Introduction
Traumatic soft tissue defects such as bedsores,
chronic skin ulcers, limb necrosis, osteonecrosis and other
ischemic orthopedic diseases are the most clinically intractable
and common issues in orthopedics due to unsatisfactory conventional
treatments (1). Interest in
therapeutic vasculogenesis has arisen as a potential solution to
these problems where endothelial progenitor cells (EPCs) or stem
cells are transplanted into the ischemic site to promote
angiogenesis (2,3). EPC-mediated angiogenesis has a unique
advantage of not being affected by the function of the original
blood vessels and native endothelial cells (ECs). EPCs are widely
present in adult bone marrow, and have sustained and pluripotent
differentiation abilities. EPCs promote the development of blood
vessels by differentiating into mature vascular ECs then secreting
vascular growth factors (4,5). EPC-mediated angiogenesis improves blood
supply to the ischemic site in both animal and clinical trials
(6,7); however, injected growth factors are
rapidly cleared from the body and thus any transplanted cells
undergo rapid cell death in large numbers due to the lack of
nutrients. This phenomenon hinders the clinical applications of
EPC-mediated therapeutic angiogenesis.
Hydrogels are promising biocompatible and
biodegradable biomaterials used as cell transplant vectors that
also have roles as drug release systems and tissue engineered cell
scaffolds (8,9). Hydrogels are water-soluble polymer
networks capable of absorbing a large amount of water to swell and,
after swelling, are capable of maintaining their three-dimensional
polymer structure due to physical or chemical cross-linking between
polymer chains (10). Due to
hydrogels displaying good biocompatibility, water permeability and
a high degree of swelling this makes them a promising biomaterial
for implantation (11). Since 2000,
arginylglycylaspartic acid (RGD) peptides have been used to modify
hydrogels in order to improve their biocompatibility (8). The RGD tripeptide is the smallest amino
acid sequence recognized by adherent proteins. This sequence binds
specifically to cell surface integrin receptors, which mediate cell
adhesion, migration and growth. Consequently, the immobilization of
RGD directly onto a biomaterial promotes the adhesion of
receptor-mediated cells to the surface of the material (8,9,12). In 2009, matrix-metalloproteinase
(MMP)-sensitive polyethylene glycol (PEG) hydrogels were used to
encapsulate human umbilical vein ECs and thymosin β-4, a human
protein that promotes tissue regeneration. It was determined that
the modified hydrogel provide an environment that supports human
umbilical vein ECs, reduces apoptosis, increases cell survival rate
and achieves a controlled release of thymosin β-4 (12).
The proliferation, differentiation, maturation, and
integration of host blood vessels with EPCs require growth factor
involvement such as basic fibroblast growth factor (bFGF) and
vascular endothelial growth factor (VEGF) (10,11,13,14).
Vascular growth factors and EPCs work synergistically to create new
blood vessels, capillaries, and the formation of capillary networks
in the ischemic area. Seliktar et al (15) combined RGD and multiple growth
factors into the PEG hydrogel backbone by covalent binding and
found that this design not only slowly releases the growth factors
but also promotes the adhesion and growth of ECs on the biomaterial
surface.
Scaffolds are critical in EPC-mediated therapeutic
vasculogenesis therefore the purpose of the present study was to
design modified PEG hydrogels with covalently bound RGD to
encapsulate EPCs alongside VEGF and bFGF. The role of the growth
factors was to maintain EPC health, integrate with the host blood
vessels, and also to ensure the sustained long-term release of
vascular growth factors into the wound.
Materials and methods
Animal study
Twenty male Sprague Dawley rats (6–8 weeks) weighing
180–200 g were obtained from the Experimental Animal Center of
Huazhong University of Science and Technology. Animals were housed
at constant room temperature with a 12-h light-dark cycle, and free
access to a standard rodent diet and water. Protocols involving the
use of the animals were approved by the Ethics Committee of Tongji
Medical College, Huazhong University of Science and Technology
(2016 Institutional Animal Care and Use Committee no. S782). Animal
experiments were performed in accordance with the Guide for the
Care and Use of Laboratory Animals.
Synthesis of four-arm-polyethylene
glycol-vinylsulfone (PEG-VS) monomer reaction
The hydrogel synthesis method was adapted from
Seliktar et al (15).
Four-arm-PEG powder (cat. no. 4ARM-PEG-OH; Shanghai Jinpan Biotech
Co., Ltd.) was dissolved in dichloromethane (DCM; analytical
reagent, purity >99.5%) to prepare a 0.2 g/ml solution. Then
PEG-OH, sodium hydride, and diVS were mixed together in a reaction
molar ratio of 1:5:50, respectively. The reactor was wrapped with
foil to keep it in the dark for the entire time. The reaction was
kept in an argon atmosphere and stirred for 3 days at room
temperature. Pure acetic acid (pH 7) was used to neutralize the
solution following completion of the reaction and all residues were
filtered using filter paper. Then cold pure acetic acid was then
added in excess to produce a precipitate and the supernatant was
discarded. The precipitate was washed using DCM, then subsequently
resuspended in DCM, and filtered again to produce a precipitate.
DCM was used to wash this precipitation twice to remove the excess
diVS. Finally, powder was obtained by overnight freeze vacuum
drying at 50°C in a lyophilizer (model no. FD-1A-50; Beijing
Boyikang Laboratory Instruments Co., Ltd.). Nuclear magnetic
resonance spectroscopy (1H-NMR; AVANCE III HDNanobay 400
MHz compact NMR system; Bruker BioSpin Corporation) was conducted
according to the basic NMR operational manual to assess the VS to
acquire a peak at 6.1–6.4.
Formation of MMP-sensitive PEG
hydrogels modified with RGD
Hydrogel formation was prepared as previously
described in the literature (12,16,17). In
brief, 330 mg of the prepared four-arm-PEG-VS monomer was dissolved
in 2.7 ml Tris base, acetic acid and ethylenediaminetetraacetic
acid (TAE; 0.3 M; pH 8) solution, with w/v of 10% at 4°C, then
stored at the same temperature. A total of 4.7 mg RGD
(Ac-GCRDGPQGIWGQDRCG-NH2; Zoonbio Biotechnology, Co.,
Ltd.) was dissolved in 3 ml TAE buffer (0.3 M; pH 8) then 4.7 mg
matrix metalloproteinase (MMP) reaction substrate peptide (Zoonbio
Biotechnology, Co., Ltd.) was dissolved in 3 ml TAE buffer (0.3 M;
pH 8). Following which, 30 µl of RGD TAE solution and 30 µl of MMP
reaction peptide TAE solution were added to 270 µl of
four-arm-PEG-VS TAE solution then mixed at 37°C for 30 min.
Finally, the mixed solution was transferred into a clean penicillin
bottle and kept at 37°C for 30 min to form a hydrogel.
Hydrogels with 5-carboxyfluorescein (cat. no. F5008;
US Everbright, Inc.)-labeled peptide encapsulated with VEGF (cat.
no. 400-31) and bFGF (cat. no. 400-29; both 1.2 ng/well; Peprotech)
were manufactured in a similar manner as the aforementioned
procedures but the RGD peptides were replaced with
5-carboxyfluorescein fluorescent-labeled peptides then hydrogels
were soaked in PBS.
Release curve
The hydrogel was soaked in PBS then ELISAs (cat. no.
E-EL-R0091 and E-EL-R0020; Elabscience Biotechnology Co., Ltd.) was
used to test the content and stability of bFGF and VEGF every 12 h.
MMP-2 (100 and 1,000 ng) and MMP-9 (100 and 1,000 ng) were added to
the samples following 72 h, then the optical density (OD450) value
was measured by ELISA. The release curve was plotted after
repeating the previous method to detect the slow-release effect of
bFGF and VEGF.
Hydrogel stability and biodegradation
test
A hydrogel stability test was conducted where
hydrogels were transferred into a 12-well plate, and then soaked in
40 ml PBS in the dark at room temperature. PBS was replaced every
day. In order to set up the standard solutions of fluorescent RGD
peptides, the concentration of RGD peptide were as follows: 2, 1,
0.5, 0.25 and 0.125 µg/ml. Fluorescence intensities at OD505 for
both standard solutions and the soaking PBS solution were measured
with a fluorescence photometer. To test the degradation behavior of
the hydrogel, wet weight hydrogel (20 mg; n=3) was dried in a
lyophilizer until the weight. Then the dried hydrogels were placed
in a penicillin bottle, soaked in PBS on a shaking bed at room
temperature. The weight of the hydrogels after lyophilization was
measured every day and was used for the degradation rate
calculation.
Hydrogel enzymatic degradation
test
The hydrogels with fluorescently labeled peptides
were digested using a bacterial collagenase solution (0, 0.5, 1, 2
active bacterial collagenase units per ml PBS; cat. no. JS10538;
Shanghai Jinsui Bio-Technology Co., Ltd.). The free
fluorescence-labeled peptide concentration in solution was
determined by fluorescence spectrophotometer to plot a hydrogel
degradation release curve. The difference between tested weight on
each day and initial weight on the first day was obtained. The
degradation rate was calculated by calculating the ratio of the
aforementioned difference and the initial weight.
EPC adhesion and proliferation
The EPCs isolated and used in the current study were
derived from the rat bone marrow where no vascular ECs are present.
EPCs were isolated from rat bone marrow cells, according to a
standard procedure (18) and then
von Willebrand factor (vWF; forward primer,
5′-GCTCCAGCAAGTTGAAGACC-3′, reverse primer,
5′-GCAAGTCACTGTGTGGCACT-3′), kinase insert domain receptor (KDR;
forward primer, 5′-CTTTTGGTAGCGGGATGAAA-3′, reverse primer,
5′-TCCATTGAAATGGGATTGGT-3′), TEK receptor tyrosine kinase (Tek;
forward primer, 5′-ACAATCGTGGACGGCTATTC-3′, reverse primer,
5′-AAGGTCTTTAGGGGCTGGAA-3′), CD34 (forward
5′-ACTTCTGTTGCCTCGGAGAA-3′; reverse 5′-ACGGTTGGGTAAGTCTGTGG-3′),
CD133 (forward 5′-CCTCTGGTGGGGTATTTCTT-3′; reverse
5′-AGGTGCTGTTCATGTTCTCC-3′), and nitric oxide synthase 3 (eNOS;
forward primer, 5′-CACAGGCATCACCAGGAAGAA-3′, reverse primer,
5′-ATACCAGTGGGTCCGAGCA-3′) gene expression were determined by
semi-quantitative PCR to confirm the success of EPC isolation
(3,19). β-actin (F240; forward primer,
5′-CACGATGGAGGGGCCGGACTCATC-3′ and R240; reverse primer,
5′-TAAAGACCTCTATGCCAACACAGT-3′) was used as the reference gene. The
25 µl PCR reaction system contained 0.5 µl 10 µM forward primer,
0.5 µl 10 µM reverse primer, 2 µl 2.5 mM dNTP (cat. no. D40300A;
Takara), 0.25 µl Ex Taq (cat. no. DRR100A; Takara), 2.5 µl 10 fold
Ex Taq E buffer, 1 µl cDNA and ddH20. The PCR conditions
were as follows: Denaturation at 95°C for 4 min, then 35 cycles of
denaturation at 95°C for 4 min, annealing at 54°C for 30 sec and
extension at 72°C for 25 sec, and a final extension step of 72°C
for 4 min. The PCR was conducted on an ABI7900 thermocycler
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and products
were detected on a 1% agarose gel with 0.5 µg/ml ethidium bromide.
Gels were observed with JY02S Gel Imager (Beijing Junyi
Electrophoresis Co., Ltd.) and the acquired gel images were
processed by Photoshop CS5 (Adobe Systems, Inc.).
To investigate EPC adhesion and proliferation on the
hydrogel, EPCs were inoculated on the RGD modified hydrogel surface
at a seeding density of 5×104 cells/cm2. EPCs
were cultivated in the incubator with 5% CO2 at 37°C in
the modified DMEM (Gibco; Thermo Fisher Scientific, Inc.) with 25
U/ml 10% FBS heparin, 2 mmol/l glutamine (both Sigma-Aldrich; Merck
KGaA), 20 µg/l VEGF, 10 µg/l bFGF, 10 µg/l leukemia inhibitory
factor and 10 µg/l epidermal growth factor (all Peprotech). The
growth of EPCs on the hydrogels were observed and counted using
inverted phase contrast microscope. Blank RGD-modified hydrogel was
used as a control.
MTT assay for cell metabolic
activity
MTT assay was used to assess the biocompatibility of
hydrogels. In brief, EPC density was adjusted to a concentration of
1×105 cells/ml and 100 µl cell suspension was added to
the surface of RGD-modified hydrogels and non-RGD-modified
hydrogels. The plates were incubated under standard conditions
(37°C; 5% CO2) overnight. Hydrogels where the cells had
been inoculated were carefully removed from the wells. Then 10 µM
MTT solution was added to samples to assess the adherent cells and
the plates were incubated at 37°C for 4 h. The supernatant was
discarded and 100 µl of DMSO was added to each well. The plates
were placed on a shaking bed for 10 min. The absorbance was read at
570–595 nm at 0, 4, 12, 24, 48, and 72 h.
EPC culture experiments encapsulated
in PEG hydrogel encapsulated with bFGF and VEGF
EPCs were seeded into the hydrogel as previously
described (20). The hydrogel was
modified according to the previous procedure. The initial EPC
seeding density was 5×105/well on a 12-well plate. bFGF
and VEGF were added into each well at a concentration of 1.2
ng/well, respectively. MMP-mediated bFGF and VEGF release rates
were detected by bFGF and VEGF ELISA kit (cat. no. E-EL-R0091 and
E-EL-R0020, respectively; Elabscience Biotechnology Co., Ltd.) with
the method adapted from Kraehenbuehl et al (12). For samples for flow cytometry
detection, collected cells were first digested by trypsin-EDTA then
washed twice with PBS. After being re-suspended with 500 µl binding
buffer, 5 ml Annexin V-FITC and 5 µl propidinm iodide were added to
the cells. The samples were then kept in dark for 5–15 min at room
temperature. Viable cells were detected by flow cytometry with by
flow cytometry with Annexin V-FITC Apoptosis kit (cat. no. KG108;
Nanjing KeyGen Biotech Co., Ltd.). The percentage analysis was
finished on Epics XL-4 Flow Cytometer with System II™ software
(version 3.0; both from Beckman Coulter, Inc.).
Reverse transcription-quantitative (RT-q)PCR was
performed to determine the expression levels of
platelet/endothelial cell adhesion molecule 1 (PECAM1), CD34, KDR,
Tek, angiopoietin-2 and protocadherin (pcdh12) genes after 7 days
of cultivation. Total RNA from rat EPCs was isolated using TRIzol
reagent (cat. no. 15596-018; Invitrogen; Thermo Fisher Scientific,
Inc.) and the converted to cDNA with the RevertAid™ First Strand
cDNA Synthesis kit (cat. no. K1622; Fermentas; Thermo Fisher
Scientific, Inc.) following manufacturer's protocol. The cDNA was
diluted 10-fold in double distilled water and the reaction
conditions were: 50°C for 2 min, followed by 40 cycles of 95°C for
30 sec and 60°C for 30 sec. qPCRs were conducted on Real PCR
machine (ABI7900; Applied Biosystems; Thermo Fisher Scientific,
Inc.) using Maxima SYBR-Green/Fluorescein qPCR Master Mix (cat. no.
K0241; Fermentas; Thermo Fisher Scientific, Inc.). The reaction
system for each gene includes 4 µl 10-fold diluted cDNA of the
target gene, 10 µl Maxima SYBR-Green/Fluorescein qPCR Master Mix
(2X), 5.2 µl H2O, 0.4 µl 100 µM forward primer (PECAM1:
5′-ATCAGCACCACCTCGAAATC-3′; CD34; Tek; KDR; angiopoietin-2:
5′-GGCGAGGAGTCCAACTACAG-3′; and pcdh12: 5′-TCTGGTGCTGACTGCCTATG-3′)
and 0.4 µl 100 µM reverse primer (PECAM1:
5′-CTTTTTGTCCACGGTCACCT-3′; CD34; Tek; KDR; angiopoietin-2:
5′-AAGTTGGAAGGACCACATGC-3′; and pcdh12:
5′-CACATGCTTGCCAAAGAAGA-3′). β-actin 240 was used as the reference
gene. The results were quantified using the 2−ΔΔCq
method (21).
The chick chorioallantoic membrane (CAM) method was
adapted from Valdes et al (22) to investigate the function of bFGF and
VEGF encapsulated within the hydrogel on angiogenesis by scoring
the newly formed angiogenic vessels (23). Chicken embryos (Wuhan Institute of
Bioloigcal Products, Co., Ltd.) with similar weights were obtained
from fresh fertilized eggs (50±5 g) after they were opened on a
cleaned surface without breaking the embryos. Experimental samples
were divided into three groups: Control, modified hydrogel without
bFGF or VEGF, and modified hydrogel with both bFGF and VEGF. Each
group had three biological replicates. The tested hydrogels were
carefully placed in an avascular region between two main blood
vessels in each chicken embryo. The hydrogels were replaced with
fresh gels every three days. After 11 days, angiogenic vessels were
counted after the removal of the transparent tested hydrogel, then
a 2.5 ml methanol and acetone mixture (equal volume) was added to
fix the vessels at room temperature for 20 min. The vessel numbers
were counted under a dissecting microscope with a 10-fold
magnification (model no. MZFLIII; Leica Microsystems GmbH).
Statistical analysis
All studies had three replicates for every tested
group. Statistical analysis was performed using SPSS v20.0
statistical software platform (IBM Corp.). The cell count data was
described by the number of cases and examined by Fisher's exact
test. Measurement data were expressed as the mean ± standard
deviation, comparisons between three groups were analyzed by ANOVA,
followed by the Tukey's Honest Significant Difference post hoc
test, and Student's t-test was used to compare the two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
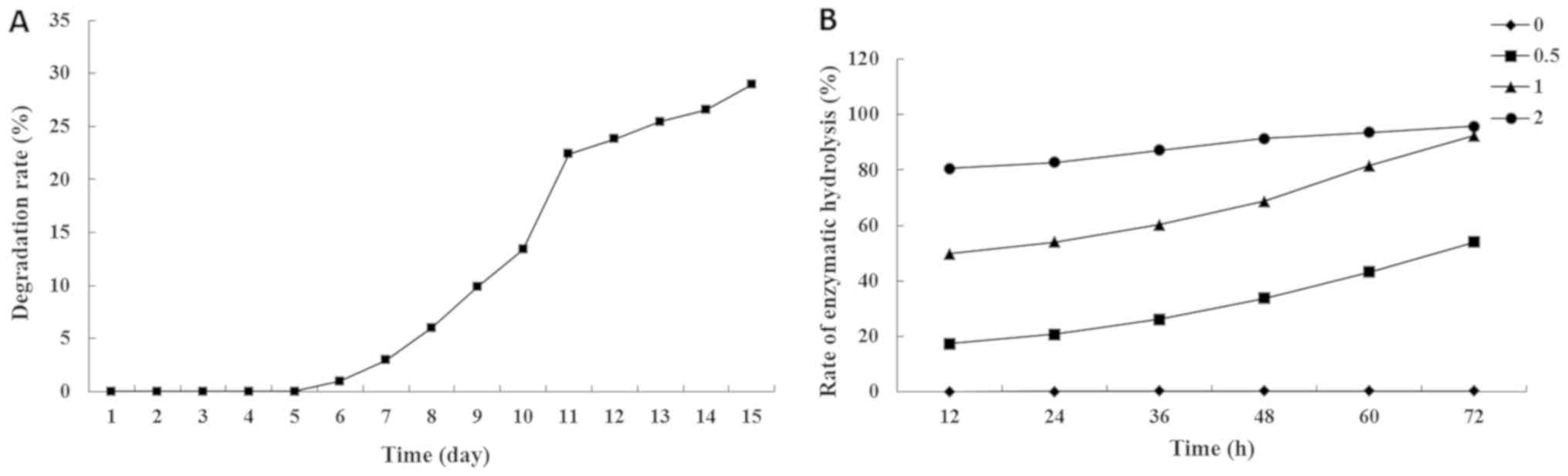
Hydrogel degradation profile
PEG hydrogels, which present non-adhesive
characteristics to proteins and cells, have been widely used to
form a thin coating on the injury site of carotid arteries in
vivo (15,24,25). The
present results demonstrated that the PEG hydrogels were stable for
6 days, then underwent gradual degradation at a relatively constant
rate with ~30% of the gels degraded by 14 days (Fig. 1A). In the enzymatic degradation test,
gels were decomposed by bacterial collagenase. The higher the
concentration of bacterial collagenase the faster the rate of
enzymatic hydrolysis. The hydrogels stored in buffer without
collagenase were not decomposed in first 72 h, and the gels kept in
the 2 active U/ml collagenase were decomposed by >95% in the
first 72 h (Fig. 1B).
RGD-modified hydrogel significantly
increases EPC adhesion and viability
EPCs were inoculated onto the surface of RGD
modified hydrogels at 5×104 cell/cm2. The
growth of the EPCs and their adhesion to the hydrogel were observed
using an inverted phase contrast microscope. The average number of
EPCs on the surface of the well in the RGD-modified hydrogel group
was significantly higher compared with that of the control group
(P<0.001; Table I). In addition,
the viability of EPCs on the gels was assessed with the MTT assay.
The number of adherent cells was higher in the RGD-modified group
compared with the control one (P<0.05). In summary, the
RGD-modified PEG hydrogel significantly promoted the adhesion and
growth of the EPCs.
 | Table I.Endothelial progenitor cell number
counted in the wells with polyethylene glycol hydrogel. |
Table I.
Endothelial progenitor cell number
counted in the wells with polyethylene glycol hydrogel.
| Well number | RGD-modified | Non
RGD-modified |
|---|
| 1 | 305 | 34 |
| 2 | 321 | 42 |
| 3 | 318 | 49 |
| Mean | 314.67 | 41.67a |
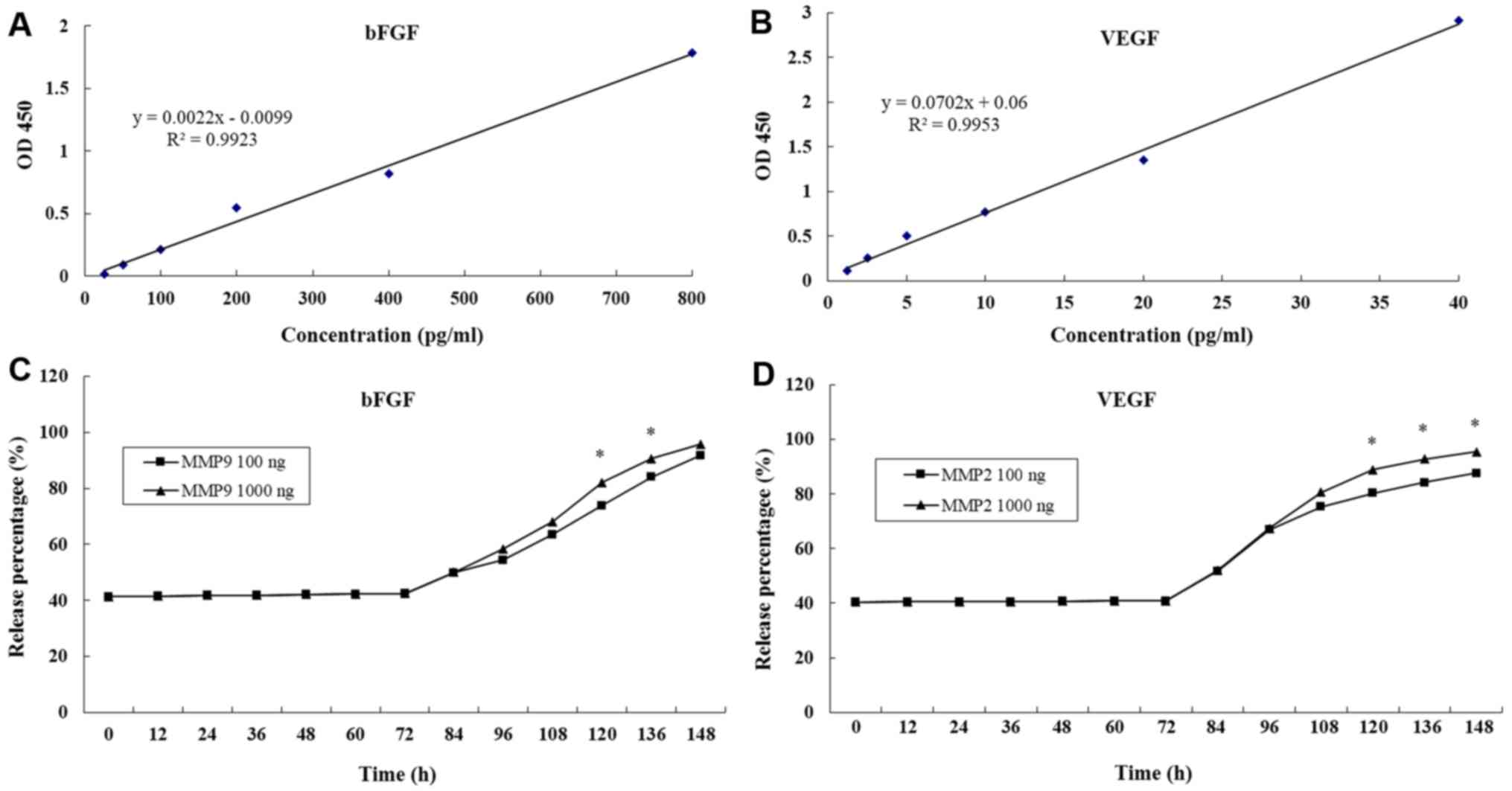
Release of bFGF and VEGF from
hydrogels
Growth factor-based cell therapies are considered to
be potentially curative treatment options (16,26). The
main limitation of growth factor application in biomedical
therapies is their high diffusibility and short half-life in
vivo (16). The present study
prepared enzyme-sensitive PEG hydrogels containing RGD using the
Michael-type addition reaction method (12). The growth factors bFGF and VEGF were
encapsulated in the hydrogel and the concentrations in the
supernatant were detected by ELISA every 12 h and compared with
standard curves. The standard curves of bFGF and VEGF were drawn by
establishing a relationship between actual OD values (the
difference between measured OD values and blank OD values) and
paired standard concentrations (Fig. 2A
and B). The release % of bFGF and VEGF were both maintained at
41% over 72 h (Fig. 2C and D).
Following the first 72 h, MMP-2 and MMP-9 were added
to the hydrogels. The release % of bFGF and VEGF both increased
steadily. At 148 h, >90% of bFGF and VEGF had been released into
the supernatant (Fig. 2C and D). In
addition, it was determined that the greater the initial MMP-2 and
MMP-9 concentration then the higher the growth factor release %
(Fig. 2). Results suggested that the
MMP-sensitive PEG hydrogels maintained a stable release of bFGF and
VEGF.
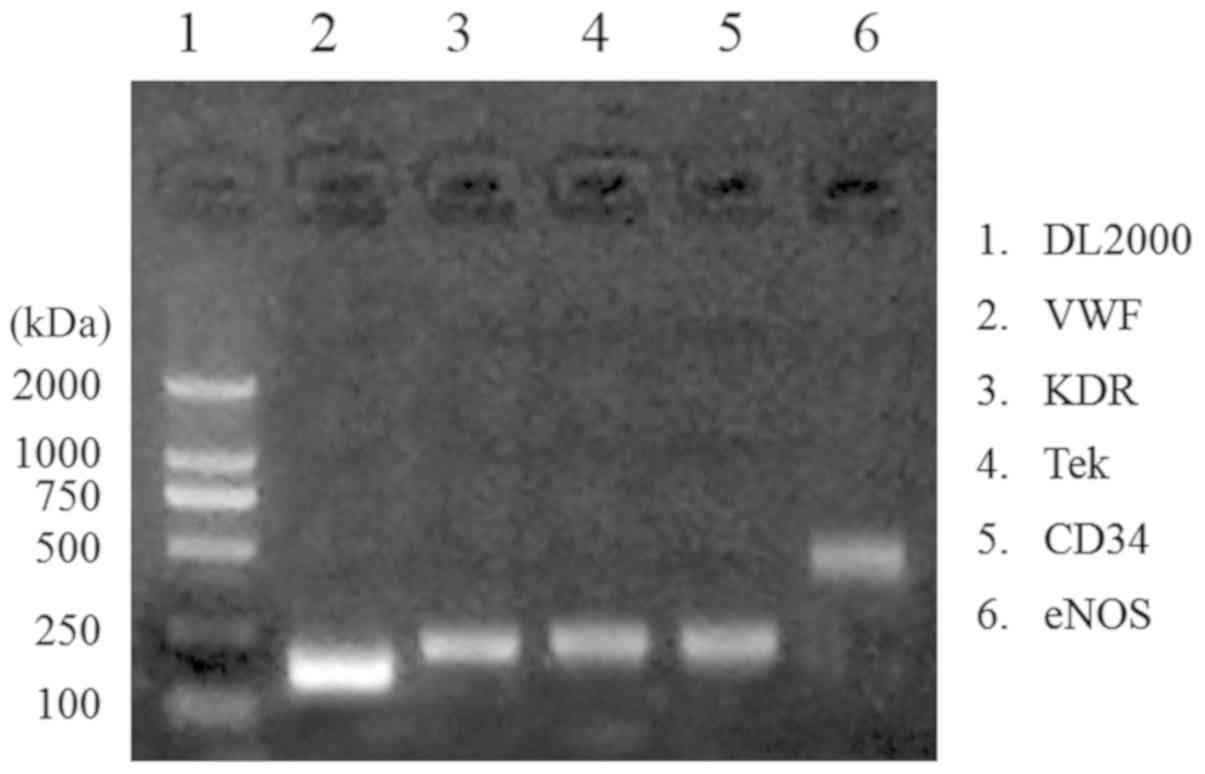
EPC isolation confirmation by
semi-quantitative gene expression
The expression of VWF, KDR, Tek, CD34 and eNOS genes
are used to determine isolation of EPCs (27). The present gene expression results
visualized by 1.5% agarose gel electrophoresis indicated that EPCs
were successfully isolated due to upregulation of the
aforementioned genes (Fig. 3).
Encapsulation of bFGF and VEGF within
hydrogels significantly increases EPC survival rate
Flow cytometry demonstrated that EPC survival rate
in hydrogels encapsulated with bFGF and VEGF was 38.69% compared
with 4.14% in hydrogels without bFGF and VEGF (P<0.05; Fig. 4). Typically, cells that do not
aggregate have a high mortality rate following the first 7 days;
however the present results demonstrated that the growth factors
promoted EPC cell survival in hydrogels.
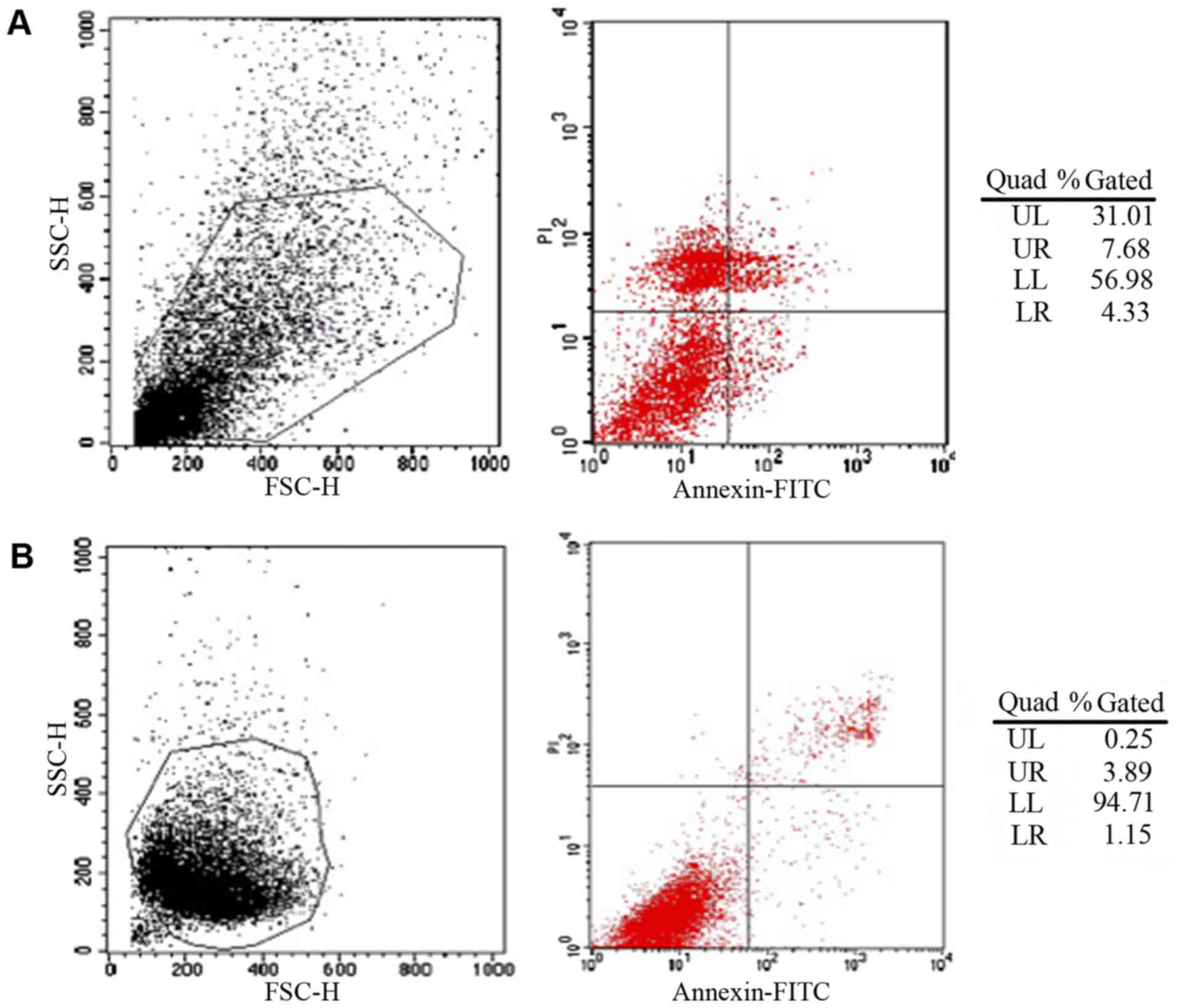 | Figure 4.Survival rates of EPCs in PEG
hydrogels determined by flow cytometry. (A) PEG hydrogels
encapsulated with EPCs, bFGF and VEGF demonstrating 38.69% surival
rate. (B) PEG hydrogels encapsulated with EPCs only demonstrating
4.14% surival rate. EPC, endothelial progenitor cells; PEG,
polyethylene glycol; bFGF, basic fibroblast growth factor; VEGF,
vascular endothelial growth factor; UL, upper left; UR, upper
right, LL, lower left; LR, lower right; SSC-H, side scatter height;
FSC-H, forward scatter height; FITC, fluorescein isothiocyanate;
PI, propidium iodide. |
Encapsulation of bFGF and VEGF within
hydrogels significantly increases EPC adhesion
The mean number of viable EPCs observed in the
hydrogel containing bFGF and VEGF was 378.3 compared with 302.3 in
the hydrogels without bFGF and VEGF in hydrogel (P<0.05).
Results suggested that the growth factors significantly increased
EPC adhesion.
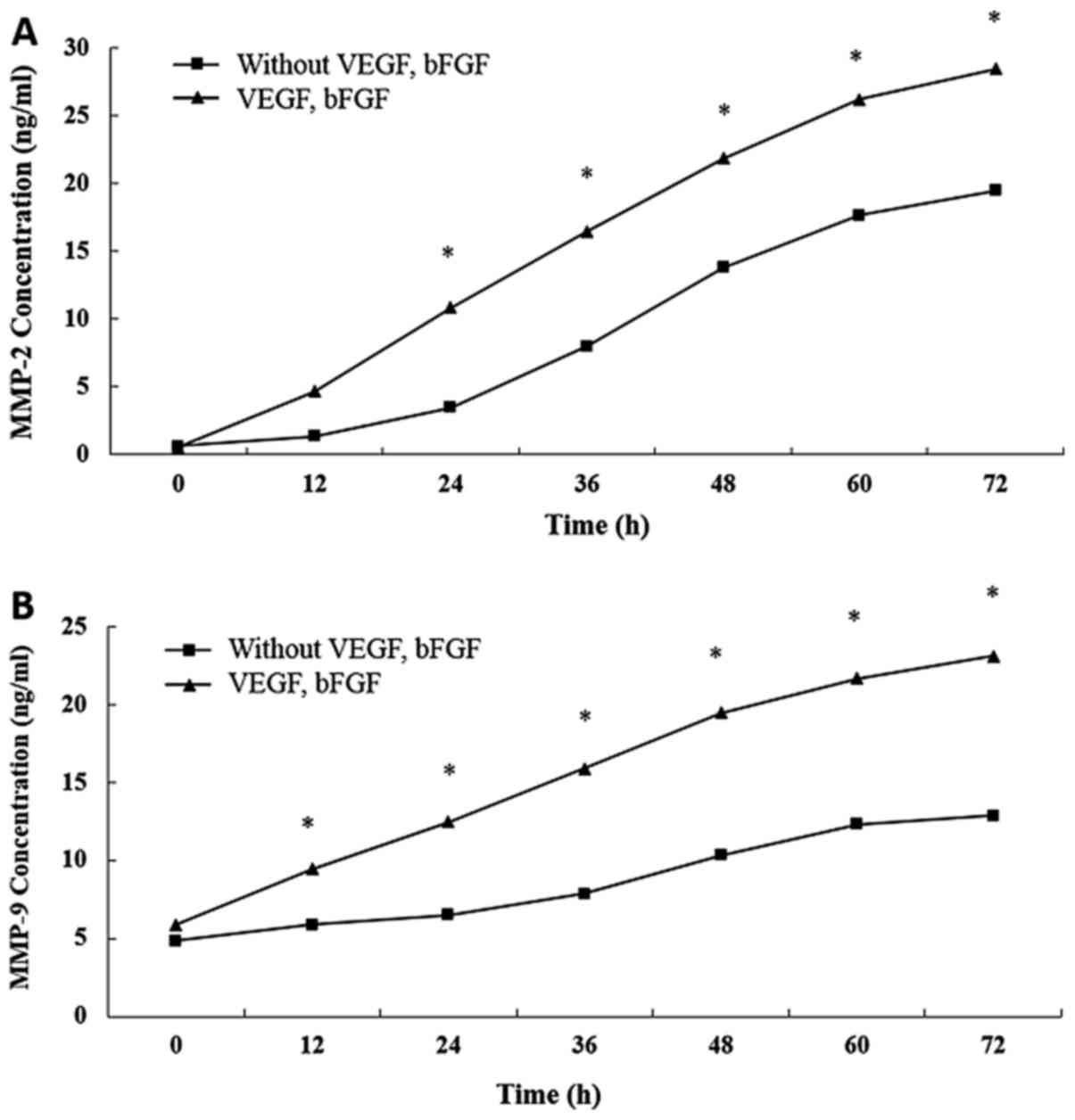
Encapsulation of bFGF and VEGF within
hydrogels significantly increases MMP release
The concentrations of both MMP-2 and MMP-9 in the
supernatant were significantly higher for the hydrogels
encapsulated with growth factors compared with those without them
(P<0.05; Fig. 5).
VEGF and bFGF promotes EPC
differentiation
Differentiation determines the success of the
implanted EPCs in the transplanted tissue. EPCs were incubated in
the hydrogels with or without bFGF and VEGF. Following 7 days of
culture, RT-qPCR was used to determine the expression of PECAM1,
CD34, KDR, Tek, angiopoietin-2 and pcdh12 genes. Results
demonstrated that hydrogels encapsulated with bFGF and VEGF had
significantly higher PECAM1, CD34, KDR, Tek, angiopoietin-2 and
pcdh12 gene expression compared with hydrogels without bFGF and
VEGF (P<0.05; Table II). The
significant difference in gene expression demonstrated that VEGF
and bFGF promoted the differentiation and maturation of EPCs.
 | Table II.Endothelial progenitor cell
differentiation-associated gene expression. |
Table II.
Endothelial progenitor cell
differentiation-associated gene expression.
| Group | PECAM1 | CD34 | KDR | Angio | Tek | Pdch12 |
|---|
| With bFGF and
VEGF | 1.024 | 1.008 | 1.032 | 0.335 | 1.032 | 1.014 |
| Without bFGF and
VEGF | 0.311a | 0.266a | 0.440a | 0.206a | 0.175a | 0.371a |
VEGF and bFGF facilitates the
neovascularization process
EPCs form new capillaries via vasculogenesis in the
center of the infarct (28). By
comparison, other primitive cells, such as hematopoietic stem
cells, secrete important growth factors such as VEGF, and stimulate
healthy small blood vessel generation in the infarcted marginal
zone by angiogenesis through ‘germination’ growth (29,30).
Vasculogenesis and angiogenesis work synergistically to establish a
new blood supply for the infarction area. In the present study, the
CAM assay was used to determine the effect of bFGF and VEGF on
angiogenesis induction in vitro. The number of vessels in
the hydrogels encapsulated with bFGF and VEGF was significantly
higher than the number of vessels in the blank group and hydrogels
without bFGF and VEGF encapsulation (P<0.05; Table III). Results indicated that the
hydrogel encapsulated with bFGF and VEGF facilitated the
neovascularization process, in which both vasculogenesis and
angiogenesis are involved.
 | Table III.Chick chorioallantoic membrane
angiogenesis assay. |
Table III.
Chick chorioallantoic membrane
angiogenesis assay.
| Group | Large blood
vessels | Medium blood
vessels | Small blood
vessels | Total |
|---|
| Blank control | 1 | 5 | 3 |
9a |
| PEG hydrogel
without bFGF and VEGF | 2 | 7 | 16 | 25a |
| PEG hydrogel with
bFGF and VEGF | 4 | 10 | 26 | 36 |
Discussion
The present study designed a MMP-sensitive PEG
hydrogel modified with RGD then encapsulated bFGF, VEGF and EPCs
within the biomaterial to observe and assess its function as a cell
transplantation vector and a release system. Hydrogels have good
biocompatibility, high water permeability and can be manufactured
to produce different microstructures and properties. Therefore,
hydrogels can be applied widely in the biomedical field (31). PEG hydrogels display good
biocompatibility, histocompatibility and the chemical structure can
be adjusted to meet the needs of different biomedical applications
(24,25,32). PEG
hydrogels also promote the integration of implant and recipient
tissue (33). In the current study,
inoculated cells were initially suspended to overcome the uneven
cell density and weak adhesion. The release of MMPs from cells
gradually degraded the hydrogel therefore cell proliferation and
hydrogel degradation had a combined effect. The modified PEG
hydrogel system serves an important role in assisting and
protecting against the above process. Results indicated that the
initial hydrogel structure was maintained for 7 days, which makes
it appropriate for a role as a sustained release carrier.
RGD is one of the smallest amino acid sequence
recognized by many adherent proteins (8). This sequence binds specifically to cell
surface integrin receptors, thereby mediating cell adhesion,
migration and growth (34–37). Directly immobilizing RGD to the
surface of a material can promote the adhesion of the
receptor-mediated cells. In the present study, EPCs were isolated
from rat bone marrow cells with this cell source excluding the
possibility of contamination from vascular ECs. Semi-quantitative
PCR for EPC related markers confirmed successful EPC isolation.
Several other robust tools for confirmation of EPC isolation
include western blot analysis, phase contrast microscopy and
morphological assessment. The average number of EPCs observed on
the surface of the RGD-modified hydrogel group was significantly
higher compared with the non-RGD modified group. In addition, EPCs
were determined to rapidly adhere to the hydrogel with the cells
and material forming contacting points. EPCs dispersed radially in
the modified hydrogels. The RGD-modified hydrogel significantly
increased the adhesion and proliferation of EPCs, which suggested
that the presence of RGD significantly promoted
biocompatibility.
Many studies have identified VEGF and bFGF as the
most important growth factors for therapeutic angiogenesis
(12,13). However, maintaining EPC activity and
integration with host blood vessels, and ensuring sustained release
of vascular growth factor in the wound long-term have not yet been
solved in angiogenesis research. Therefore, in the present study,
hydrogels were encapsulated with bFGF, VEGF and EPCs to determine
the sustained release effect of bFGF and VEGF, and also to observe
the culture of EPCs. Following addition of MMP-2 and MMP-9 at 72 h,
the release % of bFGF and VEGF increased steadily and reached
>95%. These results suggested that the MMP-sensitive PEG
hydrogels released bFGF and VEGF in a stable manner. The results
also imply that VEGF and bFGF have a synergistic role in the
formation of neovascularization. In addition, following 7 days of
culture, 38.69% of EPCs survived, suggesting that the modified
hydrogels supported EPC proliferation, reduced apoptosis and
improved the survival rate.
VEGF and bFGF participate in all aspects of
angiogenesis and promote the degradation of capillary basement
membrane, facilitate EC proliferation and migration, and the
formation of tubular structures (38). This process eventually leads to
capillary angiogenesis and microcirculation improvement (39). The effect of bFGF and VEGF on the
proliferation, differentiation, maturation and vascularization of
EPCs in hydrogels has yet to be fully elucidated therefore the
present study investigated encapsulation of EPC and growth factors
within a PEG hydrogel. RGD-modified hydrogels encapsulated with
VEGF and bFGF displayed ~40% EPCs attachment at 7 days compared
with ~4% EPC attachment for RGD hydrogels without VEGF and bFGF.
These results suggested that VEGF and bFGF enhanced the adhesion of
EPCs, and facilitated the proliferation of EPCs. Gene expression
analysis determined that EPC-related gene expression was higher in
hydrogels encapsulated with bFGF and VEGF compared with the group
without bFGF and VEGF. These results suggested that VEGF and bFGF
promoted the differentiation and maturation of EPCs.
There are several cell types suitable for
angiogenesis studies, including HUVECs and EPCs. In the present
study, EPCs were chosen due to their important roles in both
angiogenesis and vasculogenesis, as well as their ability to
migrate into the blood system stream and ability to differentiate
into a variety of mature vascular EC types. The concentration of
VEGF in vitro is positively correlated with the
proliferation rate of EPCs (40–43).
bFGF can facilitate EC proliferation and migration. When bFGF
functions together with VEGF, this synergistic pair promotes the
splitting of ECs into capillary-like structures inducing
angiogenesis in vivo (44,45). For
the present CAM study, new blood vessels grew markedly. The number
of blood vessels in the bFGF and VEGF group was significantly
higher than the control group. These results suggested that the
hydrogels containing bFGF and VEGF induced CAM angiogenesis.
Sustained release of bFGF and VEGF is capable of stimulating EC
proliferation and budding, but also capable of promoting
transplantation of EPCs hyperplasia and vascularization.
Transplanted EPCs are able to overcome the limitations of EC aging
and dysfunction, indicating that the two components in the hydrogel
have a strong synergistic association.
In conclusion, the RGD-modified hydrogel displayed
good mechanical properties suitable for a role as a sustained
release carrier. The presence of RGD in the hydrogel significantly
promoted its biocompatibility as EPC proliferation was supported,
apoptosis was reduced and EPC survival rate was improved. In
addition, hydrogels encapsulated with VEGF and bFGF enhanced the
adhesion of ECs, promoted the production of extracellular matrices
and facilitated EPC proliferation. The differentiation and
maturation of EPCs were also facilitated in the RGD-modified
hydrogels. Furthermore, the hydrogels encapsulated with bFGF and
VEGF induced angiogenesis with the two growth factors and EPCs
displaying a strong synergy. The present results may provide a
potential treatment for soft tissue defects such as bone exposure,
chronic skin ulcers, bedsores, limb necrosis, osteonecrosis and
other ischemic diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
R&D Program of China (grant no. 2017YFC1103800) and Natural
Science Fund of Hubei (grant no. 2016CFB657).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
LO, YD and DD conceived of the current study. LO and
YD designed the current study. LO, YD, ZS, GL and SY obtained the
relevant data. CY, GL and DD drafted the manuscript. CY revised the
manuscript critically for important intellectual content. All
authors analyzed and interpreted the data, and also read and
approved the final manuscript.
Ethics approval and consent to
participate
Protocols involving the use of the animals were
approved by the Ethics Committee of Tongji Medical College,
Huazhong University of Science and Technology (2016 IACUC Number:
S782). Animal experiments were performed in accordance with the
Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Winkler H: Treatment of chronic
orthopaedic infection. EFORT Open Rev. 2:110–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fadini GP, Losordo D and Dimmeler S:
Critical reevaluation of endothelial progenitor cell phenotypes for
therapeutic and diagnostic use. Circ Res. 110:624–637. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janic B and Arbab AS: The role and
therapeutic potential of endothelial progenitor cells in tumor
neovascularization. ScientificWorldJouralo. 10:1088–1099. 2010.
View Article : Google Scholar
|
|
5
|
Khoo CP, Pozzilli P and Alison MR:
Endothelial progenitor cells and their potential therapeutic
applications. Regen Med. 3:863–876. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Q, Silva EA, Wang A, Fritton JC,
Mooney DJ, Schaffler MB, Grossman PM and Rajagopalan S: Sustained
release of multiple growth factors from injectable polymeric system
as a novel therapeutic approach towards angiogenesis. Pharm Res.
27:264–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marfella R, Luongo C, Coppola A, Luongo M,
Capodanno P, Ruggiero R, Mascolo L, Ambrosino I, Sardu C, Boccardi
V, et al: Use of a non-specific immunomodulation therapy as a
therapeutic vasculogenesis strategy in no-option critical limb
ischemia patients. Atherosclerosis. 208:473–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benton JA, Fairbanks BD and Anseth KS:
Characterization of valvular interstitial cell function in three
dimensional matrix metalloproteinase degradable PEG hydrogels.
Biomaterials. 30:6593–6603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brandl F, Kastner F, Gschwind RM, Blunk T,
Tessmar J and Gopferich A: Hydrogel-based drug delivery systems:
Comparison of drug diffusivity and release kinetics. J Control
Release. 142:221–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanjaya-Putra D, Yee J, Ceci D, Truitt R,
Yee D and Gerecht S: Vascular endothelial growth factor and
substrate mechanics regulate in vitro tubulogenesis of endothelial
progenitor cells. J Cell Mol Med. 14:2436–2447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang QR, Wang BH, Huang YH, Dai G, Li WM
and Yan Q: Purification and growth of endothelial progenitor cells
from murine bone marrow mononuclear cells. J Cell Biochem.
103:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kraehenbuehl TP, Ferreira LS, Zammaretti
P, Hubbell JA and Langer R: Cell-responsive hydrogel for
encapsulation of vascular cells. Biomaterials. 30:4318–4324. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mieno S, Boodhwani M, Robich MP, Clements
RT, Sodha NR and Sellke FW: Effects of diabetes mellitus on
VEGF-induced proliferation response in bone marrow derived
endothelial progenitor cells. J Card Surg. 25:618–625. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sufen G, Xianghong Y, Yongxia C and Qian
P: bFGF and PDGF-BB have a synergistic effect on the proliferation,
migration and VEGF release of endothelial progenitor cells. Cell
Biol Int. 35:545–551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seliktar D, Zisch AH, Lutolf MP, Wrana JL
and Hubbell JA: MMP-2 sensitive, VEGF-bearing bioactive hydrogels
for promotion of vascular healing. J Biomed Mater Res A 68A.
704–716. 2004. View Article : Google Scholar
|
|
16
|
Yeo Y, Geng W, Ito T, Kohane DS, Burdick
JA and Radisic M: Photocrosslinkable hydrogel for myocyte cell
culture and injection. J Biomed Mater Res B Appl Biomater.
81:312–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kraehenbuehl TP, Zammaretti P, Van der
Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME and Hubbell JA:
Three-dimensional extracellular matrix-directed cardioprogenitor
differentiation: Systematic modulation of a synthetic
cell-responsive PEG-hydrogel. Biomaterials. 29:2757–2766. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Zhao Z, Zhang H, Hou J, Feng W,
Zhang M, Guo J, Xia J, Ge Q, Chen X and Wu X: Simultaneous
isolation of mesenchymal stem cells and endothelial progenitor
cells derived from murine bone marrow. Exp Ther Med. 16:5171–5177.
2018.PubMed/NCBI
|
|
19
|
Huizer K, Mustafa DAM, Spelt JC, Kros JM
and Sacchetti A: Improving the characterization of endothelial
progenitor cell subsets by an optimized FACS protocol. PLos One.
12:e01848952017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Camci-Unal G, Nichol JW, Bae H, Tekin H,
Bischoff J and Khademhosseini A: Hydrogel surfaces to promote
attachment and spreading of endothelial progenitor cells. J Tissue
Eng Regen Med. 7:337–347. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valdes TI, Kreutzer D and Moussy F: The
chick chorioallantoic membrane as a novel in vivo model for the
testing of biomaterials. J Biomed Mater Res. 62:273–282. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deryugina EI and Quigley JP: Chapter 2:
Chick embryo chorioallantoic membrane models to quantify
angiogenesis induced by inflammatory and tumor cells or purified
effector molecules. Methods Enzymol. 444:21–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hill-West JL, Chowdhury SM, Slepian MJ and
Hubbell JA: Inhibition of thrombosis and intimal thickening by in
situ photopolymerization of thin hydrogel barriers. Proc Natl Acad
Sci USA. 91:5967–5971. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
West JL and Hubbell JA: Separation of the
arterial wall from blood contact using hydrogel barriers reduces
intimal thickening after balloon injury in the rat: The roles of
medial and luminal factors in arterial healing. Proc Natl Acad Sci
USA. 93:13188–13193. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reinlib L and Field L: Cell
transplantation as future therapy for cardiovascular disease?: A
workshop of the National Heart, Lung, and Blood Institute.
Circulation. 101:E182–E187. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawamoto A and Losordo DW: Endothelial
progenitor cells for cardiovascular regeneration. Trends Cardiovasc
Med. 18:33–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shintani S, Murohara T, Ikeda H, Ueno T,
Honma T, Katoh A, Sasaki K, Shimada T, Oike Y and Imaizumi T:
Mobilization of endothelial progenitor cells in patients with acute
myocardial infarction. Circulation. 103:2776–2779. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Richardson TP, Peters MC, Ennett AB and
Mooney DJ: Polymeric system for dual growth factor delivery. Nat
Biotechnol. 19:1029–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arras M, Mollnau H, Strasser R, Wenz R,
Ito WD, Schaper J and Schaper W: The delivery of angiogenic factors
to the heart by microsphere therapy. Nat Biotechnol. 16:159–162.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heldman AW, Cheng L, Jenkins GM, Heller
PF, Kim DW, Ware M Jr, Nater C, Hruban RH, Rezai B, Abella BS, et
al: Paclitaxel stent coating inhibits neointimal hyperplasia at 4
weeks in a porcine model of coronary restenosis. Circulation.
103:2289–2295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Slepian MJ: Polymeric endoluminal paving:
A family of evolving methods for extending endoluminal therapeutics
beyond stenting. Cardiol Clin. 12:715–737. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Xu B, Puperi DS, Wu Y, West JL
and Grande-Allen KJ: Application of hydrogels in heart valve tissue
engineering. J Long Term Eff Med Implants. 25:105–134. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eichler W, Friedrichs U, Thies A, Tratz C
and Wiedemann P: Modulation of matrix metalloproteinase and TIMP-1
expression by cytokines in human RPE cells. Invest Ophthalmol Vis
Sci. 43:2767–2773. 2002.PubMed/NCBI
|
|
35
|
Ma C and Chegini N: Regulation of matrix
metalloproteinases (MMPs) and their tissue inhibitors in human
myometrial smooth muscle cells by TGF-beta1. Mol Hum Reprod.
5:950–954. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Overall CM, Wrana JL and Sodek J:
Transcriptional and post-transcriptional regulation of 72-kDa
gelatinase/type IV collagenase by transforming growth factor-beta 1
in human fibroblasts. Comparisons with collagenase and tissue
inhibitor of matrix metalloproteinase gene expression. J Biol Chem.
266:14064–14071. 1991.PubMed/NCBI
|
|
37
|
Wick W, Platten M and Weller M: Glioma
cell invasion: Regulation of metalloproteinase activity by
TGF-beta. J Neurooncol. 53:177–185. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guan J, Sacks MS, Beckman EJ and Wagner
WR: Synthesis, characterization, and cytocompatibility of
elastomeric, biodegradable poly(ester-urethane)ureas based on
poly(caprolactone) and putrescine. J Biomed Mater Res. 61:493–503.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferrara N and Alitalo K: Clinical
applications of angiogenic growth factors and their inhibitors. Nat
Med. 5:1359–1364. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Elbert DL and Hubbell JA: Conjugate
addition reactions combined with free-radical cross-linking for the
design of materials for tissue engineering. Biomacromolecules.
2:430–441. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Henry TD, Rocha-Singh K, Isner JM,
Kereiakes DJ, Giordano FJ, Simons M, Losordo DW, Hendel RC, Bonow
RO, Eppler SM, et al: Intracoronary administration of recombinant
human vascular endothelial growth factor to patients with coronary
artery disease. Am Heart J. 142:872–880. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee KY, Peters MC, Anderson KW and Mooney
DJ: Controlled growth factor release from synthetic extracellular
matrices. Nature. 408:998–1000. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mann BK, Gobin AS, Tsai AT, Schmedlen RH
and West JL: Smooth muscle cell growth in photopolymerized
hydrogels with cell adhesive and proteolytically degradable
domains: Synthetic ECM analogs for tissue engineering.
Biomaterials. 22:3045–3051. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lutolf MP and Hubbell JA: Synthesis and
physicochemical characterization of end-linked poly(ethylene
glycol)-co-peptide hydrogels formed by michael-type addition.
Biomacromolecules. 4:713–722. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
West JL and Hubbell JA: Polymeric
biomaterials with degradation sites for proteases involved in cell
migration. Macromolecules. 32:241–244. 1999. View Article : Google Scholar
|