Introduction
Post-stroke depression (PSD) is one of the most
frequent psychiatric complications of stroke and affects ~33% of
stroke patients (1). Patients who
have suffered a stroke who also suffer from PSD only show minor
improvement in rehabilitation compared with stroke patients without
depression. Therefore, it is necessary to investigate its
mechanisms.
The majority of current PSD theories consider PSD to
be caused by underlying problems, including hippocampal neuronal
regeneration disorder, the presence of inflammatory cytokines,
hypothalamic-pituitary-adrenal axis disorder, monoamine deficiency,
and neuroanatomical problems, for example, frontal lobe neuronal
injury. Among these, the presence of inflammatory cytokines has
been linked to the underlying mechanisms of PSD and several studies
have shown that the elevation of cytokines is a key factor in the
development of nerve cell damage leading to PSD (2,3).
However, the specific mechanism underlying the elevated cytokines
remains to be fully elucidated.
Increasing evidence has suggested that the
cerebellum may be important in cognition, behavior, and psychiatric
illnesses (4,5). Schmahmann and Sherman found isolated
cerebellar lesions in patients, which can lead to cerebellar
cognitive affective syndrome (CCAS) (4). In addition, a key feature of CCAS is
the development of dysregulation when the cerebellar fastigial
nucleus (FN) is involved in the lesions (6). Evidence from one of our previous
studies showed that depressive-like behaviors in PSD rats were
improved by FN electrical stimulation (FNS) (7). Similarly, Su et al reported that
electrical stimulation at the FN substantially alleviated symptoms
in patients with PSD (8). Taken
together, there is a possibility that cerebellar dysfunction may be
involved in the underlying mechanisms of PSD. However, few studies
have investigated the putative role of the cerebellum in PSD.
A previous study indicated that stimulation of the
cerebellar FN can provide a protective effect by inhibiting
cerebrovascular inflammation (9). In
addition, lesions of neuronal somas in bilateral FN with kainic
acid (KA) have been shown to lead to the enhanced activity of T
cells, B cells and natural killer cells (10). Our previous preliminary study also
showed that, following FNS, the expression of inflammatory
cytokines in the hippocampus was reduced significantly, thus
protecting the Purkinje cells in this region and simultaneously
improving the depressive-like behaviors of the PSD rats (7). However, there is no direct structural
connection between the cerebellum and immune system. Therefore, it
is important to examine the pathways involved in cerebellar
immunomodulation in order to better understand the mechanisms of
PSD.
It is well known that the hypothalamus is a crucial
immunoregulatory center, which modulates the immune system via the
sympathetic nervous system and the hypothalamic-pituitary-adrenal
axis (11–13). A direct cerebellar-hypothalamic
projection has been found (14,15),
which arises from cerebellar FN neurons and mainly travels along
the superior cerebellar peduncle (scp). Following crossing at the
decussation of the scp (XSCP), it travels along the contralateral
scp and terminates in the lateral hypothalamic area (LHA) (14,15).
Experiments have indicated that there are direct glutamatergic and
γ-aminobutyric acid (GABA)ergic projections from the FN to the LHA,
which convey immunomodulating information from the FN (16,17).
Accordingly, cerebellar immunoregulatory information may be
transmitted to the hypothalamus via direct cerebellar-hypothalamic
projections. Based on these findings, it was hypothesized in the
present study that direct cerebellar-hypothalamic projections may
transmit cerebellar immunoregulatory information to the
hypothalamus, through which the regulatory information is then
conveyed to the immune system involved in PSD.
In the present study, lesions of the FN or XSCP,
behavioral signs of depression, mRNA levels of tumor necrosis
factor (TNF)-α, interleukin (IL)-6, and IL-1β in the hippocampus,
and the content of GABA and glutamate in the LHA were examined. The
aim was to provide further evidence of the mechanisms suggested for
the involvement of the cerebellum in PSD.
Materials and methods
Establishment of a PSD rat model
Healthy Sprague-Dawley (SD) rats weighing 240–260 g,
which were aged 13 weeks, were provided by the Center of
Experimental Animals, Jinzhou Medical University (Jinzhou, China).
A total of 30 SD rats were randomly divided into five groups:
Sham-operated, Stroke, PSD, FN lesion, and XSCP lesion groups. The
middle cerebral artery was not occluded in animals of the Sham
group, whereas the rats in the Stroke, PSD, FN lesion, and XSCP
lesion groups underwent middle cerebral artery occlusion (MCAO).
The rats were housed (n=5/cage) in quiet a room that was maintained
at 21–22°C with 50% relative humidity and a 12/12-h light/dark
cycle. They were allowed free access to food and water. The rats in
the PSD group were subjected to isolated housing in combination
with chronic and unexpected mild stress (CUMS) comprising water
deprivation, wet litter, food deprivation, 45° cage tilt, overnight
lighting, behavioral restriction, tail clamping, electric shock to
foot, and ice-water swimming, in order to set up a depression
model. The protocol was approved by Jinzhou Medical University for
the Care and Use of Laboratory Animals.
Induction of bilateral cerebellar FN
lesions using KA
The KA (Sigma, EMD Millipore, Billerica, MA, USA)
was dissolved in saline. Following the administration of
pentobarbital (55 mg/kg, i.p.), the MCAO rats were placed in a
stereotaxic apparatus (David Kopf 902-A; David Kopf Instruments,
Tujunga, CA, USA). The KA (0.4 µg in 0.4 µl sterilized saline) was
infused into each lateral FN using the following stereotaxic
coordinates: 11.5 mm posterior to the bregma, 1.1 mm left/right of
the midline, and 6.3 mm ventral to the bregma (18). The infusion was made over 2 min and
the needle was then left in place for 3 min. Data on body weight,
consumption of sucrose water, rearing activity, locomotor activity,
and GABA and glutamate of all rats were collected during the
experiment.
Induction of XSCP lesions using an
electrode
Anesthesia and placement were performed as described
above. Electrolytic lesions were produced using a stainless steel
electrode insulated within 0.1 mm of its sharpened tip. The
electrode was inserted into the XSCP using the following
stereotaxic coordinates: 6.7–8.0 mm posterior to the bregma, 0 mm
left/right of the midline, and 7.8–8.0 mm ventral to the bregma
(18). A current of 0.5 mA was
applied and the duration was 10 sec. Data were collected as
above.
Open-field test (OFT)
The rats were placed in the front right corner of a
clear acrylic box (16×16) for 20 min. A computer operated PAS Open
Field system (San Diego Instruments, San Diego, CA, USA) was used
in accordance with the manufacturer's protocol to quantify the
locomotor and rearing activity as the total number of beam
breaks.
Dialysate collection and
high-performance liquid chromatography (HPLC)
The contents of glutamate and GABA were measured by
HPLC. Anesthesia and placement were performed as described above.
The microdialysis probes (CMA Microdialysis AB, Kista, Sweden) were
inserted into the LHA using the following stereotaxic coordinates:
−1.8–2.56 mm posterior to the bregma, 1.4–2.6 mm right of the
midline, and 7.6–8.4 mm ventral to the bregma (18). The probes were perfused with an
artificial cerebrospinal fluid at a flow rate of 2 µl/min.
Following implantation, the dialysate fractions were collected at
30-min intervals. The fractions collected in the first 1 h were
discarded to avoid parenchymal disturbance prior to a
semi-steady-state level being reached. The subsequent two fractions
were used for evaluation. The dialysates were automatically
collected with a refrigerated autosampler (CMA Microdialysis AB)
and stored at −80°C until analysis.
The dialysate samples were individually homogenized
in 0.25 ml methanol and centrifuged for 10 min (16,873 × g) at 4°C.
Briefly, 20 µl supernatant was injected into an HPLC machine
equipped with a fluorescent detector (Waters Corporation, Milford,
MA, USA) and an amino acid analysis column (Waters Corporation).
Separation of the amino acid mixture was performed using a gradient
system at a flow rate of 1 ml/min. Mobile phase A contained a
sodium acetate buffer and mobile phase B contained acetonitrile.
Fluorometric detection was performed at an excitation and emission
wavelength of 250 and 395 nm, respectively.
Optical microscopy
At the end of the experiment, the animals were
sacrificed by decapitation. The brain tissues were removed and
fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4)
for 48 h. Subsequently, the brain tissues were sectioned coronally
into 30-mm-thick sections at the FN, XSCP and LHA levels. Nissl
staining of the sections was performed to observe the locations of
the lesions and microdialysis probes under an optical microscope at
a magnification of ×200.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
TRIzol reagent (Invitrogen, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to extract the total
RNA from the tissue samples according to the manufacturer's
protocol. RT-qPCR was used to evaluate the mRNA levels of TNF-α,
IL-6, and IL-1β. Superscript II Reverse Transcriptase (Invitrogen,
Thermo Fisher Scientific, Inc.) was used to reverse transcribe the
isolated RNA into cDNA using an oligo dT18 primer in accordance
with the manufacturer's protocol. The Sequence Detection System
7900HT containing the Universal Master Mix (both Applied
Biosystems, Thermo Fisher Scientific, Inc.) was used to perform the
qPCR with specific primers (Table
I). The PCR procedure started with an initial step of 50°C for
2 min, then 95°C for 2 min, followed by 40 cycles of denaturation
at 94°C for 15 sec, annealing at 58–62°C for 15 sec and extension
at 72°C for 15 sec. Melt curve analyses (65–95°C at a rate of 0.1°C
per sec) were performed at the end of each PCR. If multiple peaks
were observed, the data were not used. Each threshold cycle (Ct)
value was calculated by taking an average of values obtained from
triplicates samples. β-actin was used as the internal control for
normalization of the mRNA expression of TNF-α IL-6, and IL-1β. The
2−ΔΔCq method (19) was
used to calculate expression of the cytokines.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
|
| Primer (5′-3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| IL-1β |
TGCAGAGTTCCCCAACTGGTACA |
GTGCTGCCTAATGTCCCCTTG |
| IL-6 |
CCGGAGAGGAGACTTCACAG |
TCCACGATTTCCCAGAGAAC |
| TNF-α |
TCAGCCTCTTCTCATTCCTG |
TGAAGAGAACCTGGGAGTAG |
| β-actin |
GATCCGTGAAGATCAAGATCATTGCT |
TGATCTTCATTTTTTACGCGTGAATT |
Statistical analysis
All data are presented as the mean ± standard
deviation. SPSS 21.0 software (IBM SPSS, Armonk, NY, USA) was used
for statistical analysis. The difference among multiple
experimental groups was detected by one-way analysis of variance.
Variance was determined using the Least-Significant Difference
t-test and Student-Newman-Keuls post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Histopathology of the FN, XSCP and LHA
lesions
In terms of the Nissl-stained cerebellar sections at
1, 4, 8, 12, 16, 20, 24 and 28 days post-injection, all animals
receiving KA injections showed a marked loss of Nissl bodies,
indicating that the neuronal bodies in the FN had been destroyed.
The interposed nucleus adjacent to the destroyed FN did not exhibit
notable neuronal loss (Fig. 1A). The
electrolytic currents applied in the experiment resulted in acute
spherical lesions centered on the tip of the electrode. The acute
electrolytic lesions were characterized by a central zone of
cavitation injury, surrounded by a sphere of carbonized tissues,
which showed that the region of XSCP had been destroyed (Fig. 1B). It was also noted that hemorrhagic
lesions were present around the microdialysis probe track, which
confirmed the correct implantation of the microdialysis probe in
the parenchyma of the LHA (Fig.
1C).
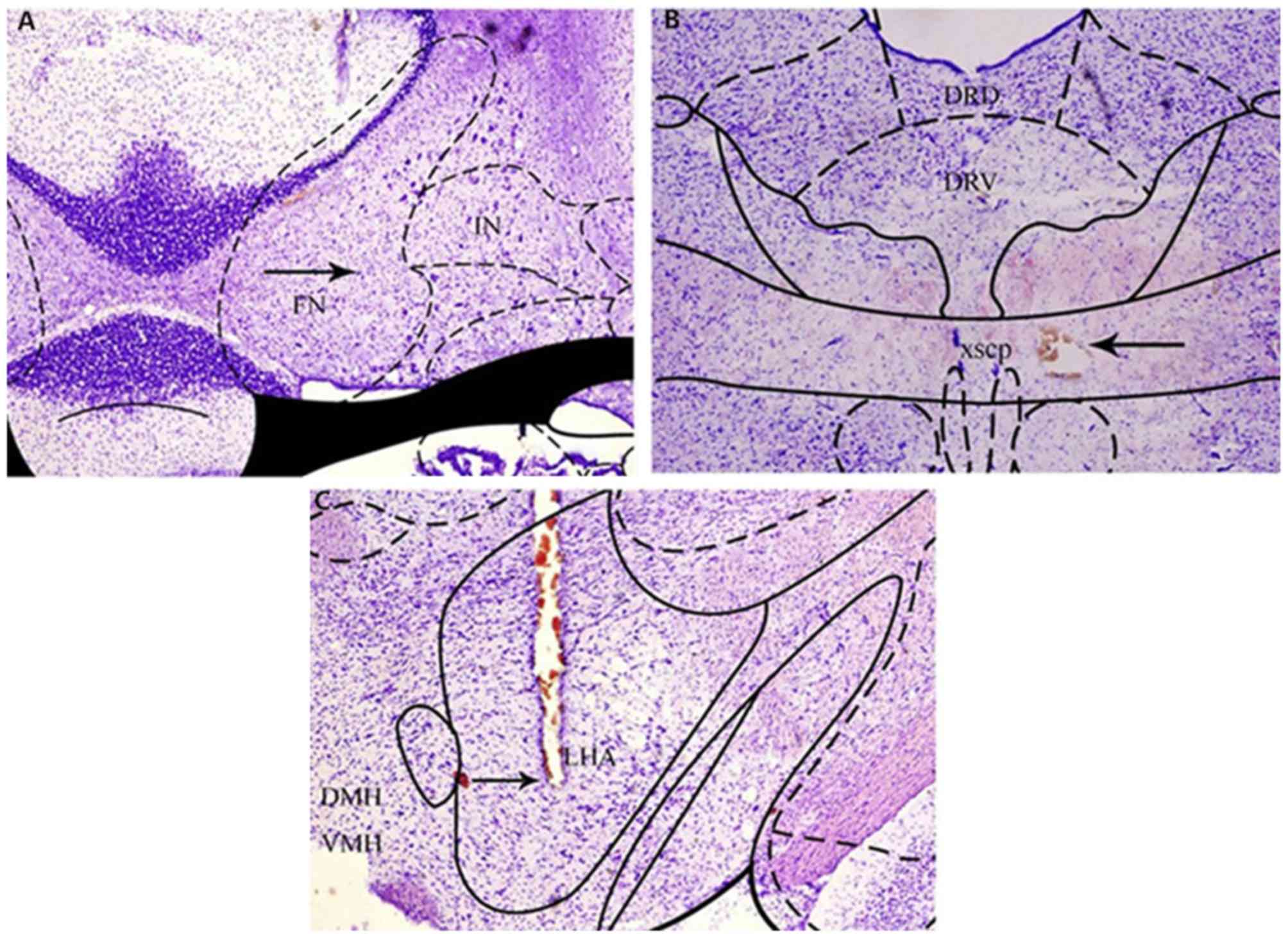 | Figure 1.Images of FN, XSCP and LHA lesions in
Nissl-stained sections. The sections were 30 µm in thickness and
were stained with cresyl violet acetate. (A) Significant neuronal
loss was present in the FN, with preservation of neurons in the
adjacent IN. (B) Round cavitation injury was present in the XSCP.
(C) Hemorrhagic lesions were present around the microdialysis probe
track in the LHA. The arrows point to lesions. FN, fastigial
nucleus; XSCP, decussation of the superior cerebellar peduncle;
LHA, lateral hypothalamic area; DRD, dorsal raphe nucleus, dorsal
region; DRV, dorsal raphe nucleus, ventral region; DMH, dorsomedial
hypothalamic nucleus; VMH, ventromedial hypothalamic nucleus. |
Establishment of a rat model of
post-stoke depression
The healthy SD rats were subjected to MACO. The rats
with PSD, FN, and XSCP lesions were further subjected to CUMS to
develop a rat depression model. The FN lesion and XSCP lesion
groups were established as described above.
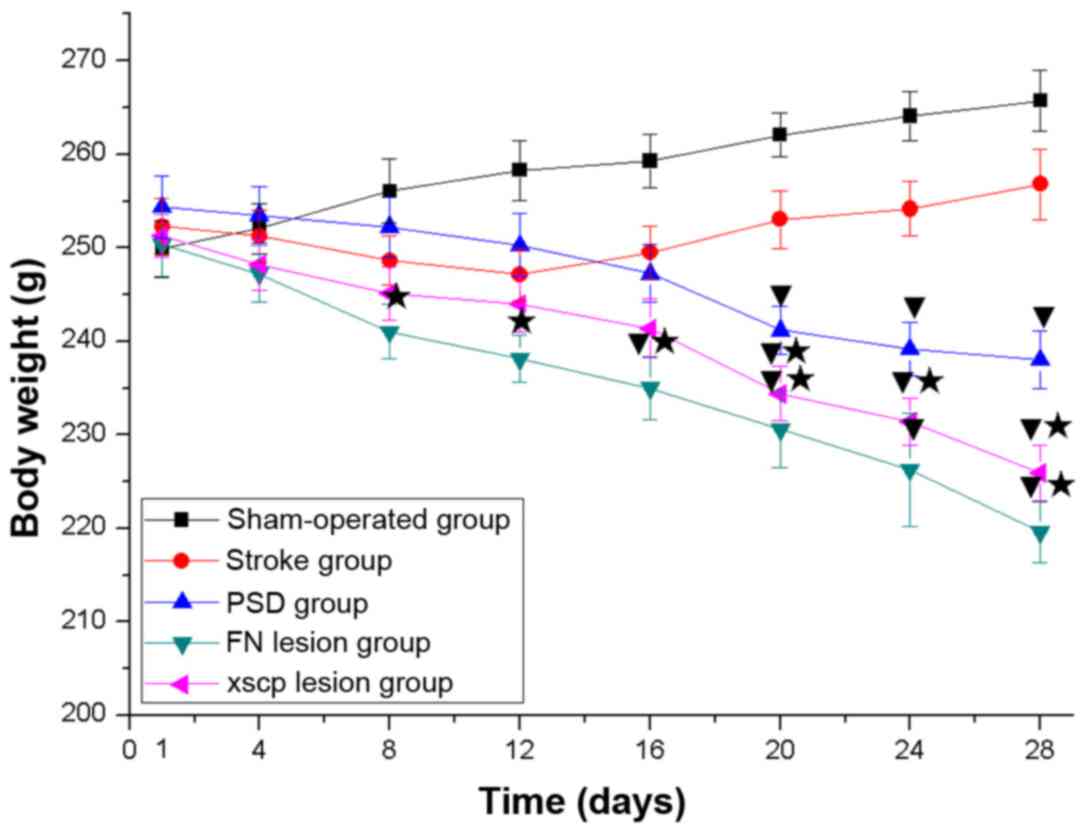
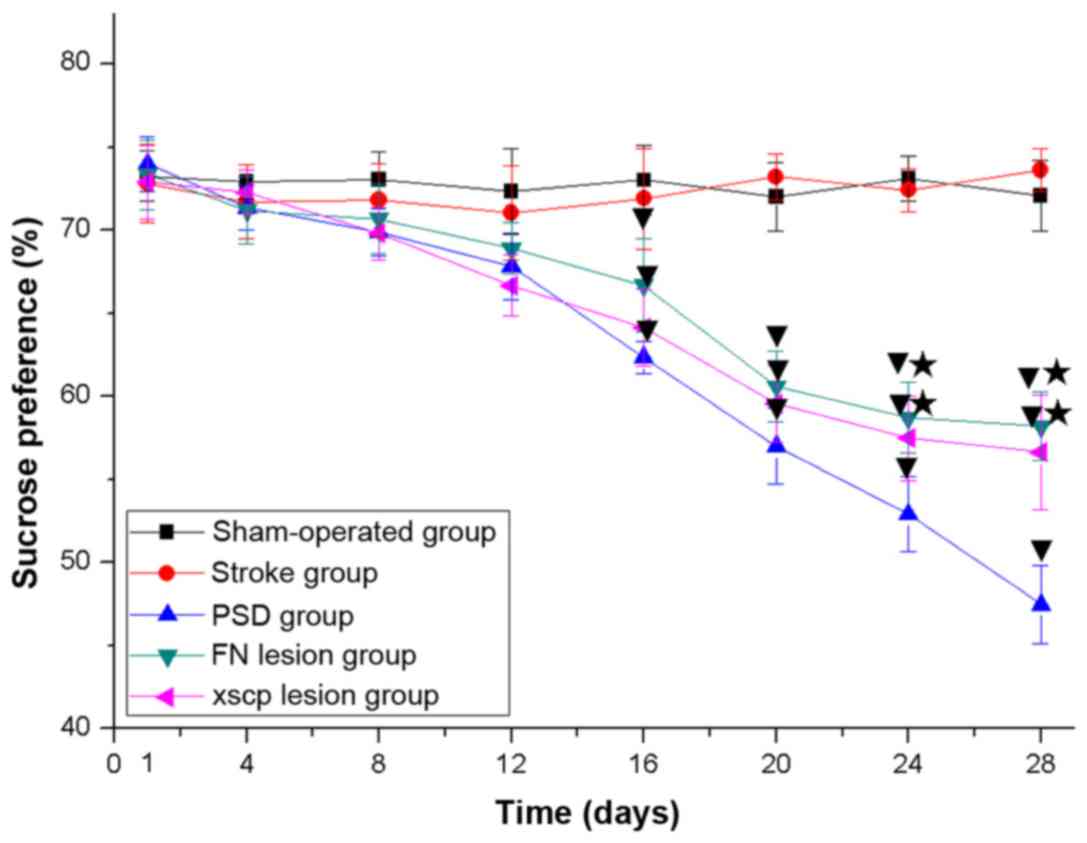
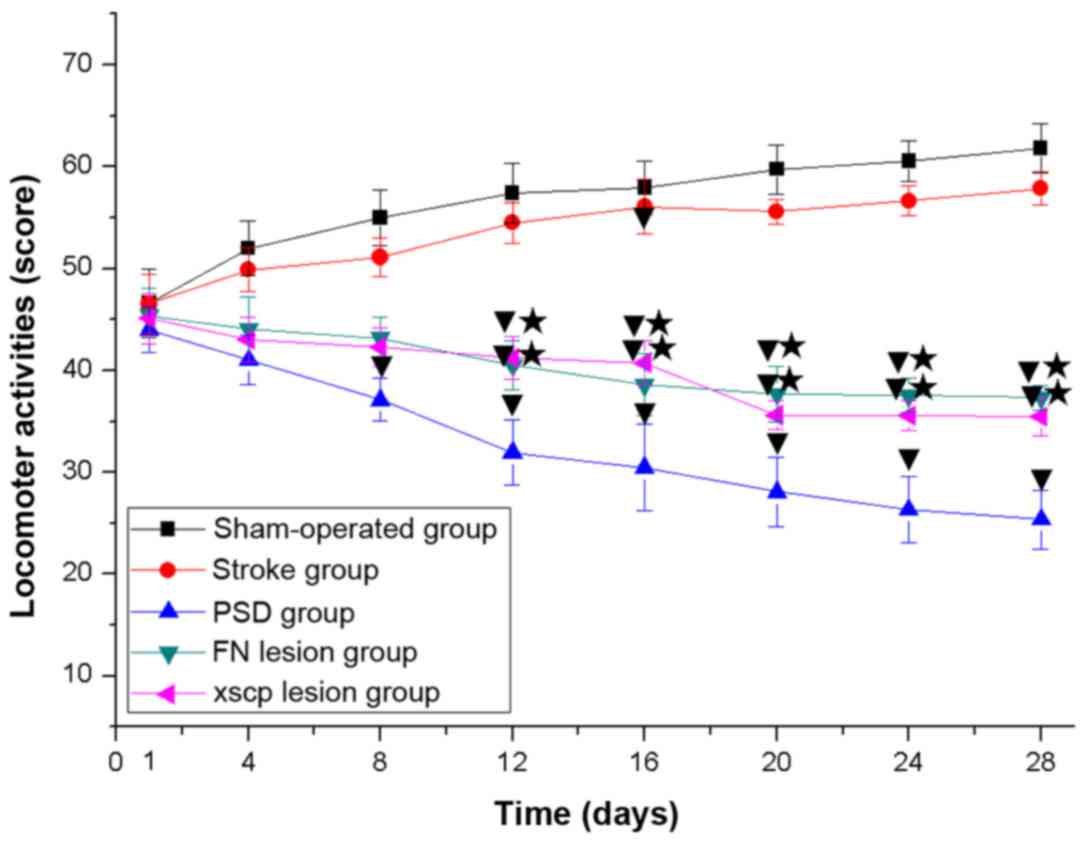
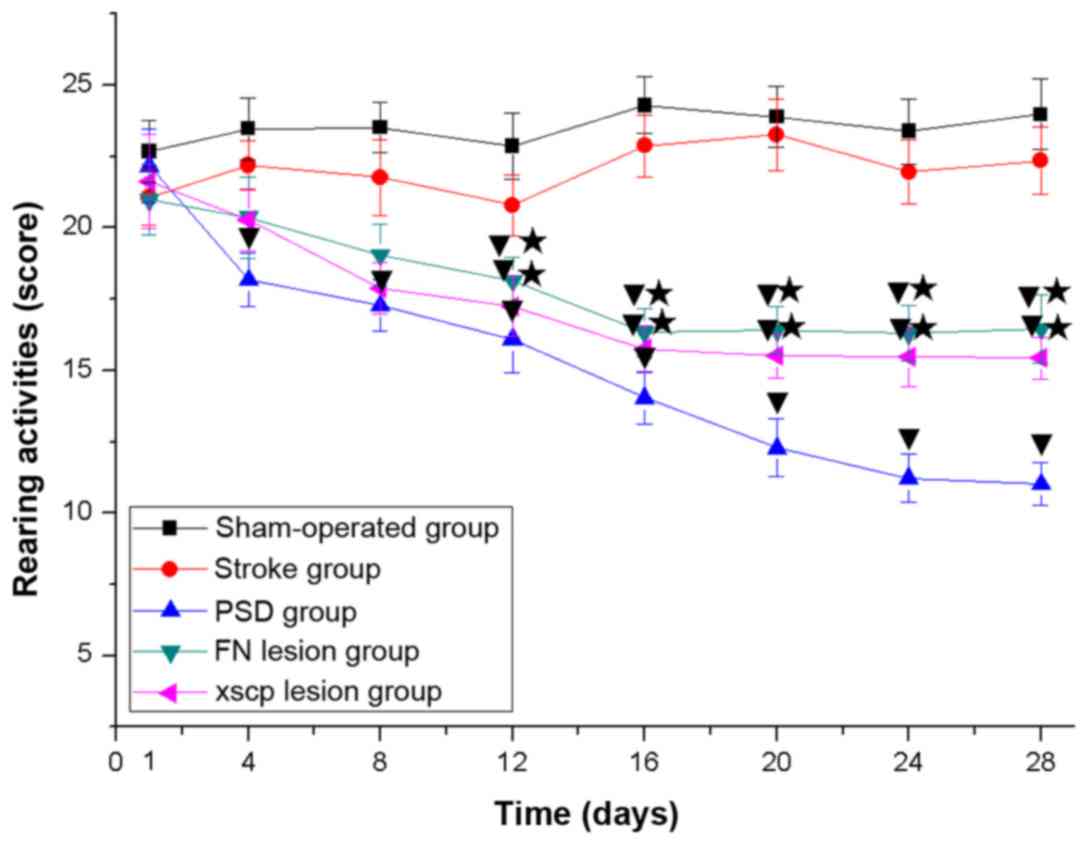
FN and XSCP lesions induce
depressive-like behaviors
In the PSD group, the rats exhibited depressive-like
behaviors, including body weight loss (Fig. 2), decreased sucrose preference
(Fig. 3), decreased locomotor
activities (Fig. 4) and decreased
rearing activities (Fig. 5) in the
OFT. Similarly, the rats with FN lesions exhibited significantly
decreased body weight, sucrose preference, locomotor activities and
rearing activities in the OFT. The rats in the XSCP lesion group
also exhibited decreased body weight, sucrose preference, locomotor
activities and rearing activities in the OFT. These data indicated
that FN lesions or XSCP lesions led to depressive-like behaviors in
the PSD rat models.
FN and XSCP lesions increase the
expression of inflammatory cytokines
Significantly higher levels of inflammatory markers
are associated with a range of depressive symptoms. In the present
study, the mRNA levels of TNF-α, IL-6, and IL-1β in hippocampal
tissues were examined (Fig. 6).
Compared with the sham rats, transient upregulation of these
cytokines was observed in the stroke rats on days 7 and 14. The PSD
rats exhibited significantly upregulated cytokine expression
between days 7 and 21. The stroke rats with the FN or XSCP lesions
exhibited significantly higher levels of cytokines, consistent with
the increase in the depressive symptoms of the FN and XSCP
rats.
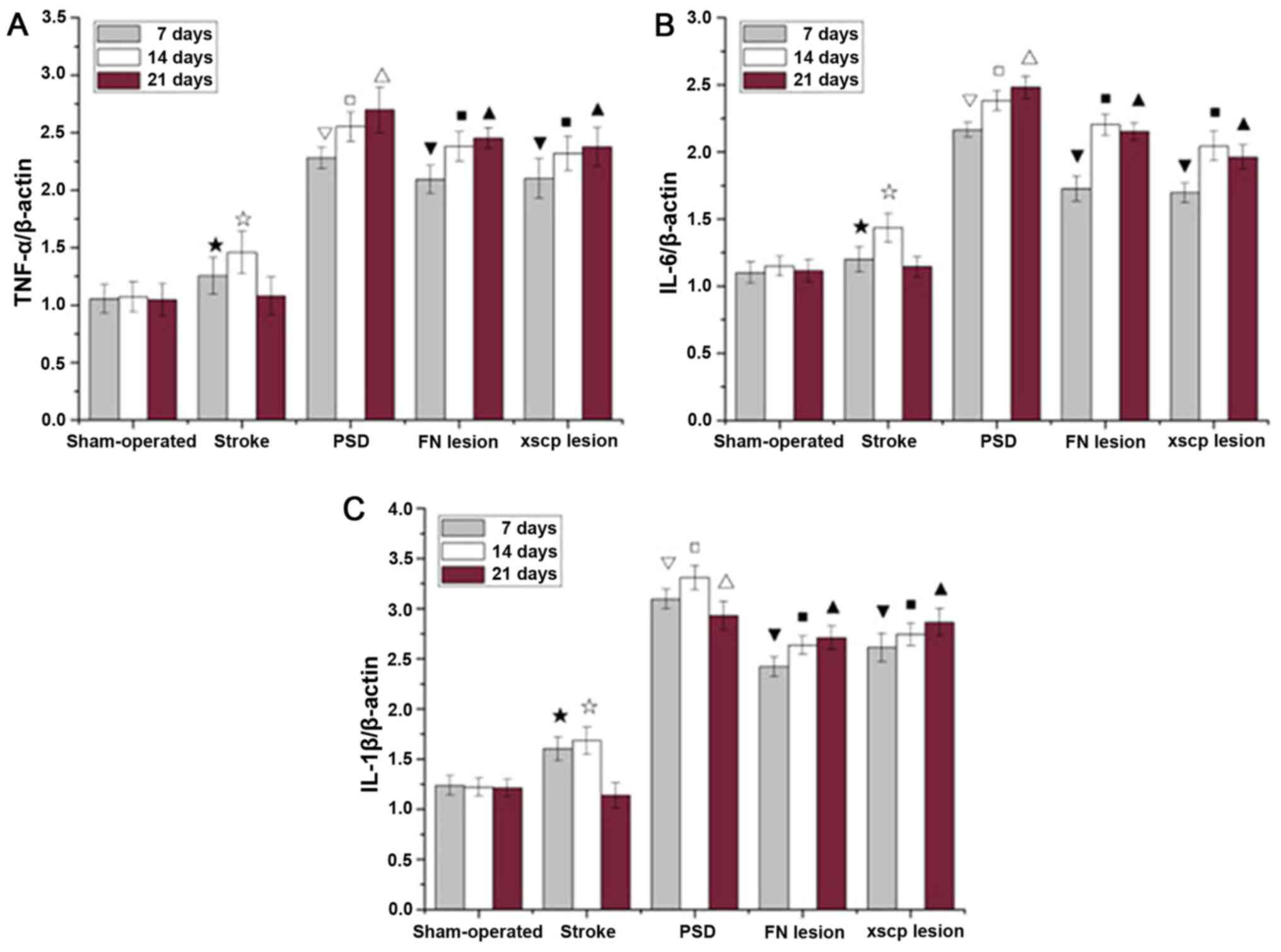 | Figure 6.Effect of FN lesions or XSCP lesions
on the expression of TNF-α, IL-6, and IL-1β in hippocampal tissues.
mRNA expression levels of (A) TNF-α, (B) IL-6, and (C) IL-1β in
hippocampal tissues of PSD rats on day 7, 14, and 21 are shown.
Compared with the Sham-operated group, the mRNA levels of cytokines
in the Stroke group increased within 14 days and then decreased to
their lowest levels on day 21. Compared with the stroke group, the
mRNA levels of cytokines in the PSD group were significantly
increased within 14 days and then remained almost stable from day
14. In the FN lesion and XSCP lesion groups, the corresponding mRNA
levels showed a similar trend to that in the PSD group, although
their absolute mRNA levels were marginally lower than those in the
PSD group. ★P<0.05, compared with the Sham group on
day 7; ☆P<0.05, compared with the Sham group on day
14; ☐P<0.01, compared with the Stroke group on day 7;
▽P<0.01, compared with the Stroke group on day 14;
ΔP<0.01, compared with the Stroke group on day 21;
▼P<0.05, compared with the PSD group on day 7;
■P<0.05, compared with the PSD group on day 14;
▲P<0.05, compared with the PSD group on day 21. The
error bars represent the standard deviation (n=6). PSD, post-stroke
depression; FN, fastigial nucleus; XSCP, decussation of the
superior cerebellar peduncle; TNF-α, tumor necrosis factor-α; IL,
interleukin. |
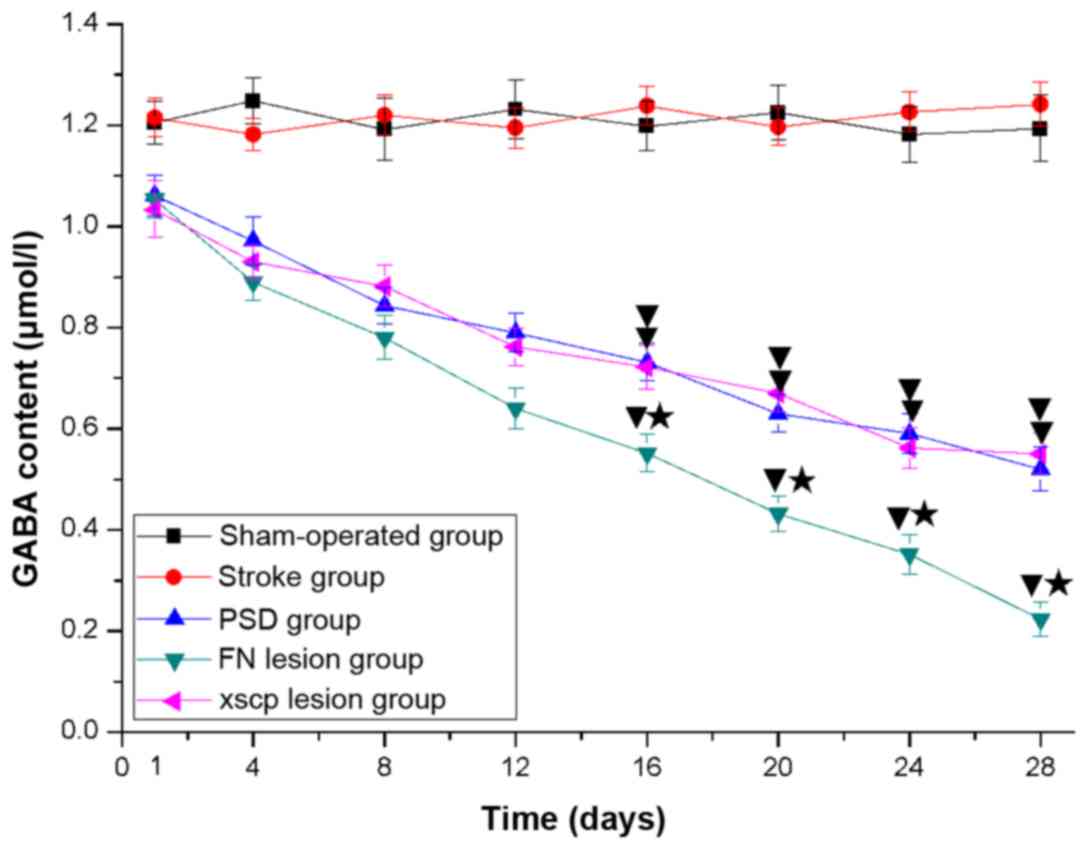
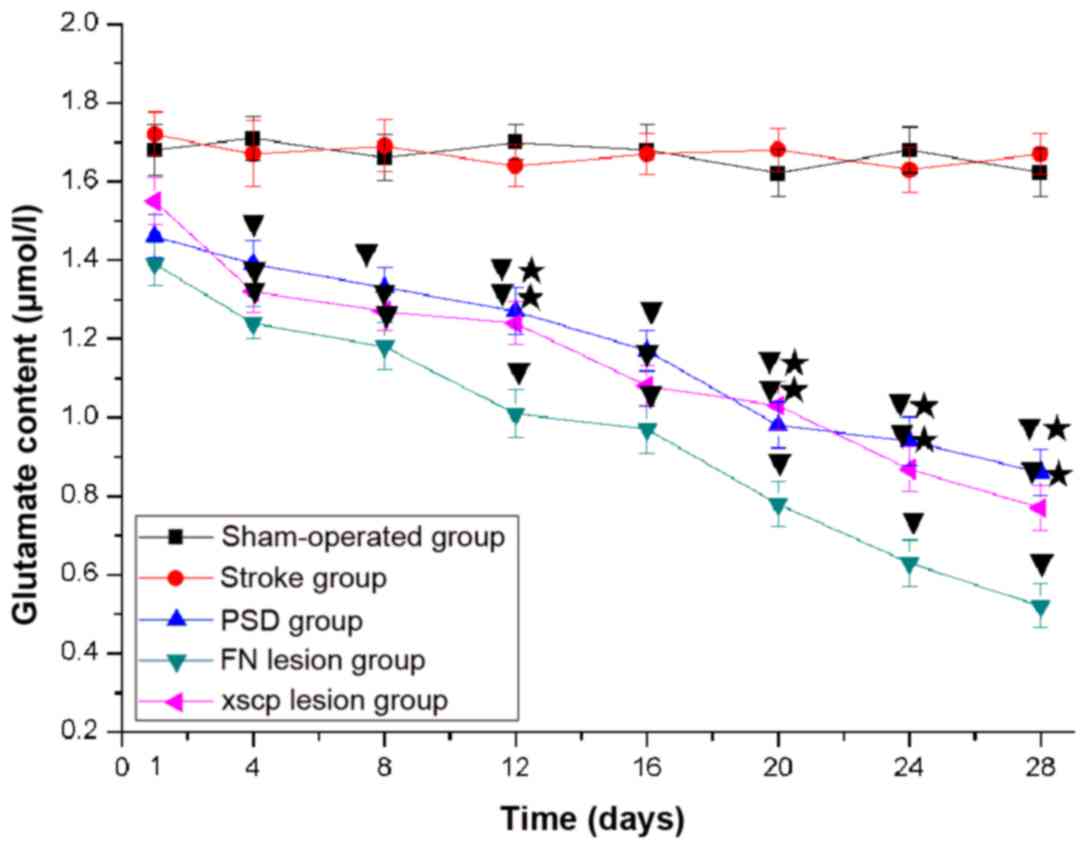
FN and XSCP lesions decrease the
content of GABA and glutamate in the LHA
The content of GABA and glutamate in the LHA is
tightly coupled to the change in the expression of inflammatory
cytokines (16,17). The present study also examined
whether FN and XSCP lesions affected the content of GABA and
glutamate. The results indicated significant loss of GABA (Fig. 7) and glutamate (Fig. 8) content in the LHA of that the rats
in the PSD group. In addition, FN and XSCP lesions significantly
decreased the content of GABA and glutamate.
Discussion
Based on previous results, MCAO and CUMS were
induced in rats to mimic post-stroke depressive-like symptoms in
patients with PSD, which mainly include the following: i) Reduced
preference for sucrose water and a putative indicator of anhedonia
(20); ii) decreased locomotor and
rearing activities suggesting changes in incentive motivation and
emotionality (21); iii) body weight
loss similar to that observed in PSD (22). In the present study, the results
showed that MCAO and CUMS decreased body weight and sucrose
preference in addition to locomotor and rearing activities in the
OFT.
As there are no KA receptors on neuronal axons, KA
has been reported to destroy neuron bodies only, without damaging
nerve fibers across the neural nuclei (23). In the present study, it was observed
that Nissl bodies, which are present in the plasma of normal
neuronal bodies and are closely associated with neuronal
degeneration and death, had almost all disappeared in the FN
infused with KA. These changes demonstrated that KA effectively
destroyed the neuronal bodies in the cerebellar FN. In addition,
the results showed that the rats in the FN lesion group exhibited
similar behavioral profiles as those observed in the PSD group
during the whole 28-day period. Cerebellar FN involvement was
suggested in previous studies, which showed that the stimulation or
damage of the FN in animals or patients ameliorated or provoked
depression (7,24,25).
Therefore, the findings of the present study support the hypothesis
that the cerebellar FN is involved in the regulation of depression.
However, how the cerebellar FN functions in PSD remains to be fully
elucidated.
The cerebellar FN is closely associated with
inflammation. Previously, it was reported that stimulation of the
cerebellar FN can inhibit cerebrovascular inflammation (9). Our preliminary study also showed that
FNS reduced the expression of inflammatory cytokines in the
hippocampus (7). In addition, FN
lesions can gradually promote the function of T cells, B cells and
natural killer cells over time (10). Therefore, in terms of the
inflammatory mechanisms underlying PSD (2), the mRNA expression levels of
inflammatory cytokines, including TNF-α, IL-6, and IL-1β, in the
hippocampus of rats were measured in the present study. The results
showed that the mRNA expression levels of TNF-α, IL-6, and IL-1β
were increased in the PSD and FN lesion groups. These results
suggested that the involvement of the cerebellar FN in PSD may be
achieved via mediating inflammatory cytokines.
As there are no direct cerebellar innervations to
lymphoid organs, cerebellar immunomodulation must be conveyed
through other pathways. Due to its direct connection with the
cerebellum and its predominant neuroimmunomodulatory effect, the
hypothalamus may be one of the most likely candidates in
transmitting immunoregulatory information from the cerebellum to
the lymphoid organs (14,15,26–28).
Previously, it was demonstrated that there were direct
glutamatergic and GABAergic projections between the FN and LHA,
which conveyed immunomodulating information from the FN (16,17). In
the present study, an electrolytic method was used to destroy the
projections from cerebellar FN neurons to the hypothalamus in the
XSCP lesion group, and a spherical lesion centered on the tip of
the electrode showed that the XSCP region was destroyed. The
present study is the first, to the best of our knowledge, to
demonstrate decreases in sucrose water consumption, body weight and
rat activities when the mRNA levels of TNF-α, IL-6, and IL-1β were
significantly increased following the formation of XSCP lesions. In
addition, the results suggested that the direct
cerebellar-hypothalamic projections may act as a potential pathway
to transmit immunoregulatory information from the cerebellar FN in
PSD.
By the anterograde and retrograde tracing of nerve
tracts in conjunction with GABA and glutamate immunohistochemistry,
previous studies have found that the direct cerebellar-hypothalamic
projections mainly contain glutamatergic and GABAergic nerve fibers
(15,16). Electrophysiological experiments have
also revealed that stimulation of the FN evokes monosynaptic
inhibitory and polysynaptic excitatory responses of LHA neurons,
suggesting that there is a direct cerebellar-hypothalamic GABAergic
and glutamatergic projection between the cerebellum and the
hypothalamus (26,27,29). In
the present study, the glutamate and GABA contents in the LHA were
decreased in the FN lesion group, indicating that the FN lesions
decreased FN GABAergic and glutamatergic transmission to the
hypothalamus. Of note, XSCP lesions also attenuated the GABA and
glutamate content in the LHA. The similar effect of FN and XSCP
lesions on the glutamate and GABA contents in the LHA showed that
the direct cerebellar-hypothalamic projection released GABA and
glutamate as neurotransmitters. Therefore, it was hypothesized that
the decrease of GABA and glutamate content in the LHA increased the
mRNA expression levels of TNF-α, IL-6, and IL-1β, suggesting that
the immune response was modulated by cerebellar FN GABAergic and
glutamatergic neurons. Previous reports (16,17) have
suggested that GABAergic and glutamatergic neurons in the
cerebellar FN regulated innate and adaptive immune responses,
consistent with the results of the present study.
In conclusion, FN and XSCP lesions resulted in
decreased levels of glutamate and GABA in the LHA, induced the
amplification of genes corresponding to inflammatory cytokines, and
ultimately caused depression-like behaviors. These findings
revealed that FN glutamatergic and GABAergic projections to the LHA
may be significant in PSD. In addition, the results suggested that
the cerebellar FN regulated mood and emotion via the direct
cerebellar-hypothalamic GABAergic and glutamatergic projections
(29).
Acknowledgements
Not applicable.
Funding
The present study was sponsored by the National
Science Foundation of China (grant nos. 81371461 and 81241050) and
the National Science Foundation of Liaoning Province (grant no.
2013022018).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL designed the study and wrote the manuscript. XZ
and BY were peformed experiments and collected data. LC performed
the statistical analysis. RS designed the study, supervised the
study and revised the manuscript.
Ethics approval and consent to
participate
All animal experiments complied with the Guidelines
on Ethical Standards (30). The current study was approved by
Jinzhou Medical University Ethics Committee for the Care and Use of
Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Towfighi A, Ovbiagele B, El Husseini N,
Hackett ML, Jorge RE, Kissela BM, Mitchell PH, Skolarus LE, Whooley
MA, Williams LS, et al: Poststroke depression: A scientific
statement for healthcare professionals from the American heart
association/American stroke association. Stroke. 48:e30–e43. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spalletta G, Bossù P, Ciaramella A, Bria
P, Caltagirone C and Robinson RG: The etiology of poststroke
depression: A review of the literature and a new hypothesis
involving inflammatory cytokines. Mol Psychiatry. 11:984–991. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiao JT, Cheng C, Ma YJ, Huang J, Dai MC,
Jiang C, Wang C and Shao JF: Association between inflammatory
cytokines and the risk of post-stroke depression, and the effect of
depression on outcomes of patients with ischemic stroke in a 2-year
prospective study. Exp Ther Med. 12:1591–1598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmahmann JD and Sherman JC: The
cerebellar cognitive affective syndrome. Brain. 121:561–579. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bugalho P, Correa B and Viana-Baptista M:
Role of the cerebellum in cognitive and behavioural control:
Scientific basis and investigation models. Acta Med Port.
19:257–267. 2006.(In Portuguese). PubMed/NCBI
|
|
6
|
Schmahmann JD, MacMore J and Vangel M:
Cerebellar stroke without motor deficit: Clinical evidence for
motor and non-motor domains within the human cerebellum.
Neuroscience. 162:852–861. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lei Z, Zhao M and Sui RB: Cerebellar
fastigial nucleus electrical stimulation alleviates depressive-like
behaviors in post-stroke depression rat model and potential
mechanisms. Cell Physiol Biochem. 41:1403–1412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su CY, Wuang YP, Lin Y and Su JH: The role
of processing speed in post-stroke cognitive dysfunction. Arch Clin
Neuropsychol. 30:148–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galea E, Glickstein SB, Feinstein DL,
Golanov EV and Reis DJ: Stimulation of cerebellar fastigial nucleus
inhibits interleukin-1beta-induced cerebrovascular inflammation. Am
J Physiol. 275:H2053–H2063. 1998.PubMed/NCBI
|
|
10
|
Ni SJ, Qiu YH, Lu JH, Cao BB and Peng YP:
Effect of cerebellar fastigial nuclear lesions on differentiation
and function of thymocytes. J Neuroimmunol. 222:40–47. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Madden KS: Catecholamines, sympathetic
innervation, and immunity. Brain Behav Immun. 17 (Suppl 1):S5–S10.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nance DM and Sanders VM: Autonomic
innervation and regulation of the immune system (1987–2007). Brain
Behav Immun. 21:736–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trotter RN, Stornetta RL, Guyenet PG and
Roberts MR: Transneuronal mapping of the CNS network controlling
sympathetic outflow to the rat thymus. Auton Neurosci. 131:9–20.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dietrichs E, Haines DE, Røste GK and Røste
LS: Hypothalamocerebellar and cerebellohypothalamic
projections-circuits for regulating nonsomatic cerebellar activity?
Histol Histopathol. 9:603–614. 1994.PubMed/NCBI
|
|
15
|
Haines DE, Dietrichs E, Mihailoff GA and
McDonald EF: The cerebellar-hypothalamic axis: Basic circuits and
clinical observations. Int Rev Neurobiol. 41:83–107. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao BB, Huang Y, Jiang YY, Qiu YH and Peng
YP: Cerebellar fastigial nuclear glutamatergic neurons regulate
immune function via hypothalamic and sympathetic pathways. J
Neuroimmune Pharmacol. 10:162–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao BB, Huang Y, Lu JH, Xu FF, Qiu YH and
Peng YP: Cerebellar fastigial nuclear GABAergic projections to the
hypothalamus modulate immune function. Brain Behav Immun. 27:80–90.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paxinos G and Watson С: The rat brain in
stereotaxic coordinates. New York. Academic1986.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Willner P, Muscat R and Papp M: Chronic
mild stress-induced anhedonia: A realistic animal model of
depression. Neurosci Biobehav Rev. 16:525–534. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roth KA and Katz RJ: Stress, behavioral
arousal, and open field activity-a reexamination of emotionality in
the rat. Neurosci Biobehav Rev. 3:247–263. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sapolsky RM, Romero LM and Munck AU: How
do glucocorticoids influence stress responses? Integrating
permissive, suppressive, stimulatory, and preparative actions.
Endocr Rev. 21:55–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Köhler C and Schwarcz R: Comparison of
ibotenate and kainate neurotoxicity in rat brain: A histological
study. Neuroscience. 8:819–835. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu JL, Li JP and Dong WW: A clinical
study of fastigial nucleus electrical stimulation on post stroke
depression. J Chin J Clin Rehabilitation. 7:1926–1927. 2003.
|
|
25
|
Schmahmann JD: The role of the cerebellum
in affect and psychosis. J Neurolinguis. 13:189–214. 2000.
View Article : Google Scholar
|
|
26
|
Wang J, Pu Y and Wang T: Influences of
cerebellar interpositus nucleus and fastigial nucleus on neuronal
activity of lateral hypothalamic area. Sci China C Life Sci.
40:176–183. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YP, Ma C, Wen YQ and Wang JJ:
Convergence of gastric vagal and cerebellar fastigial nuclear
inputs on glycemia-sensitive neurons of lateral hypothalamic area
in the rat. Neurosci Res. 45:9–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schutter DJ and Van Honk J: The cerebellum
on the rise in human emotion. Cerebellum. 4:290–294. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Min BI, Oomura Y and Katafuchi T:
Responses of rat lateral hypothalamic neuronal activity to
fastigial nucleus stimulation. J Neurophysiol. 61:1178–1184. 1989.
View Article : Google Scholar : PubMed/NCBI
|