Introduction
Cardiovascular disease (CVD) represents a major
health burden in China and throughout the world (1). Acute coronary syndrome (ACS) is the
acute manifestation of ischemic heart disease, including ST-segment
elevation myocardial infarction (STEMI), non-STEMI and unstable
angina pectoris. ACS exhibits severe manifestations in Chinese
populations and is associated with an estimated in-hospital
mortality of 5% (2). Although
percutaneous coronary intervention (PCI) is now widely available,
certain ACS patients have a significant risk for the development of
adverse cardiac events, even after PCI. Therefore, particular focus
has been directed on the identification of novel biomarkers that
may be used to predict the risk of future cardiovascular events,
and aid the implementation of appropriate medical strategies for
patients with ACS that may eventually improve disease prognosis.
ACS is characterized by unstable atherosclerotic lesions.
Inflammation has an important role in the progression of coronary
atherosclerosis, plaque rupture and the incidence of thrombosis
(3). CVD are accompanied by an
increase in several inflammatory factors, including C-reactive
protein, fibrinogen, white blood cell count, ferritin and
ceruloplasmin, and by a decrease in the levels of specific
proteins, including albumin, transferrin and anti-thrombin
(4). Previous studies have confirmed
that single inflammatory markers, including C-reactive protein
(5), neutrophils (6), platelets (7), fibrinogen (8) and albumin (9), are associated with the prognosis of
ACS, whereas the ratio of several single markers, e.g. the
neutrophil-to-lymphocyte ratio (10), may also be associated with disease
prognosis. The prognostic value of the fibrinogen-to-albumin ratio
(FAR) has been studied in certain types of cancer, including
esophageal and breast cancer (11,12).
However, to date, the association of the parameter FAR with the
clinical outcome of patients with non-ST elevation (NSTE)-ACS has
not been investigated (13). The
purpose of the present study was to explore the association between
1-year disease outcomes and FAR levels of patients admitted with
NSTE-ACS undergoing PCI.
Materials and methods
Study design and setting
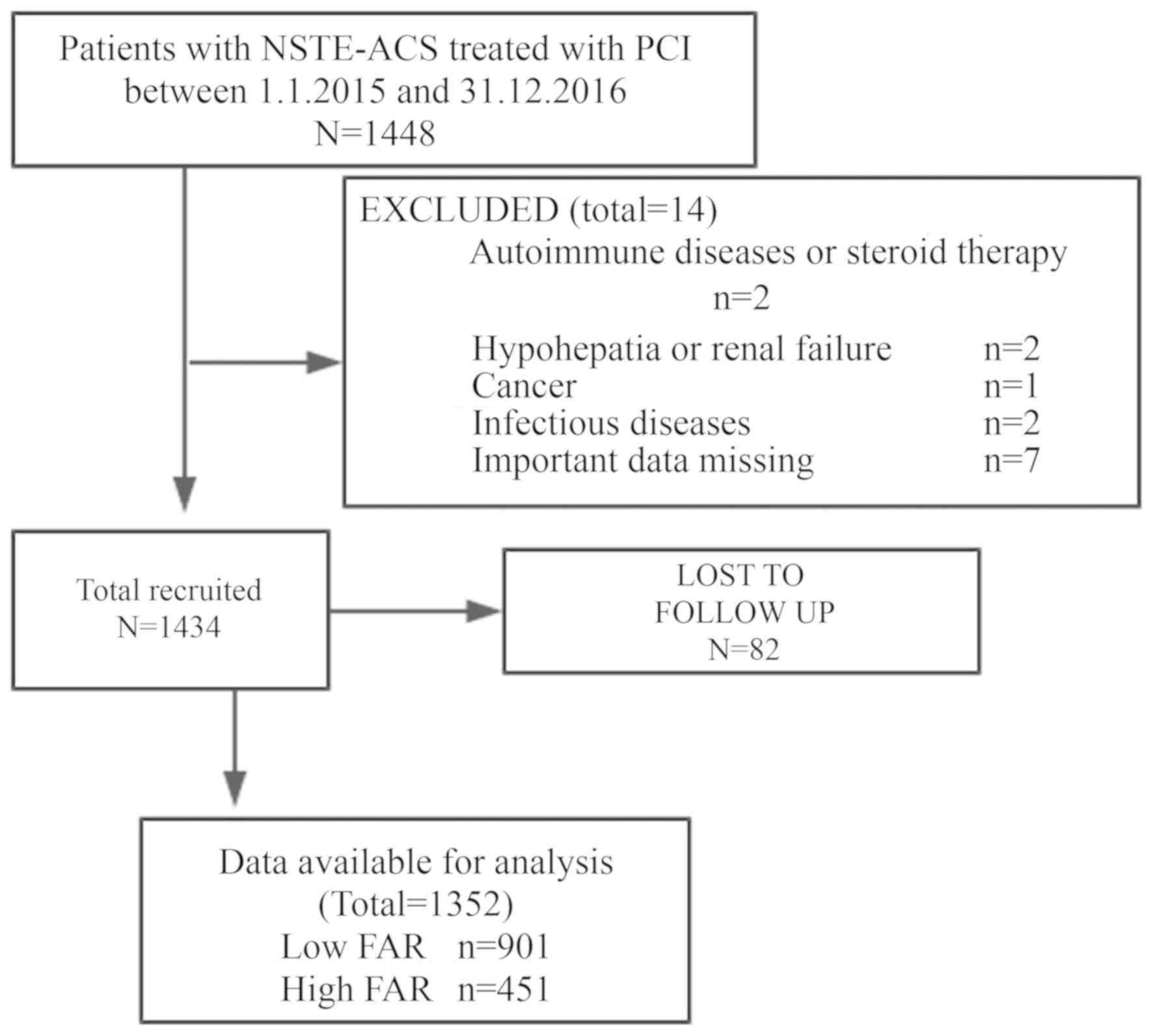
The design of the present study is illustrated in
Fig. 1. The study was based on a
prospective observational cohort. A total of 1,352 consecutive
NSTE-ACS patients (age range, 30–88 years; mean age, 62.5±10.6
years; male, 431, female, 921) were included in the cohort, who
were recruited from the 1st of January 2015 to the 31st of December
2016. The patients were hospitalized and underwent PCI at the
Shengjing Hospital of China Medical University (Shenyang, China),
located in Northeastern China. Among these patients, 632
consecutive cases were diagnosed with non-STEMI and 720 were
diagnosed with unstable angina pectoris. The clinical and
procedural data were gathered from electronic medical records,
image archives and communication systems of the interventional
imaging data, and the surgical history of the PCI cases. The venous
blood samples were obtained within 24 h of admission at room
temperature, and the samples were used for analysis within 2 h of
blood collection. The levels of albumin and fibrinogen were
measured in the clinical laboratory of the Shengjing Hospital
(Shenyang, China). Procedural data were extracted from the surgical
history of patients who underwent PCI. The angiographic parameters
were estimated visually and/or with the Picture Archiving and
Communication Systems of the interventional imaging data (PHILIPS
UNIQ FD10; http://www.philips.com.cn/, Neusoft
PACS/RIS; http://www.neusoft.com/cn?from=singlemessage).
Patients were followed up at 1, 3, 6 and 12 months after discharge.
The clinical follow-up data were obtained from an outpatient clinic
visit or telephone interviews with the patient's general
practitioner/cardiologist, the patients themselves or their family.
All enrolled patients were followed up for one year after
discharge. All events were adjudicated and classified by two
cardiologists. The present study complied with the Declaration of
Helsinki, and was granted approval by the Shengjing Hospital of the
China Medical University Research Ethics Committee (Shenyang,
China). Written informed consent was formally obtained from all
participants.
Participants and procedures
In the present study, a total of 1,448 patients with
non-STEMI and unstable angina were recruited. Patients taking
corticosteroids or cytotoxic drugs, patients receiving thrombolytic
therapy or heparin and patients with autoimmune diseases,
hypohepatia or renal failure, cancer, hematological disorders,
inflammatory or infectious diseases, pregnancy, surgery, trauma or
hemorrhagic events, who were admitted within the previous 6 months
of the initiation of the study, were excluded. The patients who
were not treated with PCI and were not followed up for blood sample
analysis were also excluded. As the use of heparin or other drugs
on admission in STEMI patients may have an impact on the results of
the study, the STEMI population was not included in the study, and
at the same time, the patients treated with coronary artery bypass
grafting (CABG) or those with prior CABG and patients unsuitable
for revascularization were not included in the study. The final
cohort consisted of 1,352 patients. FAR was defined as the
concentration ratio of fibrinogen (mg/dl) to albumin (mg/dl)
multiplied by 100. FAR levels were stratified by tertiles [first
tertile, <7.106 (n=450); second tertile, 7.106–8.713 (n=451);
third tertile, >8.713 (n=451)]. A high FAR (n=451) was defined
as a value in the third tertile (8.713) and a low FAR (n=901) was
defined as a value in the lower two tertiles (≤8.713).
Definitions
Unstable angina was defined as the presence of chest
pain originating from cardiac dysfunction that was deteriorating
(i.e., more severe, prolonged or frequent than previous episodes of
angina) or occurring at rest, without serological evidence of
myocyte necrosis (i.e., no elevation of the serum concentration of
troponin or of the levels of the MB isoenzyme of creatine kinase).
Non-STEMI was defined as the presence of cardiac chest pain with
serological evidence of myonecrosis in the absence of ST-segment
elevation.
Clinical end-points
The following endpoints were recorded: All-cause
mortality, cardiac mortality, non-fatal myocardial reinfarction and
unscheduled repeat revascularisation, which included the incidence
of unscheduled repeat PCI and/or surgical bypass of target or
non-target vessels.
Statistical analysis
Quantitative variables were expressed as the mean ±
standard deviation or median (interquartile range) and compared
with an independent-samples t-test or the Mann-Whitney U-test.
Categorical variables were represented as counts and proportions
(%) and compared with a chi-squared test. The Cox
proportional-hazard regression model was used to analyze the
effects of variables on event-free survival. Kaplan-Meier survival
curves were further constructed to assess the prognostic value of
the FAR. Multivariate Cox analysis was used for the significant
variables identified in the univariate analysis. These variables
included age, sex, history of diabetes mellitus, history of
hypertension, heart rate on admission, left ventricular ejection
fraction (LVEF), leukocyte count, neutrophil count, platelet count,
troponin-I on admission, creatinine, high-density lipoprotein
(HDL), fibrinogen-to-albumin ratio, left main disease, three-vessel
disease, and beta-blockers. The results are reported as hazard
ratios (HRs) with the corresponding 95% confidence intervals (CIs).
The accuracy of FAR for the prediction of major adverse
cardiovascular events (MACEs) was assessed according to the area
under the receiver-operating characteristic curve (ROC-AUC) with
the MedCalc software for Windows version 11.4.2.0 (MedCalc
Software). All of the tests were two-sided and P<0.05 was
considered to indicate statistical significance. All statistical
analyses were performed with the SPSS version 19 (IBM Corp.).
Results
Baseline characteristics
The sample consisted of 1,352 NSTE-ACS patients who
underwent PCI. The major clinical, demographic and angiographic
features, description of PCI and discharge medications of patients
according to their FAR are described in Table I. The patients of the high FAR group
were older, with a lower percentage of female subjects, a higher
incidence of diabetes mellitus and hypertension and a higher
percentage of non-STEMI. Furthermore, these patients exhibited a
higher incidence of left main disease and three-vessel disease and
a higher frequency of beta-blocker administration. They further
exhibited a higher heart rate on admission, a higher FAR and higher
levels of troponin-I on admission, and elevated creatinine and
fibrinogen levels. Finally, they presented with lower levels of HDL
and albumin and a lower LVEF.
 | Table I.Baseline clinical characteristics and
periprocedural details. |
Table I.
Baseline clinical characteristics and
periprocedural details.
| Variables | Low FAR (n=901) | High FAR (n
=451) | P-value |
|---|
| Age (years) | 61.5±10.4 | 64.2±10.8 |
<0.001a |
| Female
(%) | 636 (70.6) | 285 (63.2) | 0.006a |
|
Current/recent smoker | 355 (39.4) | 164 (36.4) | 0.279a |
| Diabetes
mellitus | 224 (24.9) | 158 (35.0) |
<0.001a |
|
Hypertension | 578 (64.2) | 319 (70.7) | 0.016a |
| MI | 97 (10.8) | 53 (11.8) | 0.586a |
|
PCI | 99 (11.0) | 60 (13.3) | 0.213a |
| Prior
peripheral arterial disease | 19 (2.1) | 11 (2.4) | 0.697a |
| SBP
(mmHg) | 140.4±20.2 | 139.6±22.5 | 0.462c |
| Heart
rate (bpm) | 72 (64–80) | 74 (67–83) |
<0.001b |
| LVEF
(%) | 61 (58–65) | 61 (55–64) | 0.010b |
|
Leukocyte count
(×109/l) | 7.2 (5.9–8.6) | 7.7 (6.2–9.3) | 0.001b |
|
Neutrophil count
(×109/l) | 4.7 (3.8–6.5) | 5.2 (3.9–7.5) |
<0.001b |
|
Platelet count
(×109/l) | 201.5±58.4 | 215.6±63.9 |
<0.001c |
|
Troponin-I on admission
(µg/l) | 0.0 (0.0–0.5) | 0.1 (0.0–1.0) |
<0.001b |
|
Creatinine (µmol/l) | 72.0
(59.4–83.5) | 73.0
(60.7–91.0) | 0.005b |
| Total
cholesterol (mmol/l) | 4.5±1.2 | 4.4±1.1 | 0.167c |
| HDL
(mmol/l) | 1.0 (0.8–1.2) | 0.9 (0.8–1.2) | 0.020b |
| LDL
(mmol/l) | 2.8±1.0 | 2.8±1.0 | 0.531c |
|
Triglycerides (mmol/l) | 1.6 (1.0–2.4) | 1.5 (1.0–2.1) | 0.072b |
|
Fibrinogen (g/l) | 2.9±0.5 | 4.1±0.6 |
<0.001c |
| Albumin
(g/l) | 41.2±3.3 | 37.9±3.8 |
<0.001c |
|
FAR | 7.1 (6.2–7.8) | 10.3
(9.4–11.6) |
<0.001b |
| Use of
glycoprotein IIb/IIIa inhibitor | 196 (21.8) | 83 (18.4) | 0.151a |
| Diagnosis on
admission |
|
|
|
|
Unstable Angina | 524 (58.2) | 196 (43.5) |
<0.001a |
|
NSTEMI | 377 (41.8) | 255 (56.5) |
|
| PCI details |
|
|
|
| Left
main disease | 74 (8.2) | 53 (11.8) | 0.035a |
|
Three-vessel disease | 185 (20.5) | 122 (27.1) | 0.007a |
|
Intra-aortic balloon pump | 4 (0.4) | 5 (1.1) | 0.156a |
| TIMI
flow grade 3 post PCI | 900 (99.9) | 451 (100.0) | 0.479a |
|
Aspirin | 895 (99.3) | 448 (99.3) | 0.999a |
|
Clopidogrel | 768 (85.2) | 384 (85.1) | 0.963a |
|
Ticagrelor | 124 (13.8) | 62 (13.7) | 0.994a |
|
Statin | 885 (98.2) | 441 (97.8) | 0.577a |
|
ACEI/ARBs | 518 (57.5) | 282 (62.5) | 0.076a |
|
Beta-blockers | 478 (53.1) | 266 (59.0) | 0.039a |
Clinical Outcome
The clinical outcomes at the 1-year follow-up are
provided in Table II. MACEs
occurred in 127 patients (9.4%), comprising 67 (14.1%) in the low
FAR group and 60 (13.3%) in the high FAR group. A total of 12
events of all-cause mortality, 10 events of cardiac mortality, 10
events of non-fatal myocardial reinfarction and 35 events of
unscheduled repeat revascularisation were observed in the low FAR
group. A total of 21 events of all-cause mortality, 17 events of
cardiac mortality, 6 events of non-fatal myocardial reinfarction
and 16 events of unscheduled repeat revascularisation occurred in
the high FAR group. The data indicated that all-cause mortality and
cardiac mortality were more frequent in the high FAR group
(P<0.05).
 | Table II.Major adverse cardiovascular events
during the 1-year follow-up. |
Table II.
Major adverse cardiovascular events
during the 1-year follow-up.
| Outcome | Low FAR
(n=901) | High FAR
(n=451) | P-value |
|---|
| All-cause
mortality | 12 (1.3) | 21 (4.7) | <0.001 |
| Cardiac
mortality | 10 (1.1) | 17 (3.8) | 0.001 |
| Non-fatal
reinfarction | 10 (1.1) | 6 (1.3) | 0.724 |
| Unplanned repeat
revascularization | 35 (3.9) | 16 (3.5) | 0.759 |
Prognostic performance of different
FAR levels in prediction of MACEs
The results of the Cox analysis are provided in
Table III. Univariate Cox analysis
indicated that the FAR was an independent risk factor regarding
all-cause mortality and of cardiac mortality of the NSTE-ACS
patients. Multivariate Cox analysis was performed for the
significant variables identified by the Univariate analysis. The
data demonstrated that the FAR was an independent predictor of
all-cause mortality (HR=2.223, 95% CI: 1.002–4.931, P=0.049).
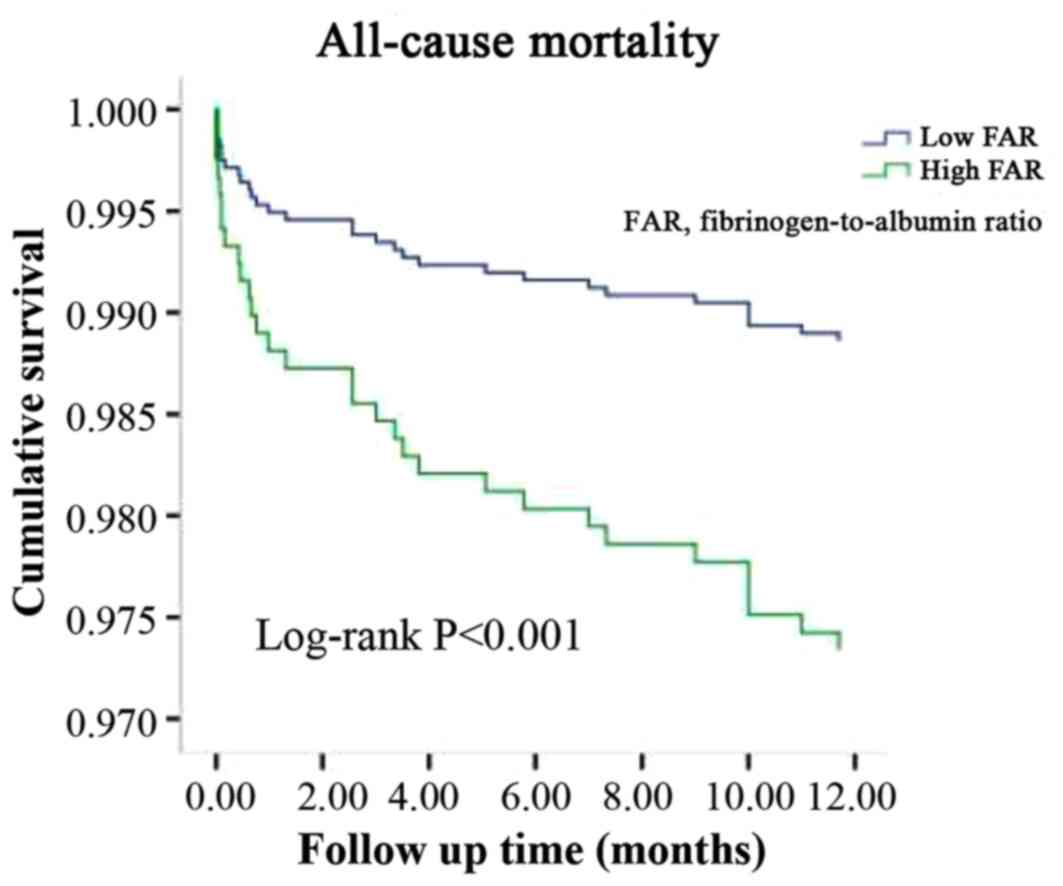
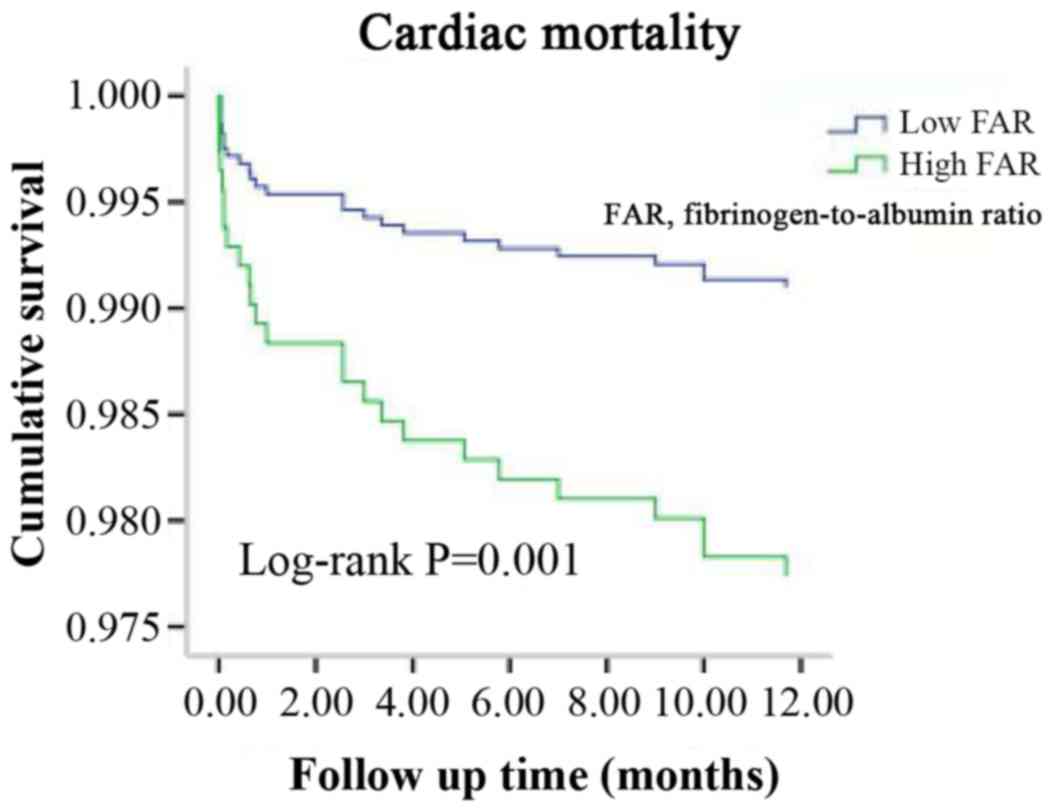
Kaplan-Meier curve analysis further revealed that patients with a
higher FAR exhibited a higher incidence of all-cause mortality
(P<0.001; Fig. 2) and cardiac
mortality (P=0.001; Fig. 3) than
those with a lower FAR (Figs. 2 and
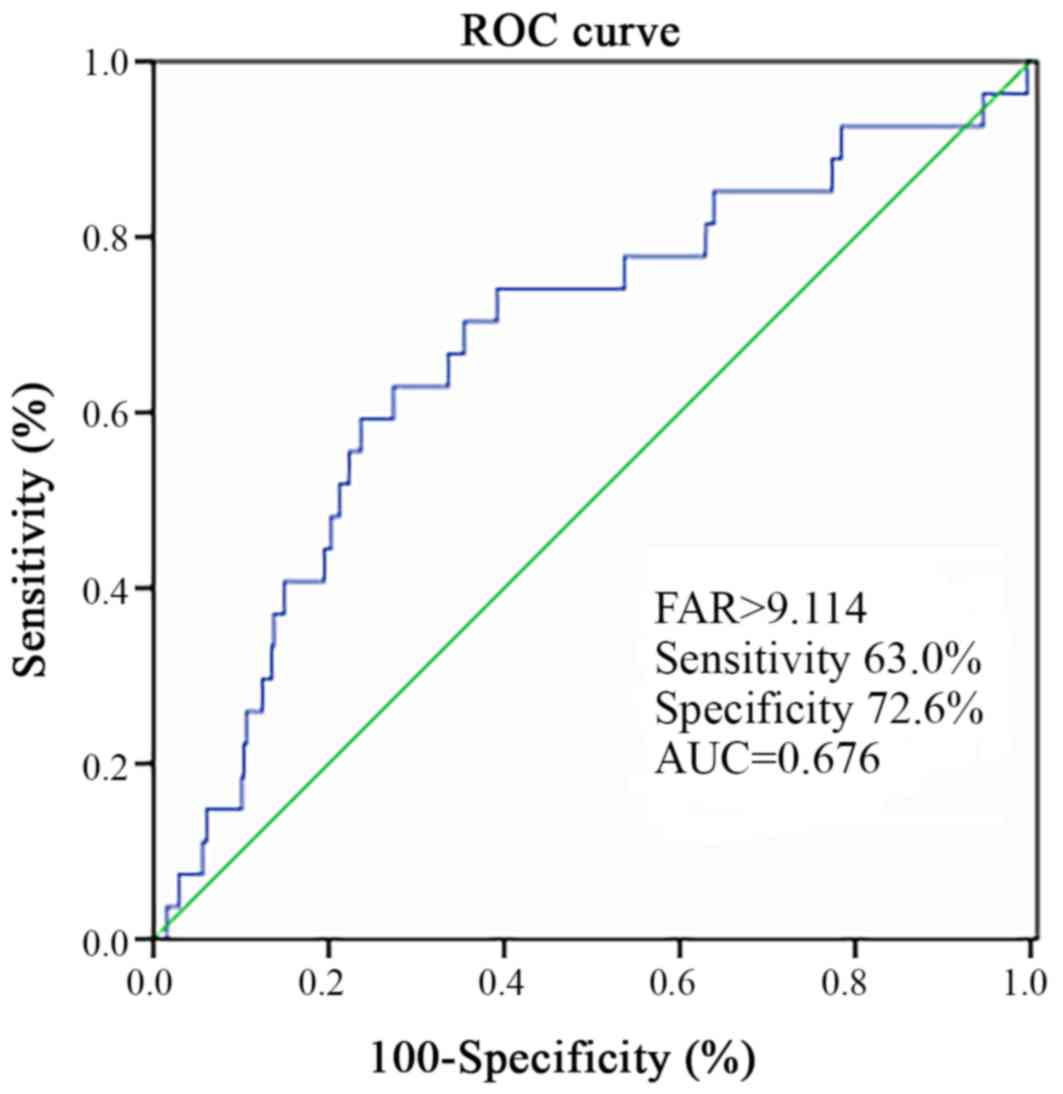
3). The cut-off points for the
sensitivity and specificity of the FAR were estimated by performing
ROC curve analysis (Fig. 4). The FAR
exhibited a sensitivity of 0.630 and a specificity of 0.726 for
predicting MACEs (Fig. 4). The
ROC-AUC was 0.676 and the cut-off value was 9.114.
 | Table III.Effects of the fibrinogen-to-albumin
ratio (third vs. first and second tertile) on clinical outcomes
according to univariate and multivariate analysis. |
Table III.
Effects of the fibrinogen-to-albumin
ratio (third vs. first and second tertile) on clinical outcomes
according to univariate and multivariate analysis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| All-cause
mortality | 3.562
(1.752,7.239) | <0.001 | 2.223
(1.002,4.931) | 0.049 |
| Cardiac
mortality | 3.450
(1.580,7.535) | 0.002 | 2.336
(0.966,5.649) | 0.060 |
| Non-fatal
reinfarction | 0.913
(0.505,1.650) | 0.763 |
|
|
| Unplanned repeat
revascularization | 1.204
(0.438,3.314) | 0.719 |
|
|
Discussion
In the present study, a prospective analysis of
1,352 consecutive patients with NSTE-ACS treated with PCI was
performed. This study found that the patients in the high FAR group
were older and with a higher incidence of diabetes mellitus and
hypertension. They exhibited higher levels of troponin-I on
admission, elevated creatinine and a lower LVEF. These patients
exhibited a higher incidence of left main disease and three-vessel
disease. A previous study indicated that hypertension, diabetes,
age, renal insufficiency and cardiac insufficiency are poor
prognostic factors of coronary heart disease (14). Furthermore, the potential association
between the FAR and MACEs in this cohort was explored. Cox analysis
suggested that the FAR was independently associated with all-cause
mortality in NSTE-ACS patients. The FAR exhibited an optimal
accuracy for predicting MACEs, and its cut-off value in patients
with NSTE-ACS undergoing PCI was 9.11, with a sensitivity of 0.630
and a specificity of 0.726. The results indicated that the FAR was
an independent predictor of the 1-year clinical prognosis of
NSTE-ACS patients. To date, a limited number of studies have been
performed with regard to the association of the FAR with the
incidence of CVD. A recent study reported that the FAR may be used
as a biomarker of infarction to evaluate the severity of coronary
artery disease in patients with STEMI (15). The FAR exhibited applicability in a
similar manner to that for the outcome-differentiator SYNTAX score
(15). To the best of our knowledge,
the association of the FAR with the prognosis of NSTE-ACS has not
been previously investigated. The present study suggested that the
FAR may serve as a prognostic marker in NSTE-ACS.
Fibrinogen is an acute-phase protein (4). Plasma fibrinogen levels rise rapidly
during the development of acute diseases, including severe
bacterial infections, trauma and major surgery (8). Chronic low-grade inflammation further
leads to elevated plasma fibrinogen levels, e.g. in the case of
atherosclerosis (16,17). Increased levels of fibrin have been
confirmed in atherosclerotic lesions (18,19).
Fibrinogen is an important factor contributing to the coagulation
process and a major component of thrombosis. In addition, this
protein is considered an important inflammatory marker. High levels
of fibrinogen are closely associated with the risk of CVD (5,20,21). The
mechanisms by which fibrinogen leads to an increase in
cardiovascular risk may be explained as follows: First, fibrinogen
promotes platelet aggregation. In addition, increased fibrinogen
levels promote the formation of fibrin and they increase plasma
viscosity (21,22). Finally, fibrinogen participates in
inflammatory reactions and its levels are increased in inflammatory
states (5,16,17,20).
Albumin is a negative acute-phase protein produced
in the liver, which is associated with the prognosis of several
diseases. A low serum albumin (SA) level is predictive of the
clinical prognosis of patients with coronary heart disease
(9,23,24).
Various studies have reported that hypoalbuminemia is associated
with adverse outcomes, particularly in patients with CVD. This
result may be explained by several mechanisms. SA exerts specific
biological functions, including anti-oxidant and anti-coagulant
activity, the maintenance of vascular integrity, the induction of
vasodilatory effects, and the activation of toxin binding and
cholesterol transport (4,25). In addition, a decreased SA
concentration may be associated with the development of
inflammatory conditions (26–28) or
inadequate nutritional intake (27).
Fibrinogen and albumin are closely associated with
inflammation, and fibrinogen may modulate the inflammatory response
by inducing the production of the pro-inflammatory cytokines
interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α from
peripheral blood mononuclear cells (8). Recently, pro-inflammatory cytokines,
particularly TNF-α and IL-1, have been reported to be associated
with the development of hypoalbuminemia in patients (28). Certain studies have indicated that
fibrinogen and albumin are closely associated with fibrinogen
levels that correlate inversely with those of SA (29,30). The
interaction between albumin and fibrinogen results in impaired
fibrinogen activity (31), while
infusion of albumin may decrease coagulation competence during
major surgery (32). Since
fibrinogen and albumin exhibit a positive and negative correlation
with the inflammatory response, respectively, their ratio may be
correlated with inflammation. Fibrinogen and albumin are two
proteins present in the serum, and it is suggested that the FAR is
more convenient and effective compared with the combination of the
two markers (fibrinogen and albumin) measured independently, and
the FAR is used in clinical applications.
The FAR may reflect information on inflammation and
may be used as a valuable serological marker. Karahan et al
(15) reported that FAR may be used
to evaluate the severity of coronary artery disease in patients
with STEMI. Recent studies have indicated that the FAR is
associated with the severity of coronary stenosis in STEMI patients
and that it is an independent predictor of all-cause mortality that
may be used as a prognostic indicator in patients with STEMI
(33). The present study suggested
that the FAR is an independent predictor of all-cause mortality in
patients with NSTE-ACS undergoing PCI. A limited number of studies
have been performed on the prognostic value of FAR in patients with
ACS. The present results should be verified in future follow-up
studies with larger sample sizes.
The present study has several limitations, including
the single-center design, which may have introduced selection bias.
Furthermore, the present study evaluated the SA and serum
fibrinogen concentration only pre-operatively, and the patient
samples were tested only once prior to PCI, which did not allow for
the time-dependent assessment of the changes noted regarding the
clinical and biochemical end-points. In addition, C-reactive
protein (CRP) a commonly studied inflammatory factor, which is
involved in multiple stress responses and serves as an important
factor for the prognosis of cardiovascular disease (5). CRP was not included in the current
study, which is a limitation. In addition, the subjects were
patients with NSTE-ACS who underwent PCI and the STEMI population
was not included. Patients treated with CABG or those who received
prior CABG or were unsuitable for revascularisation were not
included in the present study. Due to limited economic
circumstances of certain patients, intermediate lesions and
critical lesions were not subjected to fractional flow reserve
detection, did not receive any PCI treatment, conservative medical
treatment or regular follow-up and the present study did not
include such patients. For the selected patients, the criteria for
determining a successful PCI were the achievement of a minimum
stenosis diameter reduction to <20%, grade 3 thrombolysis in
myocardial infarction flow assessed by angiography, angina pectoris
eliminated or significantly alleviated, and improved abnormalities
in the electrocardiogram. At our medical center, only <15% of
the population is subjected to intravascular ultrasound validation
(34). Therefore, studies with a
larger sample size are necessary in the future. Taken together, the
results of the present study suggested that the FAR was able to
predict the 1-year prognosis of patients with NSTE-ACS treated by
PCI. Further multicenter studies are required to confirm the
present results.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DXH designed the study and drafted the manuscript.
YDJ and TTY participated in data analysis and grouping. JS, ZYW,
JKW, WLD and NS were responsible for the patient record and
follow-up. ZJS and ZQS contributed to study conception and
critically revised the manuscript. The final version of the
manuscript was read and approved by all the authors.
Ethics approval and consent to
participate
The present study complied with the Declaration of
Helsinki, and was granted approval by the Shengjing Hospital of the
China Medical University Research Ethics Committee (Shenyang,
China). Written informed consent was formally obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2016 DALYs and HALE Collaborators:
Global, regional, and national disability-adjusted life-years
(DALYs) for 333 diseases and injuries and healthy life expectancy
(HALE) for 195 countries and territories, 1990–2016, . A systematic
analysis for the Global Burden of Disease Study 2016. Lancet.
390:1260–1344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao R, Patel A, Gao W, Hu D, Huang D, Kong
L, Qi W, Wu Y, Yang Y, Harris P, et al: Prospective observational
study of acute coronary syndromes in China: Practice patterns and
outcomes. Heart. 94:554–560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Libby P: Inflammation in atherosclerosis.
Arterioscler Thromb Vasc Biol. 32:2045–2051. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmed MS, Jadhav AB, Hassan A and Meng QH:
Acute phase reactants as novel predictors of cardiovascular
disease. ISRN Inflamm. 2012:9534612012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Emerging Risk Factors Collaboration,
Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P,
Walker M, Thompson A, Sarwar N, et al: C-reactive protein,
fibrinogen, and cardiovascular disease prediction. N Engl J Med.
367:1310–1320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mayer FJ, Gruenberger D, Schillinger M,
Mannhalter C, Minar E, Koppensteiner R, Arbesú I, Niessner A and
Hoke M: Prognostic value of neutrophils in patients with
asymptomatic carotid artery disease. Atherosclerosis. 231:274–280.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iijima R, Ndrepepa G, Mehilli J, Bruskina
O, Schulz S, Schömig A and Kastrati A: Relationship between
platelet count and 30-day clinical outcomes after percutaneous
coronary interventions. Pooled analysis of four ISAR trials. Thromb
Haemost. 98:852–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jensen T, Kierulf P, Sandset PM,
Klingenberg O, Joø GB, Godal HC and Skjønsberg OH: Fibrinogen and
fibrin induce synthesis of proinflammatory cytokines from isolated
peripheral blood mononuclear cells. Thromb Haemost. 97:822–829.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurtul A, Murat SN, Yarlioglues M, Duran
M, Ocek AH, Koseoglu C, Celık IE, Kilic A and Aksoy O: Usefulness
of serum albumin concentration to predict high coronary SYNTAX
score and in-hospital mortality in patients with acute coronary
syndrome. Angiology. 67:34–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duffy BK, Gurm HS, Rajagopal V, Gupta R,
Ellis SG and Bhatt DL: Usefulness of an elevated neutrophil to
lymphocyte ratio in predicting long-term mortality after
percutaneous coronary intervention. Am J Cardiol. 97:993–996. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan Z, Zhang M, Han Q, Wen J, Luo K, Lin
P, Zhang L, Yang H and Fu J: A novel blood tool of cancer prognosis
in esophareal squamous cell carcinoma: The Fibrinogen/Albumin
Ratio. J Cancer. 8:1025–1029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hwang KT, Chung JK, Roh EY, Kim J, Oh S,
Kim YA, Rhu J and Kim S: Prognostic influence of preoperative
fibrinogen to albumin ratio for breast cancer. J Breast Cancer.
20:254–263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan D, Lawson L and Kunadian V:
Management of older patients presenting with non-ST-elevation acute
coronary syndrome. EuroIntervention. 14:e258–e260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
2012 Writing Committee Members, Jneid H,
Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE Jr, Ettinger
SM, Fesmire FM, Ganiats TG, et al: 2012 ACCF/AHA focused update of
the guideline for the management of patients with unstable
anginan/Non-ST-elevation myocardial infarction (updating the 2007
guideline and replacing the 2011 focused update): A report of the
American College of Cardiology Foundation/American Heart
Association Task Force on practice guidelines. Circulation.
126:875–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karahan O, Acet H, Ertaş F, Tezcan O,
Çalişkan A, Demir M, Kaya AF, Demirtaş S, Çevik MU and Yavuz C: The
relationship between fibrinogen to albumin ratio and severity of
coronary artery disease in patients with STEMI. Am J Emerg Med.
34:1037–1042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stone MC and Thorp JM: Plasma fibrinogen-a
major coronary risk factor. J R Coll Gen Pract. 35:565–569.
1985.PubMed/NCBI
|
|
17
|
Wilhelmsen L, Svärdsudd K, Korsan-Bengtsen
K, Larsson B, Welin L and Tibblin G: Fibrinogen as a risk factor
for stroke and myocardial infarction. N Engl J Med. 311:501–505.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoff HF, Heideman CL, Jackson RL, Bayardo
RJ, Kim HS and Gotto AM Jr: Localization patterns of plasma
apolipoproteins in human atherosclerotic lesions. Circ Res.
37:72–79. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sadoshima S and Tanaka K: Fibrinogen and
low density lipoprotein in the development of cerebral
atherosclerosis. Atherosclerosis. 34:93–103. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mahmud E, Behnamfar O, Lin F, Reeves R,
Patel M and Ang L: Elevated serum fibrinogen is associated with
12-month major adverse cardiovascular events following percutaneous
coronary intervention. J Am Coll Cardiol. 67:2556–2557. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ang L, Behnamfar O, Palakodeti S, Lin F,
Pourdjabbar A, Patel MP, Reeves RR and Mahmud E: Elevated baseline
serum fibrinogen: Effect on 2-year major adverse cardiovascular
events following percutaneous coronary intervention. J Am Heart
Assoc. 6:2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lowe GD, Fowkes FG, Dawes J, Donnan PT,
Lennie SE and Housley E: Blood viscosity, fibrinogen, and
activation of coagulation and leukocytes in peripheral arterial
disease and the normal population in the Edinburgh Artery Study.
Circulation. 87:1915–1920. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chien SC, Chen CY, Leu HB, Su CH, Yin WH,
Tseng WK, Wu YW, Lin TH, Chang KC, Wang JH, et al: Association of
low serum albumin concentration and adverse cardiovascular events
in stable coronary heart disease. Int J Cardiol. 241:1–5. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wada H, Dohi T, Miyauchi K, Shitara J,
Endo H, Doi S, Naito R, Konishi H, Tsuboi S, Ogita M, et al: Impact
of serum albumin levels on long-term outcomes in patients
undergoing percutaneous coronary intervention. Heart Vessels.
32:1085–1092. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roche M, Rondeau P, Singh NR, Tarnus E and
Bourdon E: The antioxidant properties of serum albumin. FEBS Lett.
582:1783–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nicholson JP, Wolmarans MR and Park GR:
The role of albumin in critical illness. Br J Anaesth. 85:599–610.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Don BR and Kaysen G: Serum albumin:
Relationship to inflammation and nutrition. Semin Dial. 17:432–437.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Odamaki M, Kato A, Takita T, Furuhashi M,
Maruyama Y, Yonemura K and Hishida A: Role of soluble receptors for
tumor necrosis factor alpha in the development of hypoalbuminemia
in hemodialysis patients. Am J Nephrol. 22:73–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim KJ, Yang WS, Kim SB, Lee SK and Park
JS: Fibrinogen and fibrinolytic activity in CAPD patients with
atherosclerosis and its correlation with serum albumin. Perit Dial
Int. 17:157–161. 1997.PubMed/NCBI
|
|
30
|
Kim SB, Chi HS, Park JS, Hong CD and Yang
WS: Effect of increasing serum albumin on plasma D-dimer, von
Willebrand factor, and platelet aggregation in CAPD patients. Am J
Kidney Dis. 33:312–317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Galanakis DK: Anticoagulant albumin
fragments that bind to fibrinogen/fibrin: Possible implications.
Semin Thromb Hemost. 18:44–52. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rasmussen KC, Højskov M, Johansson PI,
Kridina I, Kistorp T, Salling L, Nielsen HB, Ruhnau B, Pedersen T
and Secher NH: Impact of albumin on coagulation competence and
hemorrhage during major surgery: A randomized controlled trial.
Medicine (Baltimore). 95:e27202016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao L, Jia Y, Wang X and Huang H: The
impact of preoperative fibrinogen-albumin ratio on mortality in
patients with acute ST-segment elevation myocardial infarction
undergoing primary percutaneous coronary intervention. Clin Chim
Acta. 493:8–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smith SC, Dove JT, Jacobs AK, Kennedy JW,
Kereiakes D, Kern MJ, Kuntz RE, Popma JJ, Schaff HV, Williams DO,
et al: ACC/AHA guidelines for percutaneous coronary intervention
(revision of the 1993 PTCA guidelines)-executive summary: A report
of the American College of Cardiology/American Heart Association
task force on practice guidelines (Committee to revise the 1993
guidelines for percutaneous transluminal coronary angioplasty)
endorsed by the Society for Cardiac Angiography and Interventions.
Circulation. 103:3019–3041. 2001. View Article : Google Scholar : PubMed/NCBI
|