Introduction
Salmonella, belongs to Gram-negative bacteria
and can cause enteric diseases in humans and animals including pigs
and chickens. Salmonella enterica serovar Typhimurium (ST)
induces severe acute gastroenteritis, diarrhea, and fever in humans
and is often caused by consumption of contaminated food or water
(1). Therefore, the identification
and development of agents with anti-ST effects is of great
importance.
ST invades mammalian cells such as macrophages and
epithelial cells, and the bacteria subsequently adopt strategies
that enable their intracellular survival within the host cells.
This typically involves the identification and resistance of the
host innate immune system, which is essential for ST-induced
pathogenesis (2). ST infection can
induce a range of host immune responses for example lymphocyte
proliferation and antibody generation (3,4). The
release of cytokines including interferon (IFN)-γ, tumor necrosis
factor (TNF)-α, and interleukin (IL)-6 leads to inflammation
(5). The levels of inflammatory
cytokines and immunoglobulin (Ig)s in ST-infected hosts are
regarded as a good gauge of infection status. Conversely, a
decrease in inflammatory cytokines and Igs in circulation and in
the host organs are useful markers for evaluating potential
activity of anti-ST agents.
Ligustrum lucidum Ait (LL), Lysimachia
christinae Hance (LC), Mentha piperita Linn (MP), and
Cinnamomum cassia Presl (CC) are four edible plant foods
commonly used for cooking or folk medicine in many East Asian
countries including China, Japan and Taiwan. It is reported that
these spices contain phytochemicals such as phenolic acids and
flavonoids (6,7) that display antioxidant and
anti-inflammatory activities (8).
Furthermore, LL exerts an anti-inflammatory effect via the
inhibition of TNF-α production in mouse peritoneal macrophages
(9). In addition, MP aqueous extract
arrests the growth of bacteria such as Staphylococcus aureus,
Pseudomonas aeruginosa, and Bordetella bronchiseptica
(10). The inhibitory activities of
MP and CC essential oil extracts on ST have been previously
investigated (11,12); however, the application of aqueous
extracts against ST infection is more appropriate as the natural
properties remain unaltered.
Using aqueous extracts of common spices against
food-borne diseases should be more practical for daily life in
comparison to extracts prepared via chemical purification
processes. Currently, there are few reports regarding the
protective effects of LL, LC, MP and CC aqueous extracts against ST
infection. In order to further understand and evaluate the
application of these spices, the total content of phenolic acids
and flavonoids in these aqueous extracts was taken as indicators of
purity. An in vivo and in vitro study was conducted
to investigate the anti-ST effects of LL, LC, MP and CC aqueous
extracts.
Materials and methods
Materials
S. enterica serovar Typhimurium strain ST21
(ST21) was kindly supplied by Dr. Chao-chin Chang from National
Chung Hsing University (13).
Bacteria were grown in Luria Bertani (LB) broth to log stationary
phase (15,000 × g shaking; 37°C) for 8 h to OD600 nm of
0.8. Following harvesting by centrifugation at 8,000 × g, the
bacterial pellet was resuspended in PBS, and adjusted to a final
concentration of 1010 colony forming units (CFU)/ml in
PBS. Broth and agar were obtained from Difco Laboratories Inc. Dry
LL, LC, MP, and CC were purchased from Ka-da Spice Store. Aqueous
extracts were obtained from the dried ripe fruits of LL, dried
whole plants of LC, dried leaves of MP, and dried barks of CC
(Fig. 1). A total of 10 g of each
spice were chopped and mixed thoroughly with 400 ml
dH2O in a waring blender at room temperature for
5 min. The mixture was then boiled in dH2O for 30
min. Following cooling down to the room temperature, the aqueous
extract was collected by filtration through a Whatman No. 1 filter
paper, freeze-dried to a fine powder finally resuspended in 10 ml
dH2O.
Determination of total phenolic acid
and flavonoid contents
The method described in Sreelatha and Padma
(14) was used to determine the
total phenolic acid and flavonoid content in aqueous extract
powder. Results were expressed as gallic acid and quercetin
equivalent, respectively. This assay was repeated in
triplicate.
Anti-microbial activity
The minimum inhibitory concentrations and minimum
bactericidal concentrations of spice aqueous extracts were tested
by a two-fold serial dilution method (15). Aqueous extracts were serially diluted
with PBS to achieve concentrations of 12.5, 25, 50, 100 and 200
mg/ml. ST21 was cultured in Mueller Hinton Broth then diluted to
106 CFU/ml in PBS determined by absorbance at OD600
nm. Equal volumes of ST21 suspension and diluted spice
aqueous extract (100 µl) were mixed and added to a 96-well plate,
with an additional well containing only broth used as a negative
control. The plate was incubated at 37°C for 24 h, then the well
with the lowest concentration of spice aqueous extract that
demonstrated no visible bacterial growth was considered as the MIC.
All spice aqueous extract samples that demonstrated complete
inhibition of visual bacterial growth were identified then 10 µl of
each culture was transferred onto a LB agar plate and incubated for
16 h at 37°C. The complete visual absence of bacterial colonies on
the agar surface of samples at the lowest spice aqueous extract
concentration was defined as the MBC. Each assay was repeated in
triplicate.
Animal experiment
A total of 60 male 8-week-old Balb/c mice weighing
20–22 g were purchased from National Laboratory Animal Center. Mice
were housed in 12-h light/dark cycles with access to food and water
ad libitum. The study was approved by the China Medical
University animal care and use committee (permission no.
105-23).
Mice were randomly divided into six groups (n=10):
The infection and control group mice received the normal diet with
and without ST21 infection, respectively; the treatment group mice,
including LL, LC, MP, and CC groups, received different spice
treatments with ST21 infection. A total of 100 µl freshly prepared
aqueous extract at concentration of 50 mg/ml was applied orally to
mice by the feeding tube for 7 days, once a day. At day 7,
following a 12-h fast, each mouse was infected by oral injection of
200 µl PBS containing ST21 at 5×1010 CFU. Following
infection, aqueous extract of each spice (200 µl) was continuously
supplied for 4 days. Therefore, there was a 7-day pre-treatment
with spice aqueous extracts before the infection, and a 4-day
post-treatment following infection. Feces were collected on day
8–11. Body weight was recorded one day before the experiment (day
0), the day of infection (day 7), and the last day of treatment
(day 11). Following the 4-day treatment, mice were fasted overnight
and sacrificed in the morning. Blood (1 ml) was obtained from the
heart via microsyringe and the serum was immediately separated. The
liver, spleen and small intestine from each mouse were collected.
Each organ tissue (100 mg) was mixed with 2 ml of PBS and then
homogenized by a tissue homogenizer (Glas-Col Co.). The homogenate
was collected via filtration through a Whatman No. 1 filter paper
in preparation for further experiments.
ST21 count in feces, blood and
organs
Following ST21 infection, fecal samples were
collected daily from day 8–11 prior to treatment. The fecal matter
was processed within 2 h of sampling. ST21 per gram of feces was
determined. In brief, fecal samples (1 g) were collected in 10 ml
PBS. Fecal suspension, blood and organ homogenate at 100 µl was
serially diluted in PBS, then plated on Salmonella-Shigella
(SS) agar plates. Following incubation overnight at 37°C, CFU were
counted.
Measurement of inflammatory factor
levels
IFN-γ and IL-6 serum levels, and IFN-γ, IL-6, IL-1β,
TNF-α, and IL-12 levels in spleen homogenate of all mice and
culture supernatant collected from the H. pylori-infected
RAW264.7 cells were measured. These cytokines were analyzed using
mouse IFN-γ ELISA kit (cat. no. 88-7314-88), mouse IL-6 ELISA kit
(cat. no. 88-7064-88), mouse IL-1β ELISA kit (cat. no. 88-7013-88),
mouse TNF-α ELISA kit (cat. no. 88-7324-88) and mouse IL-12 ELISA
kit (cat. no. 88-7121-88; all Invitrogen; Thermo Fisher Scientific,
Inc.). Samples were run in duplicates according to manufacturer's
protocol.
ST21 specific Ig detection
Anti-ST21-specific IgA and IgM in the sera of
treated mice were detected using ELISA according to the method of
Chang et al (13), which was
briefly described as follows. The 96-well plates were coated with
100 µl/well of heat-killed ST21 harvested from a bacterial culture
(1×1011 CFU/ml) at 1:100 dilution in
carbonate/bicarbonate coating buffer (pH 9.6) at 4°C overnight.
Coated plates were first blocked with BSA buffer (1% of BSA in PBS)
at room temperature for 2 h, then washed 5 times in PBS containing
0.1% Tween 20 (PBST). A total of 100 µl/well of mouse serum
collected 4 days post-treatment were diluted in BSA buffer (1:10)
was then added to the coated plates for 2 h at room temperature.
Following five washes with PBST, 100 µl/well of
peroxidase-conjugated goat anti-mouse IgA (cat. no. A90-103P) or
IgM (cat. no. A90-101P) antibodies (diluted 1:5,000; Bethyl
Laboratories, Inc.) were added to each well and plates were
incubated at room temperature for 1 h. Following five washes, 100
µl/well of tetramethylbenzidine (1 mg/ml in DMSO; KPL, Inc.) was
added and incubated for 30 min at room temperature. Then 100
µl/well of stop solution (2 M N2SO4) was
added and the OD at 450 nm was measured. Each assay in this
experiment was repeated in triplicate.
Invasion of H. pylori into RAW264.7
cells
The murine monocyte/macrophage cell line RAW264.7
(cat. no. TIB-71) was obtained from American Type Culture
Collection. RAW264.7 cells were seeded into 96-well plates at a
density of 1×105 cells/well then cultured at 5%
CO2 in Dulbecco's modified Eagle's medium (DMEM; cat.
no. 12100-046; Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum for 18 h at 37°C. The culture
supernatant was removed then 100 µl of antibiotic-free DMEM was
added to each well. RAW264.7 cells were then infected with ST21 at
a multiplicity of infection (MOI) of 10. For the co-incubation
group, the cells were treated with spice aqueous extracts at a
final concentration of 5 mg/ml alongside ST21 infection for 1 h at
37°C. For the pretreatment groups, prior to infection, RAW264.7
cells (cell-pretreatment group) or ST21 (ST21-pretreatment group)
were pretreated with LL, LC, MP and CC aqueous extract at final
concentration of 5 mg/ml for 1 h at 37°C; infection was performed
as described. For TNF-α depletion, anti-mouse TNF-α capture
antibodies (1:100; cat. no. 14-7423-68A; Invitrogen; Thermo Fisher
Scientific, Inc.) were added in the cell culture treated with spice
aqueous extracts for 1 h at 37°C; the same procedure was performed
in the co-incubation group. Cell-associated bacteria were then
quantified 1 h following infection. Briefly, to determine the
number of viable intracellular bacteria, infected cells was washed
three times in PBS then incubated with 100 µg/ml of the
membrane-impermeable antibiotic gentamicin (Sigma-Aldrich; Merck
KGaA) for 1.5 h at 37°C to remove extracellular bacteria. Cell
culture supernatants were removed following centrifugation (5 min;
1,500 × g; room temperature). Cells were washed with PBS twice, and
osmotic lysis was performed to calculate the total quantity of
bacteria remaining. In brief, dH2O was added to
the infected cells following washing, the cell lysates were
re-suspended in PBS then plated using serial dilutions on the SS
agar plates (Difco; BD Biosciences). These plates were cultured
with 100 µl from each dilution at 37°C for 18 h. Bacterial cell
numbers were then determined by manual colony counting. Results
were expressed as a percentage of the invasion activity of ST21 in
comparison with the infection group. The supernatants of
ST21-infected RAW264.7 cells treated with spices were analyzed for
cytokines. The concentrations shown in Table I are also the lowest concentrations
for different batches of spices eligible for the current study.
 | Table I.Content (mg/g of dry spices) of total
phenolic acids and total flavonoids in aqueous extract of LL, LC,
MP or CC. |
Table I.
Content (mg/g of dry spices) of total
phenolic acids and total flavonoids in aqueous extract of LL, LC,
MP or CC.
| Spice | Total phenolic
acids | Total
flavonoids |
|---|
| LL | 1.05±0.12 | 2.91±0.34 |
| LC | 1.37±0.21 | 3.16±0.41 |
| MP | 1.58±0.21 | 7.65±0.86 |
| CC | 0.50±0.09 | 1.67±0.31 |
Statistical analysis
Data were analyzed using SPSS v12.0 software (SPSS,
Inc.) Statistical significance was assessed between two groups
using Student's t-test or between multiple group using one-way
analysis of variance followed by Dunnett's post hoc test. Results
were presented as the mean ± standard deviation. P<0.05 was
considered to indicate statistical significance.
Results
Total phenolic acid and flavonoid
content
In order to confirm the purity of aqueous extracts
that was prepared, the total phenolic acid and flavonoid content in
spice aqueous extracts was determined. Total phenolic acid content
in LL, LC, MP and CC aqueous extracts was in the range of 0.50–1.58
mg/g of spice (Table I). Total
flavonoid content in LL, LC, MP and CC aqueous extract was in the
range of 1.67–7.65 mg/g of spice (Table
I). MP contained the highest amount of total phenolic acids and
flavonoids whilst CC had the lowest amount.
Spice aqueous extracts exhibit
bactericidal effects
Anti-ST21 activity was examined to determine the
bactericidal activities of LL, LC, MP and CC aqueous extracts. MIC
were in the range of 125–500 mg/ml and MBC values were >500
mg/ml (Table II). LL and MP aqueous
extracts exhibited a stronger inhibitory effect on the growth of
ST21. However, it is important to note that no spice aqueous
extract was able to eradicate ST21 at concentration of <500
mg/ml.
 | Table II.MICs and MBCs of LL, LC, MP, or CC
against ST21. |
Table II.
MICs and MBCs of LL, LC, MP, or CC
against ST21.
| Spice | MIC (mg/ml) | MBC (mg/ml) |
|---|
| LL | 125 | >500 |
| LC | 500 | >500 |
| MP | 125 | >500 |
| CC | 500 | >500 |
Spice aqueous extracts maintain body
weight following infection
ST infection in mice causes murine typhoid that
results in a reduction of body weight (1,16). In
the present study, four days following ST21 infection (day 11),
infected mice had lower body weight compared with the control group
(Fig. 2; P<0.05). However,
treatment with LL, LC, MP and CC aqueous extracts significantly
increased body weight compared with the infection group (Fig. 2; P<0.05). There was no significant
difference in body weight between any groups in days 0 and 7
(Fig. 2; P>0.05).
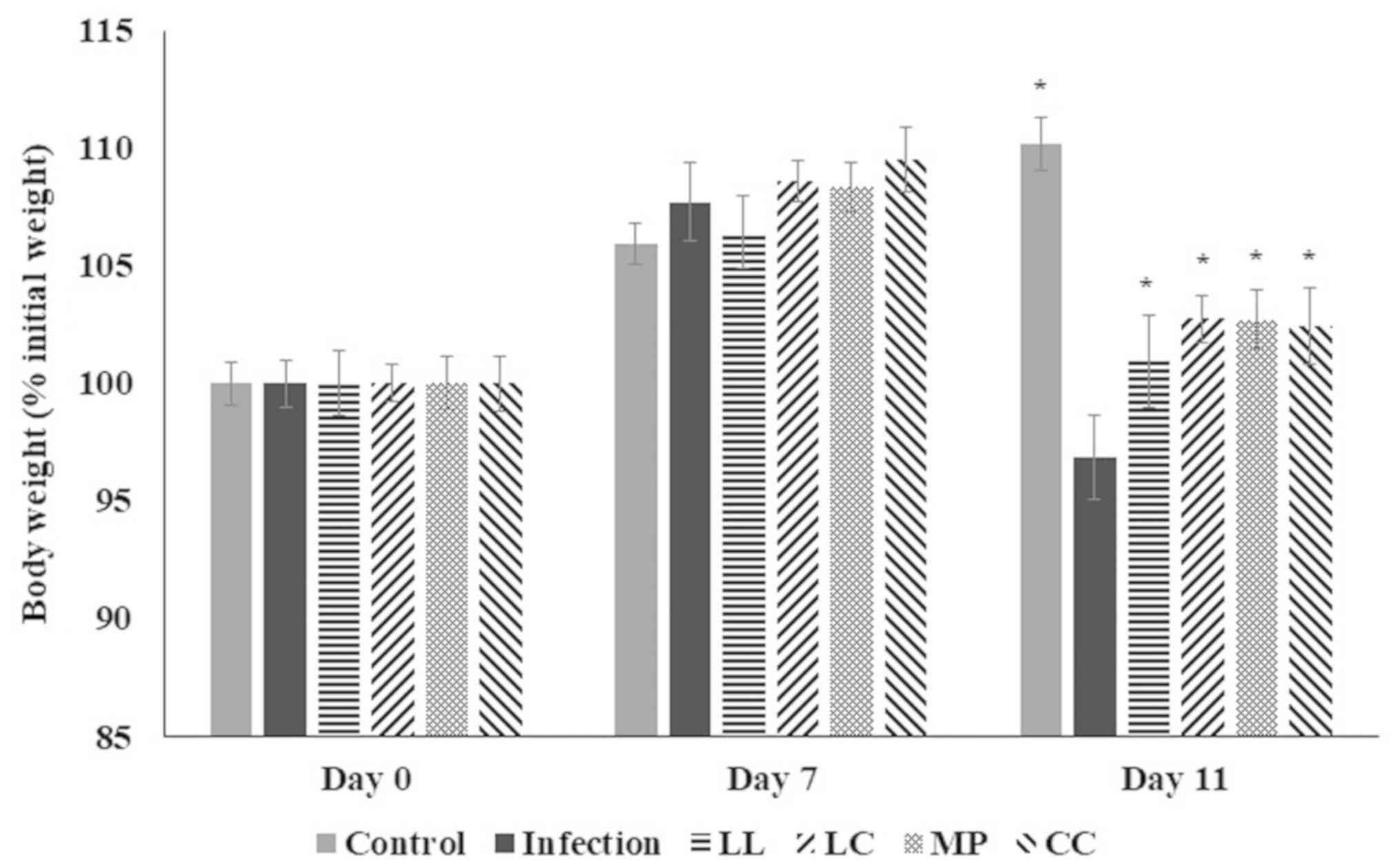 | Figure 2.Body weight of mice following
infection with ST21 then treatment with LL, LC, MP, and CC aqueous
extract. Body weight of mice without infection (control),
ST21-infected mice without any treatment (infection), or following
LL, LC, MP or CC aqueous extract treatment (n=10). *P<0.05 vs.
the infection group on the same day. ST21, Salmonella
enterica serovar Typhimurium strain ST21; LL, Ligustrum
lucidum Ait; LC, Lysimachia christinae Hance; MP,
Mentha piperita Linn; CC, Cinnamomum cassia
Presl. |
Spice aqueous extracts decrease ST21
count in feces, blood, and organs
Following oral infection of mice with ST, bacteria
replicate quickly within the guts then are subsequently eliminated
in the feces (2). ST can also spread
from the guts to the liver, spleen and blood circulatory system
(1,16). It has been reported that the ST
viable count reaches the maximum amount four days following oral
inoculation (17). In the present
study the ST21 count in feces at 1–4 days following infection (day
8–11) was increased when compared with control group (P<0.05;
Fig. 3A). Between days 8–11, fecal
ST21 counts in groups that received LL, LC, MP and CC treatment
were lower compared with the infection group (P<0.05; Fig. 3A). ST21 counts in the small
intestine, liver, spleen and blood significantly decreased
following all spice treatments (P<0.05; Fig. 3B).
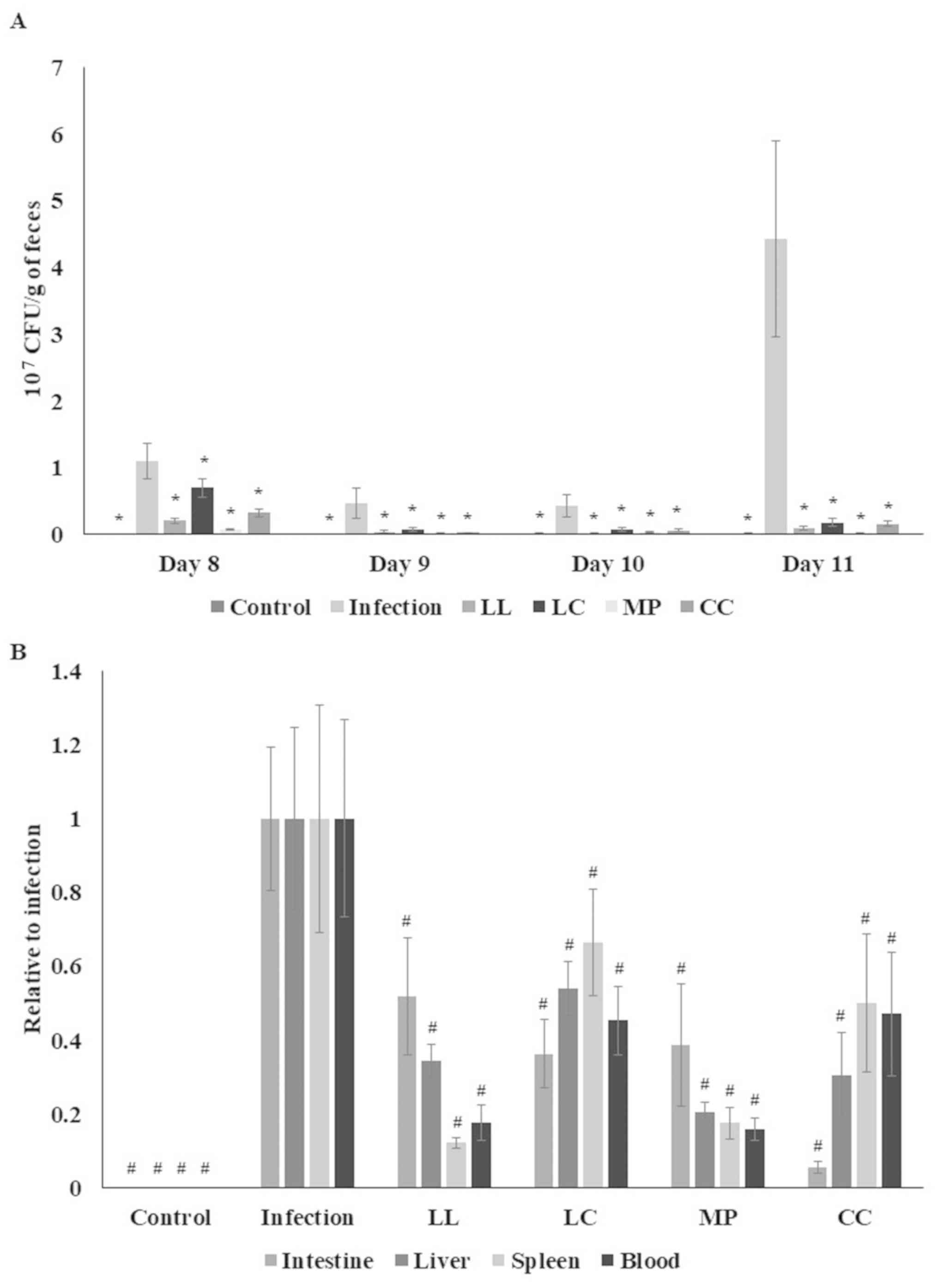 | Figure 3.Spice aqueous extracts decrease ST21
count in feces, blood, and organs. (A) ST21 counts in feces, (B)
blood, small intestine, liver and spleen of mice without infection
(control), ST21-infected mice without any treatment (infection), or
following LL, LC, MP or CC aqueous extract treatment (n=10).
*P<0.05 vs. the infection group on the same day.
#P<0.05 vs. the infection group in the same organ.
ST21, Salmonella enterica serovar Typhimurium strain ST21;
LL, Ligustrum lucidum Ait; LC, Lysimachia christinae
Hance; MP, Mentha piperita Linn; CC, Cinnamomum
cassia Presl; CFU, colony forming units. |
Spice aqueous extracts attenuate
inflammatory stress and decrease ST21 specific Igs
ST clearance involves inflammation and Ig associated
reactions therefore, monitoring inflammatory cytokines and Igs in
circulation and in host organs are necessary in evaluating
potential anti-ST activity. ST21 infection significantly increased
IFN-γ and IL-6 levels in the serum (P<0.05; Fig. 4A) whilst LL, LC, MP and CC aqueous
extract treatments significantly reduced the serum IFN-γ and IL-6
levels compared with the infection group (P<0.05; Fig. 4A). In the spleen, the spice aqueous
extract treatments significantly decreased IFN-γ, IL-6, IL-1β, and
TNF-α levels; however, there was an increase in IL-12 level
(Fig. 4B; P<0.05) whilst LC
treatment did not suppress the IFN-γ level. ST21 infection
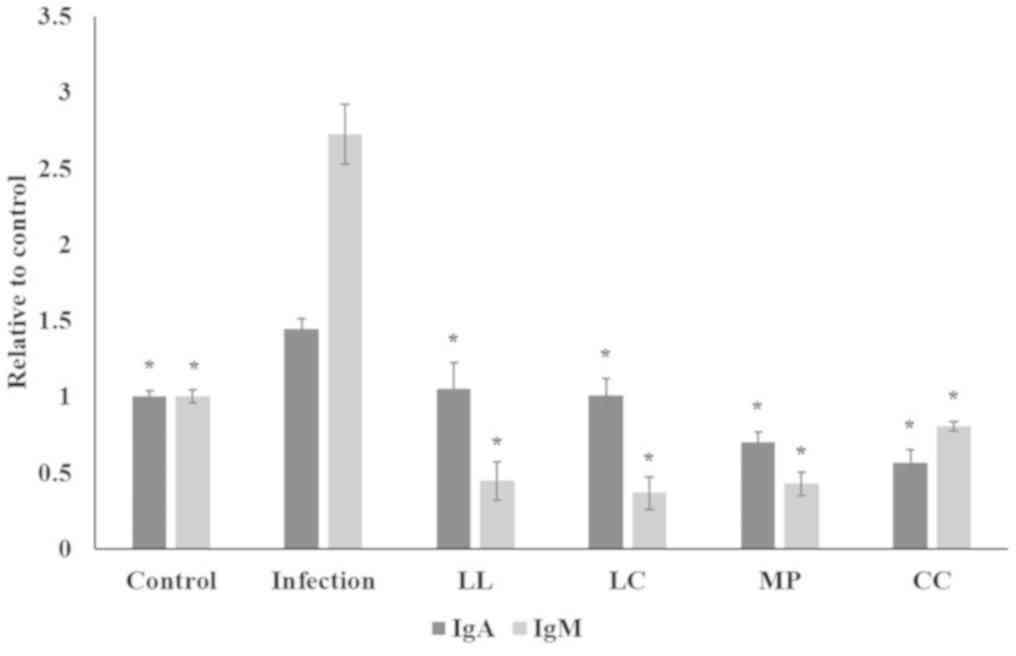
stimulated the production of ST21 specific IgA and IgM in the serum
(Fig. 5; P<0.05) whilst all spice
aqueous extract treatments significantly decreased serum IgA and
IgM levels (Fig. 5; P<0.05).
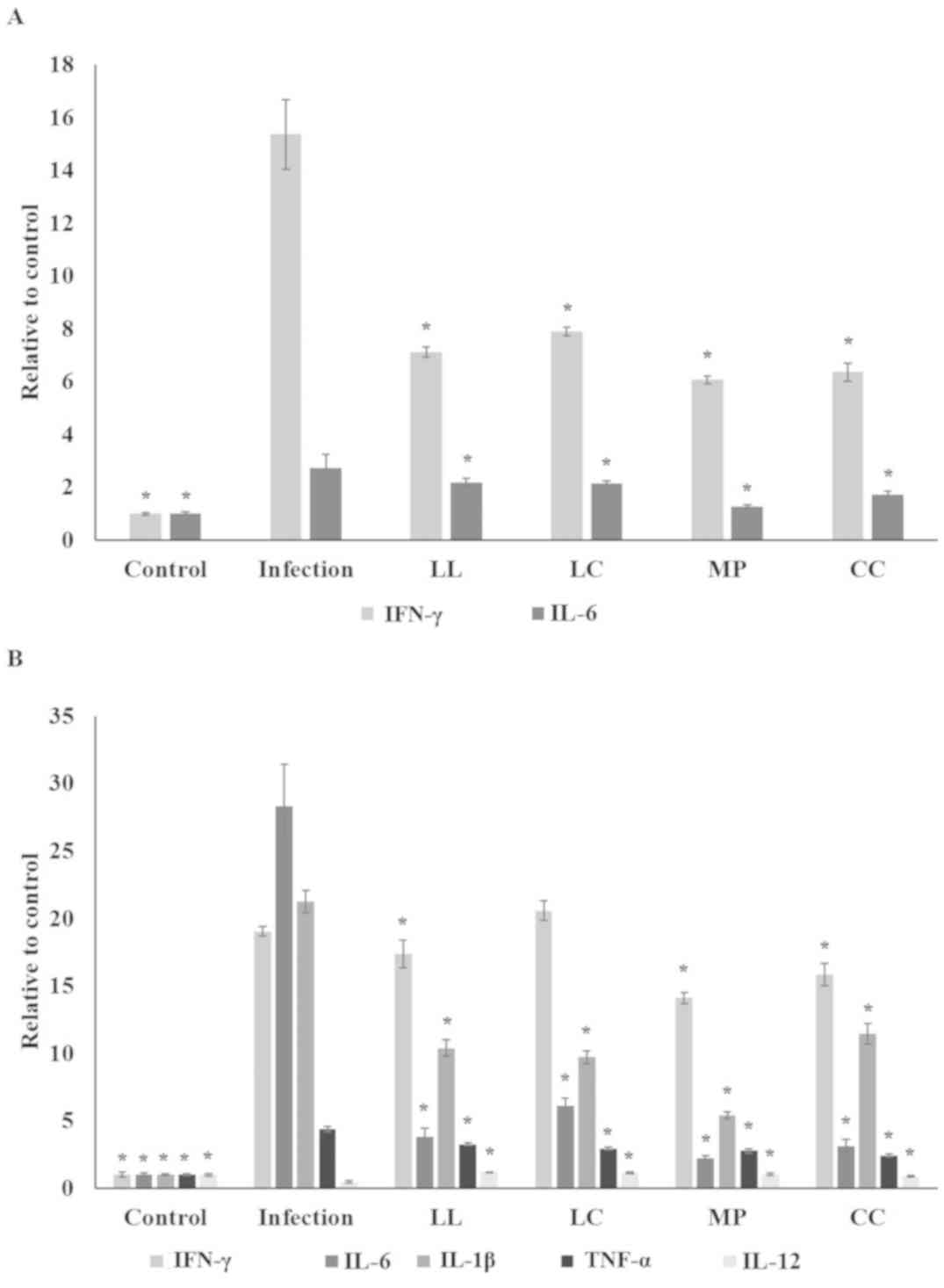 | Figure 4.Spice aqueous extract treatment
attenuates inflammation. (A) Serum levels of IFN-γ and IL-6 levels,
and (B) IFN-γ, IL-6, IL-1β, TNF-α and IL-12 levels in the spleen of
mice without infection (control), ST21-infected mice without any
treatment (infection) or following LL, LC, MP or CC aqueous extract
treatment (n=10). *P<0.05 vs. the infection group. IFN,
interferon; IL, interleukin; TNF, tumor necrosis factor; ST21,
Salmonella enterica serovar Typhimurium strain ST21; LL,
Ligustrum lucidum Ait; LC, Lysimachia christinae
Hance; MP, Mentha piperita Linn; CC, Cinnamomum
cassia Presl. |
Spice aqueous extracts reduce the
invasion ability of ST21 and decrease TNF-α levels of infected
macrophages
In order to further elucidate the possible
mechanisms of LL, LC, MP and CC aqueous extracts against ST21, a
RAW 264.7 cell infection model was established. ST21 was able to
infect RAW 264.7 cells. In the co-incubation group, all of the
spice aqueous extract treatment reduced the number of ST21 that
successfully invaded RAW 264.7 cells (Fig. 6A). To distinguish whether the
extracts affected the RAW 264.7 cells or ST21, prior to performing
the invasion assay, the cells and bacteria were pretreated with
spice aqueous extracts for 1 h. The infection process was then
performed without aqueous extracts co-incubation. In the cell
pretreatment group, the number of invading ST21 only significantly
decreased in the CC group. However, in the ST21-pretreatment group,
the invasion abilities of ST21 were significantly decreased for all
aqueous extract groups (Fig. 6A). In
order to clarify the effects of four spices in regulating TNF-α
levels for ST21 eradication, specific antibodies (TNF-α depletion
group) were added to deplete TNF-α in the cell culture (Fig. 6A). Compared with the co-incubation
group, the invasion abilities of ST21 increased in LC and infection
groups, when the level of TNF-α was depleted (Fig. 6A). All of the spice aqueous extract
only treatments reduced TNF-α levels of RAW 264.7 cells (Fig. 6B). Compared with the co-incubation
group, TNF-α levels increased in both cell- and ST21-pretreatment
groups (Fig. 6B).
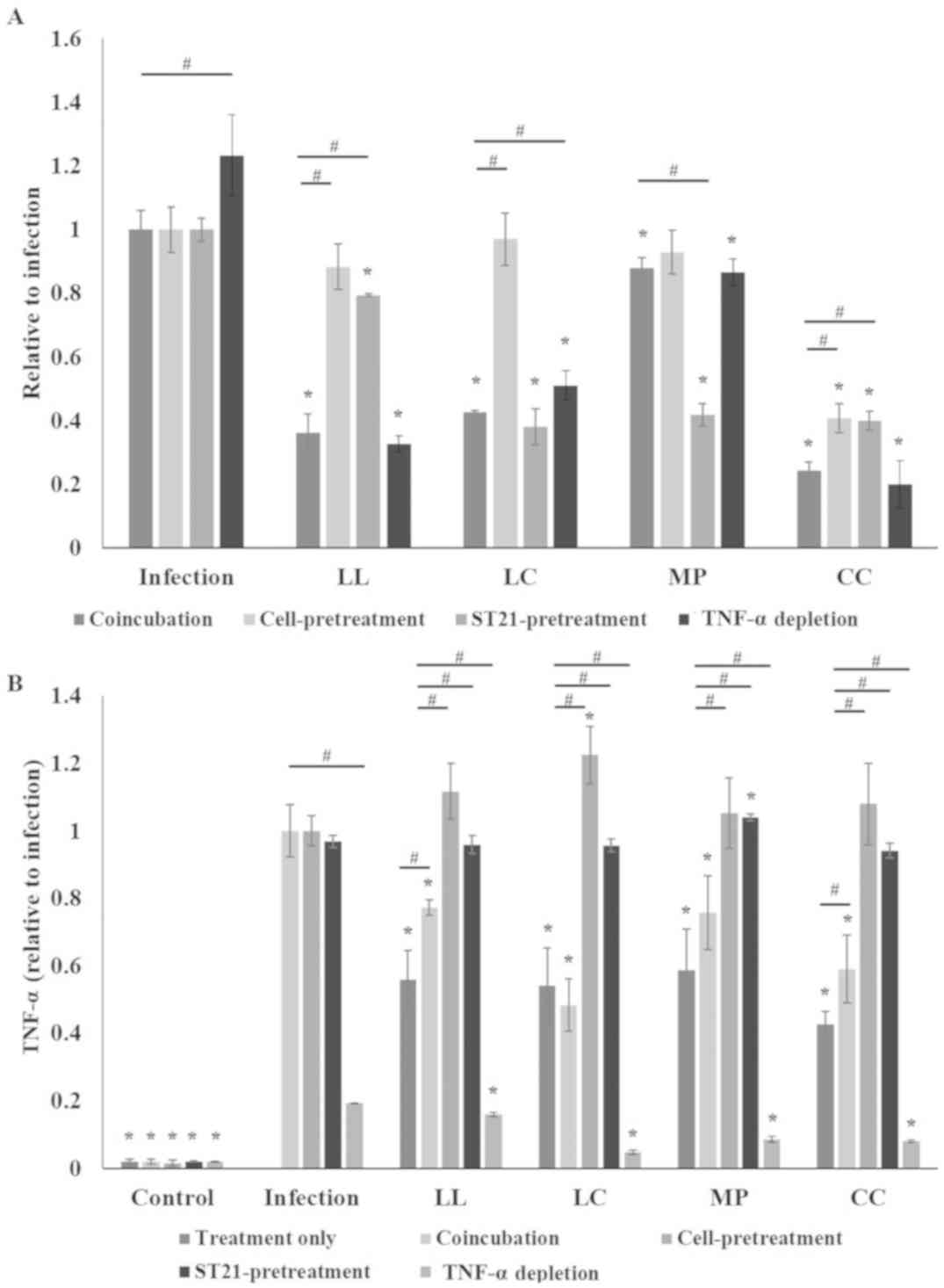 | Figure 6.Spice aqueous extracts reduce the
invasion ability of ST21 and decrease TNF-α levels of infected
macrophages in vitro. (A) ST21 invasion ability and (B)
TNF-α expression levels of ST21-infected RAW264.7 cells without any
treatment (infection), and treated with LL, LC, MP or CC aqueous
extract for 1 h. Coincubation groups refer to the cells treated
with spice aqueous extracts alongside ST21 infection.
Cell-pretreatment groups were pretreated by spice aqueous extracts
prior to ST21 infection. ST21-pretreatment groups were pretreated
with spice aqueous extracts prior to infecting cells. Treatment
only groups are the cells treated with spice aqueous extracts only
(n=3). *P<0.05 vs. the infection group. #Means
significantly different from the coincubation group with the same
treatment, P<0.05. ST21, Salmonella enterica serovar
Typhimurium strain ST21; TNF, tumor necrosis factor; LL,
Ligustrum lucidum Ait; LC, Lysimachia christinae
Hance; MP, Mentha piperita Linn; CC, Cinnamomum
cassia Presl. |
Discussion
The present study demonstrated that LL, LC, MP and
CC aqueous extracts displayed weak in vitro inhibitory and
bactericidal activity against ST21, as evidenced by the high MIC
and MBC values. However, the in vivo study determined that 7
days of pretreatment and 4 days post-treatment with spice aqueous
extracts significantly improved body weight loss, decreased ST21
count in feces, blood and organs as well as decreasing the
production of inflammatory cytokines and Igs in blood. It was
hypothesized that the spice aqueous extracts underwent in
vivo metabolism to produce the necessary bioactive compounds,
which may explain for the observed differences compared with the
in vitro model. To the best of our knowledge, the present
study was the first to demonstrate the anti-ST21 effects of LL, LC,
MP and CC aqueous extracts.
ST21 can infect the blood circulatory system and
organs, which in turn stimulates a systemic immune and inflammatory
response (1,16). The present study corroborated the
literature where it was demonstrated that greater ST21 counts and
over-production of Igs and cytokines in blood and organs were
detected following infection (18,19).
Results suggested that pretreatment with spice aqueous extracts may
result in the deposition of antibacterial compounds, that
subsequently repressed the onset of ST21 infection and decreased
fecal ST21 counts. LL and MP aqueous treatments had lower MIC
values than the other spices and decreased ST21 counts in blood,
liver and spleen. Thus, it is probable that LL and MP possessed a
higher level of anti-ST compounds and thus exhibited greater
efficiency in reducing ST21 counts. It was also determined that all
four spice aqueous extracts contained substantial levels of
phenolic acids and flavonoids. The anti-ST effect of oleanolic
acid, a triterpenoic acid, has been previously reported (20) and may be responsible for the
antioxidant and anti-inflammatory effects of LL (21). Therefore, the observed lower MIC
value of LL, and greater reduction in fecal and blood ST21 counts
in LL treated mice might be partially due to the action of
oleanolic acid. Although MP essential oil has
anti-Salmonella effects (11), the active compounds remain unknown.
It is possible that the aqueous extract used in the present study
contained the bioactive compound(s), which lowered MIC value and
induced greater ST21 clearance in feces and blood, but this needs
to be validated in future studies.
In the present study, ST21 infection triggered the
release of several inflammatory cytokines including IFN-γ, IL-6,
IL-1β and TNF-α in the blood and spleen, which revealed that
infection induced systemic inflammatory stress. However, LL, LC, MP
and CC aqueous extracts significantly decreased these inflammatory
factor levels with only a mild effect observed in spleen IFN-γ
levels. These results strongly suggested that these aqueous
extracts effectively attenuated ST21-induced inflammation in the
circulatory system and organs. It has been reported that
anti-inflammatory phytochemicals such as ursolic acid, oleanolic
acid, quercetin, cinnamic acid, rosmarinic acid and coumarin are
present in LL, LC, MP and CC (22–24). The
present study identified that these spices were rich in phenolic
acids and flavonoids and it is likely that an anti-inflammatory
effect was exerted. The subsequent effect was a decrease in IFN-γ,
IL-6, IL-1β and TNF-α levels, which alleviated ST21-induced
inflammation, except LC treatment did not suppress ST21-induced
IFN-γ expression. Chang et al (25) determined that IL-12 possesses a
potential anti-inflammatory function because the expression of
IL-10, which limits inflammatory immune responses, was conditional
on the presence of IL-12 in re-stimulated Th1 memory cells. The
present study determined that LL, LC, MP and CC aqueous treatments
significantly increased spleen IL-12 production in ST21 infected
mice, which may have enhanced the anti-inflammatory effect.
Igs, produced by the host immune system, are
involved in the response against Salmonella infection
(18,19). In the present study, the
overproduction of IgA and IgM in the blood of ST21 infected mice
indicated the activation of the immune response. LL, LC, MP and CC
aqueous extract treatments repressed the formation of IgA and IgM
in blood. It is possible that the spice aqueous extracts decreased
ST21 counts and ameliorated the inflammatory status of infected
mice, which in turn diminished the burden on the host immune system
and reduced IgA and IgM production. Maghraby and Bahgat (26) determined that coumarin, an active
component of CC, had an immunomodulatory effect at both humoral and
cellular levels in mice infected by Schistosoma mansoni. It
was also reported that MP crude leaf extract improved
Schistosoma mansoni infection in mice via an
immunomodulatory effect (27). The
spices contained phenolic acids and flavonoids therefore it is also
possible that the active compound(s) exerted immune-like actions,
which in turn alleviated the production of Igs in blood of
ST21-infected mice. However, the total content of phenolic acids
and flavonoids in blood were too low to be detected, therefore, it
is difficult to conclude the roles of phenolic acids and flavonoids
in this study.
The possible mechanisms of LL, LC, MP, and CC
against ST21 were investigated via an in vitro model. CC
aqueous extract moderated the virulence activity of ST21 and
improved the ability of macrophages against ST21 infection. CC
treatment suppressed ST21 invasion into intestinal tissues more
significantly than the other spice treatments. MP aqueous extract
exhibited both bactericidal activity and the ability to suppress
ST21 invasion, which resulted in a reduction of ST21 load in mouse
feces and organs. LL aqueous extract demonstrated a similar
mechanism against ST21 infection but with a weaker effect in
attenuating the virulence of ST21 compared with MP aqueous extract.
LC aqueous extract only moderately decreased ST21 invasion,
therefore, the treatment did not inhibit ST21 infection as
efficiently as other treatments in vivo. In the early phase
of infection, the expression of TNF-α in macrophages facilitates
the eradication of intracellular Salmonella (5). The number of intracellular ST21 was
increased compared with the coincubation group following LC aqueous
extract treatment and TNF-α depletion therefore, the protective
effect of LC against ST21 infection may involve TNF-α regulation.
Furthermore, all four spice aqueous extracts induced TNF-α
expression in macrophages, which indicated that they might trigger
the activation of macrophages through TNF-α expression regulation.
Although the bactericidal activities of LL, LC, MP, and CC aqueous
extracts were weak, they were still able to alleviate ST21 by
modulating the activation of macrophages. The mechanisms and
potential compounds involved in this infection attenuation require
further investigation.
Boiled LL, LC, MP, and CC are commonly used in food
preparation and folk medicine in many Asian countries. Therefore,
investigation into the protective effects of the aqueous extracts
is more relevant and also cheaper than isolated pure active
compounds. Although the application of these aqueous extracts is
feasible and may prove useful to prevent and attenuate
Salmonella infection, the present study did not monitor the
impact of these aqueous extracts on mouse liver and kidney
functions. For example, the hepatic toxicity of coumarin, an active
compound of CC has been reported previously (28), therefore, further in vivo
studies are necessary to further examine the metabolism, safety and
efficiency of these aqueous extracts before they can be widely used
against Salmonella infection.
In conclusion, LL, LC, MP, and CC aqueous extract
treatment attenuated ST21 infection in mice via decreasing
bacterial counts, reducing inflammatory stress and lowering Ig
production. LL and MP aqueous extract treatments demonstrated the
most significant effect on eradicating ST21 infection. All four
aqueous extracts demonstrated different mechanisms in attenuating
ST21 invasion. The present study, to the best of our knowledge, is
the first to demonstrate the protective effects of LL, LC, MP, and
CC aqueous extracts in alleviating a common food-borne disease. All
four spices are widely available and the aqueous extracts easily
prepared. The present findings suggested that LL, LC, MP and CC may
be considered as potent functional foods with
anti-Salmonella effects, but this needs to be investigated
further.
Acknowledgements
The authors would like to acknowledge Dr. Yit Lung
Khung (Department of Biological Science and Technology, China
Medical University, Taiwan) for valuable suggestions on this work
and helping the authors with the English language editing of the
manuscript.
Funding
The present study was supported by China Medical
University Hospital (grant no. DMR-106-127) and the Tainan
Municipal Hospital (grant no. 105-10).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YMH and CHC designed this study, JHF and CHS
performed experiments. MCY analyzed and interpreted the data, and
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the China Medical
University animal care and use committee (permission no.
105-23).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heithoff DM, Shimp WR, Lau PW, Badie G,
Enioutina EY, Daynes RA, Byrne BA, House JK and Mahan MJ: Human
Salmonella clinical isolates distinct from those of animal
origin. Appl Environ Microbiol. 74:1757–1766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
LaRock DL, Chaudhary A and Miller SI:
Salmonellae interactions with host processes. Nat Rev
Microbiol. 13:191–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keestra-Gounder AM, Tsolis RM and Baumler
AJ: Now you see me, now you don't: The interaction of
Salmonella with innate immune receptors. Nat Rev Microbiol.
13:206–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beal RK, Wigley P, Powers C, Barrow PA and
Smith AL: Cross-reactive cellular and humoral immune responses to
Salmonella enterica serovars Typhimurium and Enteritidis are
associated with protection to heterologous re-challenge. Vet
Immunol Immunopathol. 114:84–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lalmanach AC and Lantier F: Host cytokine
response and resistance to Salmonella infection. Microbes
Infect. 1:719–726. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krzyzanowska J, Janda B, Pecio L, Stochmal
A, Oleszek W, Czubacka A, Przybys M and Doroszewska T:
Determination of polyphenols in Mentha longifolia and M.
piperita field-grown and in vitro plant samples using
UPLC-TQ-MS. J AOAC Int. 94:43–50. 2011.PubMed/NCBI
|
|
7
|
Xia EQ, Yu YY, Xu XR, Deng GF, Guo YJ and
Li HB: Ultrasound-assisted extraction of oleanolic acid and ursolic
acid from Ligustrum lucidum Ait. Ultrason Sonochem.
19:772–776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jurikova T, Mlcek J, Skrovankova S, Balla
S, Sochor J, Baron M and Sumczynski D: Black crowberry (Empetrum
nigrum L.) flavonoids and their health promoting activity.
Molecules. 21:E16852016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
An HJ, Jeong HJ, Um JY, Park YJ, Park RK,
Kim EC, Na HJ, Shin TY, Kim HM and Hong SH: Fructus Ligustrum
lucidi inhibits inflammatory mediator release through
inhibition of nuclear factor-kappaB in mouse peritoneal
macrophages. J Pharm Pharmacol. 59:1279–1285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kozłowska M, Laudy AE, Przybył J, Ziarno M
and Majewska E: Chemical composition and antibacterial activity of
some medicinal plants from lamiaceae family. Acta Pol Pharm.
72:757–767. 2015.PubMed/NCBI
|
|
11
|
Akdemir Evrendilek G: Empirical prediction
and validation of antibacterial inhibitory effects of various plant
essential oils on common pathogenic bacteria. Int J Food Microbiol.
202:35–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alzoreky NS and Nakahara K: Antibacterial
activity of extracts from some edible plants commonly consumed in
Asia. Int J Food Microbiol. 80:223–230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang CH, Yu B, Su CH, Chen DS, Hou YC,
Chen YS and Hsu YM: Coptidis rhizome and Si Jun Zi Tang can prevent
Salmonella enterica serovar Typhimurium infection in mice.
PLoS One. 9:e1053622014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sreelatha S and Padma PR: Antioxidant
activity and total phenolic content of Moringa oleifera
leaves in two stages of maturity. Plant Foods Hum Nutr. 64:303–311.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin MC, Chang CH, Su CH, Yu B and Hsu YM:
Pteris multifida, Cortex phellodendri, and probiotics
attenuated inflammatory status and immunity in mice with a
Salmonella enterica serovar Typhimurium infection. Biosci
Biotechnol Biochem. 1–12. 2018.PubMed/NCBI
|
|
16
|
Doyle MP and Erickson MC: Reducing the
carriage of foodborne pathogens in livestock and poultry. Poult
Sci. 85:960–973. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuda T, Kosaka M, Hirano S, Kawahara M,
Sato M, Kaneshima T, Nishizawa M, Takahashi H and Kimura B: Effect
of sodium-alginate and laminaran on Salmonella Typhimurium
infection in human enterocyte-like HT-29-Luc cells and BALB/c mice.
Carbohydr Polym. 125:113–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Drago-Serrano ME, Rivera-Aguilar V,
Reséndiz-Albor AA and Campos-Rodríguez R: Lactoferrin increases
both resistance to Salmonella typhimurium infection and the
production of antibodies in mice. Immunol Lett. 134:35–46. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wijburg OL, Uren TK, Simpfendorfer K,
Johansen FE, Brandtzaeg P and Strugnell RA: Innate secretory
antibodies protect against natural Salmonella typhimurium
infection. J Exp Med. 203:21–26. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Senthilkumar PK and Reetha D: Isolation
and identification of antibacterial compound from the leaves of
Cassia auriculata. Eur Rev Med Pharmacol Sci. 15:1034–1038.
2011.PubMed/NCBI
|
|
21
|
Senthil S, Sridevi M and Pugalendi KV:
Cardioprotective effect of oleanolic acid on isoproterenol-induced
myocardial ischemia in rats. Toxicol Pathol. 35:418–423. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dorman HJ, Koşar M, Başer KH and Hiltunen
R: Phenolic profile and antioxidant evaluation of Mentha ×
piperita L. (peppermint) extracts. Nat Prod Commun. 4:535–542.
2009.PubMed/NCBI
|
|
23
|
Sandhiutami NM, Moordiani M, Laksmitawati
DR, Fauziah N, Maesaroh M and Widowati W: In vitro assesment of
anti-inflammatory activities of coumarin and Indonesian cassia
extract in RAW264.7 murine macrophage cell line. Iran J Basic Med
Sci. 20:99–106. 2017.PubMed/NCBI
|
|
24
|
Wang J, Zhang Y, Zhang Y, Cui Y, Liu J and
Zhang B: Protective effect of Lysimachia christinae against
acute alcohol-induced liver injury in mice. Biosci Trends. 6:89–97.
2012.PubMed/NCBI
|
|
25
|
Chang HD, Helbig C, Tykocinski L, Kreher
S, Koeck J, Niesner U and Radbruch A: Expression of IL-10 in Th
memory lymphocytes is conditional on IL-12 or IL-4, unless the
IL-10 gene is imprinted by GATA-3. Eur J Immunol. 37:807–817. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maghraby A and Bahgat M: Immunostimulatory
effect of coumarin derivatives before and after infection of mice
with the parasite Schistosoma mansoni.
Arzneimittelforschung. 54:545–550. 2004.PubMed/NCBI
|
|
27
|
Dejani NN, Souza LC, Oliveira SR, Neris
DM, Rodolpho JM, Correia RO, Rodrigues V, Sacramento LV, Faccioli
LH, Afonso A and Anibal FF: Immunological and parasitological
parameters in Schistosoma mansoni-infected mice treated with
crude extract from the leaves of Mentha × piperita L.
Immunobiology. 219:627–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lake BG, Evans JG, Chapuis F, Walters DG
and Price RJ: Studies on the disposition, metabolism and
hepatotoxicity of coumarin in the rat and Syrian hamster. Food Chem
Toxicol. 40:809–823. 2002. View Article : Google Scholar : PubMed/NCBI
|