Introduction
Non-small cell lung cancer (NSCLC) is a leading
cause of cancer-associated mortality worldwide, and the incidence
and mortality rates of NSCLC have significantly increased (1,2). NCSCLC
includes lung adenocarcinoma (LUAD) and lung squamous cell
carcinoma. Tumor metastasis is recognized as the major cause of
mortality of patients with NSCLC (3). Previous studies have identified a
number of genes that are involved in regulating NSCLC metastasis,
including twist family bHLH transcription factor 1 (4,5), patched
1 (PTCH1) (6) and transforming
growth factor β-induced long non-coding RNA (7). Elucidation of the molecular mechanisms
underlying NSCLC metastasis is urgently required, as this will
assist with the identification of novel therapeutic targets for
NSCLC.
A previous study has reported that RB-associated
KRAB zinc finger (RBAK) interacts with tumor suppressor
retinoblastoma 1 (8). However, the
roles of RBAK in human diseases remain largely elusive. A number of
studies have indicated that RBAK is involved in cancer progression
(9). In prostate cancer (PCa), RBAK
interacts with androgen receptor (AR) and is involved in the
regulation of the cell cycle (10).
Wan et al (9) revealed that
knockdown of RBAK inhibits PCa growth by inducing cell apoptosis. A
high expression level of RBAK has been associated with a shorter
survival time for patients with PCa following radical prostatectomy
(9). However, the prognostic value
and molecular functions of RBAK in NSCLC have remained elusive.
The present study identified that RBAK is
upregulated in NSCLC. Furthermore, the potential effects of RBAK on
NSCLC cell migration and invasion were investigated. The present
study may provide useful information that promotes the
understanding of the important roles of RBAK in NSCLC
metastasis.
Materials and methods
Analysis of public datasets
In the present study, three Gene Expression Omnibus
(GEO) datasets, including GSE19188 (11), GSE19804 (12) and GSE18842 (13), were downloaded from the National
Cancer for Biotechnology Information database for analysis of RBAK
expression in NSCLC (https://www.ncbi.nlm.nih.gov/geo/). The dataset
GSE19188 includes 91 NSCLC samples and 65 normal lung samples.
Furthermore, GSE19804 contains 60 paired NSCLC and normal samples,
and GSE18842 includes 46 NSCLC samples and 45 control samples. The
raw data of the mRNA expression profiles were downloaded and
analyzed using R language software. Background correction, quartile
data normalization and probe summarization were applied to the
original data. The limma tool in Bioconductor software (http://www.bioconductor.org/) was used to identify
differentially expressed genes (DEGs) between the NSCLC and normal
samples. The significance of each DEG was calculated by a classical
t-test and was presented as a P-value. In addition, The Cancer
Genome Atlas (TCGA) lung adenocarcinoma (LUAD) dataset, with 59
normal and 517 LUAD samples, which included clinical features such
as survival time, were downloaded from cBioPortal (http://www.cbioportal.org/).
Bioinformatics analysis
The Database for Annotation, Visualization and
Integrated Discovery (DAVID) bioinformatics tool (https://david.ncifcrf.gov/) was used to perform gene
ontology (GO) and Kyoto Encyclopedia of genes and genomes (KEGG)
pathway enrichment analyses. The present study used Search Tool for
the Retrieval of Interacting Genes (STRING; http://string-db.org/) to construct the PPI network
for target genes (minimum required interaction score. >0.4). In
addition, Cytoscape software version 3.6.0 (http://www.cytoscape.org/download-platforms.html)
was used for visualization of the PPI networks.
Cell culture
The human cell lines A549, H1299, 95D and H1975 were
purchased from the Cell Bank of the Chinese Academy of Sciences.
The NSCLC cell lines were cultured in RPMI 1640 medium containing
10% fetal bovine serum (FBS), 1% sodium pyruvate and 1%
penicillin/streptomycin (all Gibco; Thermo Fisher Scientific, Inc.)
at 37°C in a humidified atmosphere containing 5%
CO2.
Lentiviral constructs and
transfection
The RBAK short hairpin (sh)RNA
(3′-CCGGGCTGCTTGTATCAATAGCAAACTCGAGTTTGCTATTGATACAAGCAGCTTTTT-5′)
was purchased from Shanghai GeneChem Co., Ltd. Recombinant
lentiviral plasmids carrying RBAK shRNA were constructed according
to the manufacturer's protocol. The empty recombinant lentiviral
plasmids were used as negative controls. Reverse
transcription-quantitative (RT-q)PCR was performed to detect the
knockdown efficiency of RBAK shRNA.
RT-qPCR
Total RNA was extracted from the cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, 0.5–1.0
µg RNA was reverse-transcribed to complementary (c)DNA using the
RevertAid First Strand cDNA Synthesis kit (Takara Biotechnology
Co., Ltd.). qPCR was performed with the iQSYBR Green Supermix using
the ABI Prism 7900 platform (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The PCR conditions were as follows: Initial
denaturation at 95°C for 10 sec, followed by 40 cycles of 95°C for
5 sec, 57°C for 15 sec and 72°C for 20 sec. GAPDH was used as an
internal control. The relative expression levels of the target
genes were calculated using the 2−Δ∆Cq method (14). The following primers were used for
qPCR: GAPDH forward, 5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′; RBAK forward,
5′-TTACAGGGATGTGATGTTGGA-3′ and reverse,
5′-CTCTTCAGTCAGGGTTTTGCT-3′.
Wound healing assay
A total of 5,000 A549 cells were seeded into a
6-well plate. A circular hole was generated 16 h after serum
starvation of the cells using an Oris™ plate. Serum starvation
medium was RPMI 1640 containing 1% sodium pyruvate and 1%
penicillin/streptomycin (all Gibco; Thermo Fisher Scientific,
Inc.). Images were captured after 0, 24 and 48 h using a microscope
(CK40-F200; Olympus).
Transwell assay
Transwell assays were performed using 8-mm pore size
Transwell® plates (Corning Inc.), according to the
manufacturer's protocol. In brief, 50,000 A549 cells were seeded
into the upper chamber of the Transwell plate. The upper wells were
filled with serum-free RPMI 1640 and the bottom wells were filled
with RPMI 1640 containing 10% FBS. After 72 h of incubation, the
number of migrated cells was counted and images were captured.
Cells were stained with 0.1% crystal violet for 20 min at room
temperature. The invasion assay was performed using the above
protocol with the Transwell plates pre-coated with
Matrigel® basement membrane matrix (BD Biosciences).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 16.0.0; SPSS, Inc., Chicago, IL, USA). All
experiments were performed three times. Depending on the
comparison, a Student's t-test or Mann-Whitney U-test was performed
to assess the statistical significance between two groups. Kaplan
Meier and Cox regression analyses were applied to measure
disease-free survival (DFS), and log-rank test was conducted to
determine the survival difference based on RBAK expression.
P<0.05 was considered to indicate a statistically significant
difference.
Results
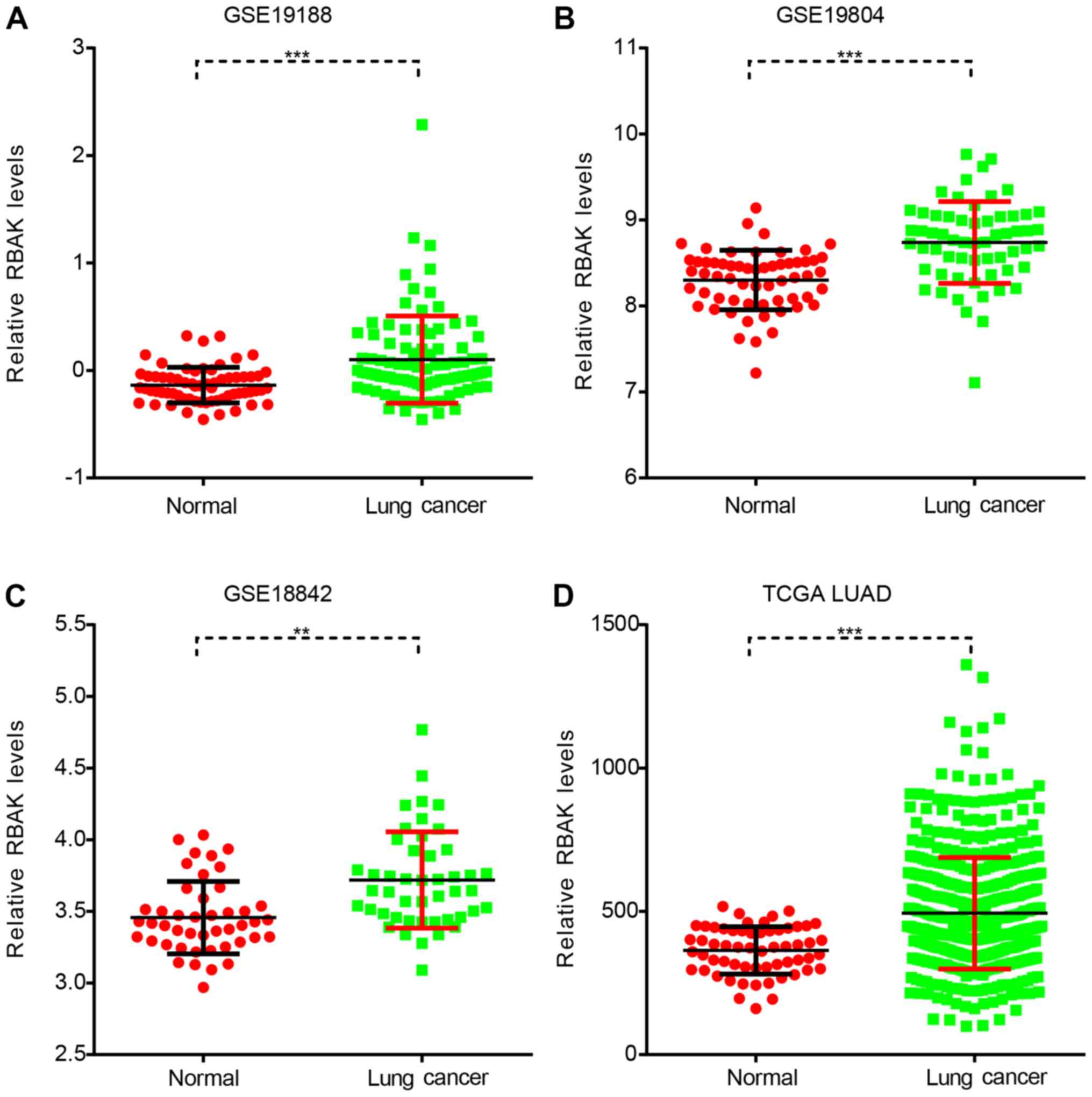
RBAK is overexpressed in NSCLC
The functional roles and expression pattern of RBAK
in human cancer, particularly NSCLC, have remained largely elusive.
The present study first analyzed RBAK expression in NSCLC using
three GEO datasets, namely GSE19188, GSE19804 and GSE18842. As
presented in Fig. 1, it was observed
that RBAK was significantly overexpressed in the NSCLC samples
compared to normal samples (Fig.
1A-C). Subsequently, TCGA LUAD dataset with 59 normal and 517
LUAD samples was analyzed. It was identified that patients with
LUAD had a higher RBAK expression level compared with that in the
normal controls (Fig. 1D).
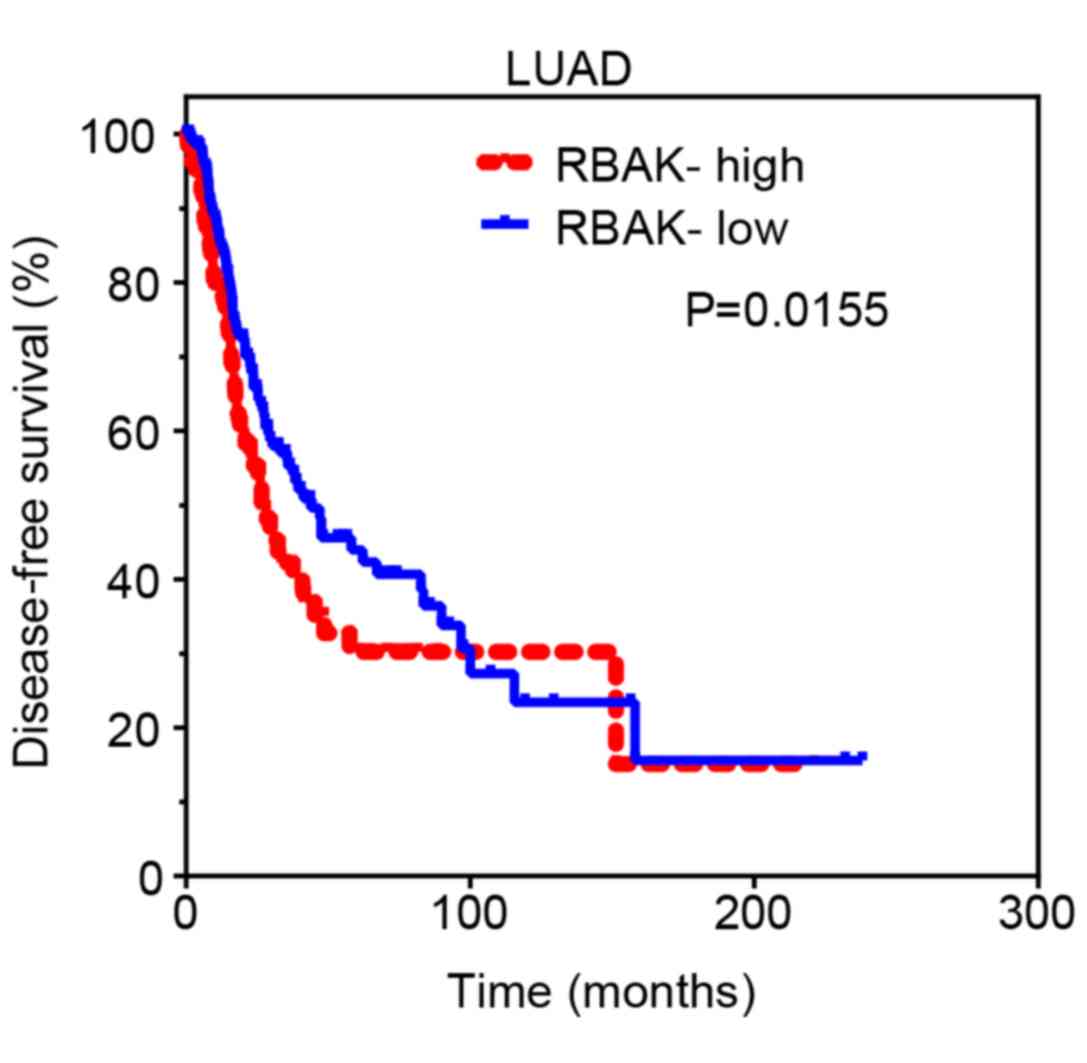
High RBAK expression is associated
with poor survival in patients with NSCLC
Subsequently, Kaplan-Meier analysis was performed to
investigate the prognostic value of RBAK in NSCLC. The cut-off
finder online system (http://molpath.charite.de/cutoff/) was used to
identify the cut-off value to stratify all NSCLC samples into
RBAK-high and RBAK-low groups. It was revealed that the DFS rate
was lower for patients with LUAD or lung squamous cell carcinoma in
the RBAK-high expression group than for those in the RBAK-low
expression group (Fig. 2). These
results suggest that RBAK may serve as a biomarker for NSCLC.
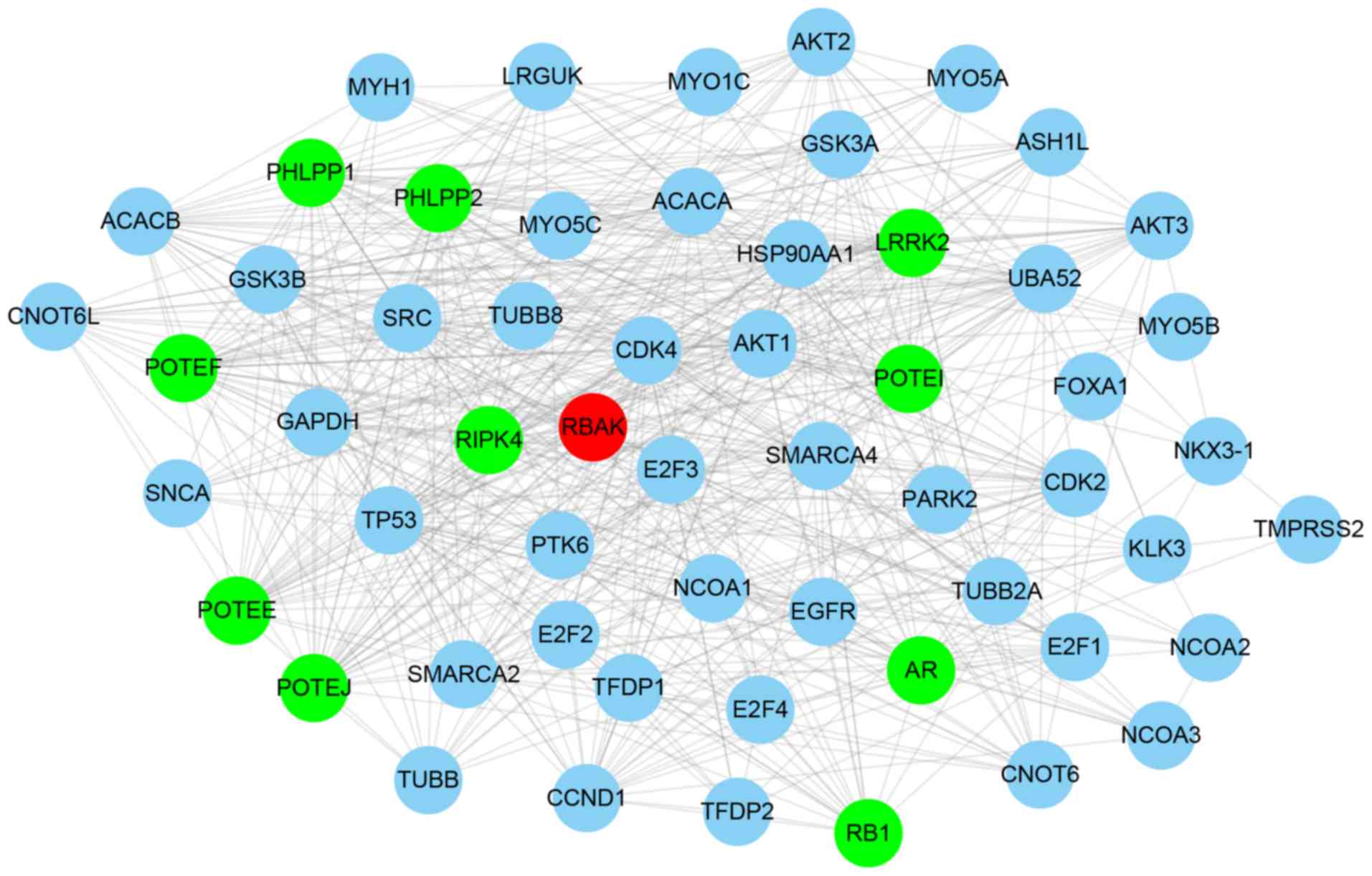
Bioinformatics analysis of RBAK in
NSCLC
Considering that the function of RBAK in NSCLC has
remained elusive, the present study performed a bioinformatics
analysis of its roles and interactions. First, an RBAK-mediated
protein-protein interaction network was constructed using the
STRING online system (https://string-db.org/cgi/network.pl). A total of 57
single nodes and 576 edges were included in the RBAK-mediated
protein-protein interaction network (Fig. 3). It was identified that the network
included a number of transcription factors, including AR, forkhead
box (FOX)A1, tumor protein (TP)53, and E2F transcription factor 1,
2 and 4, suggesting that RBAK may have a role in regulating gene
transcription. Therefore, the present study performed a
co-expression analysis for RBAK to reveal its potential roles in
NSCLC.
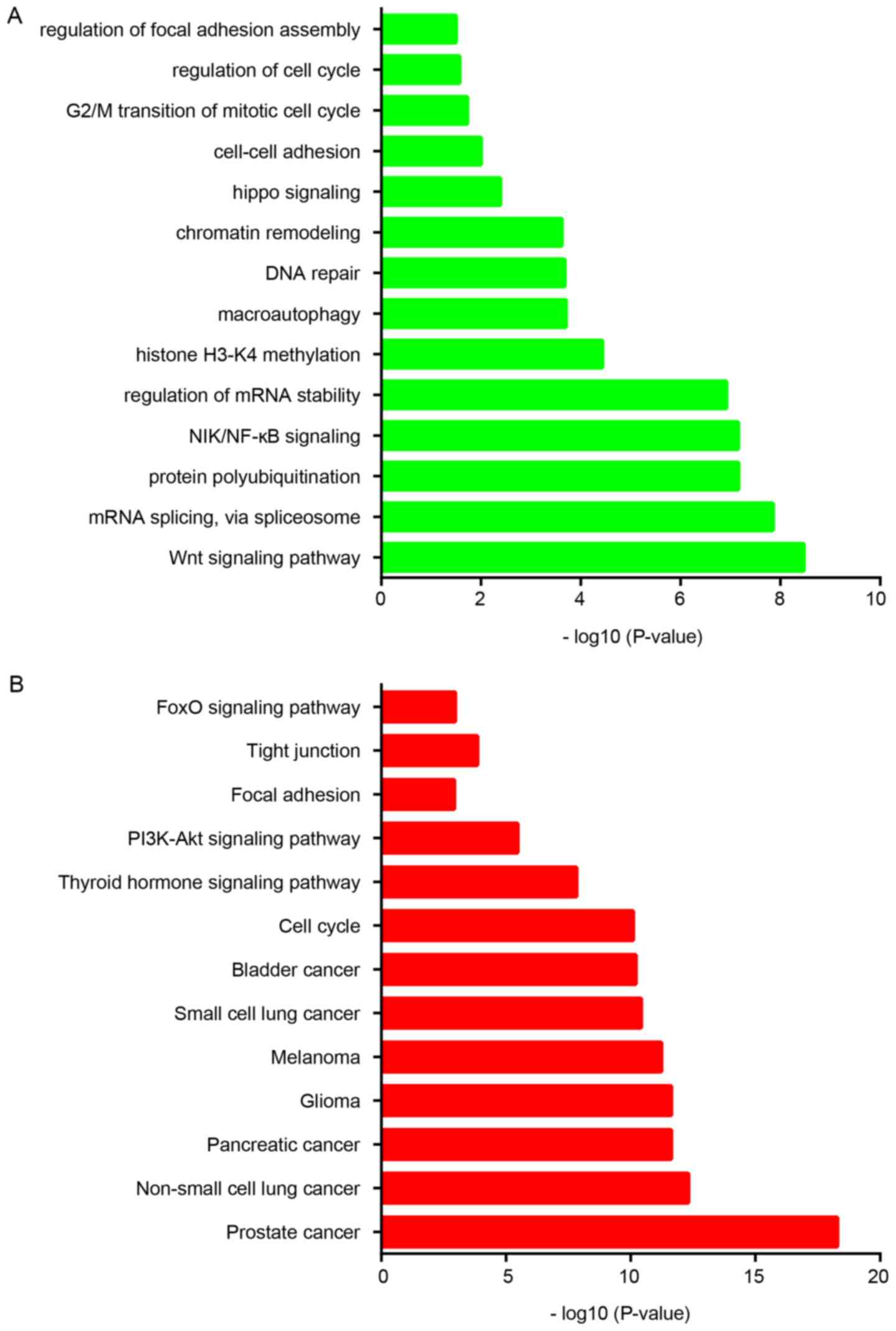
Subsequently, a functional analysis of RBAK in NSCLC
was performed based on its co-expressed genes. Gene ontology
enrichment analysis demonstrated that RBAK is involved in
regulating the Wnt signaling pathway, mRNA splicing, protein
polyubiquitination, NF-κB-inducing kinase (NIK)/NF-κB signaling,
regulation of mRNA stability, histone H3-K4 methylation,
macroautophagy, DNA repair, chromatin remodeling, Hippo signaling,
cell-cell adhesion, G2/M transition of mitotic cell cycle and focal
adhesion assembly (Fig. 4A).
Furthermore, Kyoto Encyclopedia of Genes and Genomes analysis
revealed that RBAK is significantly associated with various types
of human cancer, including NSCLC, in addition to the cell cycle,
thyroid hormone signaling pathway, tight junction and FOXO
signaling pathways (Fig. 4B).
Metastasis is a key cause of NSCLC-associated mortality. According
to the aforementioned analysis, RBAK is involved in regulating
cell-cell adhesion and focal adhesion, suggesting that RBAK may
affect the progression of metastasis in NSCLC.
Knockdown of RBAK inhibits cell
migration in NSCLC
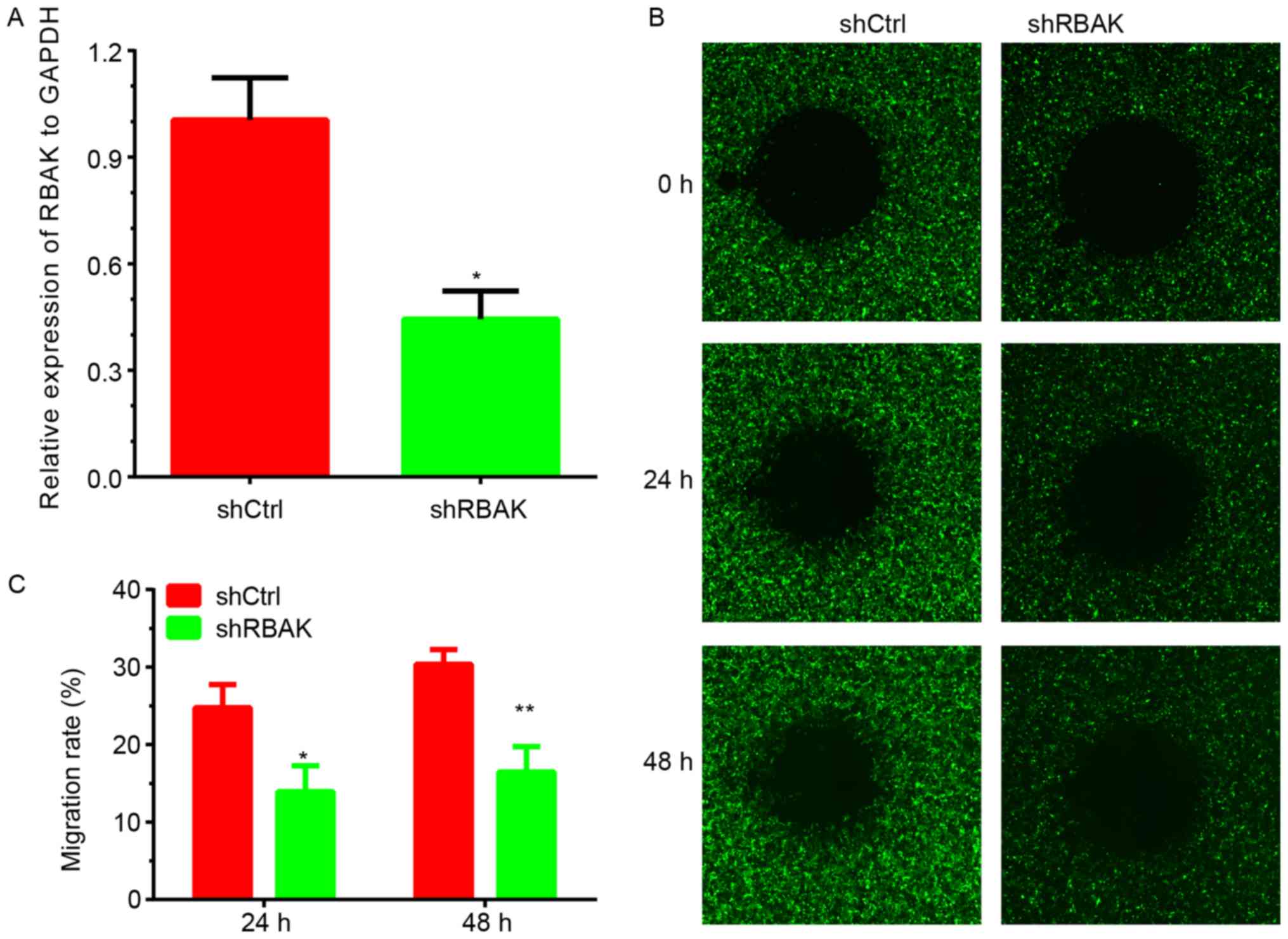
By detecting the endogenous expression of RBAK in
the human A549, H1299, 95D and H1975 cell lines, it was identified
that RBAK expression is higher in A549 cells. To further validate
the effect of RBAK on cell metastasis, the present study performed
a loss-of-function assay with NSCLC A549 cells. As presented in
Fig. 5, a wound-healing assay was
used to investigate the role of RBAK in the regulation of NSCLC
cell migration. Images of the area void of cells were obtained at
0, 24 and 48 h. After 24 h, ~25% of the wound area was repaired by
migrating cells in the control group, while only 11% of the wound
area was repaired by cells in the RBAK-knockdown group (Fig. 5B and C). After 48 h, ~30% of the
wound area was repaired by migrating cells in the control group,
while only 12% of the wound area was repaired in the RBAK-knockdown
group (Fig. 5B and C). The
transfection efficiency is presented in Fig. 5A. The knockdown efficiency is
presented in Fig. 5A and the
transfection efficiency is presented in Fig. S1.
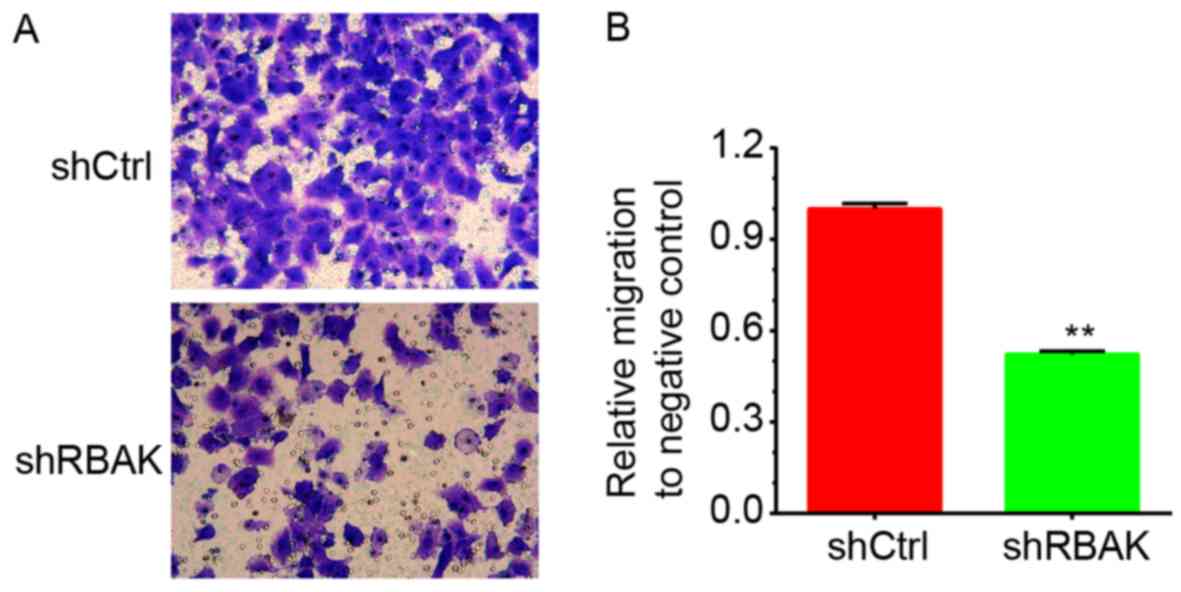
Furthermore, the present study investigated the
roles of RBAK in the regulation of NSCLC cell migration by using a
Transwell assay. As illustrated in Fig.
6, it was revealed that RBAK knockdown significantly decreased
the number of migrating A549 cells compared with that in the
negative control group (Fig. 6A and
B).
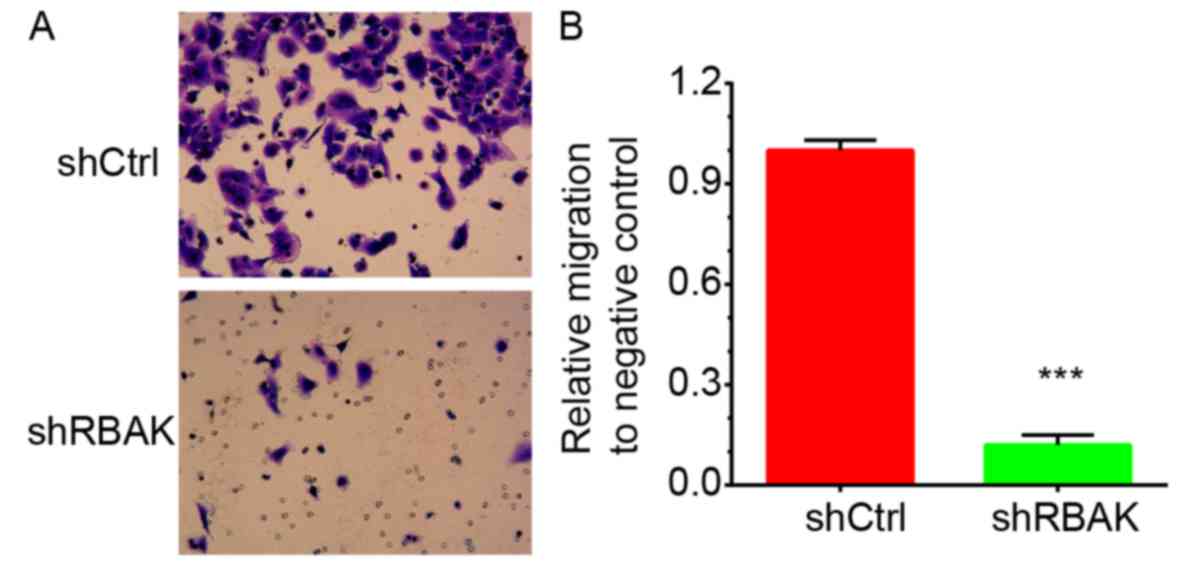
Knockdown of RBAK suppresses the
invasive ability of NSCLC cells
In addition, the roles of RBAK in the regulation of
NSCLC cell invasion were investigated. At 72 h post-transfection,
the ability of A549 cells to invade through Matrigel was assessed.
It was identified that knockdown of RBAK significantly inhibited
A549 cell invasion. RBAK knockdown decreased the number of invading
A549 cells by 88% compared with that in the negative control group
(Fig. 7A and B).
Discussion
Tumor metastasis is a major cause of mortality in
patients with NSCLC (3). The
mechanisms underlying NSCLC metastasis remain to be further
investigated. The present study was the first, to the best of our
knowledge, to identify RBAK as a novel regulator of metastasis in
NSCLC. RBAK was observed to be overexpressed in NSCLC and high
expression was associated with shorter DFS times in patients with
NSCLC. Bioinformatics analysis demonstrated that RBAK is involved
in regulating various metastasis-associated biological functions.
These roles were experimentally validated, and in vitro
assays confirmed that knockdown of RBAK suppressed NSCLC cell
migration and invasion.
A limited number of studies have indicated that RBAK
is involved in regulating cancer progression. Higher expression
levels of RBAK have been associated with a shorter survival time
for patients with PCa (9). However,
to the best of our knowledge, the expression pattern of RBAK in
NSCLC has remained largely elusive. In the present study, analysis
of public datasets revealed that the expression levels of RBAK in
NSCLC were higher compared with those in normal samples.
Furthermore, a high RBAK expression level was associated with
poorer DFS of NSCLC patients. These results suggest that RBAK may
serve as a novel prognostic marker for NSCLC.
RBAK has been identified to interact with AR and
promote cell proliferation and the cell cycle in PCa (15). In the present study, a Bioinformatics
analysis was performed to investigate the potential roles of RBAK
in NSCLC. RBAK-mediated protein-protein interaction networks were
constructed, which revealed that RBAK interacts with various key
regulators in NSCLC, including AKT1 (16), AKT2 (16), AKT3 (17), E2F1 (18), E2F2 (19), E2F3 (20), RB1 (21), cyclin D 1 (21), cyclin-dependent kinase 4 (22), epidermal growth factor receptor
(23) and TP53 (24). The present study indicated that RBAK
may be involved in regulating NSCLC progression and metastasis by
influencing cell-cell adhesion and focal adhesion. Tumor metastasis
is the major cause of mortality for patients with NSCLC. In
previous studies, a number of metastasis regulators have been
identified in NSCLC. For instance, Wan et al (6) reported that the 3′-untranslated region
of PTCH1 may promote NSCLC metastasis by activating the micro
(mi)RNA-101-3p/solute carrier family 39 member 6 axis. Ras
association domain-containing protein 1 suppresses the invasion of
NSCLC by inhibiting Yes-associated protein 1 (25). miR-483-5p promotes LUAD metastasis by
targeting Rho GDP dissociation inhibitor 1 and activated leukocyte
cell adhesion molecule (26). The
present study reported that silencing of RBAK suppresses NSCLC
migration and invasion, suggesting that RBAK promotes
metastasis-associated processes in NSCLC.
Several limitations of the present study should be
noted. First, Bioinformatics analysis demonstrated that RBAK is
involved in regulating a number of biological functions and
pathways, including the Wnt signaling pathway and mRNA splicing.
These pathways have crucial roles in NSCLC progression. Wnt
signaling regulators have widely been observed to de dysregulated
in NSCLC. Wnt signaling may regulate NSCLC cell proliferation
(27), apoptosis (28) and metastasis (29). Furthermore, the Wnt pathway may
enhance resistance to chemotherapy and radiotherapy (30). However, the effect of RBAK on these
pathways requires further validation. In addition, in vivo
experimental validation of the functions of RBAK remains to be
performed in future studies. Furthermore, the upstream regulators
of RBAK still require to be investigated. Future studies focusing
on these points may promote the understanding regarding the
important roles of RBAK in NSCLC progression
In conclusion, the present study demonstrated that
RBAK is upregulated in NSCLC vs. non-tumorous tissues samples and
may serve as a novel prognostic marker. Furthermore, the present
study was the first, to the best of our knowledge, to reveal that
RBAK is a novel regulator of cell metastasis. The present study may
serve as a basis for the development of novel potential therapeutic
approaches for NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conception and design: BH and GY. Development of
methodology and in vitro assays: BH, BW and HW. Analysis and
interpretation of data: BH, CZ, YW and LF. Writing, review and/or
revision of the manuscript: BH and GY. All authors read and
approved the final manuscript.
Ethics approval and informed consent
Not applicable.
Patients' consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small-cell lung cancer
|
|
RBAK
|
RB-associated KRAB zinc finger
|
|
PCa
|
prostate cancer
|
|
GO
|
Gene Ontology
|
|
TCGA
|
The Cancer Genome Atlas
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
LUAD
|
lung adenocarcinoma
|
References
|
1
|
Kumar MS, Hancock DC, Molina-Arcas M,
Steckel M, East P, Diefenbacher M, Armenteros-Monterroso E,
Lassailly F, Matthews N, Nye E, et al: The GATA2 transcriptional
network is requisite for RAS oncogene-driven non-small cell lung
cancer. Cell. 149:642–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji P, Diederichs S, Wang W, Böing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Millien G, Cao Y, O'Hara CJ, Tagne JB,
Hinds A, Williams MC, Ramirez MI and Kathuria H: ETS1 regulates
Twist1 transcription in a Kras (G12D) /Lkb1(−/-) metastatic lung
tumor model of non-small cell lung cancer. Clin Exp Metastasis.
35:149–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seo SK, Kim JH, Choi HN, Choe TB, Hong SI,
Yi JY, Hwang SG, Lee HG, Lee YH and Park IC: Knockdown of TWIST1
enhances arsenic trioxide- and ionizing radiation-induced cell
death in lung cancer cells by promoting mitochondrial dysfunction.
Biochem Biophys Res Commun. 449:490–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wan X, Kong Z, Chu K, Yi C, Hu J, Qin R,
Zhao C, Fu F, Wu H, Li Y and Huang Y: Co-expression analysis
revealed PTCH1-3′UTR promoted cell migration and invasion by
activating miR-101-3p/SLC39A6 axis in non-small cell lung cancer:
Implicating the novel function of PTCH1. Oncotarget. 9:4798–4813.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Z, Li Y, Che Y, Huang J, Sun S, Mao S,
Lei Y, Li N, Sun N and He J: The TGFβ-induced lncRNA TBILA promotes
non-small cell lung cancer progression in vitro and in vivo via
cis-regulating HGAL and activating S100A7/JAB1 signaling. Cancer
Lett. 432:156–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
So A, Jeske YW, Gordon RD, Duffy D,
Kelemen L and Stowasser M: No evidence for coding region mutations
in the retinoblastoma-associated Kruppel-associated box protein
gene (RBaK) causing familial hyperaldosteronism type II. Clin
Endocrinol (Oxf). 65:829–831. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wan X, Pu H, Huang W, Yang S, Zhang Y,
Kong Z, Yang Z, Zhao P, Li A, Li T and Li Y: Androgen-induced
miR-135a acts as a tumor suppressor through downregulating RBAK and
MMP11, and mediates resistance to androgen deprivation therapy.
Oncotarget. 7:51284–51300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Zhou L, Liu B, Deng Y, Wang Y,
Wang Y, Huang W, Yuan W, Wang Z, Zhu C, et al: ZNF325, a novel
human zinc finger protein with a RBaK-like RB-binding domain,
inhibits AP-1- and SRE-mediated transcriptional activity. Biochem
Biophys Res Commun. 346:1191–1199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou J, Aerts J, den Hamer B, van Ijcken W,
den Bakker M, Riegman P, van der Leest C, van der Spek P, Foekens
JA, Hoogsteden HC, et al: Gene expression-based classification of
non-small cell lung carcinomas and survival prediction. PLoS One.
5:e103122010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu TP, Tsai MH, Lee JM, Hsu CP, Chen PC,
Lin CW, Shih JY, Yang PC, Hsiao CK, Lai LC and Chuang EY:
Identification of a novel biomarker, SEMA5A, for non-small cell
lung carcinoma in nonsmoking women. Cancer Epidemiol Biomarkers
Prev. 19:2590–2597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanchez-Palencia A, Gomez-Morales M,
Gomez-Capilla JA, Pedraza V, Boyero L, Rosell R and Fárez-Vidal ME:
Gene expression profiling reveals novel biomarkers in nonsmall cell
lung cancer. Int J Cancer. 129:355–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hofman K, Swinnen JV, Claessens F,
Verhoeven G and Heyns W: The retinoblastoma protein-associated
transcription repressor RBaK interacts with the androgen receptor
and enhances its transcriptional activity. J Mol Endocrinol.
31:583–596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee MW, Kim DS, Lee JH, Lee BS, Lee SH,
Jung HL, Sung KW, Kim HT, Yoo KH and Koo HH: Roles of AKT1 and AKT2
in non-small cell lung cancer cell survival, growth, and migration.
Cancer Sci. 102:1822–1828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sung JS, Park KH, Kim ST and Kim YH:
Discovery and evaluation of polymorphisms in the AKT2 and AKT3
promoter regions for risk of Korean lung cancer. Genomics Inform.
10:167–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang T, Chen X, Qiao W, Kong L, Sun D and
Li Z: Transcription factor E2F1 promotes EMT by regulating ZEB2 in
small cell lung cancer. BMC Cancer. 17:7192017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feliciano A, Garcia-Mayea Y, Jubierre L,
Mir C, Hummel M, Castellvi J, Hernández-Losa J, Paciucci R, Sansano
I, Sun Y, et al: miR-99a reveals two novel oncogenic proteins E2F2
and EMR2 and represses stemness in lung cancer. Cell Death Dis.
8:e31412017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cooper CS, Nicholson AG, Foster C, Dodson
A, Edwards S, Fletcher A, Roe T, Clark J, Joshi A, Norman A, et al:
Nuclear overexpression of the E2F3 transcription factor in human
lung cancer. Lung Cancer. 54:155–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Betticher DC, Heighway J, Thatcher N and
Hasleton PS: Abnormal expression of CCND1 and RB1 in resection
margin epithelia of lung cancer patients. Br J Cancer.
75:1761–1768. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu A, Wu B, Guo J, Luo W, Wu D, Yang H,
Zhen Y, Yu X, Wang H, Zhou Y, et al: Elevated expression of CDK4 in
lung cancer. J Transl Med. 9:382011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yun CH, Boggon TJ, Li Y, Woo MS, Greulich
H, Meyerson M and Eck MJ: Structures of lung cancer-derived EGFR
mutants and inhibitor complexes: Mechanism of activation and
insights into differential inhibitor sensitivity. Cancer Cell.
11:217–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwaederlé M, Lazar V, Validire P,
Hansson J, Lacroix L, Soria JC, Pawitan Y and Kurzrock R: VEGF-A
expression correlates with TP53 mutations in non-small cell lung
cancer: Implications for antiangiogenesis therapy. Cancer Res.
75:1187–1190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dubois F, Keller M, Calvayrac O, Soncin F,
Hoa L, Hergovich A, Parrini MC, Mazières J, Vaisse-Lesteven M,
Camonis J, et al: RASSF1A suppresses the invasion and metastatic
potential of human non-small cell lung cancer cells by inhibiting
YAP activation through the GEF-H1/RhoB pathway. Cancer Res.
76:1627–1640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song Q, Xu Y, Yang C, Chen Z, Jia C, Chen
J, Zhang Y, Lai P, Fan X, Zhou X, et al: miR-483-5p promotes
invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1
and ALCAM. Cancer Res. 74:3031–3042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XH, Cui YX, Wang ZM and Liu J:
Down-regulation of FOXR2 inhibits non-small cell lung cancer cell
proliferation and invasion through the Wnt/beta-catenin signaling
pathway. Biochem Biophys Res Commun. 500:229–235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu C, Zhuang Y, Jiang S, Tian F, Teng Y,
Chen X, Zheng P, Liu S, Zhou J, Wu J, Wang R and Zou X:
Cinnamaldehyde induces apoptosis and reverses
epithelial-mesenchymal transition through inhibition of
Wnt/β-catenin pathway in non-small cell lung cancer. Int J Biochem
Cell Biol. 84:58–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Q, Li YD, Zhang SX and Shi YY:
Centromere protein U promotes cell proliferation, migration and
invasion involving Wnt/β-catenin signaling pathway in non-small
cell lung cancer. Eur Rev Med Pharmacol Sci. 22:7768–7777.
2018.PubMed/NCBI
|
|
30
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|