Introduction
E1A binding protein P300 (P300; also known as KAT3B)
is a member of the histone acetyltransferase family of
transcriptional co-activators. It has a variety of roles in the
transcription process and catalyzes histone acetylation through
exerting its histone acetyltransferase activity (1,2). It is
involved in various cellular processes, including DNA damage
response, cell-cycle regulation and proliferation, differentiation
and apoptosis (3).
Previous studies have indicated that P300 has a
tumour suppressor role in certain human cancer types, including
colorectal cancer (CRC) and gastric cancer (4). Wang et al (5), reported that octreotide inhibits the
proliferation of gastric cancer cells through P300 histone
acetyltransferase activation and the interaction of zinc-activated
ion channels and P300. As P300 binds to and inactivates adenovirus
E1A, it has been considered a tumour suppressor (6). At the same time, P300 activates p53 to
inhibit the proliferation of cancer cells (7). However, various studies have indicated
that P300 is a positive regulator of cancer progression and is
involved in tumorigenesis. Li et al (8) reported that overexpression of P300 is
associated with aggressive features and poor prognosis in
hepatocellular carcinoma (HCC). Isharwal et al (9), suggested that P300 may serve as a
biomarker to predict prostate cancer recurrence and is associated
with changes in the size and shape of epithelial cell nuclei. Xiao
et al (10), revealed that
high expression of P300 in breast cancer is associated with tumour
recurrence and poor prognosis. Chen et al (11), determined that high P300 expression
is associated with unfavourable survival outcomes in laryngeal
squamous cell carcinoma (LSCC) patients. Although it has been
diffusely reported, the prognostic value of P300 in various human
cancer types remains controversial. Therefore, a clinical study of
synovial sarcoma (SS) tissue samples and a meta-analysis were
herein performed to evaluate the diagnostic and prognostic value of
P300. The aim of the present study was to systematically
investigate whether a high level of P300 expression may be used as
a diagnostic and/or prognostic marker for cancer, and whether P300
may serve as a target for developing more effective therapies.
In the present two-fold study, SS samples were
analysed in a clinical study, and data from previous studies from
various countries were collected for a meta-analysis in order to
assess the prognostic value of P300 expression in cancer
patients.
Materials and methods
Specimens and clinicopathological
characteristics
The present study included retrospectively collected
paraffin-embedded samples from 43 patients with SS, including 30
biphasic SS (BSS), 10 monophasic fibrous SS (MFSS) and 3
poorly-differentiated SS (PDSS). These patients were treated at the
First Affiliated Hospital of Shihezi University School of Medicine
and the First Affiliated Hospital of Xinjiang Medical University
(Shihezi, China) between January 1968 and December 2015. SS was
diagnosed via histological and immunohistochemical (IHC) analyses
by two senior pathologists. The histopathological grading of SS was
based on the FNCLCC guidelines (12)
for soft-tissue tumors. The present study was approved by the
Internal Ethics Review Board of Shihezi University School of
Medicine (Shihezi, China).
IHC staining and scoring
IHC staining was performed with an Envision two step
kit (cat. no. PV-9003; Beijing Zhongshang Jinqiao Biotechnology
Co., Ltd.) according to the manufacturer's protocols. The following
antibodies were used: P300 (dilution, 1:500; cat. no. ab54984;
Abcam), β-catenin (dilution, 1:400; cat. no. ab32572; Abcam). The
scoring evaluation of β-catenin was performed as in a previous
study by our group (12). Cells with
nuclear staining were considered positive for P300. The P300 IHC
scoring criteria were as follows: 0–1, <5% positive tumour cells
or no staining; 2–4, 6–25% positive tumour cells and light-brown
nuclei; 5–8, 26–50% positive tumor cells and brownish yellow
nuclei; 9–12, 51–75% positive tumor cells and brown nuclei.
Statistical analysis
SPSS17.0 software (SPSS, Inc.) was used for
statistical analysis of the results of the above clinical study,
with the chi-square test or Fisher's exact test used to compare
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Publication search for the
meta-analysis and inclusion and exclusion criteria
For the meta-analysis, we the PubMed, EMBASE and Web
of Science databases were searched for entries up to 21 January
2018 to identify relevant studies. Several combinations of the
following keywords were applied: ‘cancer’, ‘carcinoma’, ‘neoplasm’,
‘tumour’, ‘P300’, ‘survival’, ‘prognosis’, ‘metastasis’ and
‘sarcoma’. Studies were considered eligible if they met all of the
following criteria: i) Patients with any malignant neoplasm
[nasopharyngeal carcinoma (NPC), non-small cell lung cancer
(NSCLC), LSCC, SCLC, oesophageal squamous cell carcinoma (OSCC),
HCC, CRC, gastroesophageal junction cancer melanoma (GEJC),
cutaneous squamous cell carcinoma (CSCC) and breast cancer]; ii)
investigation of the association between P300 expression and
prognostic factors; iii) Kaplan-Meier survival analysis of 5-year
overall survival; and iv) full-text articles written in English.
Articles were excluded based on the following criteria: i) Review
articles, laboratory studies or letters; ii) results that
overlapped with or were duplicates of previously reported data;
iii) studies lacking the key data of hazard ratios (HRs) or
confidence intervals (CIs) without survival curves.
Data extraction and quality
assessment
The data extracted mainly included the following: i)
First author name and publication year; ii) nationality, ethnicity
and size of the cohort, and type of clinical disease; iii) clinical
stages, lymph node metastasis, depth of invasion, sex, tumour size
and degree of differentiation; iv) HRs of elevated P300 expression
regarding overall survival (OS), progression-free survival (PFS),
recurrence-free survival (RFS) and disease-free survival (DFS),
along with their 95% CIs and P-values. If HRs and 95% CIs were not
directly reported in articles, and only Kaplan-Meier survival data
were available, the data were extracted from the graphical survival
plots to estimate HRs and 95% CIs. The information was carefully
and independently extracted by two authors (YL and ZH) according to
the critical review checklist of the Dutch Cochrane Centre proposed
by Meta-Analysis Of Observational Studies In Epidemiology (MOOSE)
(13). In cases of disagreement, a
consensus was reached by discussion.
Subgroup meta-analysis and influence
of clinicopathological factors
In addition to analyzing the association between
P300 expression and survival, subgroup analyses by ethnicity (Asian
vs. Caucasian) and cancer location were performed. The association
between P300 expression and clinicopathological factors, including
sex, tumour size, grade of differentiation, clinical stage, lymph
node metastasis and depth of invasion was also analysed.
Statistical methods for the
meta-analysis
HRs and 95% CIs were calculated using Cochran's Q
test and the Higgins I-squared statistics. P<0.05 was considered
to indicate statistical significance, and I2>50% was
considered to indicate heterogeneity. A random-effects model (Der
Simonian and Laird method) was applied. Otherwise, the
fixed-effects model (Mantel-Haenszel test) was used. In addition,
stratified analyses were used to minimize the influence of
heterogeneity. Publication bias was estimated via Egger' test with
a funnel plot (14). All statistical
analyses were performed using STATA software (version 12.0; Stata
Corp.).
Results
Clinical study population
The sex ratio of patients was 1.04:1 (22 males and
21 females), and the age at diagnosis ranged from 10 to 76 years
(mean, 39 years). The tumours were widely distributed; however,
most arose in the extremities (n=24, 55.8%) and trunk (n=12,
27.9%). A total of 5 tumours (11.6%) arose in the head and neck and
2 (4.7%) in the pelvis/peritoneum (data not shown).
IHC analysis of P300 expression in BSS
and MFSS
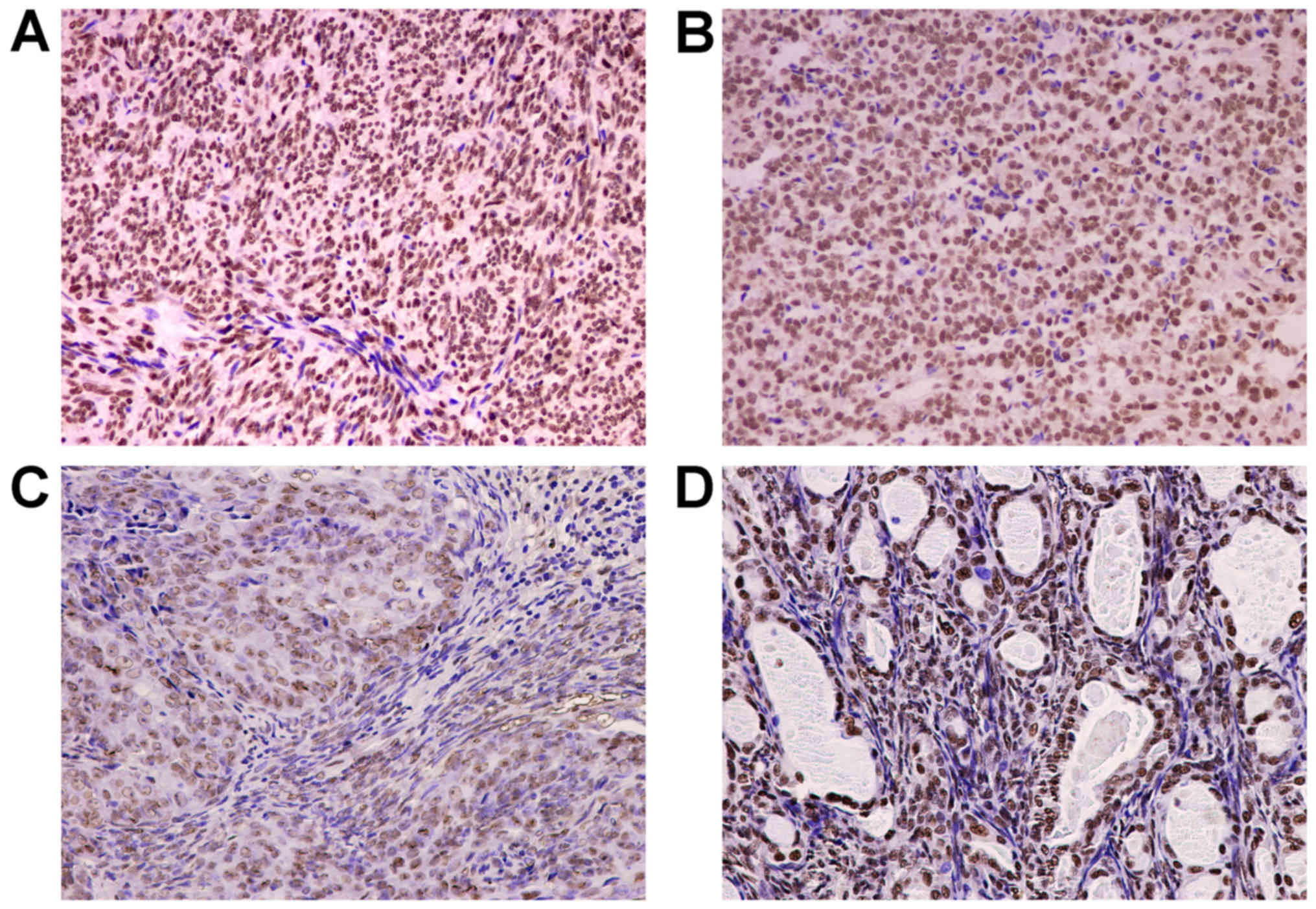
The IHC staining results are presented in Table I and representative IHC images are
provided in Fig. 1. P300 expression
was present in BSS (28/30, 93.3%) and MFSS (10/10, 100%). Strongly
positive P300 expression was noted in 10/30 (33.3%) BSS samples and
6/10 (60%) MFSS samples. Although the rate of strongly positive
P300 expression in MFSS was higher than that in BSS, this
difference was not statistically significant (P>0.05; Table I; Fig. 1A
and B). In addition, immunohistochemical expression of P300
proteins were markedly different between the epithelioid and
spindle cell components of BSS. Furthermore, P300 expression was
present in epithelial cells (29/30; 96.7%) and spindle cells
(27/30; 90%). Strongly positive P300 staining was observed in 24/30
(80%) of epithelioid cells and 9/30 (30%) of spindle cells, and the
rate of strongly positive P300 expression was significantly higher
in epithelioid cells (P<0.05; Table
I; Fig. 1C and D).
 | Table I.Differential expression of P300
proteins between BSS and MFSS. |
Table I.
Differential expression of P300
proteins between BSS and MFSS.
|
| P300 (n) |
|
|---|
|
|
|
|
|---|
| Histological
type | − | + | ++/+++ | P-value |
|---|
| BSS | 2 | 18 | 10 |
0.304a |
| MFSS | 0 | 4 | 6 |
|
| Epithelioid
cells | 1 | 5 | 24 |
<0.001b |
| Spindle cells | 3 | 18 | 9 |
|
Meta-analysis study selection and
characteristics
Initially, a total of 161 potentially relevant
articles were identified from the literature search, but 126
articles were excluded due to being meeting abstracts (n=7),
research articles on cell lines (n=89) or animal models (n=10), or
not being associated with prognosis (n=20). After reading the full
text of the studies selected, 21 articles were further excluded due
to insufficient data on survival. Ultimately, 14 studies (8,10,11,15–25)
comprising 2,517 cases were included in the final meta-analysis
(Fig. 2).
Among the 14 eligible studies included in the
meta-analysis, 12 were on Asian populations (China, Korea, Japan)
and two on Caucasian populations (British). The malignant neoplasms
in the studies included CSCC, SCLC, SCC, GEJC, NPC, NSCLC, LSCC,
HCC, OSCC, melanoma, CRC and breast cancer. Of the studies
analysed, 4 focused on PFS/RFS/DFS and 13 reported on the OS of
patients. The maximum follow-up period ranged from 50 to 250
months. The characteristics of the publications are provided in
Table II.
 | Table II.Characteristics of the studies
included in the meta-analysis. |
Table II.
Characteristics of the studies
included in the meta-analysis.
|
|
|
|
| P300 expression
status (n) | OS | PFS/RFS/DFS |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author
(year) | Cohort country of
origin | Dominant
ethnicity | Cancer type | High | Low | HR (95% CI) | P-value | HR (95% CI) | P-value | Survival
analysis | Maximum follow-up
(moths) | (Refs.) |
|---|
| Chen (2015) | China | Asian | CSCC | 86 | 79 | 2.42
(1.32,4.42) | 0.004 | 1.78
(1.08,2.93) | 0.024 | OS/RFS | 120 | (15) |
| Gao (2014) | China | Asian | SCLC | 16 | 206 | 1.49
(1.12,1.98) | 0.006 | NM | NM | OS | 120 | (16) |
| Bhandaru
(2014) | UK | White | Melanoma | 188 | 139 | 0.60
(0.44,0.83) | 0.002 | 0.59 (0.42,
0.82) | 0.002 | OS | 60 | (17) |
| Rotte (2013) | UK | White | Melanoma | 233 | 158 | 0.58
(0.43,0.78) | 0.000 | 0.59 (0.42,
0.79) | <0.001 | OS | 60 | (19) |
| Huh (2013) | Korea | Asian | CRC | 149 | 50 | NM | NM | 3.314
(1.109,9.9) | NM | DFS | 120 | (20) |
| Zhang (2013) | China | Asian | GEJC | 72 | 18 | 0.44
(0.15,1.33) | NM | NM | NM | OS | 50 | (18) |
| Liao (2012) | China | Asian | NPC | 127 | 82 | 1.83
(1.04,3.2) | 0.036 | 1.55
(0.93,2.59) | NM | OS/PFS | 250 | (21) |
| Hou (2012) | China | Asian | NSCLC | 87 | 82 | 0.55
(0.32,0.92) | 0.024 | NM | 0.041 | OS | 60 | (22) |
| Chen (2013) | China | Asian | LSCC | 40 | 40 | 4.7
(2.10,10.51) | NM | NM | NM | OS | 250 | (11) |
| Li (2011) | China | Asian | HCC | 60 | 63 | 2.08
(1.15,4.11) | 0.021 | NM | NM | OS | 70 | (8) |
| Yokomizo
(2011) | Japan | Asian | HCC | 21 | 24 | 1.54
(0.50,4.75) | NM | NM | NM | OS | 167 | (23) |
| Xiao (2011) | China | Asian | BC | 105 | 88 | 3.37
(1.60,7.76) | 0.021 | 2.05
(1.3,4.59) | 0.017 | OS/PFS | 100 | (10) |
| Li (2011) | China | Asian | ESCC | 150 | 90 | 1.658
(1.1,2.51) | 0.017 | NM | NM | OS | 120 | (24) |
| Ishihama
(2007) | Japan | Asian | CRC | 43 | 21 | 4.12
(1.05,16.2) | 0.043 | NM | NM | OS | 120 | (25) |
Meta-analysis of P300 expression and
OS
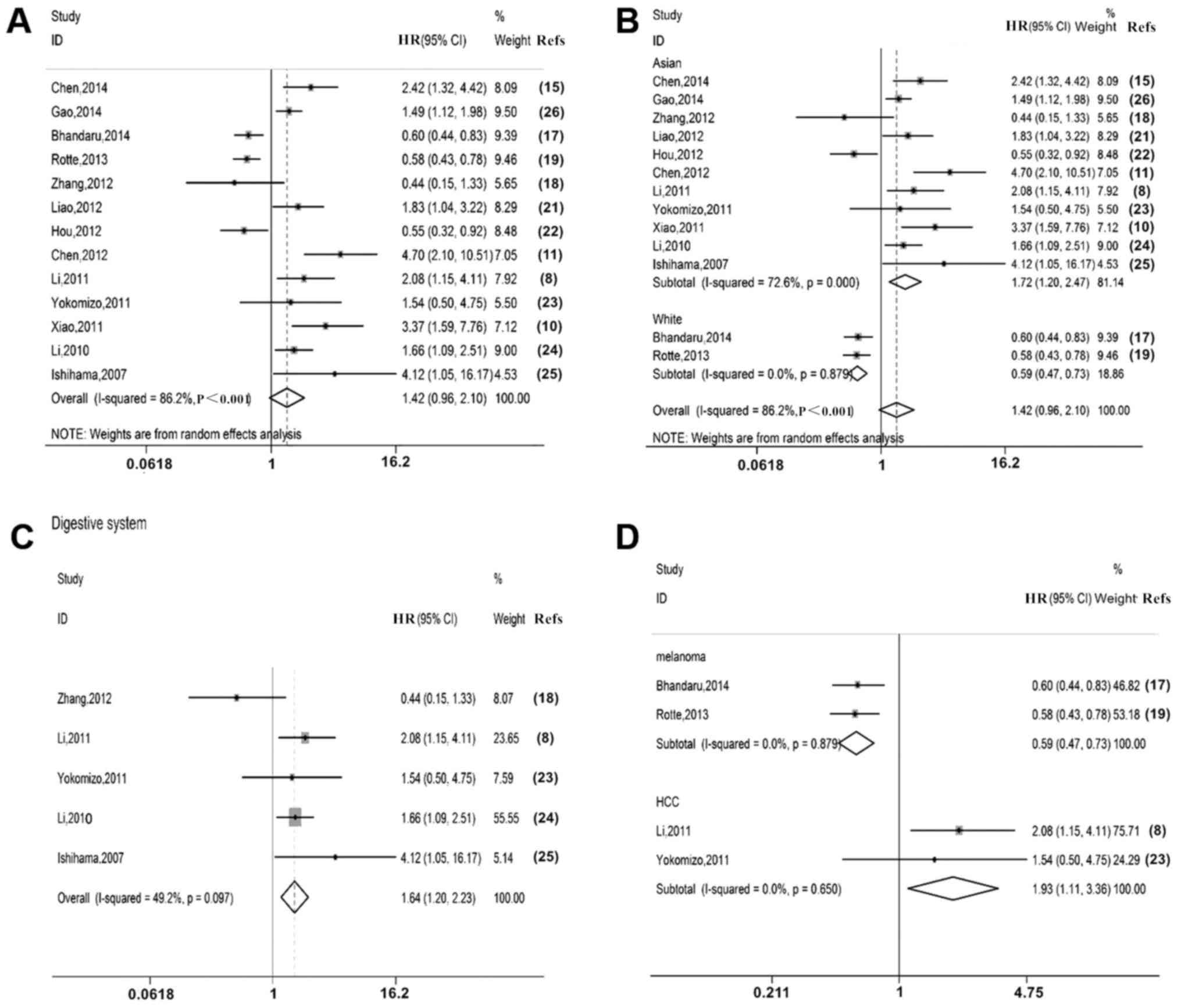
OS data were extracted from 13 studies. The
characteristics of these publications are presented in Table III. A random-effects model was used
to estimate the impact of P300 expression on OS with a pooled HR
and its 95% CI (P<0.05, I2=86.2%). The results
indicated that overexpression of P300 was not significantly
associated with poor OS (HR=1.42, 95% CI: 0.96–2.10; Fig. 3A).
 | Table III.Characteristics of the studies
included in Fig 3. |
Table III.
Characteristics of the studies
included in Fig 3.
|
|
|
|
| P300 expression
status (n) | OS |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| First author
(year) | Cohort country of
origin | Dominant
ethnicity | Cancer type | High | Low | HR (95% CI) | P-value | Survival
analysis | Maximum follow-up
(moths) | (Refs.) |
|---|
| Chen (2015) | China | Asian | CSCC | 86 | 79 | 2.42
(1.32,4.42) | 0.004 | OS | 120 | (15) |
| Gao (2014) | China | Asian | SCLC | 16 | 206 | 1.49
(1.12,1.98) | 0.006 | OS | 120 | (16) |
| Bhandaru
(2014) | UK | White | Melanoma | 188 | 139 | 0.60
(0.44,0.83) | 0.002 | OS | 60 | (17) |
| Rotte (2013) | UK | White | Melanoma | 233 | 158 | 0.58
(0.43,0.78) | 0.000 | OS | 60 | (19) |
| Zhang (2013) | China | Asian | GEJC | 72 | 18 | 0.44
(0.15,1.33) | NM | OS | 50 | (18) |
| Liao (2012) | China | Asian | NPC | 127 | 82 | 1.83
(1.04,3.2) | 0.036 | OS | 250 | (21) |
| Hou (2012) | China | Asian | NSCLC | 87 | 82 | 0.55
(0.32,0.92) | 0.024 | OS | 60 | (22) |
| Chen (2013) | China | Asian | LSCC | 40 | 40 | 4.7
(2.10,10.51) | NM | OS | 250 | (11) |
| Li (2011) | China | Asian | HCC | 60 | 63 | 2.08
(1.15,4.11) | 0.021 | OS | 70 | (8) |
| Yokomizo
(2011) | Japan | Asian | HCC | 21 | 24 | 1.54
(0.50,4.75) | NM | OS | 167 | (23) |
| Xiao (2011) | China | Asian | BC | 105 | 88 | 3.37
(1.60,7.76) | 0.021 | OS | 100 | (10) |
| Li (2011) | China | Asian | ESCC | 150 | 90 | 1.658
(1.1,2.51) | 0.017 | OS | 120 | (24) |
| Ishihama
(2007) | Japan | Asian | CRC | 43 | 21 | 4.12
(1.05,16.2) | 0.043 | OS | 120 | (25) |
A subgroup analysis by ethnicity indicated that in
the Asian populations, overexpression of P300 was significantly
associated with poor OS (HR=1.72, 95% CI: 1.20–2.47; Fig. 3B); however, overexpression of P300
predicted a favourable OS in Caucasians (HR=0.59, 95% CI:
0.47–0.73; Fig. 3B).
Furthermore, subgroup analyses by location of the
malignant neoplasms were performed. The results indicated that P300
overexpression was associated with poor OS of patients with
malignant neoplasms of the digestive system (HR=1.64, 95% CI:
1.20–2.23; Fig. 3C). In HCC, high
P300 expression was associated with poor OS (HR=1.93, 95% CI:
1.11–3.36; Fig. 3D). However, high
P300 expression was associated with favourable OS in melanoma
(HR=0.59, 95% CI: 0.47–0.73; Fig.
3D).
Meta-analysis of P300 expression and
PFS/RFS/DFS
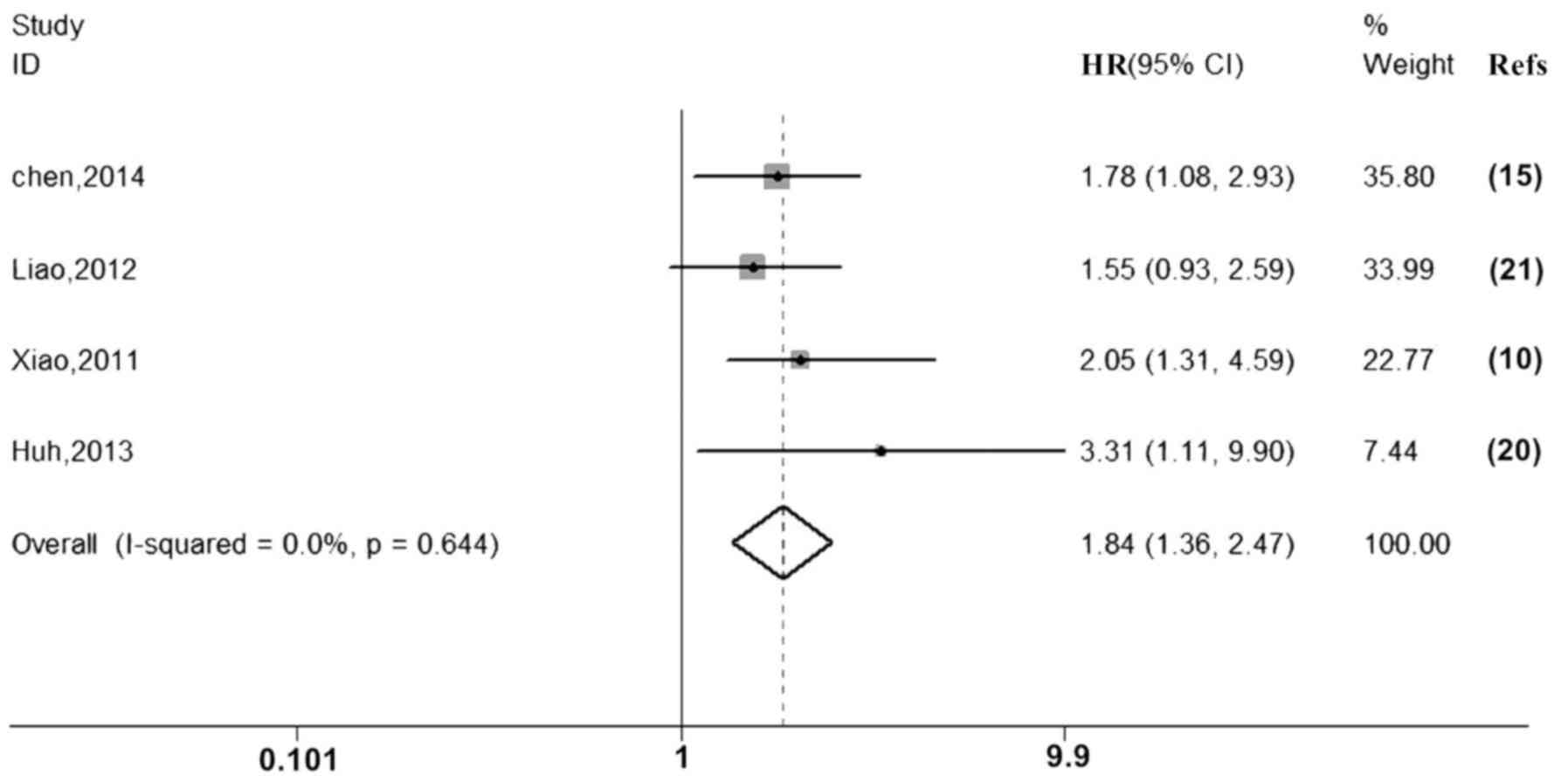
Analysis of data extracted from three studies for
PFS/RFS/DFS and meta-analysis using a fixed-effects model (P=0.644,
I2=0.0%) suggested that overexpression of P300 is
associated with poor PFS, RFS and DFS (HR=1.84, 95% CI: 1.36–2.47;
Fig. 4; Table IV).
 | Table IV.Characteristics of the studies
included in Fig. 4. |
Table IV.
Characteristics of the studies
included in Fig. 4.
|
|
|
|
| P300 expression
status (n) | PFS/RFS/DFS |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| First author
(year) | Cohort country of
origin | Dominant
ethnicity | Cancer type | High | Low | HR (95% CI) | P-value | Survival
analysis | Maximum follow-up
(moths) | (Refs.) |
|---|
| Chen (2014) | China | Asian | CSCC | 86 | 79 | 1.78
(1.08,2.93) | 0.024 | RFS | 120 | (15) |
| Huh (2013) | Korea | Asian | CRC | 149 | 50 | 3.314
(1.109,9.9) | NM | DFS | 120 | (20) |
| Liao (2012) | China | Asian | NPC | 127 | 82 | 1.55
(0.93,2.59) | NM | PFS | 250 | (21) |
| Xiao (2011) | China | Asian | BC | 105 | 88 | 2.05
(1.3,4.59) | 0.017 | PFS | 100 | (10) |
Meta-analysis of P300 expression and
clinicopathological factors
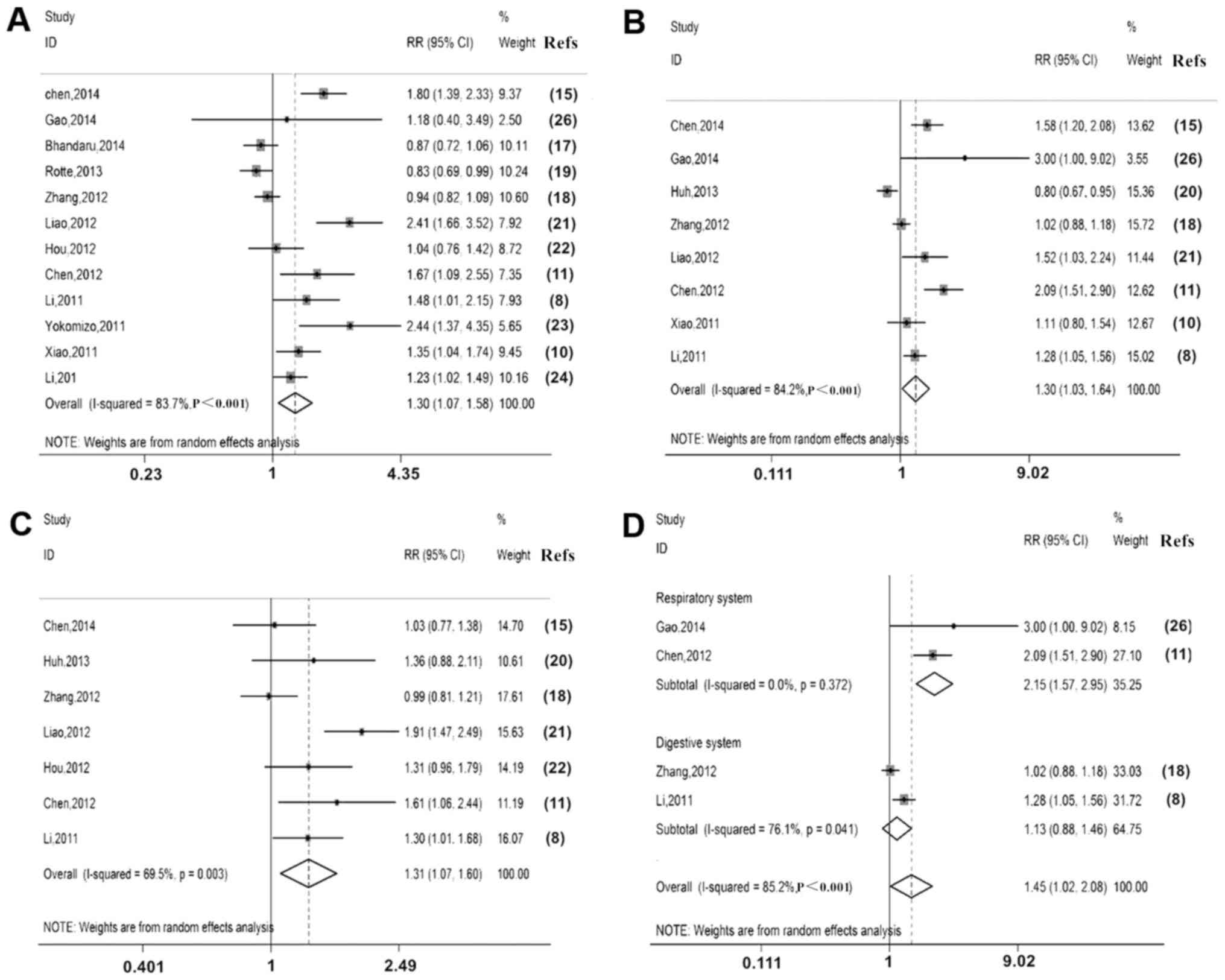
P300 expression was associated with
clinicopathological factors, including sex, tumour size, degree of
differentiation, clinical stage, lymph node metastasis and depth of
invasion (Table V). P300
overexpression was associated with clinical stage [III/IV vs. I/II,
relative Risk (RR)=1.30, 95% CI: 1.07–1.58; Fig. 5A], lymph node metastasis (M1 vs. M0,
RR=1.30, 95% CI: 1.03–1.64; Fig. 5B)
and depth of invasion (T3/T4 vs. T1/T2, RR=1.31, 95% CI: 1.07–1.60)
(Fig. 5C). However, it was not
significantly associated with sex (RR=0.97, 95% CI: 0.90–1.06),
tumour size (≤5 vs. >5 cm, RR=0.89, 95% CI: 0.67–1.19) or degree
of differentiation (well + moderate vs. poor, RR=0.86, 95% CI:
0.71–1.04; Table VI).
 | Table V.E1A binding protein P300 expression
and clinicopathological features in the studies included. |
Table V.
E1A binding protein P300 expression
and clinicopathological features in the studies included.
|
| Stage I/II | Stage III/IV | T1/T2 | T3/T4 | M0 | M1 |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| First author
(year) | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | (Refs.) |
|---|
| Chen (2015) | 52 | 69 | 34 | 10 | 39 | 37 | 47 | 42 | 63 | 71 | 23 | 8 | (15) |
| Gao (2014) | 12 | 161 | 4 | 45 | NM | NM | NM | NM | 4 | 107 | 12 | 99 | (16) |
| Bhandaru
(2014) | 117 | 76 | 71 | 63 | NM | NM | NM | NM | NM | NM | NM | NM | (17) |
| Rotte (2013) | 159 | 92 | 74 | 67 | NM | NM | NM | NM | NM | NM | NM | NM | (19) |
| Huh (2013) | NM | NM | NM | NM | 9 | 7 | 140 | 43 | 88 | 20 | 61 | 33 | (20) |
| Zhang (2013) | 41 | 7 | 103 | 25 | 19 | 4 | 125 | 28 | 41 | 7 | 60 | 9 | (18) |
| Liao (2012) | 20 | 45 | 107 | 37 | 38 | 56 | 89 | 26 | 17 | 23 | 62 | 34 | (21) |
| Hou (2012) | 60 | 58 | 27 | 24 | 66 | 70 | 21 | 12 | NM | NM | NM | NM | (22) |
| Chen (2013) | 20 | 30 | 20 | 10 | 22 | 31 | 18 | 9 | 30 | 39 | 10 | 1 | (11) |
| Li (2011) | 24 | 37 | 36 | 26 | NM | NM | NM | NM | NM | NM | NM | NM | (8) |
| Yokomizo
(2011) | 10 | 21 | 11 | 3 | NM | NM | NM | NM | NM | NM | NM | NM | (23) |
| Total events | 672 | 731 | 585 | 353 | 229 | 239 | 554 | 216 | 360 | 373 | 335 | 241 |
|
 | Table VI.RR for the association between
clinicopathological features and high expression of E1A binding
protein P300. |
Table VI.
RR for the association between
clinicopathological features and high expression of E1A binding
protein P300.
|
|
|
|
|
| Heterogeneity |
|---|
|
|
|
|
|
|
|
|---|
| Clinicopathological
feature | Number of
studies | Cases (n) | Analytical
model | Pooled RR (95%
CI) | I2
(%) | P-value |
|---|
| Tumor size (≤5 vs.
>5 cm) | 4 | 663 | Random-effects
model | 0.89
(0.67,1.19) | 86.6 | <0.001 |
| Degree of
differentiation (well + moderate vs. poor) | 9 | 1,371 | Random-effects
model | 0.86
(0.71,1.04) | 72.7 | <0.001 |
| Gender (female vs.
male) | 11 | 2,019 | Fixed-effects
model | 0.97
(0.90,1.06) | 0 |
0.849 |
A further subgroup analysis by digestive and
respiratory system malignant neoplasms indicated that
overexpression of P300 was associated with lymph node metastasis of
respiratory system malignant neoplasms (M1 vs. M0, RR=2.15, 95% CI:
1.57–2.95; Fig. 5D).
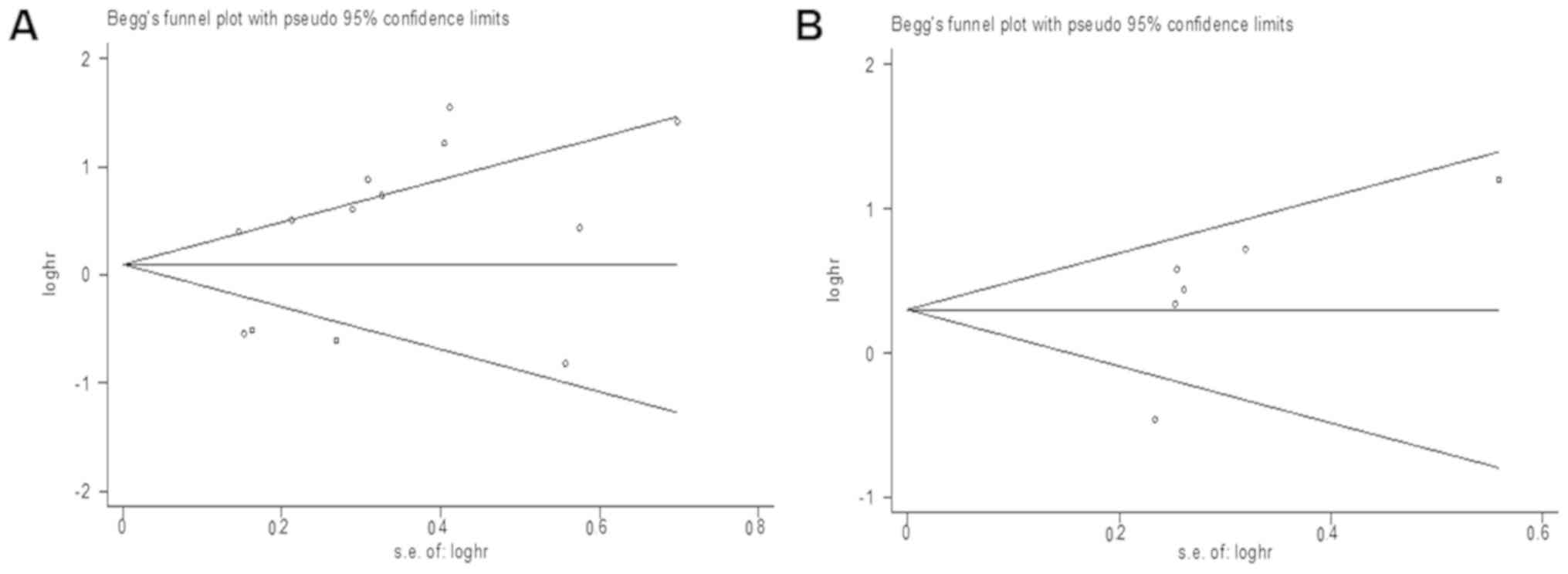
Publication bias
Publication bias for patient survival and tumour
progression was assessed by funnel plots and Egger's tests. As
expected, the funnel plots of P-values of the Egger's test were
0.102 for OS (Fig. 6A) and 0.184 for
RFS/PFS/DFS (Fig. 6B), suggesting no
publication bias.
Discussion
EP300 is an important factor in the transforming
growth factor-β signaling pathway. EP300 somatic mutations are
significantly different between the epithelial and spindle cell
components in SS, and are mainly located in the DNA-binding domain
and the gene encoding histone H3 lysine acetyltransferases
(26). The clinical analysis
included in the present study indicated that the expression of P300
was significantly different between the epithelial and spindle cell
components. In addition, the association of P300 with
epithelial-mesenchymal transition (EMT)-associated proteins that
were assessed in a previous study by our group (12) were analyzed, and it was revealed that
the expression of P300 was closely associated with the expression
of Slug (Both P300 and slug were expressed in single-phase fibrous
synovial sarcoma, and the association between the two was
analyzed), suggesting that P300 may have an important role in the
pathogenesis of SS (data not shown).
The present study further evaluated the prognostic
role of upregulated P300 expression in SS with a comprehensive and
detailed meta-analysis comprising 2,517 cases. The results
indicated that overexpression of P300 was significantly associated
with poor PFS/RFS/DFS, which is consistent with results of the
study by Xiao et al (10), in
which there was a significant association between high expression
of P300 and poor PFS. However, P300 overexpression was not
significantly associated with OS.
Although P300 has been observed as a tumour
suppressor in the majority of studies, its oncogenic role has also
been confirmed in multiple cancer types, where it is involved in
the EMT (23,27,28),
proliferation (29,30) and apoptosis (30,31). Gao
et al (16) suggested that
high expression of P300 enhanced EMT of HCC cells, and Liao et
al (27) also indicates that
P300 promotes EMT in an NPC cell line. Pifer et al (28) suggested that grainyhead-like 2
inhibits the P300 co-activator, suppressing EMT. Inagaki et
al (29), indicated that
core-binding protein/P300 histone acetyltransferase activity is
responsible for epigenetic regulation of proliferation and invasion
in HCC cells, and Dou et al (32) suggested that midazolam inhibits the
proliferation of human head and neck SCC cells by downregulating
P300 expression. Gao et al (30) reported that the P300 inhibitor C646
induces cell cycle arrest and apoptosis in acute myeloid leukemia
cells and Ono et al (31)
indicated that P300 inhibition enhances the gemcitabine-induced
apoptosis of pancreatic cancer cells.
In the present study, subgroup analyses were
performed based on ethnicity and location of the malignant
neoplasms. The ethnicity analysis demonstrated that overexpression
of P300 in Asian patients was significantly associated with poor
OS. However, overexpression of P300 was a favourable predictor of
OS in Caucasians. The differences between the Asian and Caucasian
ethnic groups demonstrate heterogeneity, and are associated with
socioeconomic status, culture, diet, environment and genetics.
Biological functions, including DNA methylation, which is a type of
epigenetic process, has a major role in the induction of genetics.
DNA methylation may cause changes of the key regulatory genes in
cancer. Genes that are differentially methylated, as observed
between Asian and Caucasian populations, are involved in oncogenic
processes, including tumour growth, tumour suppression and
metastasis (33). Langevin and
Kelsey (34) also suggested that the
oncogenic process is driven by the accumulation of genetic and
epigenetic alterations, resulting in dysregulation of tumour
suppressor genes, key oncogenes and DNA repair genes.
In the present study, P300 was associated with poor
OS of Asians with malignant neoplasms of the digestive system. The
present results are consistent with those reported by McCracken
et al (35), who reported
that compared to Caucasians, Asians are more affected by certain
cancer types, including those of the stomach, liver, oesophagus and
the uterine cervix. Chien et al (36), also demonstrated that 10–60% of Asian
Americans (Chinese and Mexican men), are likely to be diagnosed
with stage III or IV CRC compared to Caucasian men.
The association between P300 expression and
clinicopathological parameters was also analysed in the present
study. The results indicated that overexpression of P300 was
associated with clinical stage, lymph node metastasis and depth of
invasion. However, it was not significantly associated with sex,
tumour size or degree of differentiation. The present results are
consistent with those of the study by Liao et al (21), who indicated that overexpression of
P300 was positively associated with later T classification, later N
classification, distant metastasis and later clinical stage. Xiao
et al (10) also suggested
that high expression of P300 was positively correlated with higher
histological grade, advanced clinical stage and tumour recurrence.
However, the present results did not indicate any significant
association of P300 with those clinicopathological parameters.
It is important to consider the limitations of the
present meta-analysis. First, it was only possible to directly
extract the data from 9 of the 14 eligible studies, while the data
of the remaining studies were extracted from Kaplan-Meier survival
plots. Second, the cut-off values of P300 expression in the
original studies were varied, e.g. the mean values were used in
certain studies, while the median values were used in the others.
Third, some data points within the funnel plot are located outside
the funnel. Furthermore, as tissue was kept for many years, it is
possible that protein may be subject to decomposition after long
periods of storage. Furthermore, heterogeneity existed in the total
analysis of OS (I2=86.2%) and PFS/RFS/DFS
(I2=69.6%). This is likely due to the different
ethnicities, types of malignant cancer, detection methods and
follow-up periods. These factors should be considered when
evaluating the prognostic role of P300 expression in human
malignant cancers in the future. If these limitations were the be
overcome, P300 may be used as a prognostic biomarker in clinical
applications.
In conclusion, the present results indicated that
high P300 expression predicted a poor OS in Asian populations,
particularly in digestive system malignant neoplasms. However, more
comprehensive studies are required to evaluate and confirm the
prognostic value of P300 in cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81860471),
the Outstanding Youth Science and Technology Talent Cultivation
Plan of Shihezi University (grant no. 2015ZRKXJQ07), the
International cooperation projects of Shihezi University (grant no.
GJHZ201710) and the Research Project of High-Level Talents of
Shihezi University (grant no. RCZX201549).
Availability of data and materials
All data are available from the corresponding author
on reasonable request.
Authors' contributions
XL, HZ, YH and NW performed the experiments and
analyzed the data; JH, XC, JZ and YQ designed and supervised the
study. WZ, WG and LP provided crucial input for the project; ZL and
YH wrote the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and informed consent
The present study was approved by the Institutional
Ethics Review Board (IERB; no. 2013LL10) at the First Affiliated
Hospital, Shihezi University School of Medicine (Shihezi, China;
including the use of patients' tissues between 1968 and 2015).
Patients consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Kundu TK, Palhan VB, Wang Z, An W, Cole PA
and Roeder RG: Activator-dependent transcription from chromatin in
vitro involving targeted histone acetylation by p300. Mol Cell.
6:551–561. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vo N and Goodman RH: CREB-binding protein
and p300 in transcriptional regulation. J Biol Chem.
276:13505–13508. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang WC and Chen CC: Akt phosphorylation
of p300 at Ser-1834 is essential for its histone acetyltransferase
and transcriptional activity. Mol Cell Biol. 25:6592–6602. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muraoka M, Konishi M, Kikuchi-Yanoshita R,
Tanaka K, Shitara N, Chong JM, Iwama T and Miyaki M: p300 gene
alterations in colorectal and gastric carcinomas. Oncogene.
12:1565–1569. 1996.PubMed/NCBI
|
|
5
|
Wang L, Huang X, Chai Y, Zou L, Chedrawe M
and Ding Y: Octreotide inhibits the proliferation of gastric cancer
cells through P300-HAT activity and the interaction of ZAC and
P300. Oncol Rep. 37:2041–2048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gayther SA, Batley SJ, Linger L, Bannister
A, Thorpe K, Chin SF, Daigo Y, Russell P, Wilson A, Sowter HM, et
al: Mutations truncating the EP300 acetylase in human cancers. Nat
Genet. 24:300–303. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shikama N, Lee CW, France S, Delavaine L,
Lyon J, Krstic-Demonacos M and La Thangue NB: A novel cofactor for
p300 that regulates the p53 response. Mol Cell. 4:365–376. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li M, Luo RZ, Chen JW, Cao Y, Lu JB, He
JH, Wu QL and Cai MY: High expression of transcriptional
coactivator p300 correlates with aggressive features and poor
prognosis of hepatocellular carcinoma. J Transl Med. 9:52011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Isharwal SM, Miller MC, Marlow C, Makarov
DV, Partin AW and Veltri RW: p300 (histone acetyltransferase)
biomarker predicts prostate cancer biochemical recurrence and
correlates with changes in epithelia nuclear size and shape.
Prostate. 68:1097–1104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao XS, Cai MY, Chen JW, Guan XY, Kung
HF, Zeng YX and Xie D: High expression of p300 in human breast
cancer correlates with tumor recurrence and predicts adverse
prognosis. Chin J Cancer Res. 23:201–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YF, Luo RZ, Li Y, Cui BK, Song M,
Yang AK and Chen WK: High expression levels of COX-2 and P300 are
associated with unfavorable survival in laryngeal squamous cell
carcinoma. Eur Arch Otorhinolaryngol. 270:1009–1017. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi Y, Wang CC, He YL, Zou H, Liu CX, Pang
LJ, Hu JM, Jiang JF, Zhang WJ and Li F: The correlation between
morphology and the expression of TGF-β signaling pathway proteins
and epithelial-mesenchymal transition-related proteins in synovial
sarcomas. Int J Clin Exp Pathol. 6:2787–2799. 2013.PubMed/NCBI
|
|
13
|
Greenwood DC: Meta-analysis of
observational studies. Mod Methods Epidemiol. 173–189. 2012.
View Article : Google Scholar
|
|
14
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen MK, Cai MY, Luo RZ, Tian X, Liao QM,
Zhang XY and Han JD: Overexpression of p300 correlates with poor
prognosis in patients with cutaneous squamous cell carcinoma. Br J
Dermatol. 172:111–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Y, Geng J, Hong X, Qi J, Teng Y, Yang
Y, Qu D and Chen G: Expression of p300 and CBP is associated with
poor prognosis in small cell lung cancer. Int J Clin Exp Pathol.
7:760–767. 2014.PubMed/NCBI
|
|
17
|
Bhandaru M, Ardekani GS, Zhang G, Martinka
M, McElwee KJ, Li G and Rotte A: A combination of p300 and Braf
expression in the diagnosis and prognosis of melanoma. BMC Cancer.
14:3982014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang LH, Huang Q, Fan XS, Wu HY, Yang J
and Feng AN: Clinicopathological significance of SIRT1 and p300/CBP
expression in gastroesophageal junction (GEJ) cancer and the
correlation with E-cadherin and MLH1. Pathol Res Pract.
209:611–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rotte A, Bhandaru M, Cheng Y, Sjoestroem
C, Martinka M and Li G: Decreased expression of nuclear p300 is
associated with disease progression and worse prognosis of melanoma
patients. PLoS One. 8:e754052013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huh JW, Kim HC, Kim SH, Park YA, Cho YB,
Yun SH, Lee WY and Chun H: Prognostic impact of p300 expression in
patients with colorectal cancer. J Surg Oncol. 108:374–377. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liao ZW, Zhou TC, Tan XJ, Song XL, Liu Y,
Shi XY, Huang WJ, Du LL, Tu BJ and Lin XD: High expression of p300
is linked to aggressive features and poor prognosis of
nasopharyngeal carcinoma. J Transl Med. 10:1102012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ono H, Basson MD and Ito H: P300
inhibition enhances gemcitabine-induced apoptosis of pancreatic
cancer. Oncotarget. 7:51301–51310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou X, Li Y, Luo RZ, Fu JH, Zhang LJ and
Yang HX: High expression of the transcriptional co-activator p300
predicts poor survival in resectable non-small cell lung cancers.
Eur J Surg Oncol. 38:523–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Yang HX, Luo RZ, Zhang Y, Li M, Wang
X and Jia WH: High expression of p300 has an unfavorable impact on
survival in resectable esophageal squamous cell carcinoma. Ann
Thorac Surg. 91:1531–1538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishihama K, Yamakawa M, Semba S, Takeda H,
Kawata S, Kimura S and Kimura W: Expression of HDAC1 and CBP/p300
in human colorectal carcinomas. J Clin Pathol. 60:1205–1210. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi Y, Wang N, Pang LJ, Zou H, Hu JM, Zhao
J, Zhang J, Liu CX, Zhang WJ, Yuan XL and Li F: Identification of
potential mutations and genomic alterations in the epithelial and
spindle cell components of biphasic synovial sarcomas using a human
exome SNP chip. BMC Med Genomics. 8:692015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao ZW, Zhao L, Cai MY, Xi M, He LR, Yu
F, Zhou TC and Liu MZ: P300 promotes migration, invasion and
epithelial-mesenchymal transition in a nasopharyngeal carcinoma
cell line. Oncol Lett. 13:763–769. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pifer PM, Farris JC, Thomas AL, Stoilov P,
Denvir J, Smith DM and Frisch SM: Grainyhead-like 2 inhibits the
coactivator p300, suppressing tubulogenesis and the
epithelial-mesenchymal transition. Mol Biol Cell. 27:2479–2492.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inagaki Y, Shiraki K, Sugimoto K, Yada T,
Tameda M, Ogura S, Yamamoto N, Takei Y and Ito M: Epigenetic
regulation of proliferation and invasion in hepatocellular
carcinoma cells by CBP/p300 histone acetyltransferase activity. Int
J Oncol. 48:533–540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao XN, Lin J, Ning QY, Gao L, Yao YS,
Zhou JH, Li YH, Wang LL and Yu L: A histone acetyltransferase p300
inhibitor C646 induces cell cycle arrest and apoptosis selectively
in AML1-ETO-positive AML cells. PLoS One. 8:e554812013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ono H, Basson MD and Ito H: P300
inhibition enhances gemcitabine-induced apoptosis of pancreatic
cancer. Oncotarget. 7:51301–51310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dou YL, Lin JP, Liu FE, Wang LY, Shu HH,
Jiang N, Xie Y and Duan Q: Midazolam inhibits the proliferation of
human head and neck sqamous carcinoma cells by downregulating p300
expression. Tumour Biol. 35:7499–7504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mohammed SI, Springfield S and Das R: Role
of epigenetics in cancer health disparities. Methods Mol Biol.
863:395–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Langevin SM and Kelsey KT: The fate is not
always written in the genes: Epigenomics in epidemiologic studies.
Environ Mol Mutagen. 54:533–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCracken M, Olsen M, Chen MS Jr, Jemal A,
Thun M, Cokkinides V, Deapen D and Ward E: Cancer incidence,
mortality, and associated risk factors among Asian Americans of
Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA
Cancer J Clin. 57:190–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chien C, Morimoto LM, Tom J and Li CI:
Differences in colorectal carcinoma stage and survival by race and
ethnicity. Cancer. 104:629–639. 2005. View Article : Google Scholar : PubMed/NCBI
|