Introduction
High incidence of both ST-elevation (STEMI) and
non-ST-elevation acute myocardial infarction (NSTEMI) reported in
published genetic research (1)
determines the necessity of studying genetic aspects that can
contribute to the development of primary and secondary myocardial
infarction (MI) prevention programs.
The association between the 9p21.3 locus and
coronary artery disease (CAD), and MI has become one of the
important discoveries of modern cardiogenetics (1,2). The
association between this genome locus and coronary atherosclerosis,
and MI has been demonstrated in various populations (3–8).
However, the underlying mechanism remains to be elucidated. It has
been demonstrated that a non-coding RNA of locus 9p21.3 affected
cardiac expression of cyclin dependent kinase inhibitor 2 A and B
genes, and may lead to a lower index of atherosclerotic plaque
stability (9). Further studies are
necessary to enable the clinical implementation of this emerging
marker in risk stratification and outcome of patients with
cardiovascular diseases. To assess the utility of genetic markers
that may reflect the risk of MI, the existing risk calculator and
the Net Reclassification Improvement (NRI) method are usually used.
Previously, results of several large-scale projects on the
integration of genomic information into the existing CAD and MI
risk rating scales have been published, however, the results were
inconsistent (10–12).
By contrast, the clinical utility of 9p21.3 locus
genotyping to predict the outcomes for patients following MI may be
more promising compared with other loci, since it was replicated as
a genetic predictor in almost all ethnic groups and races (3,13). In
particular, for patients with acute coronary syndrome (ACS)
enrolled in the Global Registry of Acute Coronary Events (GRACE),
Buysschaert et al (13)
reported the results of inclusion of the 9p21.3 locus genotyping
data into the GRACE risk score. A positive association between the
9p21.3 locus and recurrent MI or cardiac death within the first 6
months after the primary event was revealed (13). Adrissino et al (14) proposed the use of risk allele C (SNP
rs1333040) in secondary MI prevention in people under 45
years.
Significant associations have been revealed between
the risk 9p21.3 genotypes and the outcomes in the most severe cases
of MI with multi-vessel coronary artery burden or high risk
according to the GRACE score (15,16). The
results of the present study indicated that future clinical
application of genomic data requires accurate definition of the
target group of patients. Since percutaneous coronary intervention
(PCI) can markedly alter the prognosis of patients with MI, the
present study aimed to estimate the risk of MI among patients with
different SNPs at 9p21.3 receiving differential hospital-based
treatment.
Materials and methods
Patients
The participants of the present study were patients
admitted to the two largest cardiological hospitals in Krasnoyarsk,
Russian Federation: Regional Clinical Hospital and Krasnoyarsk
Interdistrict Clinical Hospital Number 20 named after I.S. Berzon.
The inclusion criteria compromised: i) Confirmed diagnosis of MI
verified by electrocardiogram (ECG) and such clinical symptoms and
cardiac markers, including typical anginal pain lasting more than
20 min or atypical manifestations such as abdominal epigastric
pain, indigestion-like symptoms and isolated dyspnoea; ii) age
under 65 years, iii) Caucasian origin; and iv) signed informed
consent. The present study was approved by the Ethics Committee of
Professor V.F. Voino-Yasenetsky Krasnoyarsk State Medical
University.
A total of 500 patients with MI [22–65 years; median
age, 54.0; lower quartile (Q25), 48.0; upper quartile
(Q75), 59.0] were included in the present study
conducted from January 1, 2009 to the June 30, 2010. The group of
patients with MI included 411 males (82.2%) and 89 females (17.8%).
The results of 31 autopsies [24 males and 7 females, 44–65 years
(57.87±5.92 years)] with confirmed diagnosis of MI as the cause of
out-of-hospital sudden death during the same period of time were
also included in the present study.
The control group subjects were selected at random
from the Novosibirsk samples of two epidemiological studies:
Multinational Monitoring of Trends and Determinants in
Cardiovascular Disease (17), and
Health, Alcohol and Psychosocial factors In Eastern Europe (HAPIEE)
(18), conducted at the Novosibirsk
Institute of Internal and Preventive Medicine (Russian Federation)
to investigate angina and its equivalents with the use of
cardiospecific markers (troponin, creatine phosphokinase-MB
fraction). Based on the results of the Rose Angina Questionnaire, a
total of 535 individuals without CAD were selected among the HAPIEE
participants enrolled from Novosibirsk. The control group included
423 (79.1%) males and 112 (20.9%) females ≤65 years (median, 55.0;
Q25, 48.0; Q75, 61.0).
The two groups did not exhibit any significant
differences in the mean age (P=0.351) or in the presence of obesity
(including abdominal obesity) and excess weight, as presented in
Table I. In the MI group, a
statistically significant predominance of smokers, arterial
hypertension, diabetes mellitus and positive family history were
identified. However, hypercholesterolemia was significantly more
frequent in the control group. This can be due to a widespread use
of statins in the treatment of MI. Hypercholesteremia was diagnosed
if total cholesterol level was >5 mmol/l. The World Health
Organization classification based on body mass index (BMI)
calculation using the following equation: BMI=weight in
kilograms/height in meters2, was used to diagnose
obesity and excess weight. Abdominal obesity was diagnosed in the
case of waist circumference of ≥80 cm in women and ≥94 cm in men.
The presence of MI, angina pectoris or sudden cardiac death in
parents were considered as positive family history. Sex differences
in the described risk factors between the study group and the
control group are presented in Table
II.
 | Table I.General characteristics of the study
groups. |
Table I.
General characteristics of the study
groups.
| Characteristic | MI, n (%) | Control, n (%) | P-value |
|---|
| Sex |
| Male | 411 (82.2) | 423 (79.1) | 0.239 |
|
Female | 89 (17.8) | 112 (20.9) |
|
| Weight |
| BMI
18.5–24.9 | 183 (37.7) | 181 (40.0) | 0.594 |
| BMI
25–29.9 | 192 (39.6) | 181 (40.0) |
|
| BMI
≥30 | 110 (22.7) | 90 (20.0) |
|
| Overweight and
obesity |
| BMI
≥25 | 302 (62.3) | 272 (60.0) | 0.485 |
|
Abdominal obesity | 161 (48.8) | 199 (52.5) | 0.323 |
| Smoking status |
| No | 150 (30.9) | 204 (38.5) | 0.005 |
|
Yes | 305 (62.9) | 271 (51.1) |
|
|
Ex-smoker | 30 (6.2) | 55 (10.4) |
|
| Arterial
hypertension | 334 (68.9) | 300 (56.1) | <0.001 |
| Diabetes
mellitus | 52 (10.7) | 30 (6.6) | 0.026 |
|
Hypercholesterolemia | 323 (72.3) | 360 (79.5) | 0.011 |
| Positive family
history | 153 (33.6) | 107 (24.4) | 0.002 |
 | Table II.Sex differences between the study and
control groups. |
Table II.
Sex differences between the study and
control groups.
|
| Sex |
|---|
|
|
|
|---|
|
| Male | Female |
|---|
|
|
|
|
|---|
| Risk factors | MI group, n
(%) | Control group, n
(%) | P-value | MI group, n
(%) | Control group, n
(%) | P-value |
|---|
| Smoking
history |
|
Yes | 96 (23.9) | 111 (26.4) | 0.007 | 54 (64.3) | 93 (85.3) | 0.003 |
| No | 278 (69.3) | 257 (61.0) |
| 27 (32.1) | 14 (12.8) |
|
|
Ex-smoker | 27 (6.7) | 53 (12.6) |
| 3 (3.6) | 2 (1.8) |
|
| Hypertensive
disease | 272 (67.8) | 243 (57.4) | 0.002 | 62 (73.8) | 57 (50.9) | 0.001 |
| Diabetes
mellitus | 36 (9.0) | 22 (6.5) | 0.201 | 16 (19.0) | 8 (7.1) | 0.012 |
| Positive family
history | 129 (34.3) | 80 (24.2) | 0.003 | 24 (30.4) | 27 (24.8) | 0.393 |
A total of 14.5% of the study patients exhibited
NSTEMI, while the majority of the study participants (85.5%)
exhibited STEMI. ECG results revealed the presence of pathological
Q-waves in two thirds of patients. The cases of anterior and
anterior lateral MI were assigned to the anterior MI localization,
while the cases of inferior, inferior lateral and posterior MI were
assigned to the non-anterior MI localization.
The described categories of MI cases are presented
in Table III. The results are
available for 496 patients with MI as 3 patients exhibited left
bundle branch block and 1 patient had an implanted
cardiostimulator. The severity and risk of unfavorable outcomes
were assessed in each hospitalized study patient using the Global
Registry of Acute Coronary Events (GRACE) (19) and Thrombolysis in Myocardial
Infarction (TIMI) risk scores (20).
Interventional reperfusion was performed in 251 (50.2%) of 500
patients with MI. In 232 patients (92.4%) PCI involved coronary
stent insertion. A total of 249 patients (49.8%) received
conservative treatment with antithrombotics, anticoagulants,
nitrates, β-blockers and statins, including 88 patients (35.3%) who
received thrombolytic therapy.
 | Table III.Main categories of MI cases. |
Table III.
Main categories of MI cases.
| MI categories | n (%) |
|---|
| STEMI | 424 (85.5) |
| NSTEMI | 72 (14.5) |
| Anterior MI | 217 (43.7) |
| Non-anterior
MI | 241 (48.6) |
| Circular MI | 38 (7.7) |
| Q-wave | 338 (68.1) |
| non Q-wave | 158 (31.9) |
Prospective medium-term follow-up period lasted two
years. Patients were monitored three times at 6, 12 and 24 months
following discharge from the hospital. The following endpoints were
assessed: Recurrent MI, hospitalization for unstable angina (UA),
recurrent acute coronary syndrome (ACS) was defined as recurrent
MI, hospital admission for UA and repeated PCI. A total of 476
patients were discharged from the hospital. A total of 24 (5.0%)
patients were lost to follow up, and, therefore, the present study
analyzed the outcomes of the disease for 452 patients. To make a
differential estimation of the outcomes, the discharged study
participants were divided into two groups: Patients receiving the
conservative therapy (group 1) and patients who underwent PCI
(group 2).
All collected samples were marked and maintained in
freezer storage boxes until DNA extraction. DNA extraction was
performed according to the manufacturer's instructions.
Specifically, DNA was extracted from whole venous blood or
myocardial tissue samples using a phenol-chloroform extraction.
Genotypes for the rs1333049 and rs10757274 SNPs were
determined using the 5′nuclease assay with TaqMan probes (assay
identification C__1754666_10 and C__11841860_10, respectively;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cycling
conditions were 60°C for 2 min and 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 1 min. Each 10-µl
amplification reaction volume contained 1X Universal Master Mix
(ABgene Inc., Rochester, NY, USA) and 10 ng of template DNA.
Allelic discrimination was measured automatically on the ABI Prism
7900HT (Thermo Fisher Scientific, Inc) with Sequence Detection
Systems 2.1 software of the Applied Biosystems (Thermo Fisher
Scientific, Inc.).
Statistical analysis
Data were analyzed using the SPSS software (version
20.0; IBM Corp., Armonk, NY, USA). One sample Kolmogorov-Smirnov
test was used to verify the normality of the distribution of data.
The significance of differences in qualitative characteristics in
the study groups was assessed using continuity-corrected
nonparametric Pearson's chi-square test or Fisher's exact test when
the frequency of symptom occurrence was ≤5. Mann-Whitney rank test
was used to assess the significance of statistical differences in
quantitative characteristics. P<0.05 was considered to indicate
a statistically significant difference. To prove the statistical
significance of the differences between carriers of the C allele
and homozygous GG individuals the Wilcoxon signed-rank test and the
Mantel-Cox log-rank test were used. Correlation analysis was
performed using the nonparametric Spearman test. Odds ratio (OR)
was calculated to assess the risk of MI for a particular allele or
genotype. In case if one of the characteristics was equal to zero
and it was not possible to calculate the OR, the relative risk
ratios were determined for 2×2 contingency tables and confidence
intervals (CI) were calculated.
Results
Association between SNPs and MI
During the first phase of the present study, the
association between two SNPs or the 9р21.3 locus (rs1333049
and rs10757278) and MI were assessed in the Russian
population. Analysis of genotype frequencies of the studied SNPs
revealed statistically significant differences between the MI and
control groups. Frequencies in the MI group compared with the
control group were 28.6±2.0 vs. 18.4±1.7% (P<0.001) for the
rs1333049 genotype CC and 80.3±1.7 vs. 72.1±2.0% (P=0.002)
for both heterozygous and homozygous C allele carriers. For the
rs10757278 genotype, the frequencies of GG were 27.0±2.0 vs.
17.9±1.8% (P=0.002) in the MI compared with the control group and
78.1±1.8 vs. 72.4±2.1% (P=0.041) for the heterozygous G allele
carriers.
The risk of MI was 1.77 (95% CI: 1.36–2.37) for
individuals with the homozygous CC genotype of rs1333049 and
1.70 (95% CI: 1.24–2.32) for individuals with the GG genotype of
rs10757278. For the heterozygous risk allele carriers, the
OR was slightly lower than for the homozygous risk allele carriers.
Specifically, the risk of MI was 1.58 (95% CI: 1.18–2.11) for
carriers of the rs1333049 C allele and 1.36 (95% CI:
1.01–1.83) for the carriers of the rs10757278 G allele.
Both SNPs of the 9p21.3 locus (rs1333049 and
rs10757278) are sufficiently close for linkage
disequilibrium at a distance of 1,025 base pairs. The correlation
coefficient between the homozygous CC genotype at rs1333049
and the GG genotype at rs10757278 was equal to 0.943; and
0.954 between the heterozygous genotype carriers. Consequently,
these SNPs in the study population may belong to the same linkage
group. There was a direct positive correlation between MI and
homozygous risk genotypes of both SNPs, though absolute correlation
coefficient was moderate for both the rs1333049 CC genotype
(rs=0.120) and the rs10757278 GG genotype
(rs=0.108).
Regression analysis
Among the 1,035 study participants (patients with MI
and control subjects), 628 individuals (60.7%) with complete
baseline information were selected for regression analysis. The MI
status was presented as a dichotomous variable, where 0 indicated
the absence of MI and 1 the presence of MI. The genotyping results
and all risk factors evaluated in the present study, including sex,
age, presence of excess weight or obesity, abdominal obesity,
diabetes mellitus, arterial hypertension, hypercholesterolemia,
positive family history and smoking status were included into the
logistic regression analysis. Logistic regression model was built
using step-by-step inclusion of prognostic factors and
determination of the minimum set of predictors by calculating
Nagelkerke's R squared values (R2) to indicate the
effect of all model predictors on response variable dispersion.
Variables with low significance and autocorrelation
were rejected. The adjusted coefficient of determination
R2 of a combination of variables including age, smoking
status, presence of arterial hypertension, diabetes mellitus and
positive family history was 0.284 (P<0.001). Among the included
determinants, the CC genotype of rs1333049 was statistically
significant. Following the addition of rs1333049 to the
logistic regression model, adjusted R2 increased to
0.297 (P=0.006). In the logistic regression model, the
rs1333049 CC genotype exhibited an independent predictive
value (OR=1.71; 95% CI: 1.16–2.52; P=0.006).
The association between the rs1333049
genotypes and the GRACE risk score was also assessed. The CC
genotype of rs1333049 was associated with intermediate and
high GRACE risk scores in patients with STEMI. A total of 20.4±2.7%
STEMI carriers were in the low-risk group, 32.3±4.2% in the
intermediate-risk group and 28.6±7.6% in the high-risk group. There
was no association between the GRACE risk scores and
rs1333049 genotypes in patients with NSTEMI. Furthermore,
there was no association between the rs1333049 genotypes and
the TIMI score in patients with NSTEMI.
Associations between risk genotypes
and patient outcomes
The second stage of the present study was to
determine the association between risk genotypes and medium-term
outcomes in patients with MI. Within the two-year follow-up period
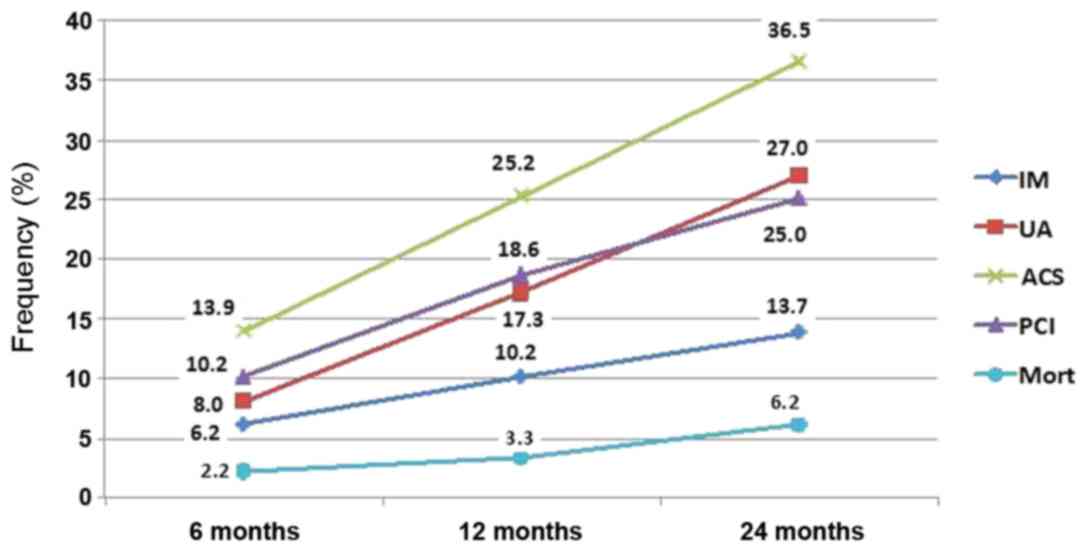
after discharge from the hospital, 36.5% of patients with MI were
re-admitted with ACS, 13.7% of patients exhibited recurrent MI and
25.0% of patients underwent a new coronary intervention. Mortality
among the discharged patients with MI was 2.2, 3.3 and 6.2% within
6, 12, and 24 months of the observation, respectively. Cumulative
frequencies of the endpoints during the two-year observation period
are presented in Fig. 1.
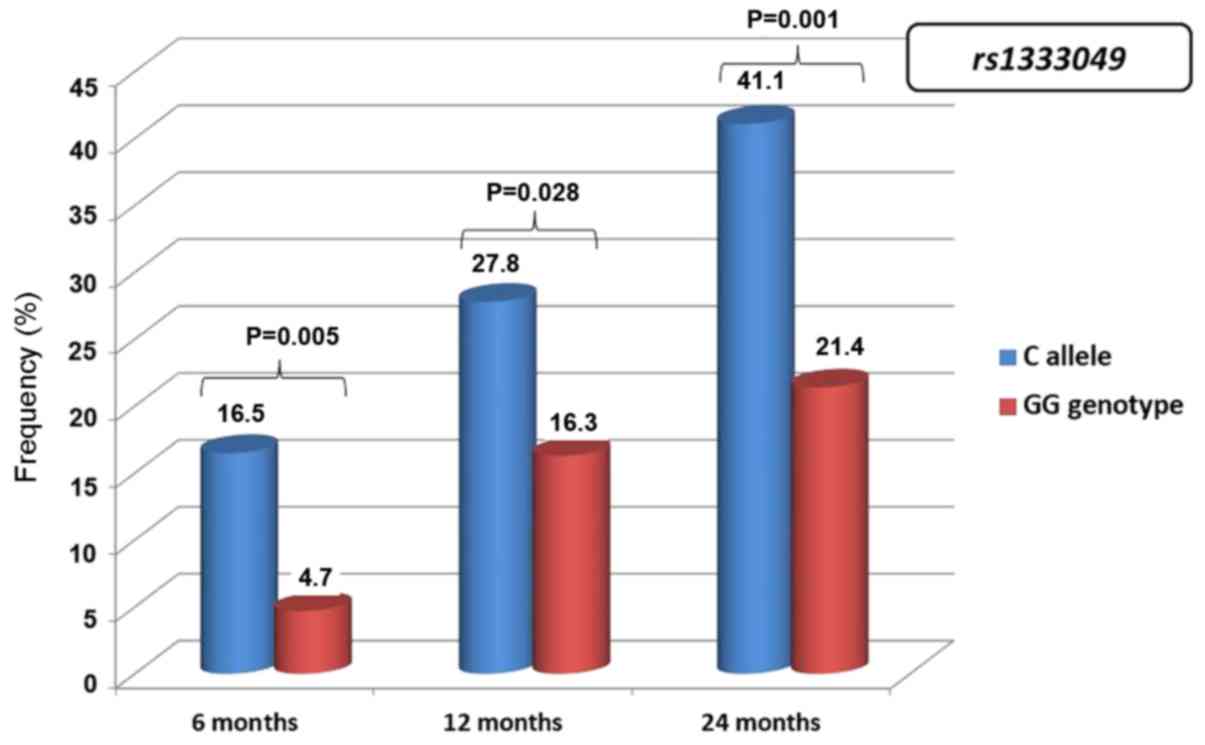
During the observation period, the cumulative ACS
frequency was significantly higher among the carriers of the CC and
CG genotypes compared with patients with homozygous GG
rs1333049 genotype. At 6 months, the cumulative ACS
frequency was 16.5±2.1% among carriers of the CC and CG genotypes
compared with 4.7±2.3% among carriers of the homozygous GG genotype
of rs1333049 (P=0.005). The difference between C allele
carriers and GG homozygotes was 27.8±2.5 vs. 16.3±4.0% (P=0.028) at
12 months and 41.1±2.7% vs. 21.4±4.4% (P=0.001) at 24 months,
respectively (Fig. 2).
Since the polymorphism at the 9p21.3 locus is known
to be associated with the severity of atherosclerotic coronary
lesions, it was hypothesized that the association between genetic
predictors and outcomes of MI could differ between groups of
patients receiving PCI and patients receiving non-interventional
procedures. In such a way, according to our hypothesis, data on
genotype variations can be used in clinical practice.
Analysis of the medium-term outcomes of MI in
different rs1333049 genotype carrier was performed. Patients
receiving the conservative therapy (group 1) and patients who
underwent PCI (group 2) exhibited no statistically significant
differences in the frequency of rs1333049 polymorphic
variants. Thus, in group 1, there were 31.8±3.0% of the CC genotype
carriers, and in group 2–27.0±2.9% (P=0.239); 79.2±2.6 and
81.3±2.5% of patients in groups 1 and 2, respectively, were C
allele carriers (P=0.553). In addition, the groups did not differ
in the total number of endpoints during the follow up period. The
frequency of recurrent ACS in group 2 compared with group 1 was
12.7±2.2 and 15.2±2.4% (P=0.428) during the 6 months after MI;
24.0±2.8 and 26. ±3.0% (P=0.550) within 12 months; and 35.7±3.2 and
38.4±3.3% (P=0.559) within two years of the follow up period,
respectively.
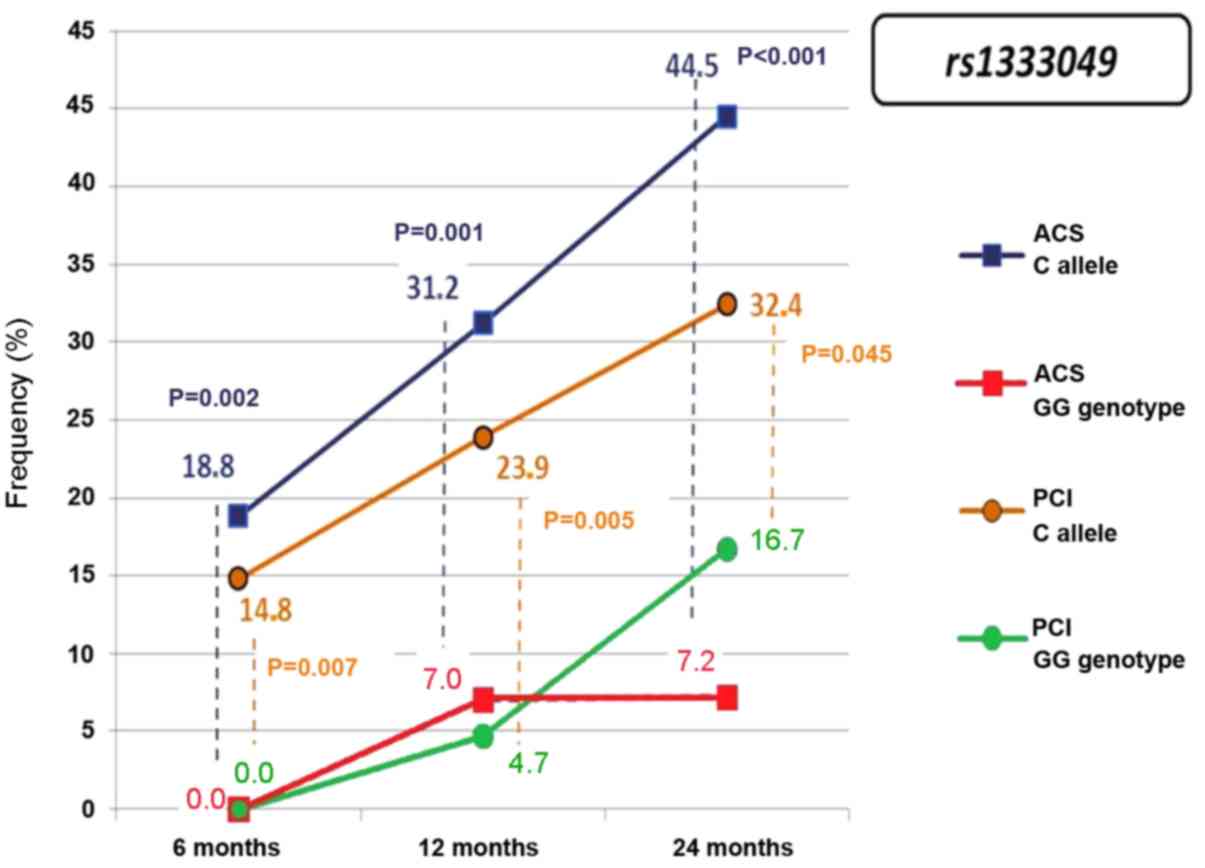
Having received conservative therapy rather than
PCI, group 1 had a statistically significant association of the C
allele at rs1333049 with recurrent ACS and subsequent PCI
(Fig. 3). The probability of
recurrent ACS in group 1 was 4.91 (95% CI: 1.45–16.66) a year after
MI and 3.77 (95% CI: 1.50–9.52) after two years. No statistically
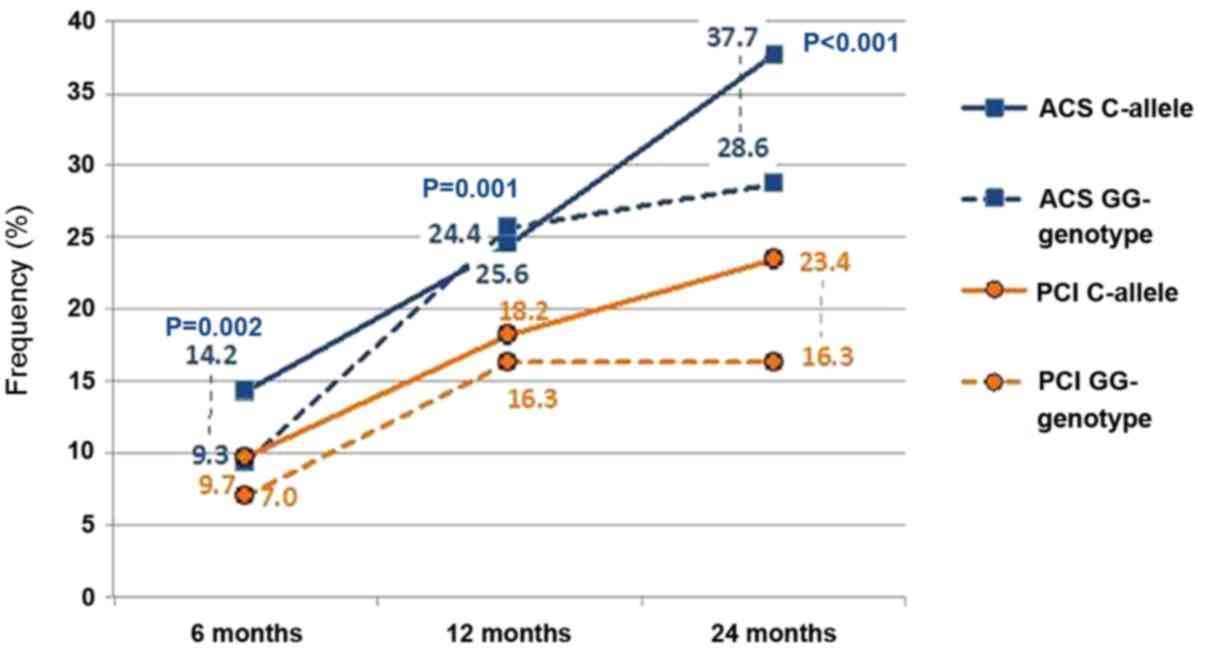
significant association between the rs1333049 polymorphic
variants and MI outcomes was revealed in the medium-term follow up
of patients who underwent PCI (Fig.
4). The frequency of recurrent MI and hospitalization for ACS
did not differ significantly within a year after MI between the
carriers of the C allele and individuals with homozygous GG
genotype of rs1333049 [11.4±2.4 vs. 11.6±4.9% (P=0.961) and
24.4±3.2 vs. 25.6±6.7% (P=0.875), respectively]. During the
two-year observation period, the frequency of recurrent MI
(15.4±2.7%) and ACS (37.7±3.7%) was higher in carriers of the
rs1333049 C risk allele compared with the carriers of the
homozygous GG genotype (11.9±4.9 and 28.6±6.9%, respectively),
however the difference was not statistically significant (P=0.563
and 0.267, respectively).
The ACS recurrence time (the time from discharge to
ACS recurrence) was 17.05±0.77 months (95% CI: 15.54–18.55) for
carriers of the homozygous CC genotype of rs1333049,
18.16±0.56 months (95% CI: 17.06–19.26) for carriers of the
heterozygous CG genotype and 20.54±0.75 months (95% CI:
19.06–22.01) for carriers of the homozygous GG genotype. These
differences between carriers of the C allele and homozygous GG
individuals were statistically significant (Mantel-Cox log-rank
test, P=0.004 and the Wilcoxon signed-rank test, P=0.005).
The frequencies of recurrent ACS in carriers of
different genotypes of rs1333049 depending on the type of
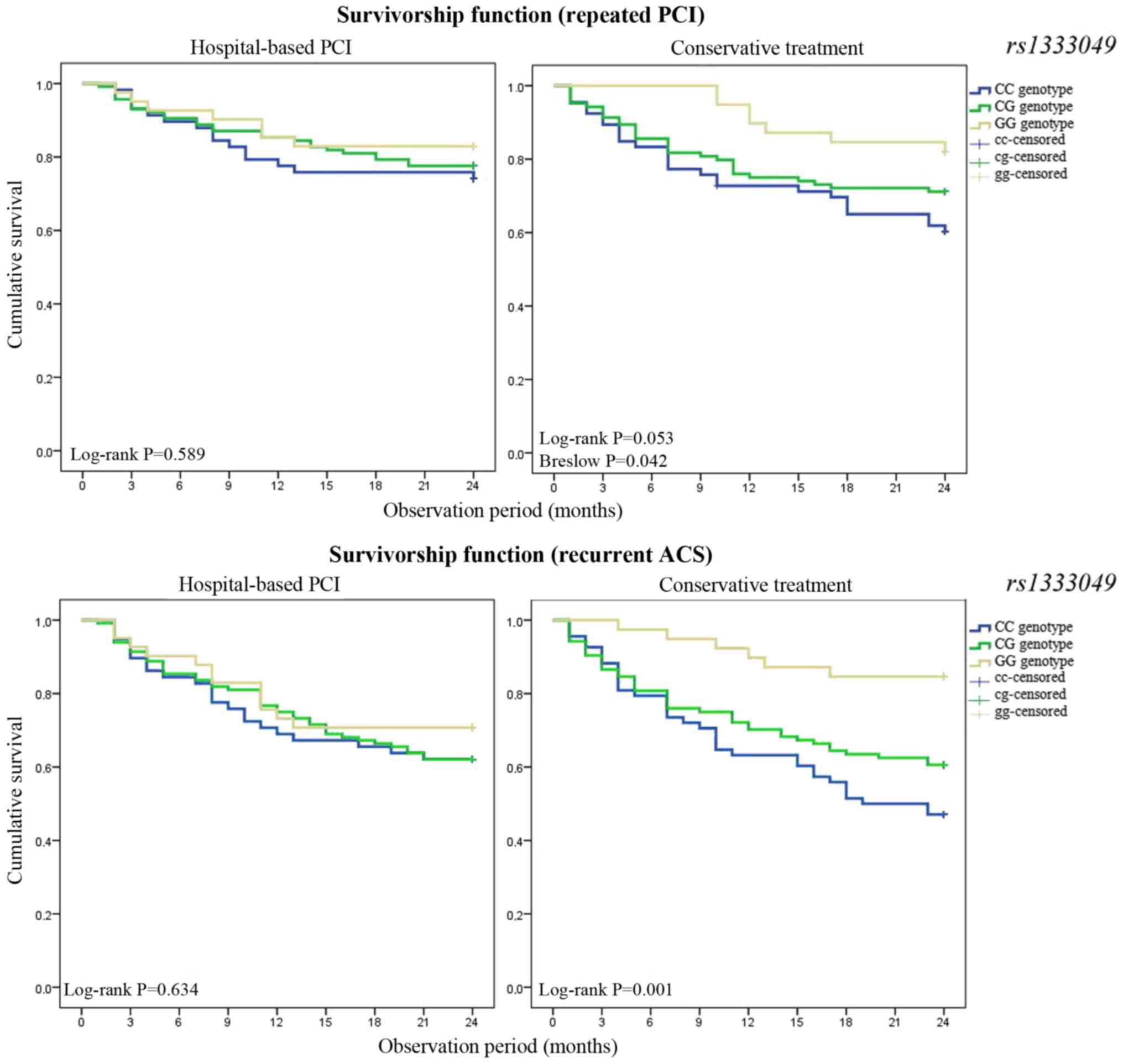
hospital care were analyzed (Fig.
5). Group 2 patients who underwent PCI during index
hospitalization demonstrated almost equivalent Kaplan-Meier Curves.
The survival time (ACS recurrence time) was 18.02±1.10 months (95%
CI: 17.10–19.99) for patients in group 1 carrying the homozygous CC
rs1333049 polymorphism, 18.54±0.74 months (95% CI:
16.86–21.58) for patients with the heterozygous CG genotype and
19.22±1.21 months (95% CI: 17.46–19.60) patients with homozygous GG
genotype.
The frequency of recurrent ACS and repeated PCI
within two years of follow-up differed significantly between group
1 patients with one or two risk C alleles at rs1333049
compared with GG heterozygotes at this locus. The survival time
(ACS recurrence time) was 16.22±1.06 months (95% CI: 14.14–18.30)
in group 2 carriers of the homozygous CC genotype of
rs1333049, 17.73±0.86 months (95% CI: 16.05–19.42) for
carriers of the heterozygous CG genotype and 21.92±0.82 months (95%
CI: 20.31–23.54) in carriers of the homozygous GG genotype.
Therefore, the interventional procedure eliminated the presumable
CAD-inducing effect of genetic predictors within a relatively short
two-year observation period. At the same time, patients who did not
receive PCI, had a strong possibility of ACS development. This
difference is due to the fact that PCI involves mechanical
atherosclerosis burden disruption and stent placement, leading to
radical changes the clinical course.
Discussion
The risk factor approach is based on the summing up
of the risk factors adverse effects. However, this approach cannot
be applied to genetic predictors, since the latter have different
genetic mechanisms. Another limiting factor in application of
genomic information in risk stratification is high frequency of
risk alleles in the population in general (21). Therefore, the use of chromosome 9p21
SNPs in the secondary prevention setting is more reasonable
compared with their use in primary prevention.
A larger investigation where the rs 1333049 C
risk allele carriership was integrated into the GRACE risk score
aimed to assess the combined end point ‘cardiac death and recurrent
MI’ within six months after ACS (13). However, the predictive value of
recurrent MI and cardiac death were not statistically significant.
From the perspective of clinical medicine, patients with ACS
represent a heterogeneous group. In-hospital and post-discharge
long-term outcomes depend on the extent of myocardial damage. One
of the limitations of the study by Buysschaert et al
(13) is the heterogeneity of the
cohort of patients with ACS. Specifically, 26.1% of patients
exhibited STEMI, 28.3% of patients exhibited UA and 41.6% patients
exhibited NSTEMI. Moreover, only half of the enrolled patients
underwent surgical intervention: PCI (47.2%) or coronary artery
bypass grafting (3.8%).
Successful application of genetic determinants as
prognostic markers was demonstrated in strictly specific groups of
patients. Szpakowicz et al (16) reported a retrospective analysis of
589 patients with STEMI. The rs1333049 and rs10757278
polymorphic variants of locus 9p21.3 were significantly associated
with overall mortality within 5 years after MI only in high-risk
patients according to the GRACE classification (GRACЕ risk score
≥155). Similarly, the Medicine, Angioplasty, or Surgery Study-II, a
randomized trial, reported an association between the 9р21.3 locus
polymorphism and overall mortality and mortality from cardiac
causes in the group of patients with the most severe cases of
multi-vessel coronary artery burden (22).
The use of interventional techniques or major
surgical procedures may affect disease outcomes for the nearest
period after MI (20). Previously,
no association was revealed between the risk genotypes of
rs1333040 and rs1333049 of the 9р21.3 locus and the
three-year follow-up outcomes in 2,028 patients with ACS treated
with PCI who underwent drug-eluting stent implantation (15). In a large prospective observational
study of acute coronary insufficiency (Osaka Acute Coronary
Insufficiency Study) an association between rs1333049 of the
9р21.3 locus and the risk of recurrent MI was investigated within a
year after discharge of 2,022 patients with ACS (23). The authors concluded that there was
no association between the risk genotype of rs1333049 and
the risk of recurrent MI in case of PCI, although the C allele had
conferred susceptibility to the first MI (ibid).
In the present study, the correlation coefficient
between homozygous CC individuals at rs1333049 and the GG
genotype at rs10757278 amounted to 0.943. For heterozygous
states at both loci the rs was equal to 0.954. These
findings are consistent with the results of other studies (3,4,6,7) and may
be due to these SNPs proximity in the genome.
However, in the logistic regression model, the
rs1333049 SNP exhibited a marginally higher predictive value
compared with rs10757278. It should be stated that, to the
best of the authors' knowledge, the association between genetic
variants and the outcomes for the carriers of the studied genotypes
who received conservative treatment for MI has not been previously
studied. Moreover, the outcomes for patients (carriers of the
studied genotypes with conservative treatment) have also not been
compared with the outcomes for patients who underwent PCI at index
hospitalization.
The presence or absence of coronary intervention
during index hospitalization should be taken into consideration
when evaluating genetic markers as predictors of medium-term
disease outcomes in patients with MI, since these predictors are
relevant only to assess the risk in patients who did not undergo
PCI. In the present study, the risk genotype exhibited an
independent predictive value in patients with MI who did not
undergo revascularization, regardless of the GRACE or TIMI risk
scores.
In conclusion, the present pilot study conducted in
the Russian Federation, confirmed the results of international
genome-wide association studies regarding the association between
the 9р21.3 locus SNPs and MI. The results of the present study
indicated that the rs1333049 genotype may be used for
medium-term disease outcome prediction in patients with MI who did
not undergo coronary intervention at index hospitalization.
However, due to a small sample size, the results of the present
study may not be generalized to other populations. Therefore,
future investigations with larger sample sizes are required to
confirm the present results.
Acknowledgements
The authors are grateful to all colleagues who
facilitated the study at the Laboratory of Molecular Genetic
Studies of Therapeutic Diseases, Institute of Internal and
Preventive Medicine of the Siberian branch of the Russian Academy
of Medical Sciences, in Krasnoyarsk Interdistrict Clinical Hospital
No. 20 named after I.S. Berzon and in Krasnoyarsk Regional Clinical
Hospital.
Funding
The authors received funding from Professor V.F.
Voino-Yasenetsky Krasnoyarsk State Medical University. No other
specific grants were received from funding agencies in the public,
commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SN developed the methodology of the research and was
involved in analyzing and interpreting the patient data. IA
participated in literature review, was involved in drafting the
manuscript, analysing and interpreting the data, and was
responsible for final text revision. PS was a major contributor in
concept development and literature review, and analyzed and
interpreted the patient data and performed the statistical data
analysis. OG revised the manuscript, and participated in data
collection, literature review and statistical analysis. VM was
responsible for carrying out molecular genetic testing (PCR
analysis) and DNA extraction. MV was involved in revising the
manuscript critically for important intellectual content and made
substantial contributions to data analysis. DB participated in
source data collection. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Professor V.F. Voino-Yasenetsky Krasnoyarsk State
Medical University. The patients signed informed consent to
participate in the study.
Patient consent for publication
The authors declare that they obtained informed
consent for publication from all the study participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Prins BP, Lagou V, Asselbergs FW, Snieder
H and Fu J: Genetics of coronary artery disease: Genome-wide
association studies and beyond. Atherosclerosis. 225:1–10. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
CARDIoGRAMplusC4D Consortium, ; Deloukas
P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR,
Ingelsson E, Saleheen D, Erdmann J, et al: Large-scale association
analysis identifies new risk loci for coronary artery disease. Nat
Genet. 45:25–33. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan K, Patel RS, Newcombe P, Nelson CP,
Qasim A, Epstein SE, Burnett S, Vaccarino VL, Zafari AM, Shah SH,
et al: Association between the chromosome 9p21 locus and
angiographic coronary artery disease burden: A collaborative
meta-analysis. J Am Coll Cardiol. 61:957–970. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Franceschini N, Carty C, Bůžková P, Reiner
A, Garrett T, Lin Y, Vöckler JS, Hindorff LA, Cole SA, Boerwinkle
E, et al: Association of genetic variants and incident coronary
heart disease in multi-ethnic cohorts. The PAGE study. Circ
Cardiovasc Genet. 4:661–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo J, Li W, Wu Z, Cheng X, Wang Y and
Chen T: Association between 9p21.3 genomic markers and coronary
artery disease in East Asians: A meta-analysis involving 9813 cases
and 10710 controls. Mol Biol Rep. 40:337–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Horne BD: Chromosome 9p21, risk
associations, and biological mechanisms in coronary heart disease.
J Am Coll Cardiol. 63:2246–2248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Munir MS, Wang Z, Alahdab F, Steffen MW,
Erwin PJ, Kullo IJ and Murad MH: The association of 9p21-3 locus
with coronary atherosclerosis: A systematic review and
meta-analysis. BMC Med Genet. 6:662014. View Article : Google Scholar
|
|
8
|
Shesternya PA, Shulman VA, Nikulina SYU,
Martynova EA, Dyomkina AI, Orlov PS, Maksimov VN and Voyevoda MI:
Predictive role of chromosome 9p21.3 polymorphisms and their
association with family history of coronary heart disease in
patients with myocardial infarction. Russian J Cardiol. 6:14–18.
2012.(In Russian).
|
|
9
|
Tajbakhsh A, Khorrami MS, Hassanian SM,
Aghasizade M, Pasdar A, Maftouh M, Tabatabai E, Parizadeh SM,
Fazeli M, Ferns GA, et al: The 9p21 locus and its potential role in
atherosclerosis susceptibility; Molecular mechanisms and clinical
implications. Curr Pharm Des. 22:5730–5737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ganna A, Magnusson PK, Pedersen NL, de
Faire U, Reilly M, Arnöv J, Sundström J, Hamsten A and Ingelsson E:
Multilocus genetic risk scores for coronary heart disease
prediction. Arterioscler Thromb Vasc Biol. 33:2267–2272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paynter NP, Chasman DI, Buring JE,
Shiffman D, Cook NR and Ridker PM: Cardiovascular disease risk
prediction with or without knowledge of genetic variation at
chromosome 9p21.3. Ann Intern Med. 150:65–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ripatti S, Tikkanen E, Orho-Melander M,
Havulinna AS, Silander K, Sharma A, Guiducci C, Perola M, Jula A,
Sinisalo J, et al: A multilocus genetic risk score for coronary
heart disease: Case-control and prospective cohort analyses.
Lancet. 376:1393–1400. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buysschaert I, Carruthers KF, Dunbar DR,
Peuteman G, Rietzschel E, Belmans A, Hedley A, De Meyer T, Budaj A,
Van de Werf F, et al: A variant at chromosome 9p21 is associated
with recurrent myocardial infarction and cardiac death after acute
coronary syndrome: The GRACE Genetics Study. Eur Heart J.
31:1132–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adrissino D, Berzuini C, Merlini PA,
Mannuccio Mannucci P, Surti A, Burtt N, Voight B, Tubaro M,
Peyvandi F, Spreafico M, et al: Influence of 9p21.3 genetic
variants on clinical and angiographic outcomes in early-onset
myocardial infarction. J Am Coll Cardiol. 58:426–434. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoppmann P, Erl A, Türk S, Tiroch K,
Mehilli J, Schömig A, Kastrati A and Koch W: No association of
chromosome 9p21.3 variation with clinical and angiographic outcomes
after placement of drug-eluting stents. JACC Cardiovasc Interv.
2:1149–1155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szpakowicz A, Kiliszek M, Pepinski W,
Waszkiewicz E, Franaszczyk M, Skawronska M, Ploski R, Niemcunowicz-
Janica A, Dobrzycki S, Opolski G, et al: Polymorphism of 9p21.3
locus is associated with 5-year survival in high-risk patients with
myocardial infarction. PLoS One. 9:e1046352014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
MONICA Monograph and Multimedia
Sourcebook, . World's largest study of heart disease, stroke, risk
factors, and population trends 1979–2002. Edited by Hugh
Tunstall-Pedoe (with 64 other contributors for the WHO MONICA
Project). WHO; Geneva: 2003
|
|
18
|
Malyutina S, Bobak M, Simonova G, Gafarov
V, Nikitin Y and Marmot M: Education, marital status, and total and
cardiovascular mortality in Novosibirsk, Russia: A prospective
cohort study. Ann Epidemiol. 14:244–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
GRACE Investigators, . Rationale and
design of the GRACE (Global Registry of Acute Coronary Events)
project: A multinational registry of patients hospitalized with
acute coronary syndromes. Am Heart J. 141:190–199. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Antman EM, Cohen M, Bernink PJ, McCabe CH,
Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D and
Braunwald E: The TIMI risk score for unstable angina/non-ST
elevation MI: A method for prognostication and therapeutic decision
making. JAMA. 284:835–842. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel RS and Ye S: Genetic determinants of
coronary heart disease: New discoveries and insights from
genome-wide association study. Heart. 97:1463–1473. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gioli-Pereira L, Santos PC, Ferreira NE,
Hueb WA, Krieger JE and Pereira AC: Higher incidence of death in
multi-vessel coronary artery disease patients associated with
polymorphisms in chromosome 9p21. BMC Cardiovasc Disord. 12:612012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hara M, Sakata Y, Nakatani D, Suna S,
Usami M, Matsumoto S, Ozaki K, Nishino M, Sato H, Kitamura T, et
al: Reduced risk of recurrent myocardial infarction in homozygous
carriers of the chromosome 9p21 rs1333049 C risk allele in the
contemporary percutaneous coronary intervention era: A prospective
observational study. BMJ Open. 4:e0054382014. View Article : Google Scholar : PubMed/NCBI
|