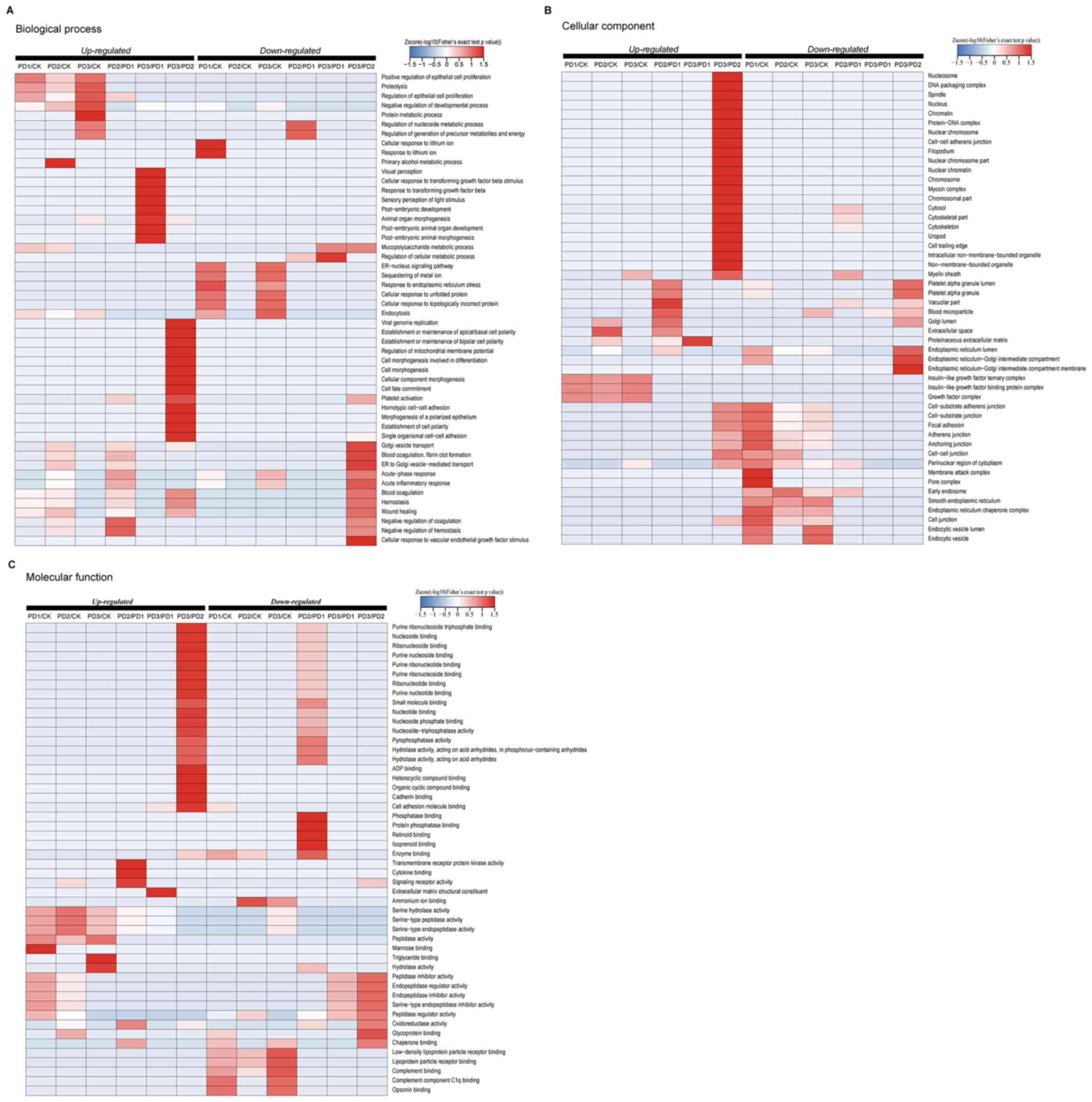
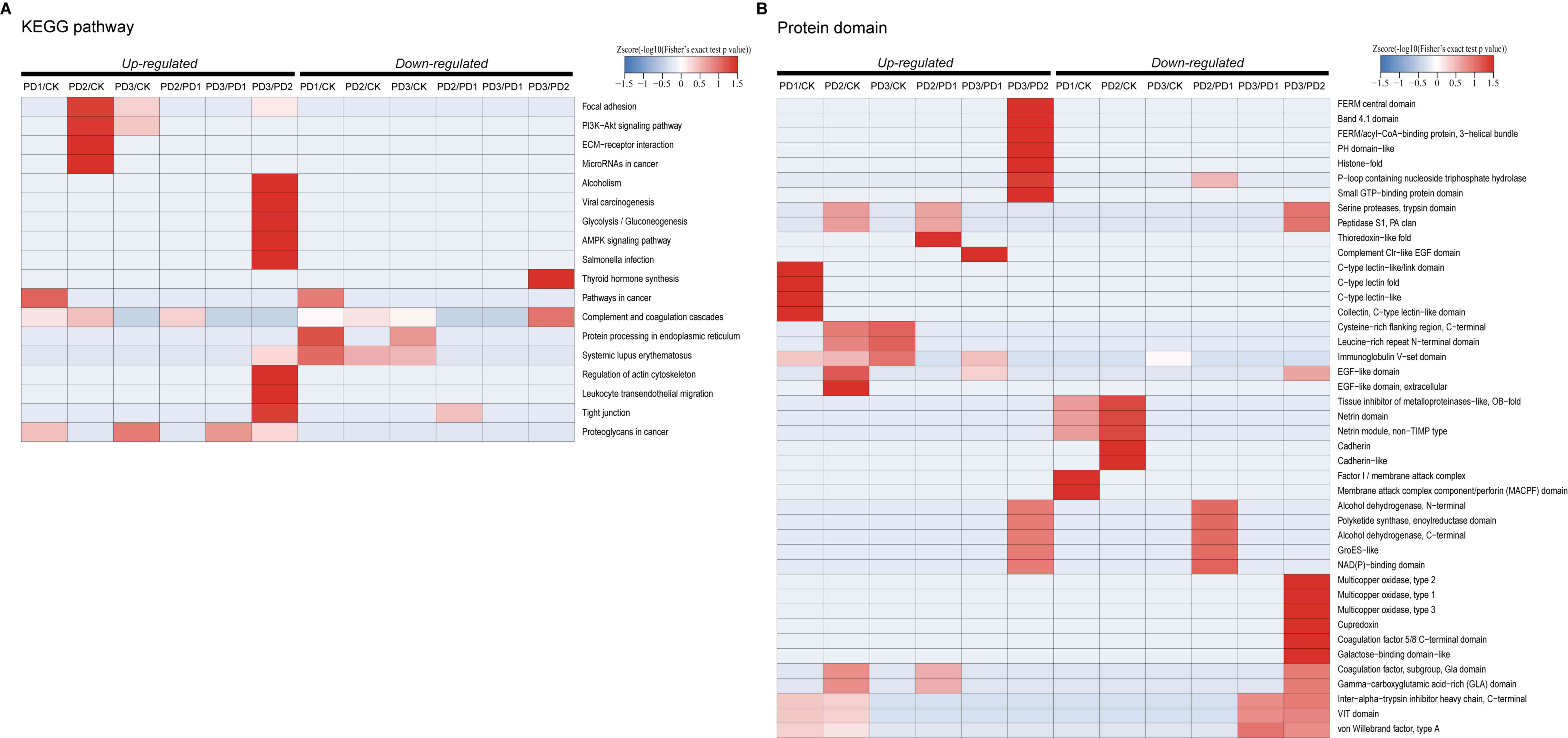
|
1
|
Ma XW and Guo RY: Dose-dependent effect of
Curcuma longa for the treatment of Parkinson's disease. Exp
Ther Med. 13:1799–1805. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuroda K, Tatara K, Takatorige T and
Shinsho F: Effect of physical exercise on mortality in patients
with Parkinson's disease. Acta Neurol Scand. 86:55–59. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan JY, Chang BL and Wu YR: Relationships
among depression, anxiety, sleep, and quality of life in patients
with Parkinson's disease in Taiwan. Parkinsons Dis.
2016:40401852016.PubMed/NCBI
|
|
4
|
Warner TT and Schapira AH: Genetic and
environmental factors in the cause of Parkinson's disease. Ann
Neurol. 53 (Suppl 3):S16–S25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cummings JL and Mendez MF: Alzheimer's
disease: Cognitive and behavioral pharmacotherapy. Conn Med.
61:543–552. 1997.PubMed/NCBI
|
|
6
|
Spitzer AL and Hakim EW: Parkinson's
disease: The interplay of medical and surgical treatment options
with physical therapy. Acute Care Perspectives. 17:82008.
|
|
7
|
Olanow CW: The pathogenesis of cell death
in Parkinson's disease-2007. Mov Disord. 22 (Suppl 17):S335–S342.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blandini F and Armentero MT: Animal models
of Parkinson's disease. FEBS J. 279:1156–1166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pienaar IS, Daniels WM and Götz J:
Neuroproteomics as a promising tool in Parkinson's disease
research. J Neural Transm (Vienna). 115:1413–1430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SI, Voshol H, van Oostrum J, Hastings
TG, Cascio M and Glucksman MJ: Neuroproteomics: Expression
profiling of the brain's proteomes in health and disease. Neurochem
Res. 29:1317–1331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monti C, Colugnat I, Lopiano L, Chiò A and
Alberio T: Network analysis identifies disease-specific pathways
for parkinson's disease. Mol Neurobiol. 55:370–381. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kiesmann M, Chanson JB, Godet J, Vogel T,
Schweiger L, Chayer S and Kaltenbach G: The movement disorders
society criteria for the diagnosis of Parkinson's disease dementia:
Their usefulness and limitations in elderly patients. J Neurol.
260:2569–2579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tyanova S, Temu T and Cox J: The MaxQuant
computational platform for mass spectrometry-based shotgun
proteomics. Nat Protoc. 11:2301–2319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okuda S, Yamada T, Hamajima M, Itoh M,
Katayama T, Bork P, Goto S and Kanehisa M: KEGG Atlas mapping for
global analysis of metabolic pathways. Nucl Acids Res 36 (Web
Server Issue). W423–W426. 2008. View Article : Google Scholar
|
|
15
|
Horton P, Park KJ, Obayashi T and Nakai K:
Protein subcellular localization prediction with WoLF PSORT. Asian
Pacific Bioinformatics Conference. 39–48. 2006.
|
|
16
|
Meincke U and Kosinski CM: Treatment of
mental disorders in patients with Parkinson's disease. Fortschr
Neurol Psychiatr. 78:279–287. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peters M, Fitzpatrick R, Doll H, Playford
D and Jenkinson C: Does self-reported well-being of patients with
Parkinson's disease influence caregiver strain and quality of life?
Parkinsonism Relat Disord. 17:348–352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schapira AH and Jenner P: Etiology and
pathogenesis of Parkinson's disease. Mov Disord. 26:1049–1055.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu H, Wang Y, Song N, Wang J, Jiang H and
Xie J: New progress on the role of glia in iron metabolism and
iron-induced degeneration of dopamine neurons in Parkinson's
disease. Front Mol Neurosci. 10:4552018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Isacson O and Chung CY: Rab3b for the
treatment and prevention of Parkinson's disease. US. 2011.
|
|
21
|
Liao W, Zhao R, Lu L, Zhang R, Zou J, Xu
T, Wu C, Tang J, Deng Y and Lu X: Overexpression of a novel
osteopetrosis-related gene CCDC154 suppresses cell proliferation by
inducing G2/M arrest. Cell Cycle. 11:3270–3279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Volles MJ and Lansbury PT Jr: Vesicle
permeabilization by protofibrillar alpha-synuclein is sensitive to
Parkinson's disease-linked mutations and occurs by a pore-like
mechanism. Biochemistry. 41:4595–4602. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Islam MS, Nolte H, Jacob W, Ziegler AB,
Pütz S, Grosjean Y, Szczepanowska K, Trifunovic A, Braun T, Heumann
H, et al: Human R1441C LRRK2 regulates the synaptic vesicle
proteome and phosphoproteome in a Drosophila model of
Parkinson's disease. Hum Mol Genet. 25:5365–5382. 2016.PubMed/NCBI
|
|
24
|
Volz TJ, Farnsworth SJ, King JL, Riddle
EL, Hanson GR and Fleckenstein AE: Methylphenidate administration
alters vesicular monoamine transporter-2 function in cytoplasmic
and membrane-associated vesicles. J Pharmacol Exp Ther.
323:738–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanji K, Kamitani T, Mori F, Kakita A,
Takahashi H and Wakabayashi K: TRIM9, a novel brain-specific E3
ubiquitin ligase, is repressed in the brain of Parkinson's disease
and dementia with Lewy bodies. Neurobiol Dis. 38:210–218. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y,
Gaston B, Palmer LA, Rockenstein EM, Zhang Z, Masliah E, et al:
Nitrosative stress linked to sporadic Parkinson's disease:
S-nitrosylation of parkin regulates its E3 ubiquitin ligase
activity. Proc Natl Acad Sci USA. 101:10810–10814. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoernke M, Koksch B and Brezesinski G:
Influence of the hydrophobic interface and transition metal ions on
the conformation of amyloidogenic model peptides. Biophys Chem.
150:64–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Medical Advisory Secretariat: Deep brain
stimulation for Parkinson's disease and other movement disorders:
An evidence-based analysis. Ont Health Technol Assess Ser. 5:1–56.
2005.
|
|
29
|
Escudero M, de la Cruz RR and
Delgado-García JM: A physiological study of vestibular and
prepositus hypoglossi neurones projecting to the abducens nucleus
in the alert cat. J Physiol. 458:539–560. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong X, Chen K, Cuevas-Diaz DR, You Y,
Sloan SA, Zhang Y, Zong S, Cao Q, Barres BA and Wu JQ:
Comprehensive identification of long non-coding RNAs in purified
cell types from the brain reveals functional LncRNA in OPC fate
determination. PLoS Genetics. 11:e10056692015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cariboni A, Davidson K, Rakic S, Maggi R,
Parnavelas JG and Ruhrberg C: Defective gonadotropin-releasing
hormone neuron migration in mice lacking SEMA3A signalling through
NRP1 and NRP2: Implications for the aetiology of hypogonadotropic
hypogonadism. Hum Mol Genet. 20:336–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Milton RH, Abeti R, Averaimo S, DeBiasi S,
Vitellaro L, Jiang L, Curmi PM, Breit SN, Duchen MR and Mazzanti M:
CLIC1 function is required for beta-amyloid-induced generation of
reactive oxygen species by microglia. J Neurosci. 28:11488–11499.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hall S, Öhrfelt A, Constantinescu R,
Andreasson U, Surova Y, Bostrom F, Nilsson C, Håkan W, Decraemer H,
Någga K, et al: Accuracy of a panel of 5 cerebrospinal fluid
biomarkers in the differential diagnosis of patients with dementia
and/or parkinsonian disorders. Arch Neurol. 69:1445–1452. 2012.
View Article : Google Scholar : PubMed/NCBI
|