Introduction
The number of individuals with diabetes is rapidly
increasing every year and by 2030, disease prevalence is expected
to reach 4.4%, with >366 million patients suffering from this
disease (1). Diabetes is a severe
disorder that increases the risk of microvascular complications.
The kidney is one of the main organs affected by microvascular
injury, and this makes diabetic nephropathy (DN) one of the most
common complications exhibited by patients with diabetes (2,3). DN is
the most common single cause of end-stage renal failure and affects
40–45% of patients entering renal replacement therapy programs
(4). The main renal histological
changes that are typical of DN are caused by the excessive
deposition of extracellular matrix (ECM). ECM accumulation results
in glomerular sclerosis and tubulointerstitial fibrosis and,
eventually, a large proportion of patients exhibiting ECM develop
chronic renal failure (5,6). The effect of the transforming growth
factor-β1 (TGF-β1)/Smad3 signaling pathway on the mediation of
renal fibrosis has been well recognized, and the inhibition of the
TGF-β1/Smad3 signaling pathway has been revealed to be an effective
approach in preventing DN progression (7,8).
In the Chinese Pharmacopoeia, herbal drug Radix
Astragali Mongolici is described as the dried root of the Mongolian
leguminous Astragalus plants (9). The constituents of Radix Astragali
Mongolici include saponins, polysaccharides and flavonoids, and
>40 constituents have been identified in the Astragalus
root (10,11). Astragalosides, the major active
components of Radix Astragali Mongolici, consist of numerous
triterpene saponins, including astragalosides I–IV (12). Astragaloside IV (ASI) is the major
active constituent of Astragalus membranaceus (also known as
huangqi) and has been widely used for the treatment of
cardiovascular diseases, hepatitis, kidney disease and skin
diseases in China (13,14). The hepatoprotective and antifibrotic
effects of ASI have been supported in previous studies (15,16).
Despite the increase in the clinical usage of ASI, the mechanisms
underlying its therapeutic effects on DN remain not clearly
understood and require further investigation.
Based on the results of previous studies, it can be
hypothesized that the therapeutic and antifibrotic effects of ASI
on DN are associated with the TGF-β1/Smad signaling pathway
(13–16). Therefore, the current study
established a rat mesangial cell (RMC) model, induced by high
glucose (HG), and a DN rat model to assess this. Cells were
cultured in different ASI doses and rats were administered ASI via
gavage, to determine the underlying mechanisms of the therapeutic
effects of ASI in DN.
Materials and methods
Animals, cells and reagents
A total of 24 adult Sprague-Dawley (SD) rats
(male/female 1:1, age, 9 weeks; weight, 200–220 g) were purchased
from the Animal Research Center of Southern Medical University. The
rats were housed in an Specific pathogen Free (SPF)-grade
laboratory with a temperature-controlled of (25°C±2°C) and a 12-h
light/dark cycle with free access to food and water. All efforts
were made to minimize animal suffering and reduce the number of
animals used. Rat mesangial cells (RMCs; cat. no. CRL-2573) were
obtained from the American Type Culture Collection. The cells were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C in a 5% CO2 humidified atmosphere. ASI was
purchased from Shanghai Jizhi Biochemical Technology Co., Ltd.
(cat. no. A50670; purity ≥99%, as determined by the manufacturer;
HPLC).
In vitro experiment
Near-confluent (80% confluent) RMCs were incubated
with DMEM supplemented with 2% FBS at 37°C for 24 h, to arrest and
synchronize cell growth, synchronized RMCs were cultured at 37°C in
normal DMEM with 5.5 nM glucose (low-glucose, LG; Sigma-Aldrich;
Merck KGaA; cat. no. G8270) or 30 nM glucose (high-glucose, HG) for
72 h, and RMCs were then collected for reverse
transcription-quantitative (RT-q) PCR assay. The synchronized RMCs
were co-cultured at 37°C in the presence of HG and ASI (0, 10, 20,
50, and 100 µg/ml) for 72 h and subsequently used for the
assessment of miR-192 expression and cell proliferation. RMCs
cultured with LG, HG, and a combination of HG and 50 µg/ml ASI for
72 h, were used in the western blot analysis.
RNA extraction and RT-qPCR
Total RNA was extracted from RMCs using TRIzol
reagent (Thermo Fisher Scientific, Inc.), and a cDNA Synthesis kit
(Takara Biotechnology Co., Ltd.) was subsequently used to
synthesize cDNA. RT-qPCR was performed to assess microRNA (miRNA)
and mRNA expression using a LightCycler 480 detection system (Roche
Diagnostics) and SYBR Green dye (cat. no. S7567; Invitrogen; Thermo
Fisher Scientific, Inc.). cDNA reverse transcription (RT) was
performed using a Reverse Transcriptase kit (cat. no. D2639A;
Takara Biotechnology Co., Ltd.) at 37°C for 15 min. The
thermocycling conditions were as follows: Initial denaturation at
92°C for 4 min, followed by 40 cycles of 90°C for 15 sec and 60°C
for 30 sec. The primers used are listed in Table I. Primers were designed using Primer
Express version 2.0 software (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primer specificities were confirmed using
The National Center for Biotechnology Information Primer-BLAST web
tool (17). GAPDH mRNA and U6 small
nuclear RNA levels were used for normalization. The RT-qPCR data
were analyzed and expressed as relative miRNA or mRNA levels using
cycle threshold values, which were subsequently converted to fold
change values using the 2−ΔΔCq method (18).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
|
| Primer sequence
(5′-3′) |
|---|
|
|
|
|---|
| Primer name | Forward | Reverse |
|---|
| H-miR-192 | RT primer:
CUGACCUAUGAAUUGACAGCCGTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGGCTGTCA |
|
|
GGGCTGACCTATGAATTG |
CAGTGCGTGTCGTGGAGT |
| H-U6 | RT primer:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAAAT |
|
|
ACGATGCACCTGTACGATCA |
TCTTTCAACACGCAGGACAG |
| H-TGF-β1 |
GGACCAGTGGGGAACACTAC |
AGAGTCCCTGCATCTCAGAGT |
| H-Smad3 |
GTGCTGGGGTTAGGTCACTG |
GAATGTCGCATCCTGTGGGA |
| H-Smad7 |
GGAGGTCATGTTCGCTCCTT |
GTTTGGTCCTGAACATGCGG |
| H-α-SMA |
GAGGGAAGGTCCTAACAGCC |
TAGTCCCGGGGATAGGCAAA |
| H-Col1 |
GCTCGTGGAAATGATGGTGC |
ACCCTGGGGACCTTCAGAG |
| H-GAPDH |
GAAAGCCTGCCGGTGACTAA |
AGGAAAAGCATCACCCGGAG |
| R-miR-192 | RT:
CUGCCAGUUCCAUAGGUCACAGGTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACCTGTGACC |
|
|
GCGCTGCCAGTTCCATAGG |
CAGTGCGTGTCGTGGAGT |
| R-U6 | RT:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAAAT |
|
|
ACGATGCACCTGTACGATCA |
TCTTTCAACACGCAGGACAG |
| R-TGF-β1 |
GACTCTCCACCTGCAAGACC |
GGACTGGCGAGCCTTAGTTT |
| R-Smad3 |
CTGGGCAAGTTCTCCAGAGTT |
AAGGGCAGGATGGACGACAT |
| R-Smad7 |
GAGTCTCGGAGGAAGAGGCT |
CTGCTCGCATAAGCTGCTGG |
| R-α-SMA |
CATCACCAACTGGGACGACA |
TCCGTTAGCAAGGTCGGATG |
| R-Col1 |
GTACATCAGCCCAAACCCCA |
CAGGATCGGAACCTTCGCTT |
| R-GAPDH |
AGTGCCAGCCTCGTCTCATA |
GGTAACCAGGCGTCCGATAC |
Cell proliferation assay
RMCs at 80% confluence were incubated with DMEM
supplemented with 2% FBS 37°C for 24 h, to arrest and synchronize
cell growth. The synchronized RMCs were seeded in triplicate in
96-well plates (1×103 cells/well) and co-cultured at
37°C in the presence of HG and ASI (0, 10, 20, 50, and 100 µg/ml)
with DMEM supplemented with 10% FBS for 0, 24, 48 and 72 h
respectively. After culture, 10 µl of Cell Counting kit-8 reagent
(Beyotime Institute of Biotechnology) was added to the medium, and
the cells were incubated for 1 h. Cell proliferation was assessed
at 24, 48 and 72 h by measuring the absorbance at 450 nm using a
microplate reader (680 Microplate Reader; Bio-Rad Laboratories,
Inc.). Assays were repeated three times.
In vivo experiment
After one week of adaptation, diabetes was induced
via a single intraperitoneal injection of streptozotocin
(Sigma-Aldrich; Merck KGaA) diluted in citrate buffer (0.1 mol/l;
pH 4.0) at a dose of 45 mg/kg (19).
The sham group (6 rats) were intraperitoneally injected with an
equal volume of 0.1 mol/l citrate buffer without streptozotocin.
The rats were then fed a normal diet for an additional three weeks;
during this time, blood glucose was maintained at >16.7 mmol/l,
the 24 h urine output was >150% above normal levels and urinary
protein excretion was >30 mg/24 h, which indicated the
successful establishment of the DN rat model (6). Six rats failed to meet the above
requirements and were eliminated from the current study. The model
rats were randomly divided into two groups: The model group and the
model + ASI group, with 6 rats in each. The model + ASI group rats
were subjected to daily intragastric administration of 40 mg/kg ASI
for 8 weeks, and the sham group and model group rats were orally
administered 40 mg/kg saline for 8 weeks. The diet, exercise and
mental state (assessed via visual inspection of behaviors including
depression and hyperactivity) of all rats were monitored daily.
After 8 weeks of treatment, urine was collected and after weighing,
rats were euthanized, and blood samples were collected via cardiac
puncture. The kidney tissue was immediately isolated, weighed and
frozen at −80°C for subsequent use in western blot analysis,
RT-qPCR, histological examination and immunohistochemistry.
Biochemical analysis
The levels of urine protein were determined using a
urinary protein kit (cat. no. C035-2; Nanjing Jiancheng
Bioengineering Institute). Creatinine and blood urea nitrogen
levels were determined using a creatinine assay kit (cat. no.
C011-2; Nanjing Jiancheng Bioengineering Institute) and a urea
assay kit (cat. no. C013-2; Nanjing Jiancheng Bioengineering
Institute).
Western blot analysis
Collected kidney tissues or RMCs were lysed in
radioimmunoprecipitation assay buffer (Roche Diagnostics) using
manufacturer's protocol. Protein concentration was measured using
the Micro BCA protein assay kit (Youdi Bio-technology Co., Ltd,
China). Total proteins (50 µg) were loaded into each lane and 12%
SDS-PAGE was performed. Cells were then transferred to PVDF
membranes (Thermo Fisher Scientific, Inc.). The PVDF membranes were
blocked with 6% non-fat dry milk at 37°C for 1 h. Immunoblotting
was performed using anti-TGF-β1 (1:200; cat. no. ab92486),
anti-Smad3 (1:1,000; cat. no. ab40854), anti-Smad7 (1:500; cat. no.
ab227309), anti-collagen type 1 (col1; 1:500; cat. no. ab6308),
anti-α-smooth muscle actin (anti-α-SMA; 1:1,000; cat. no. ab32575)
and anti-GAPDH (1:1,000; cat. no. ab8245) at 37°C for 2 h, followed
by incubation with anti-rabbit/mouse horseradish
peroxidase-conjugated IgG secondary antibodies (1:5,000; cat. nos.
ab6721 and ab190475, respectively) at 37°C for 1 h. All antibodies
were from Abcam. Antibody incubation was followed the detection of
immunoblots and visualization using enhanced chemiluminescence
Western Blotting Substrate (Pierce; Thermo Fisher Scientific, Inc.;
cat. no. 32106). GAPDH levels were used for normalization. Protein
bands were scanned and quantified using a ChemiDoc MP Image
analysis system (cat. no. 170-8280; Bio-Rad Laboratories,
Inc.).
Renal histological examination
The kidney tissues were fixed in 4% (w/v)
paraformaldehyde in PBS at room temperature for 20 min and then
sliced into 3-µm-thick sections after paraffin embedding. The
paraffin sections were deparaffinized and rehydrated prior to
staining with hematoxylin and eosin (H&E) (room temperature; 20
min) for the examination of kidney cellular structure and with
Masson's trichrome stain (room temperature; 10 min) for the
detection of collagen deposition in the renal interstitium. Visual
analysis was performed using an Olympus inverted microscope
(magnification, ×400; cat. no. CX71; Olympus Corporation
Immunohistochemistry
The aforementioned 3-µm-thick kidney tissue sections
were stained immunohistochemically with antibodies against TGF-β1
(1:1,000; cat. no. ab92486, Abcam), Smad3 (1:500; cat. no. ab40845;
Abcam) and α-SMA (1:500; cat. no. bs-10196R; BIOSS). After
deparaffin (xylene and gradient ethanol) and rehydration, slices
were boiled for 15 min using microwave irradiation for antigen
retrieval in citrate buffer (0.01 mol/l; pH 6.0). Slices were then
washed three times with PBS and blocked with 5% bovine serum
albumin (Gibco; Thermo Fisher Scientific, Inc.) for 30 min at room
temperature. The slides were incubated with the aforementioned
primary antibodies for 2 h at room temperature. Goat anti-rabbit
IgG peroxidase conjugate (1:500; cat. no. bs-0295G, BIOSS) was used
as the secondary antibody. Cells were incubated with secondary
antibodies for 1 h and room temperature. 3,39-diaminobenzidine,
nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate
(all, Sigma-Aldrich; Merck KGaA) were used as enzyme substrates.
Finally, after dehydration with gradient ethanol and
permeabilization with xylene, the slides were sealed and
photographed. Visual analysis was performed using an Olympus
inverted light microscope (magnification, ×400; cat. no. CX71;
Olympus Corporation). The mean optical density (MOD) was calculated
using Image Pro Plus 6.0 software (Media Cybernetics, Inc.).
Statistical analysis
Data were analyzed and graphed using GraphPad Prism
5 (GraphPad Software, Inc.). All results are presented as the mean
± standard deviation. Statistically significant differences between
groups were determined using a Student's t-test. Multiple
comparisons were made among three or more groups using one-way
ANOVA followed by the Bonferroni post-hoc test. The nonparametric
Mann-Whitney U test was used if data were not normally distributed.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ASI downregulates the expression of
miR-192, which is induced by HG in vitro
Compared with the LG group, miR-192 expression in
RMCs was significantly upregulated in the HG group (Fig. 1A). Furthermore, low concentration (10
g/ml) of ASI exhibited no significant effect on miR-192 expression,
the greater the ASI concentration, the more obvious the effect. The
results revealed that ASI downregulated miR-192 expression in a
dose-dependent manner (Fig. 1B).
These results demonstrated that HG upregulated and ASI
downregulated miR-192 expression in vitro.
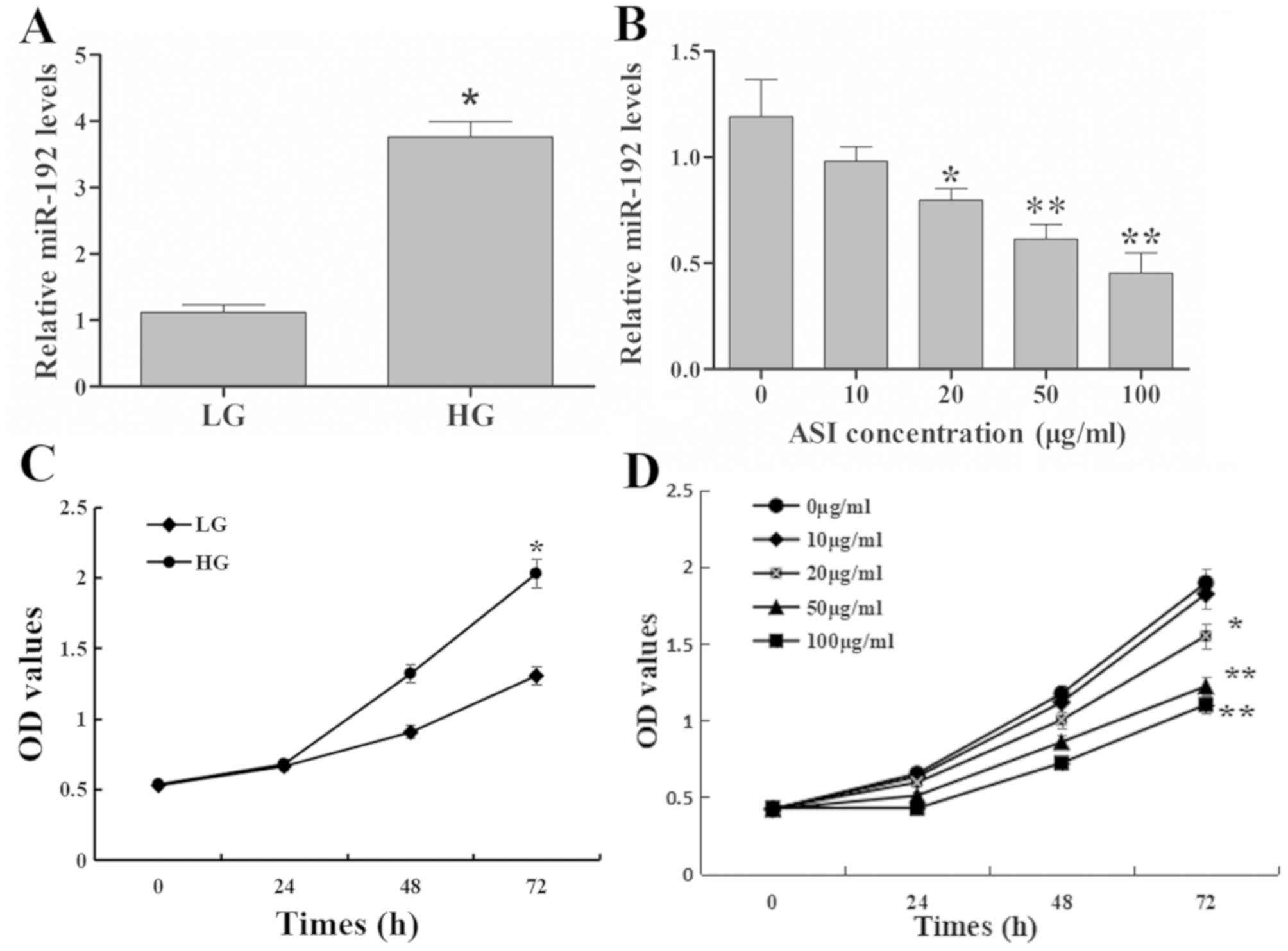 | Figure 1.Effects of ASI on miR-192 expression
and RMC proliferation. (A) miR-192 expression exhibited by the LG
and HG treatment groups. *P<0.05 vs. the LG group. (B) miR-192
expression following treatment with ASI at 0, 10, 20, 50, and 100
µg/ml. *P<0.05 vs. 0 µg/ml ASI group and **P<0.01 vs. 0 µg/ml
ASI group. (C) Cell proliferation following treatment with LG and
HG. *P<0.05 vs. the LG group. (D) Cell proliferation following
treatment with ASI at 0, 10, 20, 50, and 100 µg/ml at 0, 24, 48 and
72 h. *P<0.05 vs. 0 µg/ml ASI group and **P<0.01 vs. 0 µg/ml
ASI group. Data are presented as the mean ± standard deviation
(n=3). Each bar represents the mean of three independent
experiments, and experiments were performed in triplicate. ASI,
astragaloside IV; miR, microRNA; RMC, rat mesangial cells; LG, low
glucose; HG, high glucose; OD, optical density. |
ASI inhibits HG-induced excessive
proliferation in RMCs
The results of the cell proliferation assay revealed
that the OD values of the HG group were significantly higher
compared with those in the LG group at 72 h (Fig. 1C), which indicated that HG increased
the excessive proliferation of RMCs. Treatment with ASI decreased
the OD values in a dose-dependent manner compared with untreated
cells (Fig. 1D). These results
indicated that ASI inhibited the HG-induced excessive proliferation
of RMCs in a dose-dependent manner.
ASI alleviates HG-induced fibrosis in
RMCs
Using RT-qPCR and western blot analysis, TGF-β1,
Smad3, col1 and α-SMA mRNA and protein expression was revealed to
be significantly increased and Smad7 mRNA and protein expression
was decreased in the HG group compared with the LG group. After ASI
treatment, the mRNA and protein levels of TGF-β1, Smad3, col1, and
α-SMA were significantly decreased and those of Smad7 were
increased in the HG + ASI group compared with the HG group
(Fig. 2). These results indicated
that ASI alleviated HG-induced fibrosis in RMCs.
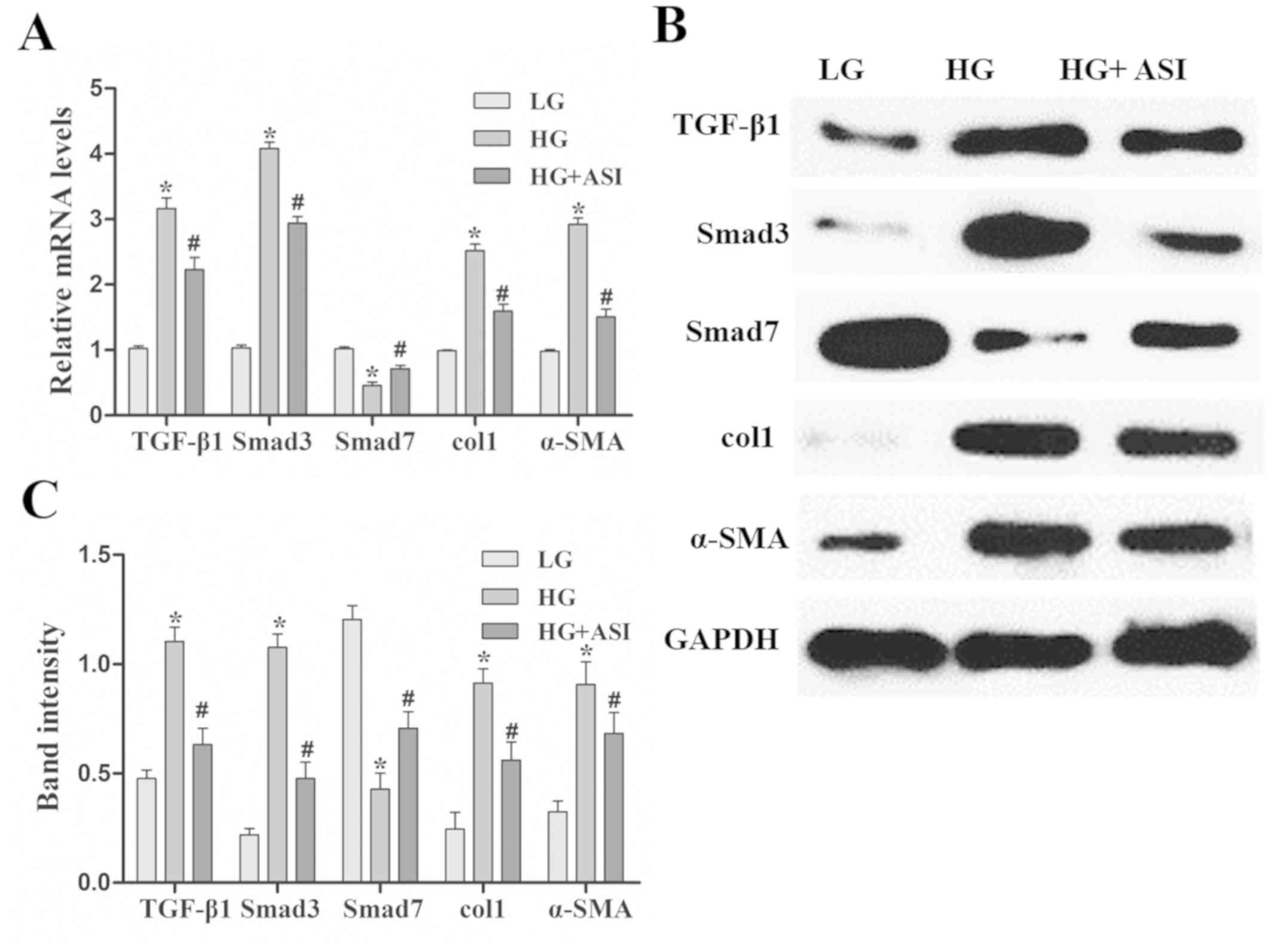 | Figure 2.Effects of ASI on the TGF-β1/Smad
signaling pathway in RMCs. (A) Reverse transcription-quantitative
PCR analysis of TGF-β1, Smad3, col1, α-SMA and Smad7 mRNA in LG, HG
and HG + ASI groups. (B) Western blot and (C) subsequent
quantification and analysis of TGF-β1, Smad3, col1, α-SMA and Smad7
mRNA in LG, HG and HG + ASI groups. Data are presented as the mean
± standard deviation (n=3). Each bar represents the mean of three
independent experiments, and experiments were performed in
triplicate. *P<0.05 vs. LG group; #P<0.05 vs. HG
group. ASI, astragaloside IV; TGF-β1, transforming growth
factor-β1; RMC, rat mesangial cells; col1, collagen type 1; α-SMA,
α-smooth muscle actin; HG, high glucose. |
ASI exhibits therapeutic effects on DN
injury
The model group exhibited a significantly lower
weight and elevated blood glucose levels compared with the sham
group, and ASI treatment was demonstrated to alleviate weight loss
and reduce blood glucose levels (Table
II). Furthermore, relative kidney weight was significantly
increased in the model group compared with the sham group, whereas
ASI treatment led to a significant reduction in kidney weight/body
weight ratio compared with the model group (Table II). The results of biochemical
analysis demonstrated that urine protein, creatinine and blood urea
nitrogen levels in the model group were significantly higher
compared with the sham group, and were reduced after ASI treatment
(Table II).
 | Table II.Blood glucose, body weight, KW/BW,
urine protein, creatinine and blood urea nitrogen after ASI
treatment for 8 weeks (n=6). |
Table II.
Blood glucose, body weight, KW/BW,
urine protein, creatinine and blood urea nitrogen after ASI
treatment for 8 weeks (n=6).
| Group | Sham | Model | Model + ASI |
|---|
| Body weight
(g) | 326.97±18.83 |
212.33±13.74a |
283.22±13.65b |
| Blood glucose
(mg/dl) | 103.1±6.06 |
315.85±12.17a |
225.86±20.33b |
| KW/BW (mg/g) | 8.13±0.47 |
13.47±1.36a |
9.93±0.98b |
| Urine protein
(mg) | 11.83±1.99 |
62.32±4.54a |
33.37±2.57b |
| Creatinine
(µmol/l) | 34.38±2.88 |
90.73±3.69a |
56.95±4.73b |
| Blood urea nitrogen
(mmol/l) | 10.07±2.34 |
28.56±1.70a |
21.37±2.92b |
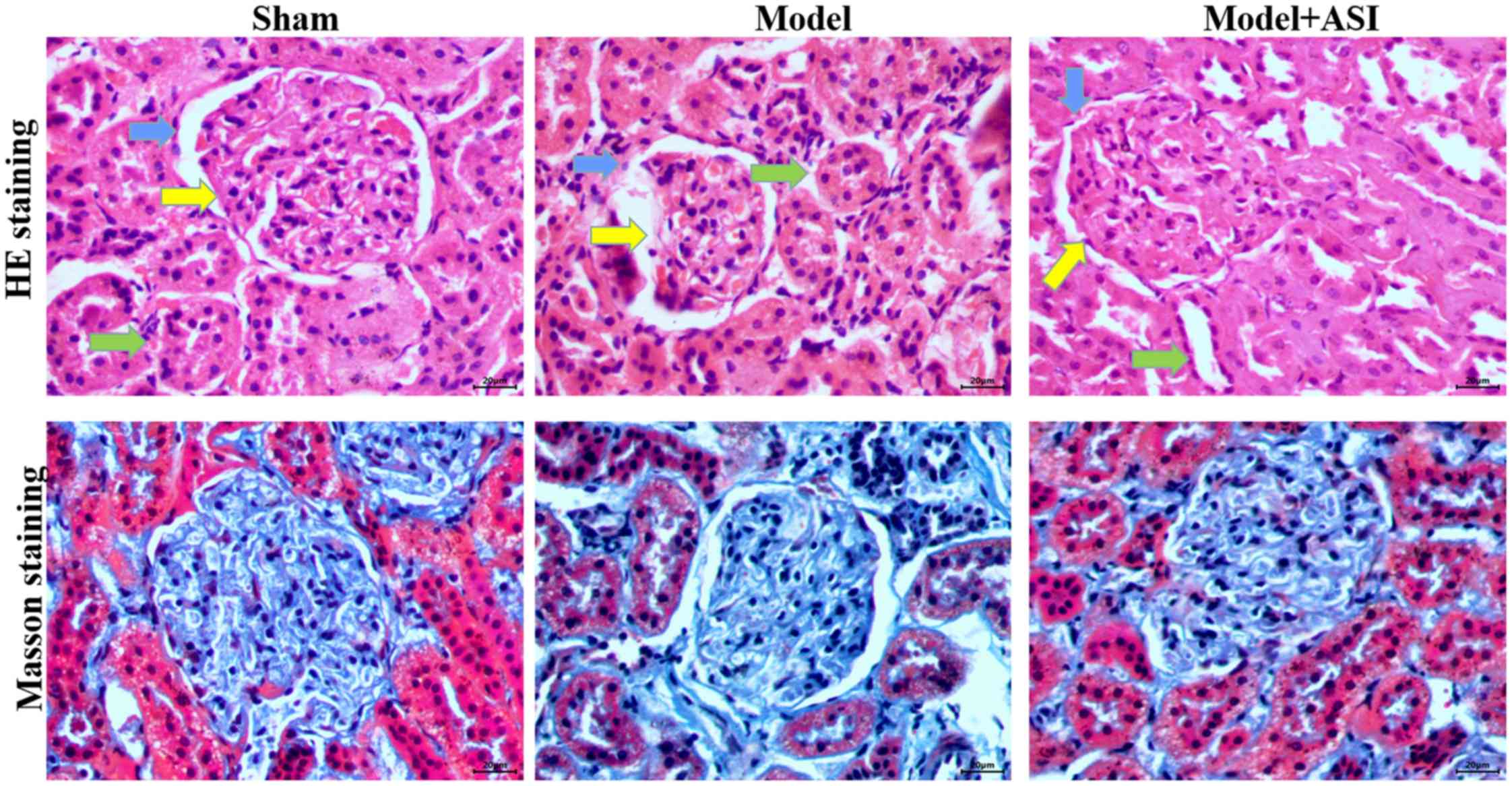
In the renal histological examination, H&E
staining revealed basement membrane thinning (blue arrows),
glomerular atrophy (yellow arrows) and proximal convoluted tubule
damage (green arrows) in the model group, and these pathological
structures were improved after ASI treatment (Fig. 3). Masson staining revealed
interstitial fibrosis and glomerular sclerosis (blue areas) in the
model group, and a reduction in interstitial fibrosis after ASI
treatment (Fig. 3). These
comparisons indicated that ASI exhibited therapeutic effects on
DN-induced cell injury.
ASI exhibits therapeutic effects on
renal fibrosis in mice
As presented in Fig.
4, the miR-192 expression levels and mRNA and protein
expression levels of TGF-β1, Smad3, col1 and α-SMA were
significantly increased, and those of Smad7 were decreased, in the
model group compared with the sham group. After ASI treatment, the
miR-192 expression levels and mRNA and protein expression levels of
TGF-β1, Smad3, col1 and α-SMA were significantly decreased and
those of Smad7 were increased in the model + ASI group compared
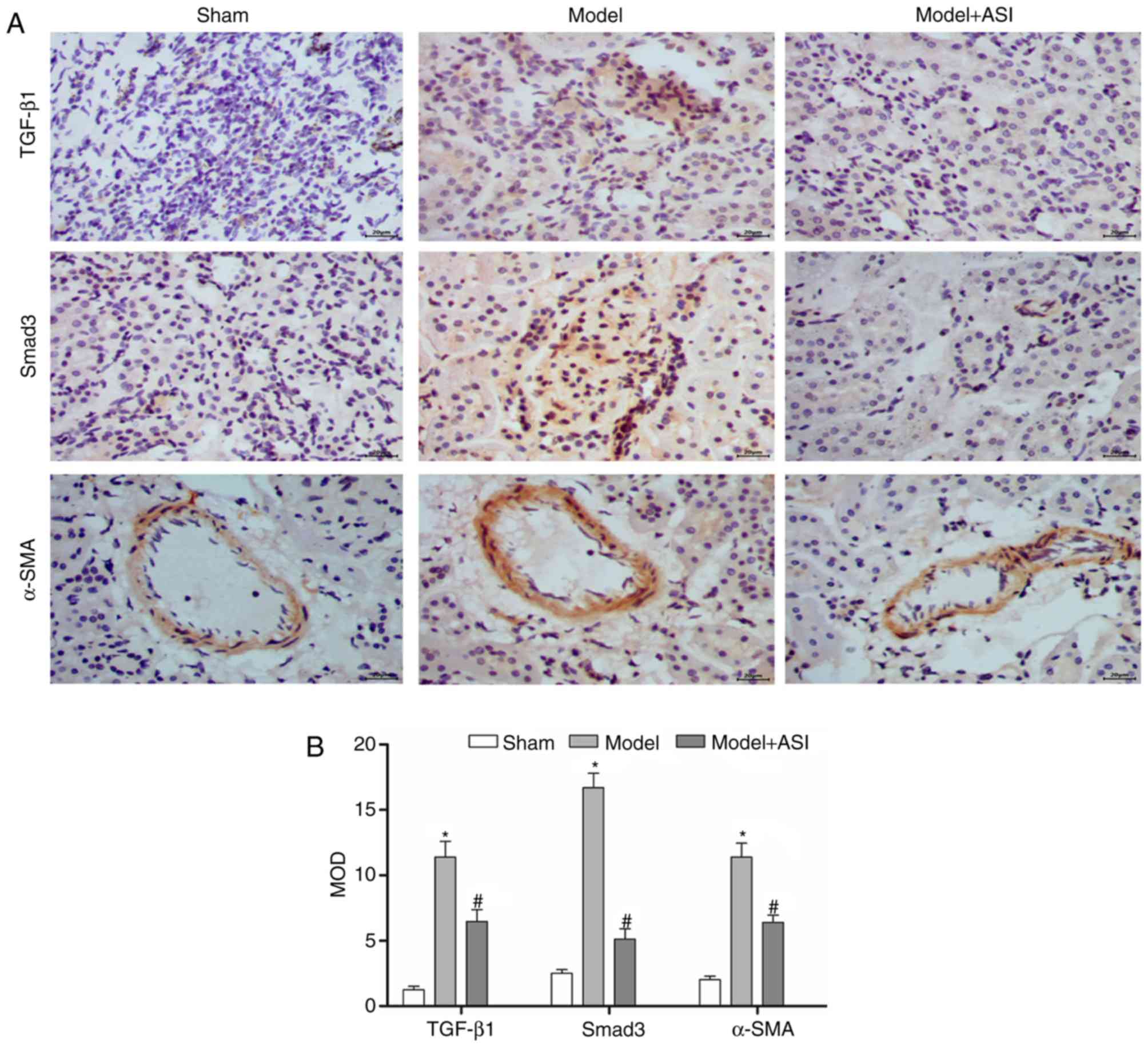
with the model group. Additionally, immunohistochemical assays of
TGF-β1, Smad3 and α-SMA supported these previous results (Fig. 5). These results indicated that ASI
alleviated renal fibrosis in DN model rats.
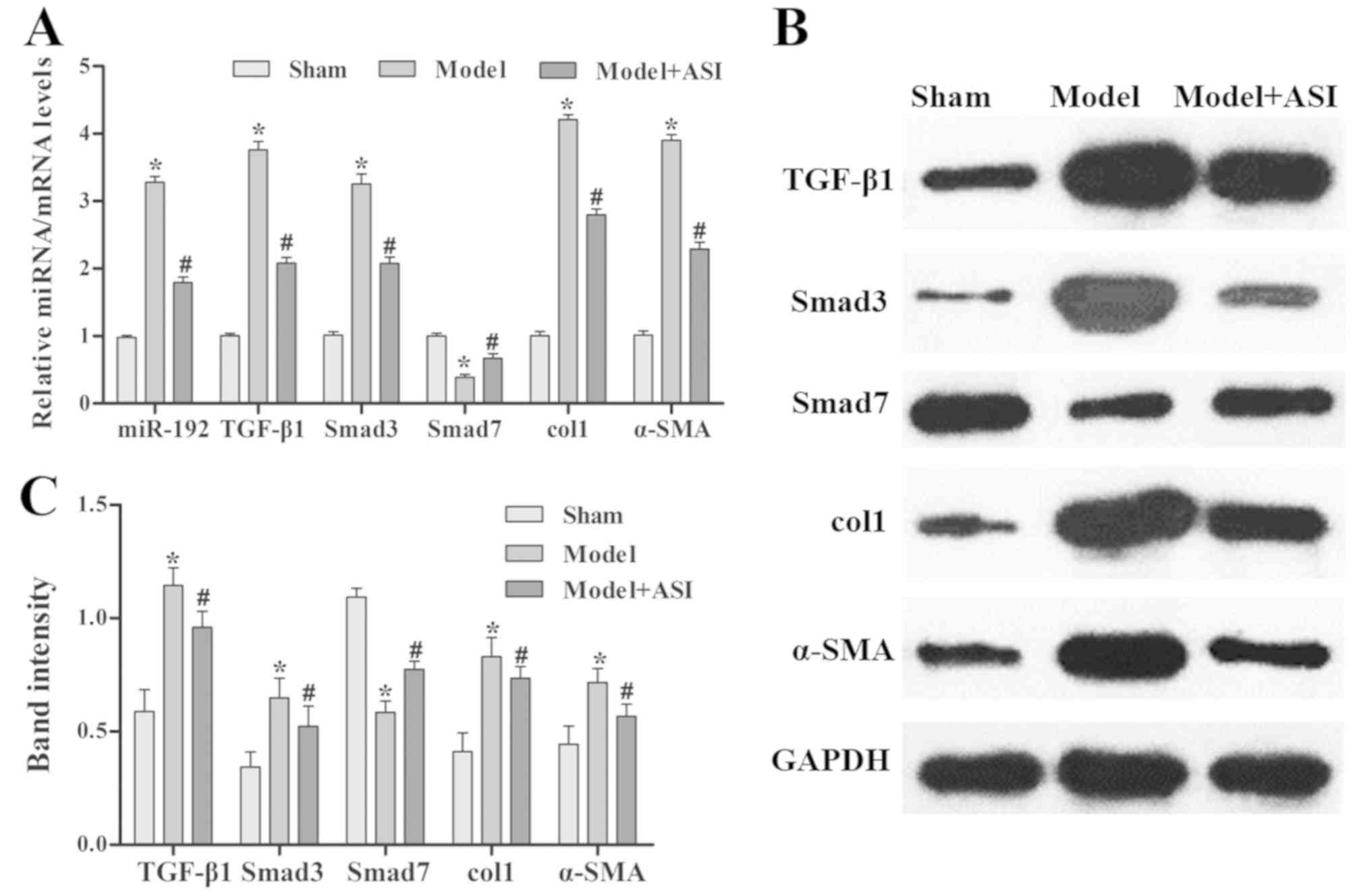 | Figure 4.Effects of ASI on the
TGF-β1/Smad/miR-192 signaling pathway in rat models of diabetic
neuropathy. (A) Reverse transcription-quantitative PCR analysis of
relative miR-192, TGF-β1, Smad3, col1, α-SMA and Smad7 expression
levels in the sham, model and model + ASI groups. (B) Western blot
and (C) subsequent quantification and analysis of TGF-β1, Smad3,
col1, α-SMA and Smad7 protein levels in the sham, model and model +
ASI groups. Data are presented as the mean ± standard deviation
(n=6). *P<0.05 vs. sham group; #P<0.05 vs. model
group. ASI, astragaloside IV; TGF-β1, transforming growth
factor-β1; miR, microRNA; col1, collagen type 1; α-SMA, α-smooth
muscle actin. |
Discussion
Radix Astragali has been used as a medicine in China
for >2,000 years, and its pharmacological effects and underlying
mechanisms are being extensively studied (20). A variety of studies have demonstrated
that astragalosides, the major active components of Radix
Astragali, exhibit diverse pharmacological effects, including
anti-inflammatory, antihypertensive, antidiabetic and myocardial
protective effects, in vitro and in vivo (21–23). The
streptozotocin-induced diabetic rat model is characterized by
hyperinsulinemia, which results in an increase in blood glucose, a
marked reduction in body weight and polyuria (7). The current study assessed the in
vitro and in vivo effects of ASI on DN. After ASI
treatment for 8 weeks, blood glucose levels, body weight and kidney
weight significant increased compared with the untreated model
group. Biochemical indexes (urine protein, creatinine and blood
urea nitrogen) of kidney function were also improved, and the
pathological changes in the kidney were reduced. In summary, the
results of the current study indicated that ASI exhibited
therapeutic effects on DN.
TGF-β1 is a major profibrotic factor that drives
kidney fibrosis and DN development (7). TGF-β1 activates Smad2 and Smad3, which
are two critical downstream mediators that are negatively regulated
by Smad7, to perform biological activities, including the
production of ECM (24). Some
studies have demonstrated that blocking the TGF-β1/Smad3 signaling
pathway is an effective therapy for DN (7,8).
Additionally, it has been established that excessive collagen
deposition is a main characteristic of renal fibrosis and TGF-β1
signaling serves an important role in the stimulation of col1
expression (25,26). During DN progression, α-SMA, which is
expressed by myofibroblasts, is located in the renal interstitium
and is associated with mesangial proliferation (27). The present study indicated that ASI
inhibited the excessive proliferation of HG-induced RMCs, decreased
TGF-β1, Smad3, col1 and α-SMA mRNA and protein expression and
increased Smad7 mRNA and protein expression in vitro and
in vivo. Therefore, it can be concluded that ASI may be used
an effective therapy for DN, and its underlying mechanism may
involve the inhibition of excessive proliferation and renal
fibrosis via the TGF-β1/Smad signaling pathway.
Previous studies have revealed that miR-192 is
associated with the pathogenesis of DN (28,29) and
that miR-192 is enriched in mesangial cells, renal tubular
epithelial cells and kidney tissues in experimental diabetes
(30). Furthermore, Chung et
al (31) reported that miR-192
is an important mediator of TGF-β1 signaling in renal fibrosis
in vitro, and is tightly regulated by TGF-β1, via Smad3,
during renal fibrosis. Kato et al (32) demonstrated that miR-192 regulated the
expression of Smad-interacting protein 1 (an important factor in
the TGF-β1/Smad signaling pathway). The results of the current
study revealed that miR-192 expression was upregulated in
HG-induced RMCs and streptozotocin-induced DN model rats and that
ASI downregulated miR-192 expression in a dose-dependent manner.
This was accompanied by a decrease in DN injury and expression of
fibrosis-related protein, including TGF-β1, Smad3, col1 and α-SMA.
The results of the present study indicated that ASI regulated renal
fibrosis, resulting from DN, via the miR-192 and TGF-β1/Smad
signaling pathways.
In conclusion, the present study demonstrated that
ASI exhibits therapeutic effects on DN, which are achieved by
inhibiting excessive mesangial proliferation and renal fibrosis via
the TGF-β1/Smad/miR-192 signaling pathway. In the future, studies
should focus on determining the regulatory relationships between
miR-192 and the TGF-β1/Smad signaling pathway, and attempt to
identify other relevant molecular mechanisms in DN, such as those
involved in signaling pathways that mediate anti-inflammatory
effects.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Health
Technology Innovation Project of Jilin Province (grant no.
2017J076).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and QM conceived and designed the experiments.
CC, XC and QM performed the experiments. HL and SZ performed the
statistical analysis. CC and XC wrote the manuscript. All authors
gave final approval of the version to be published.
Ethics approval
The experimental studies were approved by The
Institutional Animal Care and Use Committee of Guangzhou Youdi
Bio-technology Co., Ltd. (approval no. Y20170623).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coughlan MT, Cooper ME and Forbes JM:
Renal microvascular injury in diabetes: RAGE and redox signaling.
Antioxid Redox Signl. 9:331–342. 2007. View Article : Google Scholar
|
|
3
|
Battisti WP, Palmisano J and Keane WE:
Dyslipidemia in patients with type 2 diabetes. Relationships
between lipids, kidney disease and cardiovascular disease. Clin
Chem Lab Med. 41:1174–1181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bergrem H and Leivestad T: Diabetic
nephropathy and end-stage renal failure: The Norwegian story. Adv
Ren Replace Ther. 8:4–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kolset SO, Reinholt FP and Jenssen T:
Diabetic nephropathy and extracellular matrix. J Histochem
Cytochem. 60:976–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu R, Mao J, Yang Y, Zhang Y, Tian Y and
Zhu J: Protective effects of calcitriol on diabetic nephropathy are
mediated by down regulation of TGF-β1 and CIP4 in diabetic
nephropathy rat. Int J Clin Exp Pathol. 8:3503–3512.
2015.PubMed/NCBI
|
|
7
|
Al-Rasheed NM, Al-Rasheed NM, Al-Amin MA,
Hasan IH, Al-Ajmi HN, Mohammad RN and Attia HA: Fenofibrate
attenuates diabetic nephropathy in experimental diabetic rat's
model via suppression of augmented TGF-β1/Smad3 Signaling pathway.
Arch Physiol Biochem. 122:186–194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato M, Muragaki Y, Saika S, Roberts AB
and Ooshima A: Targeted disruption of TGF-beta1/Smad3 signaling
protects against renal tubulointerstitial fibrosis induced by
unilateral ureteral obstruction. J Clin Invest. 112:1486–1494.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
State Pharmacopoeia Commission.
Pharmacopoeia of the People's Republic of China. 2005
editionBeijing: Chemical Industry Press; 2005. pp. 212. 2005
|
|
10
|
Wu HW, Fang J, Tang LY, Lu P, Xu H, Zhao
Y, Li D, Zhang Y, Fu M and Yang H: Quality evaluation of Astragali
radix based on DPPH radical scavenging activity and chemical
analysis. Chin Herbal Med. 6:282–289. 2014. View Article : Google Scholar
|
|
11
|
He JX, Mou QQ, Zhang JQ, Tian Q, He C, Yin
R and Li H: Rapid identification of Astragali radix from different
origins by UPLC combined with chemometrics methods. Chin
Traditional Herbal Drugs. 48:179–184. 2017.
|
|
12
|
Yan MM, Wei L, Fu YJ, Zu YG, Chen CY and
Luo M: Optimisation of the microwave-assisted extraction process
for four main astragalosides in radix Astragali. Food Chem.
119:1663–1670. 2010. View Article : Google Scholar
|
|
13
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of Astragaloside IV: A literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang WD, Chen H, Zhang C, Liu RH, Li HL
and Chen HZ: Astragaloside IV from Astragalus membranaceus
shows cardioprotection during myocardial ischemia in vivo and in
vitro. Planta Med. 72:4–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Wei W, Sun WY and Li X: Protective
effects of astragaloside IV on porcine-serum-induced hepatic
fibrosis in rats and in vitro effects on hepatic stellate cells. J
Ethnopharmacol. 122:502–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan X, Gong Z, Wang B, Guo X, Yang L, Li
D and Zhang Y: Astragaloside inhibits hepatic fibrosis by
modulation of TGF-β1/Smad signaling pathway. Evid Based Complement
Alternat Med. 2018:32316472018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye J, Coulouris G, Zaretskaya I,
Cutcutache I, Rozen S and Madden T: Primer-BLAST: A tool to design
target-specific primers for polymerase chain reaction. BMC
Bioinformatics. 13:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shivananjappa MM and Muralidhara:
Abrogation of maternal and fetal oxidative stress in the
streptozotocin-induced diabetic rat by dietary supplements of
Tinospora cordifolia. Nutrition. 28:581–587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun XY and Sun FY: Shen Nong Ben Cao
JingTaiyuan: Shanxi Science and Technology Press; pp. 112–113.
2010
|
|
21
|
He Y, Du M, Gao Y, Liu H, Wang H, Wu X and
Wang Z: Astragaloside IV Attenuates experimental autoimmune
encephalomyelitis of mice by counteracting oxidative stress at
multiple levels. PLoS One. 8:e764952013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang ZS, Xiong F, Xie XH, Chen D, Pan JH
and Cheng L: Astragaloside IV attenuates proteinuria in
streptozotocin-induced diabetic nephropathy via the inhibition of
endoplasmic reticulum stress. BMC Nephrol. 16:442015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang N, Wang XH, Mao SL and Zhao F:
Astragaloside IV improves metabolic syndrome and endothelium
dysfunction in Fructose-fed rats. Molecules. 16:3896–3907. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang D, Zhang G, Chen X, Wei T, Liu C,
Chen C, Gong Y and Wei Q: Sitagliptin ameliorates diabetic
nephropathy by blocking TGF-β1/Smad signaling pathway. Int J Mol
Med. 41:2784–2792. 2018.PubMed/NCBI
|
|
25
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Izumi N, Mizuguchi S, Inagaki Y, Saika S,
Kawada N, Nakajima Y, Inoue K, Suehiro S, Friedman SL and Ikeda K:
BMP-7 opposes TGF-beta 1-mediated collagen induction in mouse
pulmonary myofibroblasts through Id2. Am J Physiol Lung Cell Mol
Physiol. 290:L120–L126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Qu X and Bertram JF:
Endothelial-myofibroblast transition contributes to the early
development of diabetic renal interstitial fibrosis in
streptozotocin-induced diabetic mice. Am J Pathol. 175:1380–1388.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma X, Lu C, Lv C, Wu C and Wang Q: The
expression of miR-192 and its significance in diabetic nephropathy
patients with different urine albumin creatinine ratio. J Diabetes
Res. 2016:67894022016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu F, Zhang ZP, Xin GD, Guo LH, Jiang Q
and Wang ZX: miR-192 prevents renal tubulointerstitial fibrosis in
diabetic nephropathy by targeting Egr1. Eur Rev Med Pharmacol Sci.
22:4252–4260. 2018.PubMed/NCBI
|
|
30
|
Wang B, Herman-Edelstein M, Koh P, Burns
W, Jandeleit-Dahm K, Watson A, Saleem M, Goodall GJ, Twigg SM,
Cooper ME and Kantharidis P: E-cadherin expression is regulated by
miR-192/215 by a mechanism that is independent of the profibrotic
effects of transforming growth factor-beta. Diabetes. 59:1794–1802.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chung AC, Huang XR, Meng X and Lan HY:
miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc
Nephrol. 21:1317–1325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kato M, Zhang J, Wang M, Lanting L, Yuan
H, Rossi JJ and Natarajan R: MicroRNA-192 in diabetic kidney
glomeruli and its function in TGF-beta-induced collagen expression
via inhibition of E-box repressors. Proc Natl Acad Sci USA.
104:3432–3437. 2007. View Article : Google Scholar : PubMed/NCBI
|