Introduction
Diagnostics in aortic pathology require more
attention (1,2) and little is known about the
histological patterns of aortic pathology with regard to medial
degeneration (MD), atherosclerosis (ATS) and aortitis in relation
to potential risk factors as well as to their distribution in
different aortic segments. While MD is reported to be the leading
histological finding in cases of aneurysm, the roles of ATS and
inflammatory processes seem to be underestimated (3,4). A
clinical manifestation of all these aortic lesions is an aortic
aneurysm. As a permanent dilatation of the aortic wall, an aortic
aneurysm may affect all aortic segments, including the thoracic
aorta and abdominal aorta (5). These
aneurysms are classified according with involved aortic segments,
such as aortic root, ascending aorta, arch, or descending aorta for
thoracic aneurysms and suprarenal, juxtarenal and infrarenal aorta
for abdominal aneurysms (1). The
etiology, natural history, histology and treatment differ for each
of these aneurysms segments, because the aorta is subject to a
variety of diseases, degenerative, inflammatory, infectious and
idiopathic (3).
The aim of the present study was to assess aortic
aneurysms, including MD, ATS and inflammatory processes, according
to the newest consensus documents on non-inflammatory and
inflammatory aortic pathology (6–8) in
association with potential risk factors.
Materials and methods
Patient characteristics
The intraoperative specimens of the entire aorta
were obtained from 52 patients recruited between March 2015 and
September 2016. The specimens included 39 thoracic aortic aneurysm
(TAA) and 13 abdominal aortic aneurysm (AAA) samples from 38 men
(73%) and 14 women (27%) with ages ranging between 19 and 80 years
(average, 61.7 years). The specimens were recovered during open
aortic surgery. The patients with TAA and severe aortic valve
incompetence underwent ascending aortic replacement. Surgically,
the sinotubular junction was remodelated using Dacron grafts and
the aortic valve plane was relocated. The basic goal of surgical
repair of AAA was the exclusion of the aortic aneurysm from the
systemic circulation with preservation of blood flow to the pelvis
and legs via an implanted new bifurcated graft (Hemashield
Platinum).
The individual clinical data [age, sex, hypertension
(HTA), smoking, history of the aortic disease and previous
inflammatory processes] were obtained from medical records. The
present study was conducted according to the Helsinki Declaration
and approved by the Institutional Review Committee at The ‘Prof.
Dr. George Georgescu’, Institute of Cardiovascular Diseases.
Written informed consent was signed by all patients.
Histological analysis
For histological studies, AAT and AAA were fixed
with 10% neutral buffered formalin solution (at room temperature,
24 h) and embedded in paraffin. Cross-sections of aortic tissue (4
µm) were stained with hematoxylin and eosin for tissue morphology.
Elastic Van Gieson's staining (at room temperature, 55 min) was
used to analyze elastic fibers. The histological assessment was
performed by an experienced pathologist using an optical microscope
(CX41; Olympus Corporation; magnifications, ×40, ×100, ×200 and
×400). The measurements were visualized using color image analysis
software (QuickPHOTO MICRO 3.0; PROMICRA, s.r.o.).
To optimize the histological diagnosis, standardized
nomenclature was applied, according to consensus documentation
referring to unified nomenclature for a variety of non-inflammatory
degenerative aortic lesions (8) for
the histological analysis of the aorta specimens. ATS lesions were
present in both TAA and AAA cases, presenting various degrees of
severity with consequent aortic wall changes. The severity of the
atherosclerotic lesions in surgically resected segments of the
aorta was graded according to Stone et al (7): i) 1, mild-1; ii) 2, moderate; and iii)
3, severe.
Statistical analysis
Data were analyzed using IBM SPSS Statistics 21
Software and results were expressed as mean ± standard deviation
and percentages. The correlations between non-inflammatory
degenerative aortic lesions and potential atherosclerotic risk
factors (age>65 years, male sex, smoking, arterial hypertension,
and bicuspid aortic valve) were evaluated by Kendall's tau-b
coefficient correlation with P<0.05 being significant.
Results
An aneurysm is defined as a dilatation
of ≥50% above the normal diameter of an artery
Of the total number of patients with aortic
aneurysms who underwent aortic dilation, thoracic aorta was the
most commonly involved segment (n=39), while abdominal aorta was
only identified in one-third of cases (n=13); the two aortic
segments demonstrated different etiologies. Aortic aneurysms have a
multifactorial etiology. The multiple risk factors included
advanced age, male, smoking, HTA, ATS and bicuspid aortic valve
(BAV) (Table I).
 | Table I.Comparative analysis of risk factors
and histological characteristics in patients with surgical aortic
aneurysms. |
Table I.
Comparative analysis of risk factors
and histological characteristics in patients with surgical aortic
aneurysms.
| Histological
lesions | Patients n (%) | Age (years) | Male n (%) | HTA n (%) | Smoking n (%) | BAV n (%) |
|---|
| TAA | 39 (100) | 54.6±13.7 | 31 (79.5) | 13 (33.3) | 22 (56.4) | 5 (12.8) |
| Medial
degenerative | 32 (82.1) | 53.7±14.1 | 28 (87.5) | 12 (37.5) | 19 (59.4) | 5 (15.6) |
| Mild | 3
(7.7) | 48.7±23.0 | 3
(100) | 1
(33.3) | 2
(66.7) | 0 (0) |
|
Moderate | 17 (43.6) | 52.9±16.1 | 14 (82.4) | 5
(29.4) | 8
(47.1) | 3 (17.6) |
|
Severe | 12 (30.8) | 56.1±8.2 | 11 (91.7) | 6
(50.0) | 9
(52.9) | 2 (16.7) |
| Atherosclerotic | 9
(23.1) | 57.2±12.1 | 6
(66.6) | 1
(33.3) | 1
(16.7) | 0 (0) |
| Mild | 6
(15.4) | 57.0±14.1 | 4
(66.7) | 0
(0) | 1
(16.7) | 0 (0) |
|
Moderate | 3
(7.7) | 57.8±8.7 | 2
(33.3) | 1
(33.3) | 0
(0) | 0 (0) |
| Inflammatory | 5
(12.8) | 54.4±12.6 | 3
(60) | 5
(100) | 4
(80) | 0 (0) |
| TA | 1
(2.6) | 36 | 0
(0) | 1
(100) | 1
(100) | 0 (0) |
|
Syphilitic aortitis | 1
(2.6) | 56 | 1
(100) | 1
(100) | 1
(100) | 0 (0) |
| GCA | 3
(7.7) | 60.0±10.0 | 2
(66.7) | 3
(100) | 2
(66.7) | 0 (0) |
| AAA | 13 (100) | 70.2±8.0 | 9
(69.2) | 3
(23.1) | 6
(53.8) | 0 (0) |
| Atherosclerotic
lesions | 13 (100) | 70.2±8.0 | 9
(69.2) | 3
(23.1) | 6
(53.8) | 0 (0) |
|
Severe | 13 (100) | 70.2±8.0 | 9
(69.2) | 3
(23.1) | 6
(53.8) | 0 (0) |
The average patient age for TAA was 55.6 years in
males and 50.6 years in females and the average patient age for AAA
was 69.7 years in males and 71.2 years in females. From the entire
aortic aneurysm group (n=52), males were predominant in both the
TAA group (79.5%; 31 of 39 patients) and the AAA group (69.2%; 9 of
13 patients).
Of the risk factors, smoking was a more common
finding in TAA (56.4%; n=22) than in AAA (46.2%; n=6). Similarly,
HTA registered higher values in TAA (33.3%; n=13) than in AAA
(23.1%; n=3). BAV was found only in five of the TAA cases (12.8%)
in association with aortic regurgitation. No genetic disorder
history was identified in the study group patients.
Histopathological examination revealed only MD
lesions (64.1%, 25 of 39 patients), ATS lesions (23.1%, 9 of 39
patients), mixed lesions, MD and ATS (17.9%, 7 of 39 patients) in
patients with TAA (n=39), and microscopically proven aortitis
(12.8%, 5 of 39 patients) in TAA, while in patients with AAA (n=13)
only ATS lesions (100%) were present (Table I).
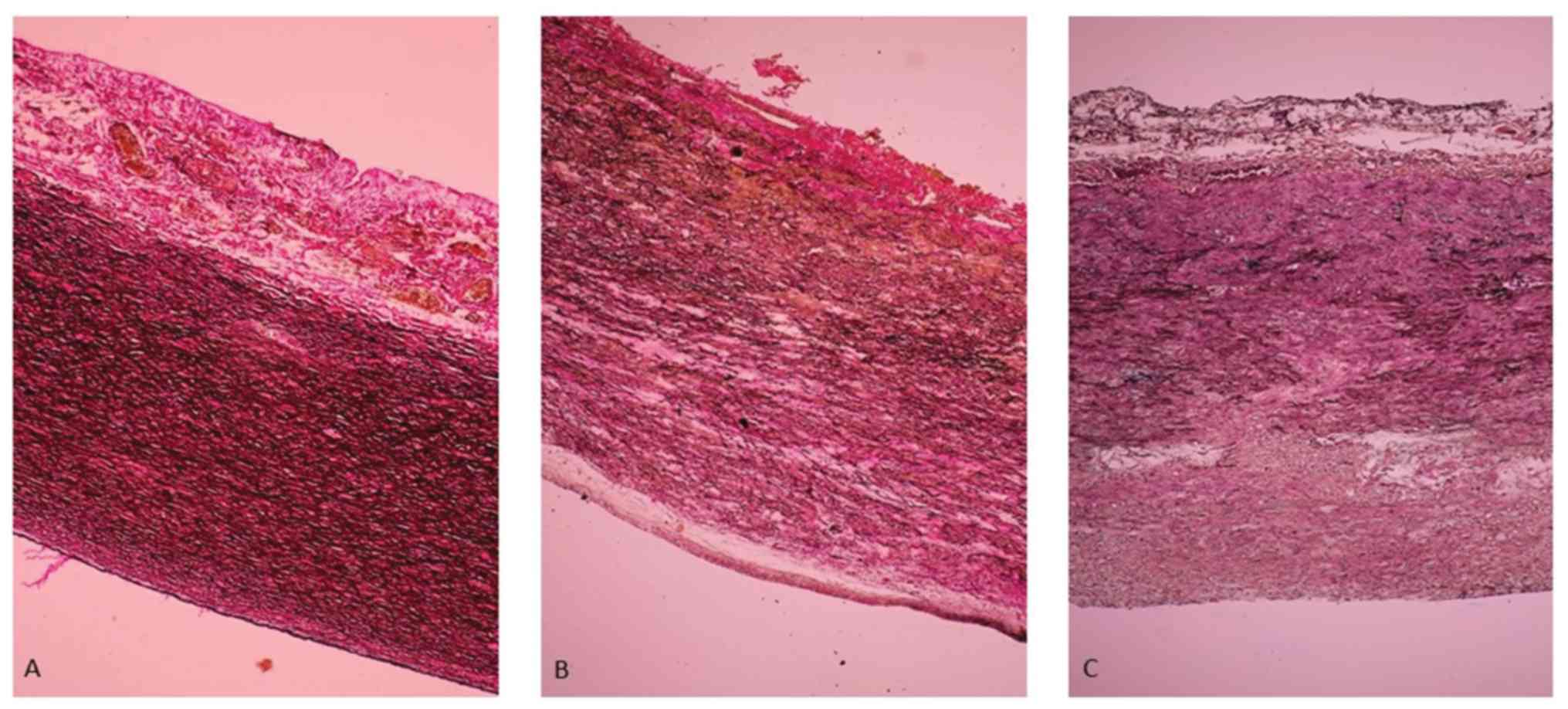
MD lesions in aortic aneurysms
A total of 32 (82.1%) cases of MD (only lesions or
together with ATS lesions) were identified in the patients with
TAA. Of all these lesions, 3 (12%) were with mild medial
degeneration (mMD), 17 (43.6%) with moderate medial degeneration
(MMD) and 12 (48%) with severe medial degeneration (SMD) (Fig. 1).
In the patients with TAA with mMD aortic lesions
(grade 1), the aortic medial wall showed a multifocal decrease in
the number of smooth muscle cells (SMCs) with minimal collagen
deposition, a decrease in number of the elastic fibers (EF) and
multifocal, mild EF fragmentation associated with focal, mild
intralamellar mucoid accumulation (Fig.
1A).
In the patients with TAA with MMD aortic lesions
(grade 2), the aortic wall had a decreased number of SMCs and
multifocal mild substitutive fibrosis, a reduction in the number of
EFs and multifocal moderate EF fragmentation with formation of
multifocal, moderate intralamellar and focal, translamellar mucoid
accumulation (Fig. 1B).
In the patients with TAA with SMD aortic lesions
(grade 3) the medial aortic wall showed extensive, band-like SMC
loss, multifocal moderate EF fragmentation and multifocal severe
translamellar mucoid accumulation (Fig.
1C).
In our study, the medial degenerative aortic lesions
(mild=1, moderate=2, and severe=3) significantly correlated with
advanced age (>65 years) (r=−0.39, P<0.01) and male sex
(r=0.27, P<0.05; Table II).
 | Table II.Kendall's Tau-b correlation
coefficient of degenerative, atherosclerotic and inflammatory
lesions with potential risk factors in aortic aneurysm (n=52). |
Table II.
Kendall's Tau-b correlation
coefficient of degenerative, atherosclerotic and inflammatory
lesions with potential risk factors in aortic aneurysm (n=52).
|
| Medial degenerative
(mild, moderate, severe) | Atherosclerotic
(mild, moderate, severe) |
|---|
|
|
|
|
|---|
| Co-variables | r-value | P-value | r-value | P-value |
|---|
| Sex (Male) | 0.27 | 0.03a | −0.08 | 0.53 |
| Age (>65
years) | −0.39 |
<0.01a | 0.40 | 0.02a |
| Arterial
hypertension | 0.22 | 0.10 | −0.18 | 0.17 |
| Smoking | 0.13 | 0.34 | 0.29 | 0.03a |
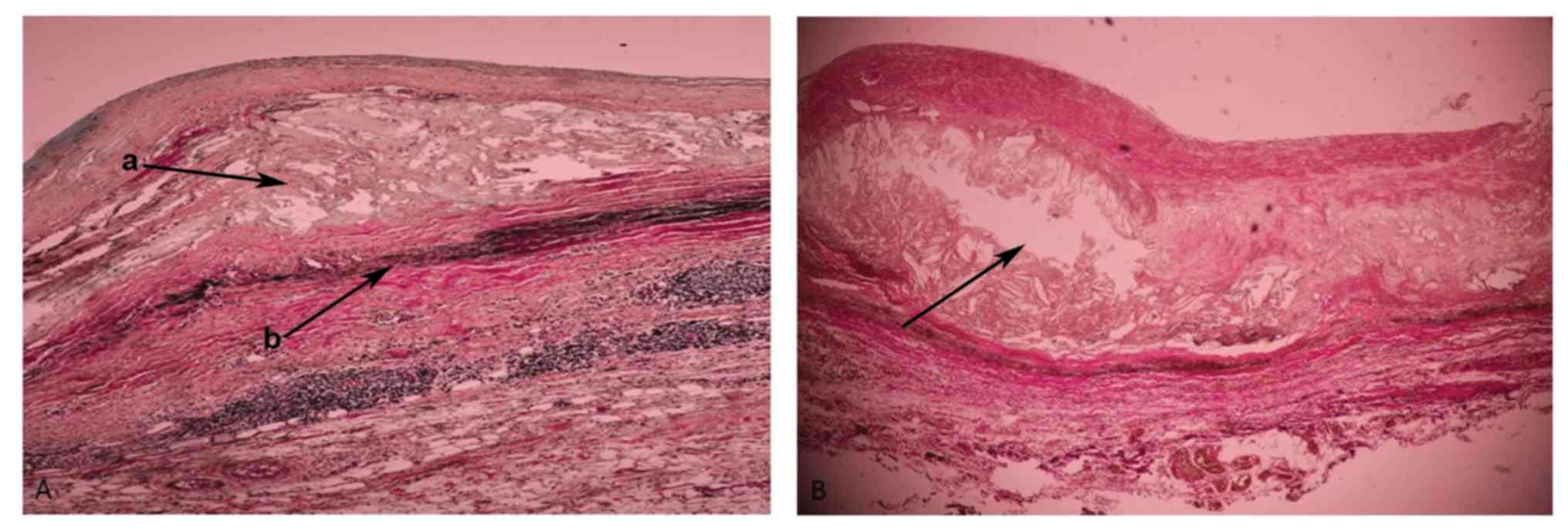
Atherosclerotic lesions in aortic
aneurysms
The atherosclerotic aneurysms were found in 22
patients, 13 (9 males, 4 females) were abdominal and 9 (6 males, 3
females) were thoracic aneurysms. In the AAAs, all atherosclerotic
lesions were severe (grade 3), with fibrotic changes of the entire
wall, with or without associated complications. In the TAAs, most
cases had MMD or SMD associated with intimal atherosclerotic
lesions of different types (grades 1–2), with the formation of
mixed, degenerative-atherosclerotic aneurysms (7 cases). A total of
2 patients with TAA with pure ATS lesions (mild) were identified.
In most AAA cases, adventitial inflammation was also observed. The
present results revealed early atherosclerotic lesions in the
thoracic aorta (Fig. 2A) and
advanced atherosclerotic lesions in the abdominal aorta (Fig. 2B).
Kendall's tau-b correlation shows that the
atherosclerotic aortic lesions (1, mild; 2, moderate; and 3,
severe) significantly correlated with advanced age (>65 years)
(r=−0.40, P<0.01) and smoking (r=−0.29, P<0.05; Table II).
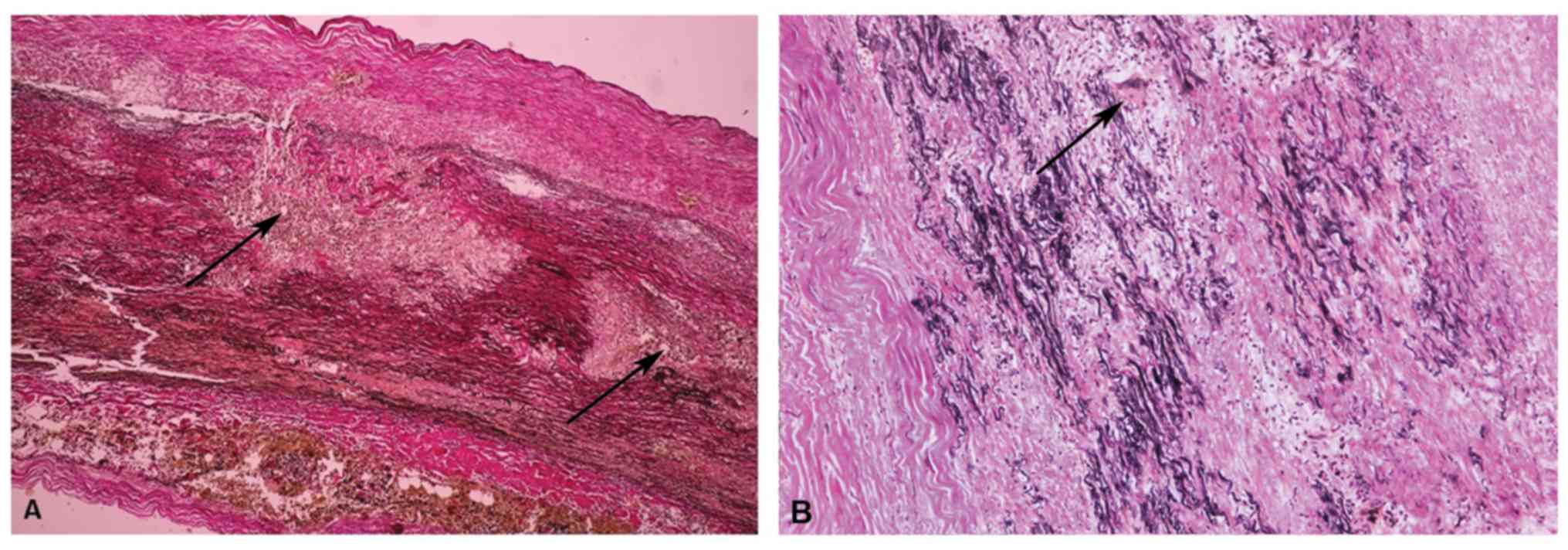
Inflammation in aortic aneurysms
In total, 5 patients with TAA had aortitis,
determined by histological examination, including three giant cell
aortitis (GCA) cases, one Takayasu aortitis (TA) case and one
patient with syphilitic aortitis. The aortitis group included 3 men
and 2 women. The GCA cases consisted of 2 males and 1 female with
an average age of 62.5 years (ranging between 52 and 70 years); the
patient with TA was a 36-year-old female and the patient with
syphilitic aortitis was a 56-year-old male.
In all five TAA aortitis cases, the mean
pre-operative erythrocyte sedimentation rate was 31.5 mm/h, and for
C-reactive protein was 21.6 mg/d. Of the 5 patients, 1 (20%) needed
coronary artery bypass grafting, 3 (60%) underwent aortic valve
surgery (1 aortic valve repair and 2 aortic valve replacement), and
all 5 (100%) required aortic surgery (ascending aortic
replacement).
In most aortitis cases (60%) the diagnosis was made
after the operation based on histopathologic examination, except TA
where the examination suggested pre-operatory TA disease, and
syphilitic aortitis where the patient had a history of
syphilis.
The common histological features in granulomatous
aortitis were: i) Diffuse intimal hyperplasia, granulomatous
inflammation in the media comprised of epithelioid macrophages and
occasional giant cells related by ‘laminar medial necrosis’, areas
of smooth muscle cell loss with collapsed elastic fibers, fibrotic
scars and an adventitial accompanying lymphoplasmacytic infiltrate
in GCA (Fig. 3A); and ii) diffuse
intimal hyperplasia, compact granulomas and medial necrosis,
scarring in the tunica media with disruption and disorganization of
the remaining elastic fibers, and dense adventitial fibrosis in TA
(Fig. 3B).
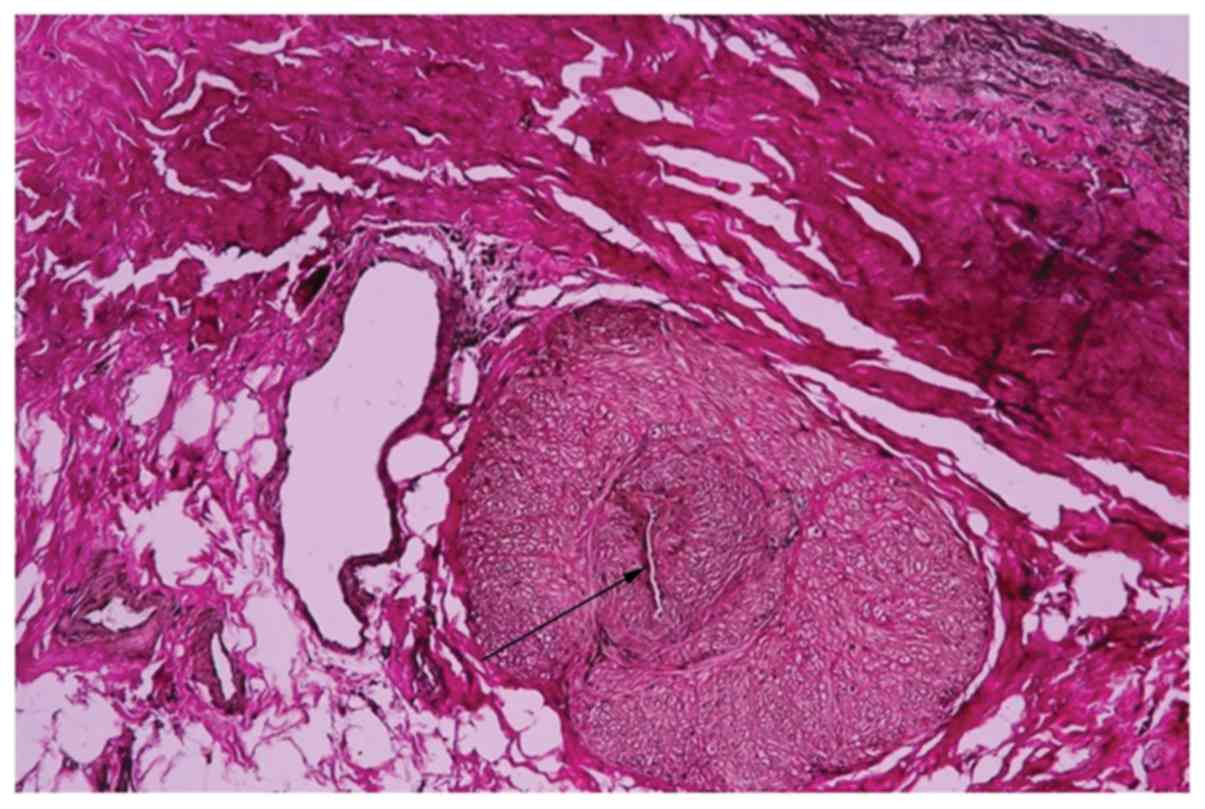
Among the 5 aortitis cases, one was diagnosed with
syphilitic aortitis on the basis of the pathological features and
patient history. A man in his 50 s, who presented valvular
regurgitation, aneurysmal disease and myocardial ischemia, also
required coronary artery bypass grafting surgery. All these lesions
were cardiovascular complications of syphilis involving the aorta
(leading to the formation of aneurysms and aortic-valve
incompetence) and coronary artery, causing coronary ostial stenosis
(angina pectoris complaints). The histopathological examination
revealed a chronic mesoaortitis, characterized by normal media
replacement with fibrous scars and adventitial endarteritis of the
vasa vasorum (Fig. 4).
Discussion
An aortic aneurysm is defined as a dilatation of an
aortic segment with a diameter of at least one and one-half times
the normal diameter (8). Aortic
aneurysms can involve the ascending aorta or descending aorta. In
the present study, the aortic aneurysms included 39 (75%) thoracic
and 13 (25%) abdominal cases. Other previous epidemiology studies
reported that AAA involves mainly older patients, while TAA
involves mainly two populations: One of old age with no special
history (9) and another of young age
suffering from connective tissue disorders, including Marfan's
syndrome (10). Marfan's syndrome or
connective tissue disorders were not detected in the young patients
with TAA in the present study (11,12).
Good familial history, histopathological examination and genetic
screening are important factors that require consideration.
In agreement with Hiratzka et al (13), the present study identified that the
most common risk factors for the development of aortic aneurysm
were advanced age (>65 years old), male, smoking, high blood
pressure and family history. The potential risk factors identified
to be associated with aortic aneurysm development were advanced
age, HTA and BAV in TAA, and advanced age, male, smoking and ATS in
AAA.
Previous studies reported that TAAs are most often
associated with MD, which can be regarded as a structural
alteration responsible for the development of aortic aneurysms in
relation to other risk factors (8,14). In
the present study, the histopathological profile showed that MD was
the most common histopathological substrate in TAA (82.1%),
granulomatous aortitis (10.25%) was a rare cause of TAA and
syphilitic aortitis (2.5%) was a very rare encountered case, and
ATS was a common histopathological substrate in AAA (100%).
In patients with aortic aneurysms, histological
analysis of the aortic resection specimens provides important
diagnostic information on the MD lesions, describing a definite
pattern of distribution of MD severity within the aortic wall.
Moreover, aortitis was an unexpected diagnosis during the
histological examination of surgically resected aortic thoracic
aneurysms in patients without clinical signs or symptoms of
systemic vasculitis. Aortic thoracic aneurysm is in most cases a
fatal condition, especially in association with more severe
degenerative changes in the aortic media.
GCA and TA, diagnosed in the present study, are
considered the most common causes of non-infectious aortitis
(8). Garcia-Martinez et al
(15) observed that ≥20% of patients
with GCA and 50% of patients with TA would develop changes
consistent with aneurysmal aortitis, while in the present study, it
was identified that only 7.6% of patients with GCA had TAA and 2.5%
patients with TA had TAA, the remaining one TAA case having
syphilitic aortitis.
Histopathological examination is not sufficient to
distinguish between the two conditions, and, together with clinical
data, it must be integrated within a diagnostic algorithm. In the
final diagnosis, American College of Rheumatology (ACR) criteria
were applied and at least three ACR criteria, including clinical,
imagistic and histological data, were present to confirm the two
granulomatous aortitis (16,17).
In the present study, syphilitic aortitis was a rare
cause of AA from all the TAA cases. In agreement with Vaideeswar
(18), the complications in the form
of aortic aneurysms and insufficiency in association with coronary
ostial stenosis frequently coexist in the tertiary syphilis. For
diagnosis, not only the pathological findings but also the clinical
features and serology should be taken into consideration.
The present study also included 20 cases of
atherosclerotic lesions. The clinical analysis of the 20 AAs
revealed two types of aneurysmal diseases, 7 (35%) mixed TAAs and
13 (65%) ATS AAAs. The present results are in agreement with Singh
et al (19), who noted that
abdominal aneurysms account for the majority of aortic aneurysms.
In the present study, the histopathological examination revealed
intimal atheroma and parietal thrombosis in both AAA and aortic
ATS, similar to the consensus statement on surgical pathology
(20). The presence of ATS lesions
in both groups may suggest the need for ATS medical therapy in all
patients with TAA and AAA. Due to the highest degree of severity of
the ATS AAA lesions, it was suggested that ATS plays an important
role in AAA development.
In the present study, mixed TAA was associated with
two lesions, MD and atherosclerotic lesions. However, additional
marked elastin fragmentation and adventitial chronic inflammation
were mainly restricted to AAA. Peshkova et al (21) identified that ATS changes in the
aortic wall underlie AAA formation, where local inflammation
contributes to aortic wall thickening. From the present results, it
was hypothesized that abundant inflammatory infiltrate represents a
common trigger for ATS and AAA development and extension.
Additionally, the present results suggested that inflammation is a
risk factor in AAA rupture, revealing the importance of
anti-inflammatory medication in reducing AAA complications in
patients.
The present study had a number of limitations,
including a small group of patients with advanced aortic disease
and no genetic studies. The small number of patients did not allow
for more extensive statistical studies. The present study reflects
morphoclinical particularities of aortic aneurysms in the
geographical area. Further studies with larger biomarker panels are
required to determine associations with other factors.
In conclusion, the present study provided insight
for the development of aortic aneurysm evaluation for detecting and
monitoring AAA and TAA in affected patients. The present study
provides insight for the inclusion of histopathological examination
in the final diagnosis, in consensus with the late knowledge and
classification of aortic surgical pathology for diagnosis
optimization. Prospective studies using large study groups will be
necessary to further histologically evaluate the extent and
severity of the disease and its progression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GT and DB conceived and designed the current study.
DB, GT, MD and BGI performed the experiments. DB, MD, REH and VM
collected and analyzed the data. DB, GT and VM wrote the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the ethical standards of the Declaration of Helsinki and was
approved by the Research Ethics Committee of Professor George
Georgescu Institute of Cardiovascular Diseases, Iasi, Romania
(approval no. 974/17.02.2015). Informed consent was obtained from
all individual participants included in the current study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davies MJ: Aortic aneurysm formation:
lessons from human studies and experimental models. Circulation.
98:193–195. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elefteriades JA: Natural history of
thoracic aortic aneurysms: Indications for surgery and surgical
versus nonsurgical risks. Ann Thorac Surg. 74 (Suppl):S1877–S1880;
discussion S1892-S1898. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pomerance A, Yacoub MH and Gula G: The
surgical pathology of thoracic aortic aneurysms. Histopathology.
1:257–276. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stary HC, Chandler AB, Dinsmore RE, Fuster
V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD and
Wissler RW: A definition of advanced types of atherosclerotic
lesions and a histological classification of atherosclerosis. A
report from the Committee on Vascular Lesions of the Council on
Arteriosclerosis, American Heart Association. Arterioscler Thromb
Vasc Biol. 15:1512–1531. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nesi G, Anichini C, Tozzini S, Boddi V,
Calamai G and Gori F: Pathology of the thoracic aorta: A
morphologic review of 338 surgical specimens over a 7-year period.
Cardiovasc Pathol. 18:134–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Halushka MK, Angelini A, Bartoloni G,
Basso C, Batoroeva L, Bruneval P, Buja LM, Butany J, d'Amati G,
Fallon JT, et al: Consensus statement on surgical pathology of the
aorta from the Society for Cardiovascular Pathology and the
Association For European Cardiovascular Pathology: II.
Noninflammatory degenerative diseases-nomenclature and diagnostic
criteria. Cardiovasc Pathol. 25:247–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stone JR, Bruneval P, Angelini A,
Bartoloni G, Basso C, Batoroeva L, Buja LM, Butany J, d'Amati G,
Fallon JT, et al: Consensus statement on surgical pathology of the
aorta from the Society for Cardiovascular Pathology and the
Association for European Cardiovascular Pathology: I. Inflammatory
diseases. Cardiovasc Pathol. 24:267–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leone O, Agozzino L, Angelini A, Bartoloni
G, Basso C, Caruso G, D'Amati G, Pucci A, Thiene G and Gallo P:
Criteria for histopathologic diagnosis of aortic disease consensus
statement from the SIAPEC-IAP study group of ‘cardiovascular
pathology’ in collaboration with the association for Italian
cardiovascular pathology. Pathologica. 104:1–33. 2012.PubMed/NCBI
|
|
9
|
Fritze O, Romero B, Schleicher M, Jacob
MP, Oh DY, Starcher B, Schenke-Layland K, Bujan J and Stock UA:
Age-related changes in the elastic tissue of the human aorta. J
Vasc Res. 49:77–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iams HD: Diagnosis and management of
Marfan syndrome. Curr Sports Med Rep. 9:93–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Topel I, Zorger N and Steinbauer M:
Inflammatory diseases of the aorta: Part 1: Non-infectious
aortitis. Gefasschirurgie. 21 (Suppl 2):S80–S86. 2016. View Article : Google Scholar
|
|
12
|
Topel I, Zorger N and Steinbauer M:
Inflammatory diseases of the aorta: Part 2: Infectious aortitis.
Gefasschirurgie. 21 (Suppl 2):S87–S93. 2016. View Article : Google Scholar
|
|
13
|
Hiratzka LF, Bakris GL, Beckman JA, Bersin
RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM,
Kazerooni EA, et al: 2010
ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the
diagnosis and management of patients with thoracic aortic disease:
A report of the American College of Cardiology Foundation/American
Heart Association Task Force on Practice Guidelines, American
Association for Thoracic Surgery, American College of Radiology,
American Stroke Association, Society of Cardiovascular
Anesthesiologists, Society for Cardiovascular Angiography and
Interventions, Society of Interventional Radiology, Society of
Thoracic Surgeons, and Society for Vascular Medicine. Circulation.
121:e266–e369. 2010.PubMed/NCBI
|
|
14
|
Butcovan D: New frontiers in the
histological diagnosis of degenerative aortopathies manifested as
aortic aneurysms and dissections. Rev Med Chir Soc Med Nat.
121:89–94. 2017.
|
|
15
|
Garcia-Martinez A, Hernandez-Rodriguez J,
Arguis P, Paredes P, Segarra M, Lozano E, Nicolau C, Ramírez J,
Lomeña F, Josa M, et al: Development of aortic aneurysm/dilatation
during the followup of patients with giant cell arteritis: A
cross-sectional screening of fifty-four prospectively followed
patients. Arthritis Rheum. 59:422–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park MC, Lee SW, Park YB, Chung NS and Lee
SK: Clinical characteristics and outcomes of Takayasu's arteritis:
Analysis of 108 patients using standardized criteria for diagnosis,
activity assessment, and angiographic classification. Scand J
Rheumatol. 34:284–292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ness T, Bley TA, Schmidt WA and Lamprecht
P: The diagnosis and treatment of giant cell arteritis. Dtsch
Arztebl Int. 110:376–386. 2013.PubMed/NCBI
|
|
18
|
Vaideeswar P: Syphilitic aortitis: Rearing
of the ugly head. Indian J Pathol Microbiol. 53:624–627. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh K, Bonaa KH, Jacobsen BK, Bjørk L
and Solberg S: Prevalence of and risk factors for abdominal aortic
aneurysms in a population-based study: The Tromsø study. Am J
Epidemiol. 154:236–244. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stone JR, Basso C, Baandrup UT, Bruneval
P, Butany J, Gallagher PJ, Halushka MK, Miller DV, Padera RF, Radio
SJ, et al: Recommendations for processing cardiovascular surgical
pathology specimens: A consensus statement from the standards and
definitions Committee of the society for cardiovascular pathology
and the association for European cardiovascular pathology.
Cardiovasc Pathol. 21:2–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peshkova IO, Schaefer G and Koltsova EK:
Atherosclerosis and aortic aneurysm-is inflammation a common
denominator? FEBS J. 283:1636–1652. 2016. View Article : Google Scholar : PubMed/NCBI
|