Introduction
Cardiac fibrosis is a cardiac disorder induced by
excess deposition of extracellular matrix in the cardiac muscle
(1). This pathological change causes
pressure or volume overload, which in turn leads to maladaptive
cardiac remodeling (2). Maladaptive
cardiac remodeling is usually followed by the development of
MYOCARDIAL stiffness, which is characterized by wall thickening and
increased left ventricular mass; once myocardial stiffness occurs,
electrical conduction is impaired, and the risk of arrhythmias and
heart failure is increased (3).
The occurrence and development of myocardial
fibrosis requires the involvement of multiple signaling pathways
(4). Transforming growth factor β1
(TGF-β1) serves an important role in fibroblast proliferation and
collagen synthesis in the myocardium, which contributes to the
development of myocardial fibrosis (5). Therefore, activation of TGF-β1
signaling is frequently observed in patients with myocardial
fibrosis (5). A growing body of
literature has demonstrated that inhibition of TGF-β1 may serve as
a potential therapeutic target for myocardial fibrosis (6–8). TGF-β1
achieves signal transduction not only through proteins, but also by
interacting with non-coding RNAs, such as microRNAs (miRNAs)
(9–11). miRNAs are a group of short non-coding
RNAs with crucial functions in physiological and pathological
processes (12). During the
development of myocardial fibrosis, miRNAs directly target a large
number of downstream target genes to participate in the development
of myocardial fibrosis, and regulation of miRNA expression has
exhibited potential in the clinical treatment of myocardial
fibrosis (4). miRNA-663 directly
targets TGF-β1 expression in multiple human diseases (13,14), but
its involvement in myocardial fibrosis is currently unknown. The
present study aimed to determine whether miRNA-663 participates in
myocardial fibrosis through interaction with TGF-β1.
Materials and methods
Cell line and patients
AC16 human hybrid cardiomyocytes (cat. no. SCC109)
were purchased from EMD Millipore. Cells were cultivated under in
sodium bicarbonate buffered Medium 199 (Cellgro M-199, Mediatech,
Inc; Corning) under the conditions of 37°C, 5% CO2 and
95% humidity. For TGF-β1 treatment, 104 cells cells were
cultured in 2 ml sodium bicarbonate buffered Medium 199 containing
0, 10, 20 or 40 ng/ml TGF-β1 (Sigma-Aldrich; Merck KGaA) for 24 h
prior to subsequent experiments.
Endomyocardial biopsies were obtained from 34
patients with myocardial fibrosis (patient group) admitted to the
Second Hospital of Lanzhou University between January 2015 and May
2018. Necropsies were obtained from 19 individuals (control group)
recently deceased following a car accident, in whom no cardiac
lesions were observed. Inclusion criteria of the patient group: i)
Patients diagnosed by endomyocardial biopsies; ii) patients willing
to join the study. Exclusion criteria: i) Patients diagnosed with
multiple diseases; ii) patients who received treatment within 90
days before admission. The patient group comprised 20 males and 14
females, and their age ranged from 30 to 67 years (mean age,
48.8±6.1 years). The control group comprised 11 males and 8
females, and their age ranged from 29 to 66 years (mean age,
48.0±5.6 years). This study was approved by the Ethics Committee of
the Second Hospital of Lanzhou University. The subjects or their
families signed informed consent. General information on the two
groups of participants is presented in Table I.
 | Table I.General information of the two groups
of participants. |
Table I.
General information of the two groups
of participants.
| Characteristic | Patients (n=34) | Controls (n=19) |
|---|
| Sex (n) |
|
|
| Male | 20 | 11 |
|
Female | 14 | 8 |
| Age range
(years) | 30–67 | 29–66 |
| Mean age (years) | 48.8±6.1 | 48.0±5.6 |
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA and miRNA were extracted from tissue and
cells using a MPure™ Total RNA Extraction kit (cat. no. 117022160;
MP Biomedicals, LLC) and miRNeasy Mini kit (cat. no. 217004; Qiagen
China Co., Ltd.), respectively. Total RNA was reverse transcribed
into cDNA using Tetro Reverse Transcriptase kit (Bioline Inc.) with
the following conditions: 52°C for 20 min and 75°C for 10 min.
PowerUp SYBR™ Green Master Mix (Thermo Fisher Scientific, Inc.) was
used to prepare qPCR mixtures and PCR was performed using a 7500
Fast Real Time PCR System (Applied Biosystems) with β-actin or U6
as the endogenous control. Detection of miRNA-663 and U6 was
performed using TaqMan assays (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 50 sec at 95°C, followed
by 40 cycles of 12 sec at 95°C and 32 sec at 57°C. The primers used
were: β-actin forward, 5′-GCACCACACCTTCTACAATG-3′ and reverse,
5′-TGCTTGCTGATCCACATCTG-3′; TGF-β1 forward,
5′-TACCATGCCAACTTCTGTCTGGGA-3′ and reverse,
5′-ATGTTGGACAACTGCTCCACCTTG-3′; miRNA-663a forward,
5′-AGGCGGGGCGCCGCGGGACCGC-3′; miRNA-663a reverse primer and U6
primers were included in the kit. Data were processed using the
2−ΔΔCq method (15).
Cell transfection
MISSION® miRNA Mimic hsa-miR-663 (cat.
no. HMI0895) and MISSION® miRNA Negative Control 1 (cat.
no. HMC0002) were purchased from Sigma-Aldrich (Merck KGaA).
Lipofectamine® 3000 reagent (Thermo Fisher Scientific,
Inc.) was used according to manufacturer's instructions to
transfect miRNAs into AC16 cells at a concentration of 15 nM.
Untransfected cells were used as control cells. Cells transfected
with MISSION® miRNA Negative Control 1 were negative
control cells. An miRNA-663 overexpression rate of 200% was
considered to indicate a successful transfection.
Western blotting
Total protein was extracted from successfully
transfected AC16 cells using CelLytic™ MEM Protein Extraction kit
(Sigma-Aldrich; Merck KGaA). Protein was quantified using a BCA kit
(Sangon Biotech Co., Ltd.) followed by electrophoresis using 10%
SDS-PAGE (30 µg per lane) and gel transfer (PVDF membranes),
membranes were incubated with rabbit anti-human TGF-β1 (cat. no.
ab92486; 1:1,200; Abcam), plasminogen activator inhibitor-1 (PAI-1;
cat. no. ab28207; 1:1,200; Abcam), TIMP metallopeptidase inhibitor
1 (TIMP-1; cat. no. ab109125; 1:1,200; Abcam) and GAPDH (cat. no.
ab9485; 1:1,200; Abcam) primary antibodies (4°C for 18 h), followed
by incubation with horseradish peroxidase-conjugated anti-rabbit
immunoglobulin G (cat. no. MBS435036; 1:1,200; MyBioSource, Inc.)
goat secondary antibody (24°C for 2 h). Signals were developed
using Pierce ECL Western Blotting Substrate (Thermo Fisher
Scientific, Inc.). Densitometry was performed using Image J v1.46
software (National Institutes of Health). TGF-β1, PAI-1 and TIMP-1
expression was normalized to GAPDH.
Statistical analysis
Data are presented as mean ± standard and were
processed using GraphPad Prism 6 software (GraphPad Software,
Inc.). Correlations between the expression levels of miRNA-663 and
TGF-β1 were analyzed by Pearson correlation coefficient.
Comparisons between two groups were performed using Student's
t-test. Comparisons among multiple groups were performed by one-way
ANOVA followed by Tukey's test. Receiver operating characteristic
(ROC) curve analysis was performed with the patient group as true
positive cases and the control group as true negative cases.
P<0.05 was considered to indicate a statistically significant
difference.
Results
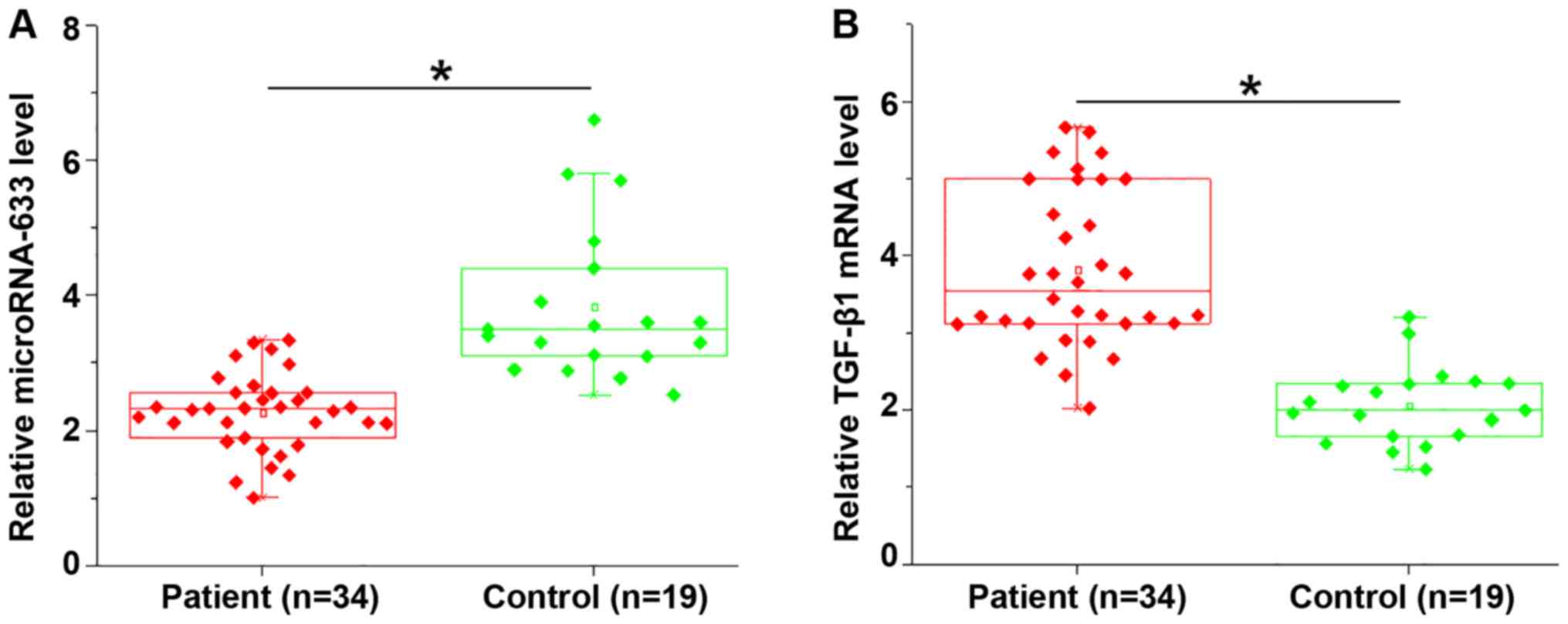
Expression levels of miRNA-663 and
TGF-β1 are altered in endomyocardial biopsies of patients with
myocardial fibrosis
Expression levels of miRNA-663 and TGF-β1 mRNA in
endomyocardial biopsies of patients with myocardial fibrosis and
necropsies of control subjects were detected by RT-qPCR. Compared
with the control group, miRNA-663 was significantly downregulated
(P=0.0124; Fig. 1A), whereas TGF-β1
was significantly upregulated (P=0.0111; Fig. 1B) in endomyocardial biopsies of
patients with myocardial fibrosis.
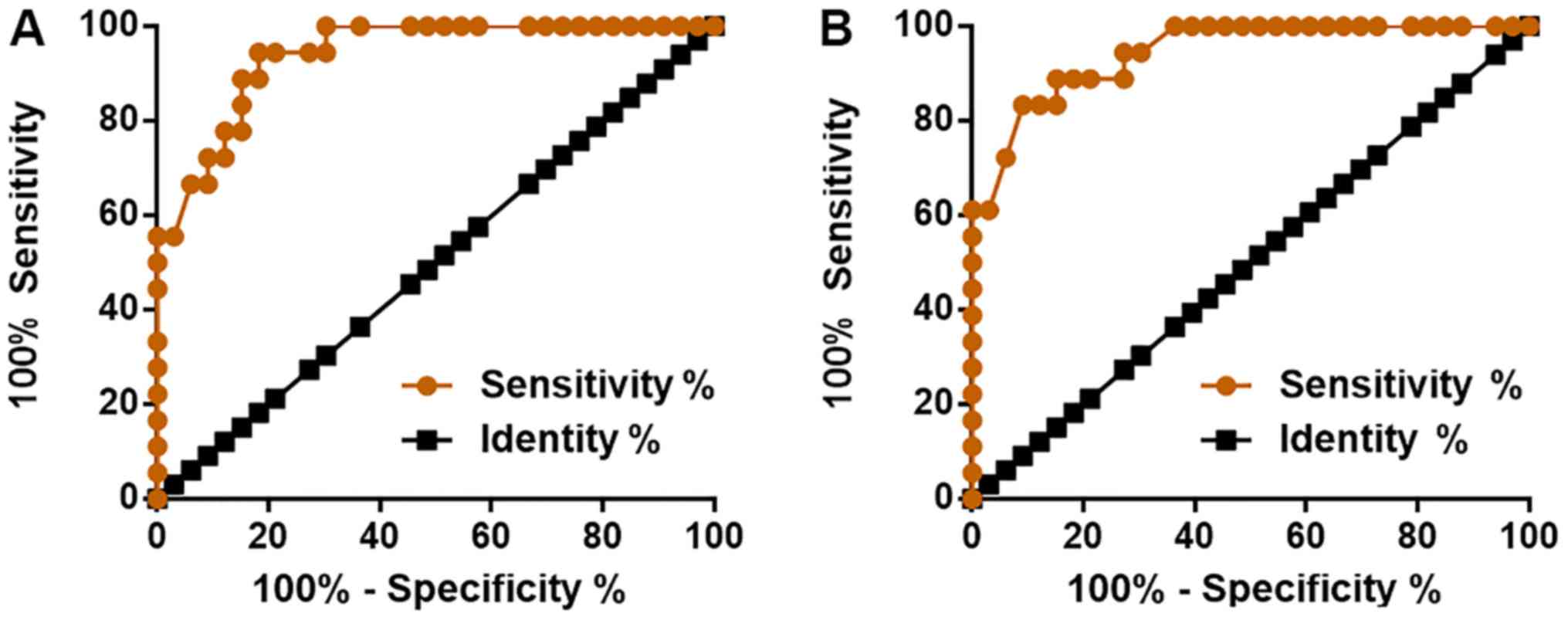
Altered expression of miRNA-663 and
TGF-β1 distinguishes myocardial fibrosis patients from
controls
To evaluate the diagnostic values of miRNA-663 and
TGF-β1 for myocardial fibrosis, ROC curve analysis was performed
using the patient group as true positive cases and the control
group as true negative cases. For miRNA-663, the area under the
curve was 0.9394 (SEM, 0.0303; 95% CI, 0.8800–0.9987; P<0.0001;
Fig. 2A). For TGF-β1, the area under
the curve was 0.9444 (SEM, 0.0296; 95% CI, 0.8865–1.0020;
P<0.0001; Fig. 2B).
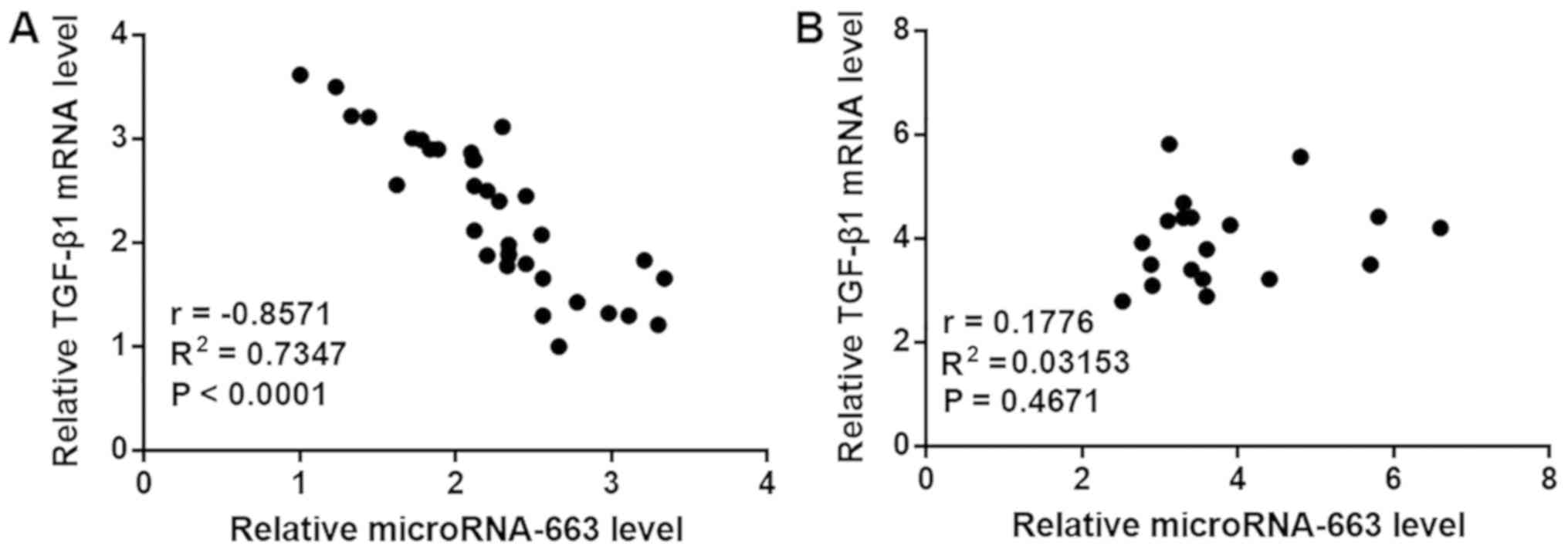
Expression of miRNA-663 is negatively
correlated with TGF-β1 in the patient group, but not in the control
group
Correlations between expression levels of miRNA-663
and TGF-β1 were analyzed by Pearson correlation coefficient. A
significant negative correlation between the expression levels of
miRNA-663 and TGF-β1 was observed in the patient group (Fig. 3A). However, no correlation was
observed between the expression levels of miRNA-663 and TGF-β1 in
the control group (Fig. 3B).
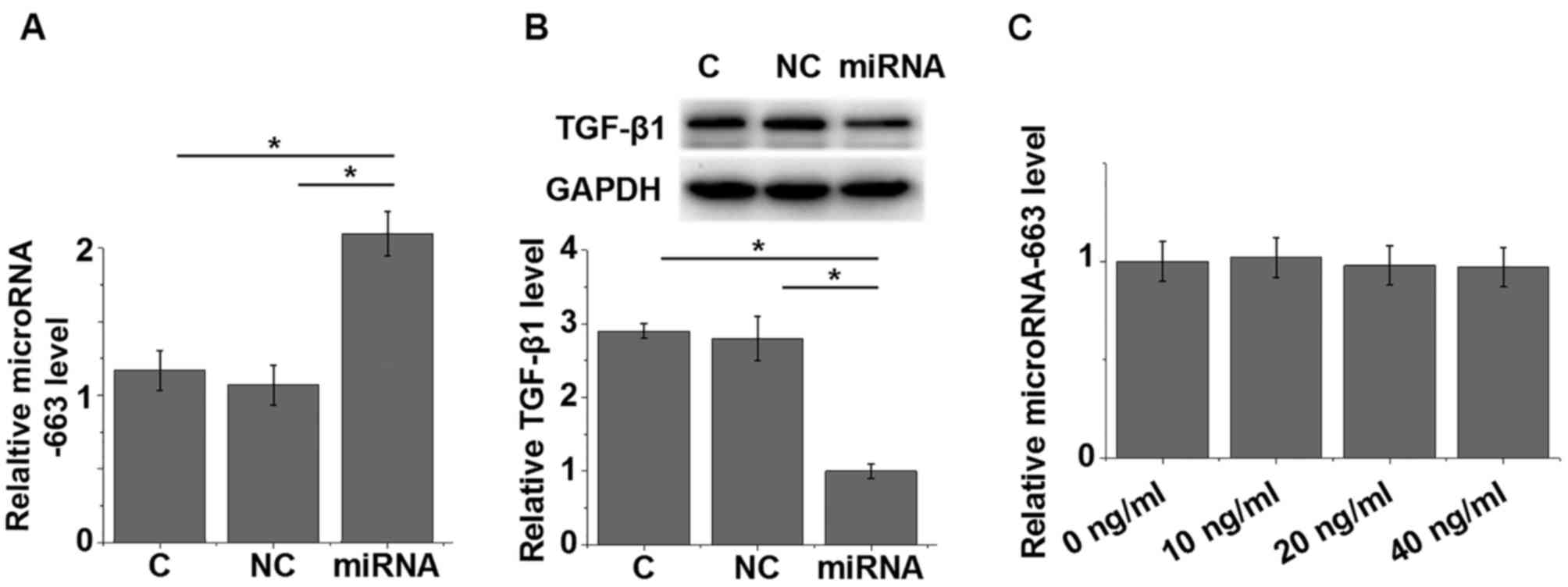
Overexpression of miRNA-663 mediates
the downregulation of TGF-β1 and myocardial fibrosis markers in
AC16 cells
Cells of the AC16 human cardiomyocyte cell line were
successfully transfected with miRNA-663 mimic (Fig. 4A). The expression of TGF-β1 was
detected by western blotting; compared with the control and
negative control groups, TGF-β1 protein expression was
significantly reduced in AC16 cells transfected with miRNA-663
mimic (P=0.0123; Fig. 4B). By
contrast, treatment with 10, 20 and 40 ng/ml TGF-β1 did not
significantly change the expression of miRNA-663 in AC16 cells
(Fig. 4C). In addition,
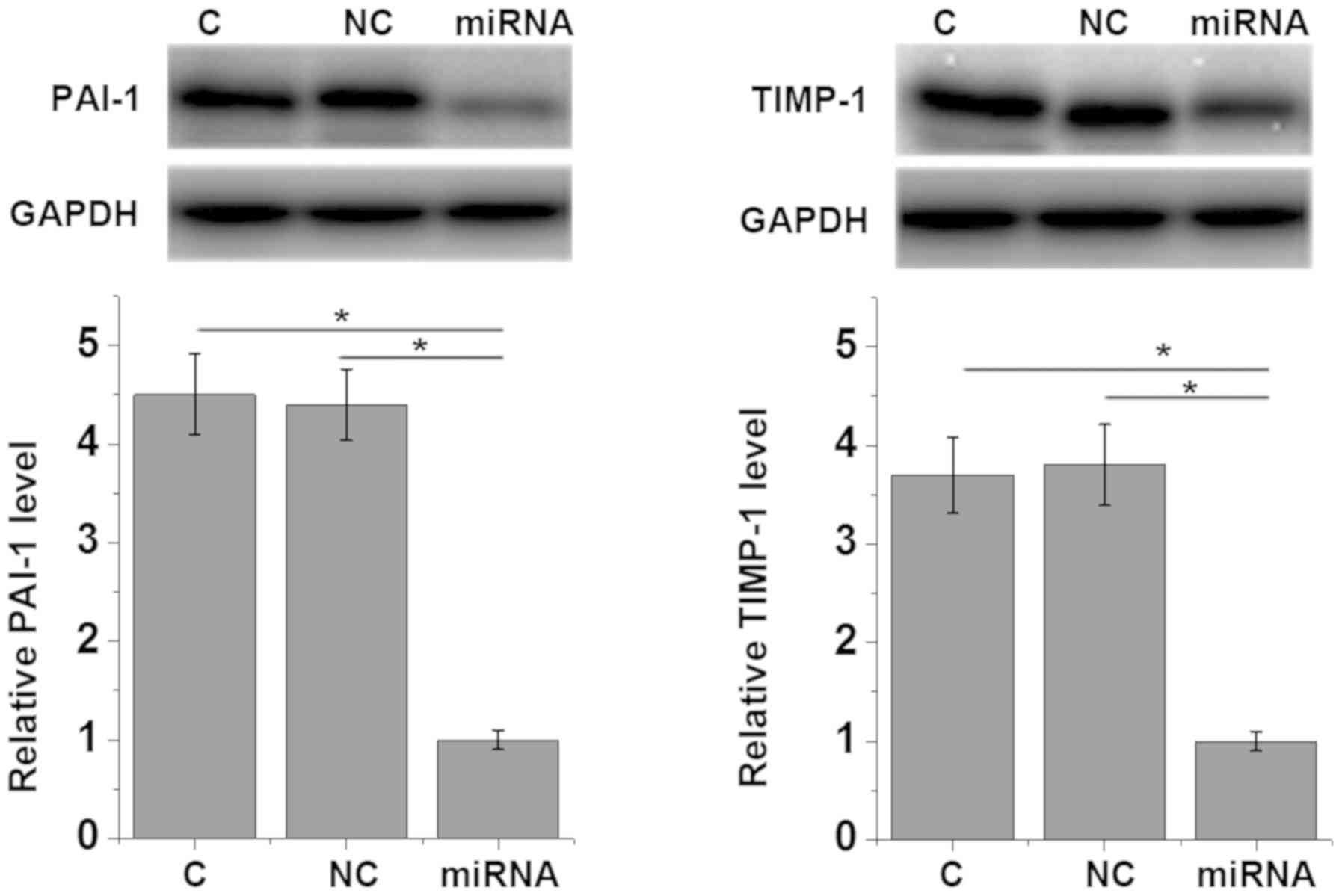
overexpression of miRNA-663 led to the reduced expression of
myocardial fibrosis markers PAI-1 (P=0.0292) and TIMP-1 (P=0.0189)
(1) compared with the control and
negative control groups (Fig. 5),
which further supported the hypothesis that miRNA-663 exhibits
inhibitory effects on myocardial fibrosis.
Discussion
miRNA-663 is a well-characterized tumor suppressor
miRNA in various types of human malignancies, including
glioblastoma, papillary thyroid carcinoma and lung cancer (13,14,16),
whereas its involvement in other human diseases is largely unknown.
The present study demonstrated that miRNA-663 may regulate the
expression of TGF-β1 to participate in myocardial fibrosis.
The development of myocardial fibrosis is
accompanied by changes in expression levels of a large number of
genes, including miRNAs (17).
Altered expression levels of certain miRNAs may reflect the
progression of myocardial fibrosis (18). As a tumor suppressor, miRNA-633 is
downregulated in different types of cancer, and the downregulation
of miRNA-633 promotes cancer cell proliferation and tumor growth
(13,14,16). In
the present study, significantly reduced expression of miRNA-663
was observed in patients with myocardial fibrosis compared with
controls. The development of myocardial fibrosis involves the
abnormal proliferation of cardiac fibroblasts (19); therefore, miRNA-663 downregulation
may be involved in the dysregulation of the proliferation of
cardiac fibroblasts. Further studies are required to test this
possibility.
The activation of TGF-β1 promotes fibroblast
proliferation and collagen synthesis in the myocardium, which in
turn contributes to the development of myocardial fibrosis
(5). TGF-β1 is overexpressed in the
majority of patients with myocardial fibrosis (20). Consistent with previous studies, the
present study observed significantly increased TGF-β1 mRNA
expression levels in patients with myocardial fibrosis compared
with those in controls. Previous studies have demonstrated that the
expression of TGF-β1 in human diseases can be regulated by miRNAs
(9–11). In the present study, a significant
reverse correlation between TGF-β1 and miRNA-663 was observed in
patients with myocardial fibrosis. The results of the in
vitro experiments using the AC16 human cardiomyocyte cell line
also demonstrated that TGF-β1 may act upstream of miRNA-663 as an
inhibitor in cardiomyocytes due to the following observations: i)
miRNA-663 reduced TGF-β1 expression; ii) exogenous TGF-β1 treatment
failed to significantly alter the expression of miRNA-663. However,
the expression levels of TGF-β1 and miRNA-663 were not
significantly correlated in controls group tissues. This is
potentially due to the in vitro overexpression system not
fully reflecting the in vivo conditions. In addition,
disease-related factors may mediate the regulation of TGF-β1
expression by miRNA-663. Overexpression of miRNA-663 also led to
the downregulated expression of myocardial fibrosis markers PAI-1
and TIMP-1, which suggests that miRNA-663 exhibits inhibitory
effects of on myocardial fibrosis.
In conclusion, miRNA-663 was significantly
downregulated, whereas TGF-β1 was significantly upregulated in
patients with myocardial fibrosis. miRNA-663 overexpression led to
downregulated TGF-β1 expression in cardiomyocytes. Therefore,
miRNA-663 overexpression may serve as a potential therapeutic
target for myocardial fibrosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the LongYuan
Youth Innovative Talents Support Program (grant no. 2109901) and
Doctoral Research Initiation Fund of Lanzhou University Second
Hospital (grant no. ynbskyjj2015-2-6).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BRG guaranteed the integrity of the entire study.
XYW, JZ and BRG designed the study concepts. BRG defined
intellectual content. XYW and JZ conducted literature research.
XYW, YLW and WSC conducted the clinical studies. JZ, XQG and YCZ
conducted the experimental studies. XYW, YLW, XQG and YCZ acquired
data. JZ, XQG and YCZ analyzed data. XYW and JZ performed
statistical analysis. XYW and JZ prepared the manuscript, and BRG
edited and reviewed the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Hospital of Lanzhou University. All
patients or their families provided written informed consent prior
to their inclusion in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gourdie RG, Dimmeler S and Kohl P: Novel
therapeutic strategies targeting fibroblasts and fibrosis in heart
disease. Nat Rev Drug Discov. 15:620–638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kong P, Christia P and Frangogiannis NG:
The pathogenesis of cardiac fibrosis. Cell Mol Life Sci.
71:549–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Talman V and Ruskoaho H: Cardiac fibrosis
in myocardial infarction-from repair and remodeling to
regeneration. Cell Tissue Res. 365:563–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo Y, Gupte M, Umbarkar P, Singh AP, Sui
JY, Force T and Lal H: Entanglement of GSK-3β, β-catenin and TGF-β1
signaling network to regulate myocardial fibrosis. J Mol Cell
Cardiol. 110:109–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seeland U, Haeuseler C, Hinrichs R,
Rosenkranz S, Pfitzner T, Scharffetter-Kochanek K and Böhm M:
Myocardial fibrosis in transforming growth factor-beta(1)
(TGF-beta(1)) transgenic mice is associated with inhibition of
interstitial collagenase. Eur J Clin Invest. 32:295–303. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao M, Zheng S, Yang J, Wu Y, Ren Y, Kong
X, Li W and Xuan J: Suppression of TGF-β1/Smad signaling pathway by
sesamin contributes to the attenuation of myocardial fibrosis in
spontaneously hypertensive rats. PLoS One. 10:e01213122015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang W, Wang C, Li L and Sun P: Inhibition
of TGF-β1 might be a novel therapeutic target in the treatment of
cardiac fibrosis. Int J Cardiol. 256:192018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Shao L, Ma A, Guan G, Wang J,
Wang Y and Tian G: Telmisartan delays myocardial fibrosis in rats
with hypertensive left ventricular hypertrophy by TGF-β1/Smad
signal pathway. Hypertens Res. 37:43–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pezzolesi MG, Satake E, McDonnell KP,
Major M, Smiles AM and Krolewski AS: Circulating TGF-β1-regulated
miRNAs and the risk of rapid progression to ESRD in type 1
diabetes. Diabetes. 64:3285–3293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Qiu Y, Shi NW, Zhao JN, Wang YC,
Jiang H and Qian HB: microRNA-21 mediates the TGF-β1-induced
migration of keratinocytes via targeting PTEN. Eur Rev Med
Pharmacol Sci. 20:3748–3759. 2016.PubMed/NCBI
|
|
11
|
Castro NE, Kato M, Park JT and Natarajan
R: Transforming growth factor β1 (TGF-β1) enhances expression of
profibrotic genes through a novel signaling cascade and microRNAs
in renal mesangial cells. J Biol Chem. 289:29001–29013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q, Cheng Q, Chen Z, Peng R, Chen R, Ma
Z, Wan X, Liu J, Meng M, Peng Z and Jiang B: MicroRNA-663 inhibits
the proliferation, migration and invasion of glioblastoma cells via
targeting TGF-β1. Oncol Rep. 35:1125–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Zhang H, Zhang P, Dong W and He L:
MicroRNA-663 suppresses cell invasion and migration by targeting
transforming growth factor beta 1 in papillary thyroid carcinoma.
Tumuor Biol. 37:7633–7644. 2016. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu ZY, Zhang GL, Wang MM, Xiong YN and
Cui HQ: MicroRNA-663 targets TGFB1 and regulates lung cancer
proliferation. Asian Pac J Cancer Prev. 12:2819–2823.
2011.PubMed/NCBI
|
|
17
|
Van Rooij E, Sutherland LB, Thatcher JE,
DiMaio JM, Naseem RH, Marshall WS, Hill JA and Olson EN:
Dysregulation of microRNAs after myocardial infarction reveals a
role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA.
105:13027–13032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang L, Ellims AH, Moore XL, White DA,
Taylor AJ, Chin-Dusting J and Dart AM: Circulating microRNAs as
biomarkers for diffuse myocardial fibrosis in patients with
hypertrophic cardiomyopathy. J Transl Med. 13:3142015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Turner NA: Inflammatory and fibrotic
responses of cardiac fibroblasts to myocardial damage associated
molecular patterns (DAMPs). J Mol Cell Cardiol. 94:189–200. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Travers JG, Kamal FA, Robbins J, Yutzey KE
and Blaxall BC: Cardiac fibrosis: The fibroblast awakens. Circ Res.
118:1021–1040. 2016. View Article : Google Scholar : PubMed/NCBI
|