Introduction
Septic shock is one of the leading causes of death
in critical care medicine with an incidence that increases year by
year (1). Although technology for
the treatment of septic shock continues to advance, disease
mortality rate remains high (2). The
pathogenesis of sepsis is extremely complex, involving many systems
such as immunity, neuroendocrine and coagulation systems (3–5).
Inflammatory reactions and immune dysfunction are important
parameters in patients with septic shock. A previous study
determined that septic shock occurs as a result of excessive
deterioration of the systemic inflammatory response with the
dynamic balance of pro-inflammatory and anti-inflammatory mediators
determining the occurrence and development of septic shock
(6). The excessive uncontrolled
activation of inflammatory factors and disturbances in immune
function can lead to multiple organ failure and death.
Interleukin (IL)-6, a pro-inflammatory mediator, is
an important stimulator of acute protein synthesis by the liver
during sepsis (7). It has a long
metabolism time and is released in large quantities by stimulated
endothelial cells and macrophages. IL-6 is considered an important
marker of sepsis (8). C-reactive
protein (CRP) is a non-specific systemic inflammatory marker
protein (9), which is a typical
acute-phase reaction protein produced and secreted by hepatocytes
(10). The level of CRP is
associated with the inflammatory response and repair level of the
body, when inflammation occurs, CRP level rises rapidly in a short
time, with the recovery and remission of the disease, CRP can
quickly return to normal level (11). Interferon-γ (IFN-γ) is a type of
interferon that regulates immune function, resists viruses,
prevents tumorigenesis and inhibits the secretion of cells
(12–14).
Glucocorticoids are an important class of regulatory
molecules in the body (15). They
have important regulatory roles in development, growth, metabolism
and immune function (16–18). In clinical practice, they are widely
used as effective anti-inflammatory and immunosuppressive agents
(19). However, possible adverse
reactions include secondary infections, gastrointestinal bleeding,
and hyperglycemia (20). Therefore,
further research into glucocorticoids is required. In the present
study, hydrocortisone was administered to high-inflammation and
low-inflammation rats to assess its treatment effect and its
protective effect on the liver. This may provide a theoretical
basis for the scientific and rational application of
glucocorticoids for the treatment of septic shock.
Materials and methods
Establishment of septic shock rat
model
A total of 60 Sprague-Dawley rats (male; age, 6–8
weeks; weight, 190±10 g). were purchased from Beijing Weitong Lihua
Experimental Animal Technology Co., Ltd. Rats were housed in SPF
animal room at 20–26°C, relative humidity was 50–60% and 12-h
light/dark cycle. Rats have free access to food and drink.
Rats were randomly divided into control (n=12) and
model groups (n=48). They were fasted overnight prior to surgery
with free access to drinking water. Rats were anesthetized with an
intraperitoneal injection of 1% pentobarbital sodium (40 mg/kg;
Jiangsu Hengrui Medicine Co., Ltd.). Following supine fixation, an
incision was made in the right groin to separate the femoral
artery. The end of the heart was ligated with silk thread (size 0)
and a heparinized 22G cannula was inserted proximally. The silk
thread was ligated and fixed. The other end of the cannula was
connected to a blood pressure monitor and the mean arterial
pressure (MAP) was measured. The left external jugular vein was
isolated and placed in the tube as an injection drug route.
Following completion of the above surgery, animals were stabilized
for 10 min prior to drug injection.
Model group rats were injected with
lipopolysaccharide (10 mg/kg; Sigma-Aldrich; Merck KGaA) for 10
min. The control group was injected with an equal volume of normal
saline calculated from rat body weight (average 0.2 ml). The vital
signs monitor (Indus Instruments) continuously monitored changed in
MAP and heart rate. Rats were observed for the presence or absence
of weakness, chills, bloating, diarrhea and erect hairs.
Establishment of a successful shock model was considered when MAP
declined by at least 50% and rats were observed for the presence of
weakness, chills, bloating, diarrhea and erect hairs. Following
successful modeling, fluid resuscitation commenced. The
resuscitation fluid was treated with a compound sodium chloride
injection (30 ml/kg/h) and femoral vein quick rehydration was used
to restore normal blood pressure. A muscular injection of
penicillin (100,000 U/Kg) (North China Pharmaceutical Company,
Ltd.) was then administered. All experiments were performed in
accordance with the principles established by the Affiliated Yantai
Yuhuangding Hospital of Qingdao University Animal Ethics Committee
(project no. YHDQD1632; ethical approval date, 15/03/2017).
Experimental groups
Following resuscitation and penicillin treatment,
rats were anesthetized with an intraperitoneal injection of 1%
pentobarbital sodium (40 mg/kg), following which 0.5 ml carotid
artery blood was collected and serum was obtained via
centrifugation (1,000 × g for 5 min, at room temperature). IL-6
(cat. no. SEKR-0005-96T; Solarbio Science & Technology Co.,
Ltd.), CRP (cat. no. ABIN368062; antibodies-online) and IFN-γ (cat.
no. DY585; R&D Systems, Inc.) content in the serum of rats were
detected via ELISA. Following resuscitation and penicillin
treatment, prior to glucocorticoid treatment and according to the
levels of IL-6, CRP and IFN-γ, rats were equally divided into a
high-inflammation group (CRP ≥500 ng/ml; IL-6 ≥50 pg/ml; IFN-γ ≥150
pg/ml; n=24) and the content of IL-6, CRP and IFN-γ below the
previous parameters were low-inflammation group (n=24). The high
and low-inflammation groups were then equally divided into a
glucocorticoid therapy group (n=12) and a non-glucocorticoid
therapy group (n=12), and intervention therapy was also performed.
The glucocorticoid-treated group was injected with hydrocortisone
(6 mg/kg; Sigma-Aldrich; Merck KGaA) in the external jugular vein
and the non-glucocorticoid-treated group was injected with the same
amount of saline. The treatment was administered 6 h the rats
resuscitation, once a day for 7 days.
Monitoring of arterial pressure and
heart rate
The dietary activities (eat and drink) of rats were
observed in each group. The vital sign detector (Indus Instruments)
was used to continuously monitor the arterial pressure and heart
rate changes in each group of rats during modeling and treatment
for 7 days.
Liver function test
According to the manufacturer's instructions
(Beijing Solarbio Science & Technology Co., Ltd.), the contents
of serum alanine aminotransferase (ALT; cat. no. BC1555) and
aspartate aminotransferase (AST; cat. no. BC1565) were measured
using an automatic biochemical analyzer (Beckman Coulter,
Inc.).
ELISA detection of serum IL-6, CRP and
IFN-γ content
Arterial blood was collected from each group of rats
and the serum was separated via centrifugation at 1,000 × g for 10
min, at room temperature. ELISA kits were used to detect the
changes of serum IL-6, CRP and IFN-γ contents according to the
manufacturer's protocol.
Hematoxylin and eosin (H&E)
staining
Liver tissue was removed, fixed in 4%
paraformaldehyde at 4°C for 12 h, embedded in paraffin and cut into
4 µm sections. Samples were rinsed twice with xylene for 10 min and
dehydrated with a descending alcohol series (100% alcohol for 5
min; 95% alcohol for 5 min; 75% alcohol for 5 min). Tissue was then
stained with hematoxylin for 10 min and eosin for 5 min at room
temperature (Beijing Solarbio Science & Technology Co., Ltd.).
Samples were washed with 100% alcohol and rinsed twice with xylene
for 5 min each. Neutral gum was used for sealing. Light microscopy
(Olympus Corporation; magnification, ×400) was used to observe the
pathological features of liver tissue.
Western blot analysis
Liver tissue was extracted using protein extraction
reagents (cat. no. C0481; Sigma-Aldrich; Merck KGaA). The
concentration of protein was detected using BCA protein assay kit
(Thermo Fisher Scientific, Inc.). A total of 30 µg of protein was
added to 50 µl of Laemmli sample buffer (Sigma-Aldrich; Merck KGaA)
and boiled for 5 min. Proteins (30 µg per lane) were then separated
on 10–20% SDS-PAGE and electrophoresed at 20V for 3 h. The proteins
were separated according to their molecular weight. Protein samples
were transferred to polyvinylidene difluoride membranes (EMD
Millipore), which were subsequently blocked with 5% skimmed milk at
4°C overnight. Membranes were incubated with primary antibodies
anti-phosphorylated (p)-p38 mitogen-activated protein kinase (MAPK;
1:1,000; cat. no. ab195049; Abcam), anti-p38MAPK (1:1,000; cat. no.
ab31828; Abcam), anti-p-NF-κB-p65 (1:2,000; cat. no. ab86299;
Abcam) and anti-NF-κB-p65 (1:1,000; cat. no. ab16502; Abcam)
overnight at 4°C. Membranes were washed with PBS three times then
incubated with horseradish peroxidase-labeled goat anti-rabbit IgG
(1:2,000; cat. no. ab6721; Abcam) at 37°C for 1 h. Membranes were
washed with PBS three times and protein band were visualized using
enhanced chemiluminescence detection system (Thermo Fisher
Scientific, Inc.). Gray-scale scanning and quantification were
performed using Image J software (version 1.32; National Institutes
of Health) with protein levels normalized to β-actin.
Statistical analysis
Data were analyzed using SPSS19.0 statistical
software (SPSS, Inc.). Data were expressed as the mean ± standard
deviation. Comparisons between two groups were analyzed using
Student's t-test. One-way analysis of variance was used for data
analysis between multiple groups with a Least Significant
Difference post-hoc test. P<0.05 was considered to indicate
statistical significance.
Results
Rat serum inflammatory factor content
increases during inflammation
According to the levels of CRP, IL-6 and IFN-γ in
the model groups, 48 rats were divided into a high-inflammation
treatment group (HT group), a high-inflammation non-treatment group
(HNT group), a low-inflammation treatment group (LT group) and a
low-inflammation non-treatment group (LNT group). Rats were classed
as high-inflammation when levels of CRP ≥500 ng/ml, IL-6 ≥50 pg/ml
and IFN-γ ≥150 pg/ml. CRP, IL-6 and IFN-γ levels in all model
groups were significantly increased compared with the control group
(P<0.01; Table I). The serum
contents of CRP, IL-6 and IFN-γ in the low inflammation group were
significantly lower than those of the high inflammation group
(P<0.05; Table I).
 | Table I.Content of CRP, Il-6, IFN-γ in serum
of rats following septic shock model establishment. |
Table I.
Content of CRP, Il-6, IFN-γ in serum
of rats following septic shock model establishment.
| Parameters | Control | HT group | HNT group | LT group | LNT group |
|---|
| CRP (ng/ml) | 303.02±16.21 |
604.47±15.09a |
604.33±19.47a |
466.54±19.81a,b |
459.23±17.79a,b |
| IL-6 (pg/ml) | 14.64±2.83 |
61.86±2.48a |
61.67±2.92a |
46.31±2.58a,b |
45.73±2.73a,b |
| IFN-γ (pg/ml) | 56.28±4.90 |
349.75±8.56a |
354.52±9.65a |
127.53±7.05a,b |
128.51±7.58a,b |
Liver damage indicators increase
during inflammation
The arterial pressures of all model groups were
significantly lower compared with the control group (P<0.01;
Table II). Similarly, heart rate,
ALT and AST levels were significantly higher compared with the
control group (P<0.01; Table
II). However, there was no significant difference amongst
inflammation groups of the model group (P>0.05; Table II).
 | Table II.Changes of the rats arterial pressure,
heart rate, ALT and AST. |
Table II.
Changes of the rats arterial pressure,
heart rate, ALT and AST.
| Parameters | Control | HT group | HNT group | LT group | LNT group |
|---|
| Arterial
pressure | 102.01±5.15 |
56.86±6.94a |
58.46±6.38a |
55.73±7.07a |
52.79±7.17a |
| Heart rate | 314.58±11.16 |
496.17±13.11a |
496.42±13.45a |
491.92±10.75a |
493.42±11.24a |
| ALT (U/l) | 41.66±2.87 |
94.15±5.47a |
95.20±5.32a |
81.19±1.98a |
82.68±3.53a |
| AST (U/l) | 116.30±4.96 |
181.77±6.33a |
184.55±7.09a |
165.72±6.15a |
167.15±4.53a |
Serum CRP, IL-6 and IFN-γ levels decrease in
high-inflammation septic shock rats following glucocorticoid
therapy. There were no marked differences in serum CRP, IL-6 and
IFN-γ levels between the HT group and control group following 7
days of treatment (P>0.05; Fig.
1). Before 7 days of treatment, the CRP, IL-6 and IFN-γ levels
in the four model groups were significantly higher than control
group. In the LT group, following 7 days of treatment, the content
of serum CRP, IL-6 and IFN-γ was significantly higher compared with
the control group (P<0.01; Fig.
1). The content of CRP, IL-6 and IFN-γ in HNT group at each
time point was significantly higher compared with the control and
HT groups (P<0.01; Fig. 1).
However, no marked differences were identified between the LNT
group and LT group (P>0.05). These results demonstrated that
steroid glucocorticoid therapy was more effective in attenuating
inflammation in the high-inflammation group compared with the
low-inflammation group.
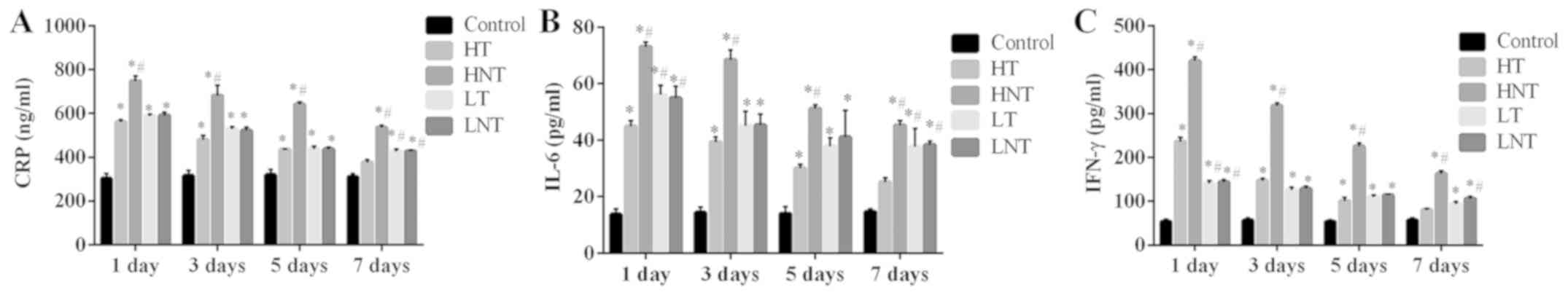 | Figure 1.Serum levels of CRP, IL-6 and IFN-γ
are decreased in high-inflammation septic shock model rats
following glucocorticoid therapy. (A) CRP, (B) IL-6 and (C) IFN-γ
levels were measured on days 1, 3, 5 and 7 following the
establishment of a septic shock model (n=12). *P<0.01 vs. the
control group; #P<0.01 vs. the HT group. CRP,
C-reactive protein; IL, interleukin; IFN, interferon; HT,
high-inflammation treatment; HNT, high-inflammation non-treatment;
LT, low-inflammation treatment; LNT, low-inflammation
non-treatment. |
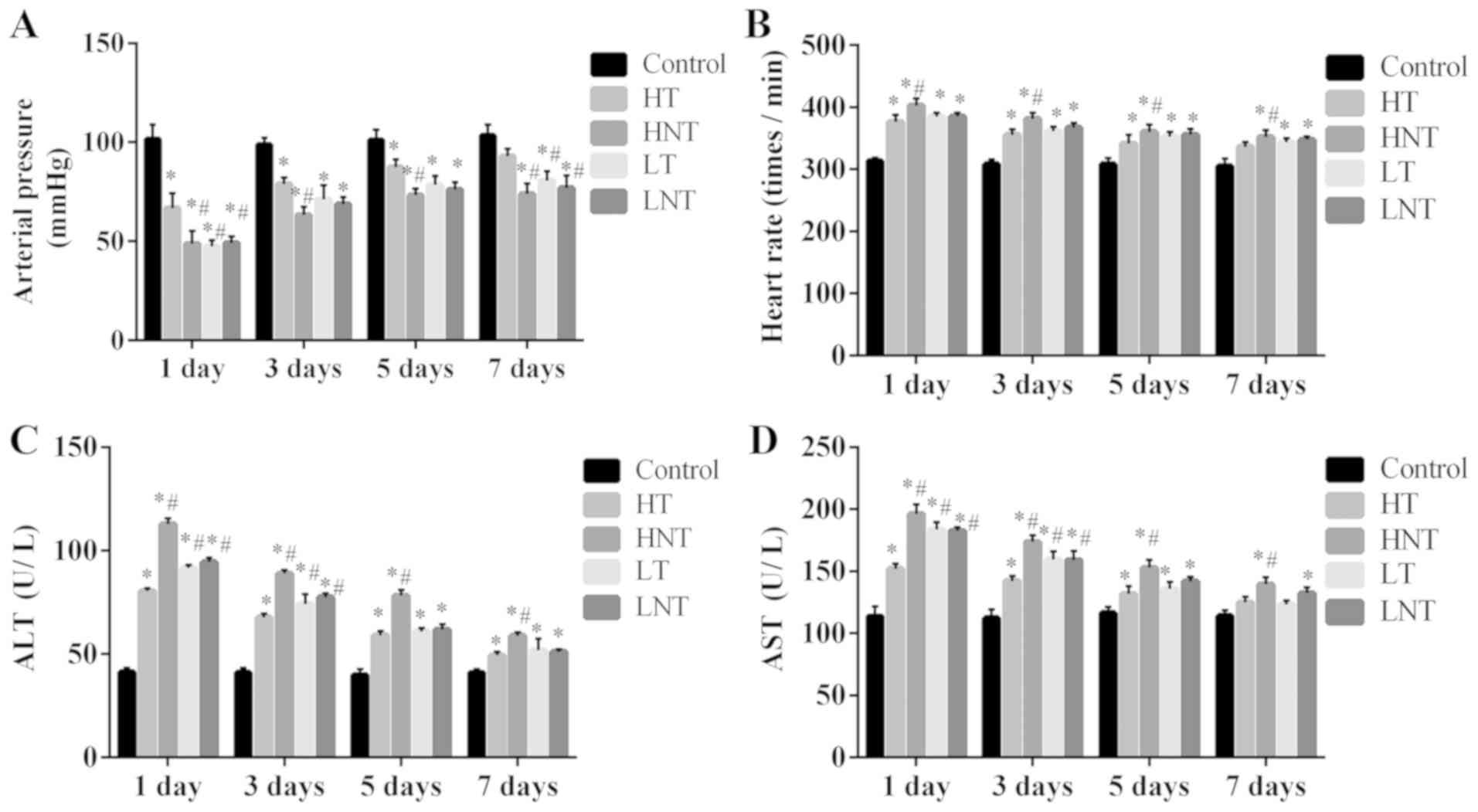
Arterial pressure and liver function
increases and heart rate decreases following the glucocorticoid
therapy of high-inflammation septic shock model rats
Following 7 days of treatment, the arterial pressure
of HT rats was significantly increased compared with the other
model groups (P<0.01; Fig. 2A),
but revealed no significant difference compared with the control
group (P>0.05; Fig. 2A). The
arterial pressure of rats in LT group was also significantly lower
than those of the HT group (P<0.01; Fig. 2A). Heart rate decreased for HT and LT
group after treatment and there were no marked differences for HT
rats compared with the control group following 7 days of treatment
(P>0.05; Fig. 2B). At day 1,
levels of serum ALT and AST in each model group were significantly
increased compared with the control group. Furthermore, the content
of ALT and AST in the LT group was significantly increased compared
with the HT group at day 1 (P<0.01; Fig. 2C and D). Following 7 days of
treatment, in comparison with control group, serum ALT in HT group
was significantly higher (P<0.01), whilst AST levels was no
significant difference (P>0.05). ALT was slightly higher in the
LT group compared with the HT group, but not significantly
(P>0.05; Fig. 2C and D).
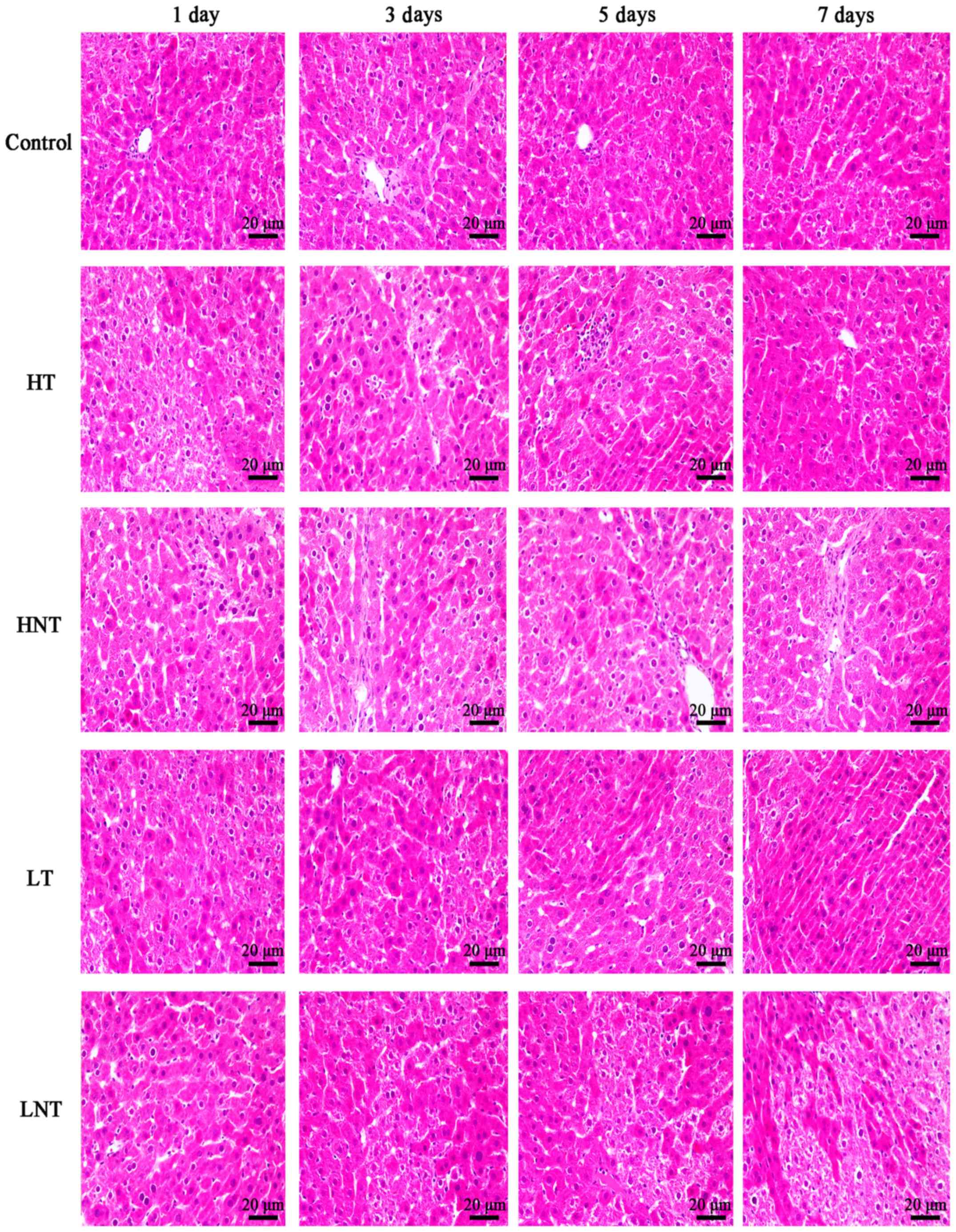
Pathological changes of septic shock
model rats
Fig. 3 demonstrates
that the liver tissue structure of the control rats was intact and
clear, without edema or inflammation. In the HT group, the hepatic
sinusoids were dilated with local congestion. At day 3, there was
hepatic sinusoidal dilatation and portal area fibrosis. At day 5,
hepatic lobular hepatic sinusoidal micro dilatation and
microthrombus formation was observed. Hepatocytes were uniform in
size and comparable to normal tissues on 7 days. In the HNT group,
hepatic sinusoids expanded with necrosis at day 1. Hepatic
sinusoidal expansion, necrosis, hepatocytes size were heterogeneous
at day 3. On 5 days there were areas of necrosis and collapse of
hepatic lobular structure, inflammatory cell infiltration.
Following 7 days, there were areas of necrosis and fibrosis in
portal area and hepatic sinusoidal dilation. In the LT group,
hepatic sinusoids extended, hepatocytes were dead and portal area
enlarged at day 1. On day 3, the structure of hepatic lobule was
abnormal and there was hepatic sinusoidal dilation and necrosis. At
day 5, hepatic lobular structure disappeared, hepatic sinusoids
expanded and necrosis was apparent. Hepatic lobule structure was
abnormal, hepatic sinusoidal dilatation occurred, portal area was
enlarged and necrosis was present at 7 days. In LNT group, hepatic
sinusoidal dilatation occurred, portal area was enlarged, hepatic
cell underwent vacuolization at day 1. Hepatic sinusoids dilated,
hepatocyte swelled, necrosis occurred and microthrombus formed at
day 3. Hepatic sinusoidal dilation was present, hepatic cord
disappeared and microthrombus formation occurred at day 5. Hepatic
lobule structure had almost disappeared, hepatic sinusoid expanded
and sheet necrosis was observed at day 7.
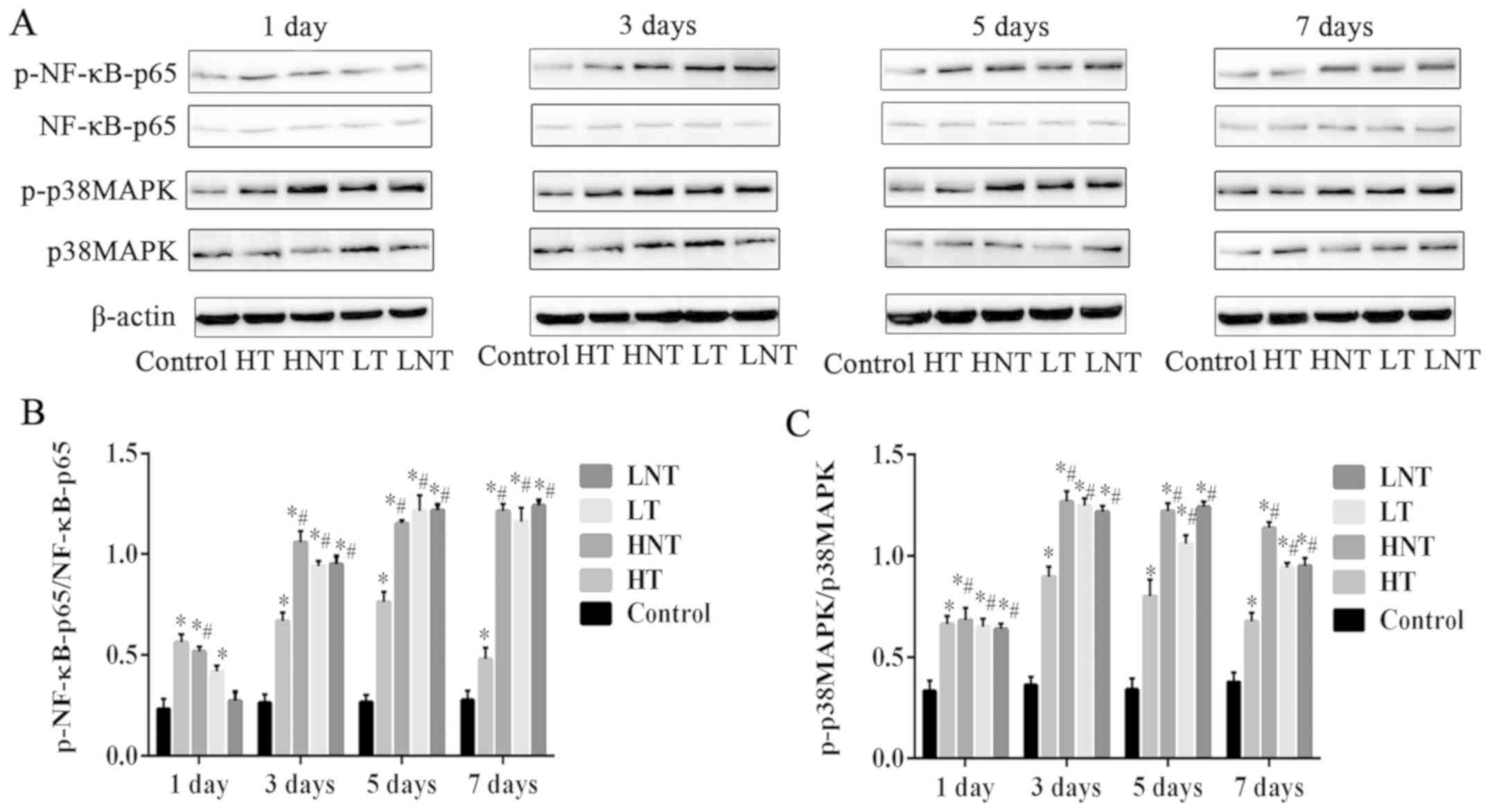
Phosphorylation of NF-κB-p65 and
p38MAPK decreases following glucocorticoid treatment of
high-inflammation septic shock model rats
From day 3 onwards, the phosphorylation levels of
NF-κB-p65 (Fig. 4A and B) and
p38MAPK (Fig. 4A and C) proteins in
the HT group were significantly decreased compared with the other
model groups (P<0.01). LT, LNT and HNT groups displayed
significantly higher phosphorylation compared with the control
group (P<0.01; Fig. 4). The level
of NF-κB-p65 protein phosphorylation was highest in LNT group, and
the level of p38MAPK protein phosphorylation was highest in HNT
group, whilst there was no obvious difference between LT group and
LNT group at day 7. These results demonstrated that glucocorticoid
treatment had more beneficial effect in the high-inflammation group
compared with the low-inflammation group.
Discussion
Glucocorticoids are considered to be the most
effective anti-inflammatory drugs (21), but their therapeutic role in sepsis
remains controversial, particularly regarding the proper dose of
them. A large number of clinical studies have determined that the
concentration of inflammatory factors in low in the blood of sepsis
patients receiving hydrocortisone (22,23).
Furthermore, glucocorticoids are immunosuppressive agents, and
different degrees of immune function inhibition may occur in the
course of application. A previous study demonstrated that high
doses of glucocorticoids increases the possibility of double
infection and mortality, therefore the use of high doses of
glucocorticoids was prohibited (24). Hydrocortisone treatment significantly
attenuates proinflammatory cytokines in patients (25). The early application of low-dose
hydrocortisone can improve patient prognosis (26).
The present study grouped rats according to the
level of blood inflammatory factors to evaluate the effectiveness
of hydrocortisone in the treatment of high-inflammation and
low-inflammation. The results demonstrated that CRP, IL-6 and IFN-γ
HT rat serum levels decreased significantly following 7 days of
glucocorticoid treatment, with the arterial pressure, heart rate
and liver function of rats ameliorated compared with the
low-inflammation treatment group. There was no obvious difference
between HT treatment compared with the control group following 7
days of treatment. The results of H&E staining determined that
following 7 days of treatment with hydrocortisone in the HT, rat
liver tissue structure was recovered significantly compared with
rats in the LT group. Taken together, these results demonstrated
that the treatment effect of hydrocortisone on the
high-inflammation group was superior to the low-inflammatory
group.
Current research on glucocorticoid treatment for
patients with severe sepsis recommends early treatment (27); however, there is no clear standard
for ‘early’. The early clinical manifestations of patients with
severe sepsis and the corresponding monitoring of immune
inflammatory factors in the blood are factors used to determine the
use of glucocorticoid therapy. Domestic and international studies
on the timing of glucocorticoid use in patients with severe sepsis
are rare. Therefore, the timing of glucocorticoid adjuvant therapy
for patients with septic shock is currently inconclusive.
Therefore, the present study based its results on the level of
inflammatory factors.
The p38 MAPK signaling pathway is crucial in cell
signal transduction, disease development and the progression of
sepsis. It is one of the key pathways leading to the occurrence of
multiple organ dysfunction (28).
NF-κB serves a central role in the development of inflammatory
reactions. The activation and overexpression of NF-κB causes an
imbalance of cytokines and produces a cascade effect of
inflammatory responses (29,30). Studies have also demonstrated that
the p38MAPK/NF-κB signaling pathway is activated in the course of
various diseases, including sepsis and certain inflammatory
reactions (31,32). Additionally, its excessive activation
is closely associated with the development and severity of the
disease. The present study examined the expression of p38MAPK and
NF-κB-p65 in different inflammation groups and evaluated the effect
of glucocorticoid treatment on the p38MAPK/NF-κB signaling pathway.
The results determined that the phosphorylation levels of p38MAPK
protein and NF-κB-p65 protein in all the model groups were
significantly increased compared with the control. Following
treatment with hydrocortisone, the protein expression in the HT
group decreased significantly, and the phosphorylation level was
significantly inhibited after 7 days of treatment. The present
results determined that glucocorticoids have a better protective
effect on the liver of septic shock rats with high-inflammation
compared with low inflammation. The present study hypothesized that
this effect may be due to the strong anti-inflammatory effects of
glucocorticoids quickly inhibiting high-level inflammation. A
limitation of the present study is that the pathological changes
were only observed in the liver. Future work will investigate
whether glucocorticoids exert the same protective effects on lung
and kidney tissue in high inflammatory septic rats and will
elucidate the underlying reaction mechanism.
In conclusion, the present study analyzed the
effects of glucocorticoids on inflammatory markers, liver function
and tissue changes in septic shock rats. The findings provided a
theoretical basis for the use of glucocorticoids in the treatment
of septic shock according to the level of inflammation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, ML and YL designed the study. XL, ML, LL, XT and
YL performed experiments. XL, ML, LL and XT performed data
analysis, interpreted the data and acquired samples. XL and ML
contributed to pathological analysis. XL, ML and YL wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
of the Affiliated Yantai Yuhuangding Hospital of Qingdao
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schulte W, Bernhagen J and Bucala R:
Cytokines in Sepsis: Potent immunoregulators and potential
therapeutic targets-an updated view. Mediators Inflamm.
2013:1659742013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan J and Li S and Li S: The role of the
liver in sepsis. Int Rev Immunol. 33:498–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ryan T, Coakley JD and Martinloeches I:
Defects in innate and adaptive immunity in patients with sepsis and
health care associated infection. Ann Transl Med. 5:4472017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dewitte A, Lepreux S, Villeneuve J,
Rigothier C, Combe C, Ouattara A and Ripoche J: Blood platelets and
sepsis pathophysiology: A new therapeutic prospect in critical
[corrected] ill patients? Ann Intensive Care. 7:1152017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi LL and Han YQ: Research advance of
sepsis related coagulation and inflammation biomarkers. Medical
Recapitulate. 5:873–877. 2016.(In Chinese).
|
|
6
|
Spoto S, Valeriani E and Costantino S:
Nosography of systemic inflammatory response syndrome, sepsis,
severe sepsis, septic shock and multiple organ dysfunction syndrome
in internal medicine patients. Italian J Med. 9:243–251. 2015.
View Article : Google Scholar
|
|
7
|
Michalek J, Svetlikova P, Fedora M,
Klimovic M, Klapacova L, Bartosova D, Hrstkova H and Hubacek JA:
Interleukin-6 gene variants and the risk of sepsis development in
children. Hum Immunol. 68:756–760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bozza FA, Salluh JI, Japiassu AM, Soares
M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC and Bozza PT:
Cytokine profiles as markers of disease severity in sepsis: A
multiplex analysis. Crit Care. 11:R492007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alexandrov PN, Kruck TP and Lukiw WJ:
Nanomolar aluminum induces expression of the inflammatory systemic
biomarker C-reactive protein (CRP) in human brain microvessel
endothelial cells (hBMECs). J Inorg Biochem. 152:210–213. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mayer CM, Gruben HJ, Land EM, Scharnag H
and Marz W: W12-P-038 Statin induced inhibition of CRP expression
in primary human hepatocytes. Atherosclerosis. 6:70–71. 2005.
View Article : Google Scholar
|
|
11
|
Memiş D, Gursoy O, Tasdogan M, Süt N, Kurt
I, Türe M and Karamanlioğlu B: High C-reactive protein and low
cholesterol levels are prognostic markers of survival in severe
sepsis. J Clin Anesth. 19:186–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji Y, Zheng MF, Ye SG, Wu XB and Chen JY:
Agrocybe aegerita polysaccharide combined with chemotherapy
improves tumor necrosis factor-α and interferon-γ levels in rat
esophageal carcinoma. Dis Esophagus. 26:859–863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valentine L, Potts R and Premenko-Lanier
M: CD8+ T cell derived IFN-γ prevents infection by a second
heterologous virus. J Immunol. 189:5841–5848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin CF, Lin CM, Lee KY, Wu SY, Feng PH,
Chen KY, Chuang HC, Chen CL, Wang YC, Tseng PC and Tsai TT: Escape
from IFN-γ-dependent immunosurveillance in tumorigenesis. J Biomed
Sci. 24:102017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conway-Campbell BL, George CL, Pooley JR,
Knight DM, Norman MR, Hager GL and Lightman SL: The HSP90 molecular
chaperone cycle regulates cyclical transcriptional dynamics of the
glucocorticoid receptor and its coregulatory molecules CBP/p300
during ultradian ligand treatment. Mol Endocrinol. 25:944–954.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oppert M, Schindler R, Husung C, Offermann
K, Gräf KJ, Boenisch O, Barckow D, Frei U and Eckardt KU: Low-dose
hydrocortisone improves shock reversal and reduces cytokine levels
in early hyperdy-namic septic shock. Crit Care Med. 33:2457–2464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiang G, Fan M, Ma Y, Wang M, Gao J, Chen
J, Li X, Xue W, Wang Y, Gao H, et al: Anti-inflammatory actions of
Caesalpinin M2 in experimental colitis as a selective glucocoricoid
receptor modulator. Biochem Pharmacol. 150:150–159. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Annane D, Bellissant E, Bollaert PE,
Briegel J, Confalonieri M, De Gaudio R, Keh D, Kupfer Y, Oppert M
and Meduri GU: Corticosteroids in thetreatment of severe sepsis and
septic shock in adults: A systematic review. JAMA. 301:2362–2375.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Laviolle B, Annane D, Fougerou C and
Bellissant E: Gluco- and mineralocorticoid biological effects of a
7-day treatment with low doses of hydrocortisone and
fludrocortisone in septic shock. Intensive Care Med. 38:1306–1314.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shapiro NI, Trzeciak S, Hollander JE,
Birkhahn R, Otero R, Osborn TM, Moretti E, Nguyen HB, Gunnerson K,
Milzman D, et al: The diagnostic accuracy of plasma neutrophil
gelatinase-associated lipocalin in the prediction of acute kidney
injury in emergency department patients with suspected sepsis. Ann
Emerg Med. 56:52–59.e1. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M,
Fang Q, Xu Q, Wang D, Jin Y, et al: Epidemiology of severe sepsis
in critically ill surgical patients in ten university hospitals in
China. Crit Care Med. 35:2538–2546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu H, Chi D, Wang S and Liu B: Effect of
early goal-directed therapy on mortality in patients with severe
sepsis or septic shock: A meta-analysis of randomised controlled
trials. BMJ Open. 6:e0083302016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Walton E, Oliveros H and Villamor E:
Hemoglobin concentration and parasitemia on hospital admission
predict risk of multiple organ dysfunction syndrome among adults
with malaria. Am J Trop Med Hyg. 91:50–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bone R, Fisher CJ Jr, Clemmer TP, Slotman
GJ, Metz CA and Balk RA: A controlled trial of high-dose
methylprednisolone in the treatment of severe sepsis and septic
shock. N Engl J Med. 317:653–658. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Y and Ding C: Effects of
hydrocortisone on regulating inflammation, hemodynamic stability,
and preventing shock in severe sepsis patients. Med Sci Monit.
24:3612–3619. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Greenberg SB and Coursin DB: Timing of
corticosteroids in refractory septic shock: A key or wishful
thinking? Crit Care Med. 42:1733–1735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu J, Wang X, Chen Q, Chen M, Cheng L, Dai
L, Jiang H and Sun Z: The effect of early goal-directed therapy on
mortality in patients with severe sepsis and septic shock: A
meta-analysis. J Surg Res. 202:389–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yousif NG, Hadi NR, Alamran F and Zigam
QA: Cardioprotective effects of irbesartan in polymicrobial sepsis:
The role of the p38MAPK/NF-κB signaling pathway. Herz. 43:140–145.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cuzzocrea S, Crisafulli C, Mazzon E,
Esposito E, Muià C, Abdelrahman M, Di Paola R and Thiemermann C:
Inhibition of glycogen synthase kinase-3beta attenuates the
development of carrageenan-induced lung injury in mice. Br J
Pharmacol. 149:687–702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eisenhut M: Comment on ‘Duration and
intensity of NF-kappa B activity determine the severity of
endotoxin-induced acute lung injury’. J Immunol. 177:20382006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim SH and Shin TY: Anti-inflammatory
effect of leaves of Eriobotrya japonica, correlating with
attenuation of p38 MAPK, ERK and NF-kappaB activation in mast
cells. Toxicol In Vitro. 23:1215–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ishibashi Y, Matsui T, Ueda S, Fukami K
and Yamagishi SI: Advanced glycation end products potentiate
citrated plasma-evoked oxidative and inflammatory reactions in
endothelial cells by up-regulating protease-activated receptor-1
expression. Cardiovasc Diabetol. 13:602014. View Article : Google Scholar : PubMed/NCBI
|