal device to
evaluate cytotoxicity, which employ the impedance intensity of cell
adhesion and not enzyme activity in target cells such as the MTT
assay. IC50 values of anticancer agents calculated by
the RTCA system were significantly correlated with IC50
levels calculated by the conventional endpoint assay. Furthermore,
the RTCA system obtained measurements automatically in real-time
from immediately after treatment with anticancer agents.
Actually, RTCA system cannot evaluate directly
concrete cytotoxicity such as cell death or apoptosis and so on,
because cell index were calculated based on impedance. That is why,
the combination assay between RTCA system and cell death
measurement might be effective method in case of evaluation of
concrete cell reaction such as cell death or apoptosis precisely.
For example, Annexin V staining or expression of caspase-3 assay
for detection of apoptosis, Lactate Dehydrogenase (LDH) release
assay for detection of cell death might be effective methods
(32,33). Furthermore, it might be useful to
evaluate expression of inflammatory protein in the normal cells for
detection of side effect when anti-cancer reagent was added to the
normal cells seeded on E-plate. Dynamic real-time monitoring during
cell culture including anticancer reagents can provide valuable
insights for the early detection of therapeutic efficiency and side
effect, which cannot evaluate in conventional methods in end-point
assay. So, IC50 value calculated by RTCA system might be
useful parameter for such as screening from a standpoint of
real-time measurement in automated, avoidance of colorimetrically
problems or contamination. Furthermore, RTCA system allows the
analysis of the whole period of the experiment and does not require
the labeling that can negatively affect cell culture
experiments.
The novelty of this study is that RTCA system could
detect cytotoxicity high sensitivity compared with a conventional
method, WST-8 assay. Furthermore, RTCA system could evaluate
IC50 value preciously in real-time without restriction
of experimental period for at least 72 h after addition of
anticancer reagents.
In conclusion, our results demonstrated that RTCA
systems are useful to assay cytotoxicity and could be used in
future development of chemotherapeutic agents using cancer cells
and evaluation of their side effects in normal cells. Additionally,
an RTCA system can be used to evaluate the cell reaction profiles,
such as combined therapy and antibody-cell or drug-drug
interactions, of anticancer reagents.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MH and MN conceived and designed the study. MH and
TN performed the experiments and acquired the data. MH analyzed the
data, prepared the figures and drafted the manuscript. MH, TKY and
MN interpreted the results. MH and TKY edited and revised the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xing JZ, Zhu L, Jackson JA, Gabos S, Sun
XJ, Wang XB and Xu X: Dynamic monitoring of cytotoxicity on
microelectronic sensors. Chem Res Toxicol. 18:154–161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xing JZ, Zhu LJ, Gabos S and Xie L:
Microelectronic cell sensor assay for detection of cytotoxicity and
prediction of acute toxicity. Toxicol In Vitro. 20:995–1004. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu J, Wang X, Xu X and Abassi YA: Dynamic
and label-free monitoring of natural killer cell cytotoxic activity
using electronic cell sensor arrays. J Immunol Methods. 309:25–33.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xi B, Yu N, Wang X, Xu X and Abassi YA:
The application of cell-based label-free technology in drug
discovery. Biotechnol J. 3:484–495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Türker Sener L, Albeniz G, Dinc B and
Albeniz I: iCELLigence real-time cell analysis system for examining
the cytotoxicity of drugs to cancer cell lines. Exp Ther Med.
14:1866–1870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morioka M, Hazekawa M, Kawakubo-Yasukochi
T, Nishinakagawa T, Nakamura S and Nakashima M: Effect of collagen
type I or human fibronectin on imatinib cytotoxicity in oral
squamous cell carcinoma. Pharmacol Pharm. 7:255–263. 2016.
View Article : Google Scholar
|
|
7
|
Hazekawa M, Morioka M, Nishinakagawa T,
Kawakubo- Yasukochi T, Nakamura S and Nakashima M: Assessment of
cytotoxicity of imatinib for oral squamous cell carcinoma by a
real-time cell analysis system. eJBio. 13:56–62. 2017.
|
|
8
|
McEneny-King A, Edginton AN and Rao PP:
Investigating the binding interactions of the anti-Alzheimer's drug
donepezil with CYP3A4 and P-glycoprotein. Bioorg Med Chem Lett.
25:297–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ota T, Shinotoh H, Fukushi K, Kikuchi T,
Sato K, Tanaka N, Shimada H, Hirano S, Miyoshi M, Arai H, et al:
Esyimation of plasma IC50 of donepezil for cerebral
acetylcholinesterase inhibition in patients with Alzheimer disease
using positron emission tomography. Clin Neurophaemacol. 33:74–78.
2010. View Article : Google Scholar
|
|
10
|
Kawakubo-Yasukochi T, Morioka M, Hayashi
Y, Nishinakagawa T, Hazekawa M, Kawano S Nakamura S and Nakashima
M: The SQUU-B cell line spreads its metastatic properties to
nonmetastatic clone SQUU-A from the same patient through exosomes.
J Oral Biosci. 58:33–38. 2016. View Article : Google Scholar
|
|
11
|
Morioka M, Kawakubo-Yasukochi T, Hayashi
Y, Hazekawa M, Nishinakagawa T, Ono K, Kawano S, Nakamura S and
Nakashima M: Exosomes from oral squamous carcinoma cell lines,
SQUU-A and SQUU-B, define the tropism of lymphatic dissemination. J
Oral Biosci. 58:180–184. 2016. View Article : Google Scholar
|
|
12
|
Kawakubo-Yasukochi T, Morioka M, Hazekawa
M, Yasukochi A, Nishinakagawa T, Ono K, Kawano S, Nakamura S and
Nakashima M: miR-200c-3p spreads invasive capacity in human oral
squamous cell carcinoma microenvironment. Mol Carcinog. 57:295–302.
2018. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi K, Kanazawa H, Akiyama Y, Tazaki
S, Takahara M, Muto T, Tanzaawa H and Sato K: Establishment and
characterization of a cell line (SAS) from poorly differentiated
human squamous cell carcinoma of the tongue. J Jpn Stomatol Soc.
38:20–28. 1989.
|
|
14
|
Yoshiya M: A fibronectin-producing cell
line established from a human squamous cell carcinoma of the tongue
and its characterization. Jpn J Oral Maxillofac Surg. 36:868–880.
1990. View Article : Google Scholar
|
|
15
|
Tanaka H, Toyoshima T, Sonoda K, Kitamura
R, Sasaguri M, Kawano S, Matsubara R, Goto Y and Nakamura S:
Apoptotic function of tumor-associated antigen RCAS1 in oral
squamous cell carcinoma. J Transl Med. 12:1122014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chien MH, Chang WM, Lee WJ, Chang YC, Lai
TC, Chan DV, Sharma R, Lin YF and Hsiao M: A Fas ligand
(FasL)-fused humanized antibody against tumor-associated
glycoprotein 72 selectively exhibits the cytotoxic effect against
oral cancer cells with a low FasL/Fas ratio. Mol Cancer Ther.
16:1102–1113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takaoka S, Iwase M, Uchida M, Yoshida S,
Kondo G, Watanabe H, Ohashi M, Nagumo M and Shintani S: Effect of
combining epidermal growth factor receptor inhibitors and cisplatin
on proliferation and apoptosis of oral squamous cell carcinoma
cells. Int J Oncol. 30:1460–1476. 2007.
|
|
18
|
Morifuji M, Taniguchi S, Sakai H,
Nakabeppu Y and Ohishi M: Differential expression of cytokeratin
after orthotopic implantation of newly established human tongue
cancer cell lines of defined metastatic ability. Am J Pathol.
156:1317–1326. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hung CM, Chang CC, Lin CW, Ko SY and Hsu
YC: Cucurbitacin E as inducer of cell death and apoptosis in human
oral squamous cell carcinoma cell line SAS. Int J Mol Sci.
14:17147–17156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen YW, Huang HS, Shieh YS, Ma KH, Huang
SH, Hueng DY, Sytwu HK and Lin GJ: A novel compound NSC745885
exerts anti-tumor effect on tongue cancer SAS cells in vitro and in
vivo. PLoS One. 9:e1047032014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Casale F, Canaparo R, Serpe L, Muntoni E,
Pepa CD, Costa M, Marirone L, Zara GP, Fornari G and Eandi M:
Plasma concentrations of 5-fluorouracil and its metabolites in
colon cancer patients. Pharmacol Res. 50:173–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Der Heyden SA, Highley MS, De Bruijin
EA, Tjaden UR, Reeuwijk HJ, Van Slooten H, Van Oosterom AT and Maes
RA: Pharmacokinetics and bioavailability of oral
5′-deoxy-5-fluorouridine in cancer patients. Br J Clin Pharmecol.
47:351–356. 1999. View Article : Google Scholar
|
|
23
|
Kern W, Braess J, Friedrichsen S, Kaufmann
CC, Schleyer E and Hiddemann W: Carboplatin pharmacokinetics in
patients receiving carboplatin and paclitaxel/docetaxel for
advanced lung cancers: Impact of age and renal function on area
under the curve. J Cancer Res Clin Oncol. 127:64–68. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kenmotsu H and Tanigawara Y:
Pharmacokinetics, dynamics and toxicity of docetaxel: Why the
Japanese dose differs from the western dose. Cancer Sci.
106:497–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Minami H, Kawada K, Sasaki Y, Igarashi T,
Saeki T, Tahara M, Itoh K and Fujii H: Pharmacokinetics and
pharmacodynamics of protein-unbound docetaxel in cancer patients.
Cancer Sci. 97:235–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao P, Guo S, Tu Z, Di L, Zha X, Zhou H
and Zhang X: Gihl3 induces human epithelial tumor cell migration
and invasion via downregulation of E-cadherin. Acta Biochim Biophys
Sin (Shanghai). 48:266–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Szulccek R, Bogaard HJ and van Nieuw
Amerogen GP: Electric cell-substrate impedance sensing for the
quantification of endothelial proliferation, barrier function, and
motility. J Vis Exp. Mar 28–2014. View
Article : Google Scholar
|
|
28
|
Ku M, Kang M, Suh JS and Yang J: Effects
for sequential treatment of siAkt and paclitaxel on gastric cancer
cell lines. Int J Med Sci. 13:708–716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng Z, Wang Z, Yang M, Zhou L and Bao Y:
Polysaccharopeptide exerts immunoregulatory effects via
MyD88-dependent signaling pathway. Int J Biol Macromol. 82:201–207.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Genc G, Kilinc V, Bedir A and Ozkaya O:
Effect of creatine and pioglitazone on Hk-2 cell line cisplatin
nephrotoxicity. Ren Fail. 36:1104–1107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ng NS, Wu MJ, Myers SJ and Aldrich-Wright
JR: The in vitro renal cell toxicity of some unconventional
anticancer phenanthroline-based platinum (II) cpmplexes. J lnorg
Biochem. 179:97–106. 2018. View Article : Google Scholar
|
|
32
|
Ma K, Zhang C, huang MY, Guo YX and Hu GQ:
Crosstalk between Beclin-1-dependent autophagy and
caspase-dependent apoptosis induced by transhinone IIA in human
osteosarcoma MG-63 cells. Oncol Rep. 36:1807–1818. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Augustine D, Rao RS, Anbu J and Chidambara
Murthy KN: In vitro cytotoxic and apoptotic induction effect of
earthworm coelomic fluid of Eudrilus eugeniae, Eisenia foetida, and
Perionyx excavatus on human oral squamous cell carcinoma-9 cell
line. Toxicol Rep. 6:347–357. 2019. View Article : Google Scholar : PubMed/NCBI
|