Introduction
Brain glioma is the most common primary malignant
tumor of intracranial tumors, accounting for approximately 45% of
all intracranial tumors. The prognosis of brain glioma patients is
generally poor, and with the increase of malignancy, the prognosis
is worse (1,2). Findings have shown that the median
survival time of patients with grade IV glioblastoma multiform is
only 1 year, and the 5-year survival rate is less than 5%, being
one of the malignant tumors with the highest human mortality
(3). Surgical treatment is the most
effective treatment for brain gliomas. With the development of
molecular-targeted therapy, the survival of brain glioma patients
has been improved effectively, but the overall therapeutic effect
is still not ideal and the occurrence and development mechanism of
brain glioma is not clear yet (4,5).
Therefore, it is very important to continue to study the changes of
molecular level in brain gliomas and to find new therapeutic
targets for the clinical treatment of gliomas.
In 2008, it was reported that miR-124 could inhibit
the proliferation of glioblastoma multiform by affecting CDK6 to
promote the differentiation of brain glioma stem cells (6). However, little further research has
been carried out on this issue. Thus, the mechanism of miR-124
regulating brain glioma stem cell differentiation is not fully
understood, and its role has not been further verified. The Nogo
family has three subtypes, Nogo-A, Nogo-B and Nogo-C, which can
inhibit the growth and reconnection of synapses in central nervous
system (CNS) diseases. Nogo-A is the longest isomer, enriched in
CNS, while NgR is the receptor of Nogo (7). Nogo/NgR signaling pathway has been
reported to be related to brain injury, and the inhibition of its
activation can reduce the death of microglial cells (8). Effect of miR-124 on the proliferation
and differentiation of U87 glioma stem cells and its effect on
Nogo/NgR signaling pathway were analyzed in this study to explore
its mechanism of action.
Materials and methods
Cell sources
Brain glioma cell U87 MG-Luc2 was purchased from
ATCC (ATCCHTB-14-LUC2), hereinafter referred to as U87 cells and
has been authenticated by STR profiling (Beijing Microread Genetics
Co., Ltd.). The cells were cultured in DMEM complete medium
containing 10% fetal calf serum (Shanghai Zhong Qiao Xin Zhou
Biotechnology Co., Ltd.) with a culture condition of 37°C, 5%
CO2 and 95% relative humidity.
This study was approved by the Ethics Committee of
Xiangyang Central Hospital, Affiliated Hospital of Hubei University
of Art and Science (Xiangyang, China).
Cell passage
When cell attachment growth density of U87 reached
90%, the cells were digested with 0.25% trypsin. U87 cells were
transferred into DMEM medium and cultured in incubators at 37°C, 5%
CO2 then synaptic retraction was observed under a
microscope. The culture was collected for the third generation
use.
Isolation of U87 brain glioma stem
cells
U87 cells with an attachment growth density of 90%
were collected and digested with 0.25% trypsin. The U87 brain
glioma stem cells were sorted by adding tumor stem cell culture
medium (Jiangsu Promocell Biotechnology Co., Ltd.). The medium was
replaced every two days, centrifugation was performed at 800 × g
for 5 min at 4°C before replacement, and then half of the medium
was replaced. After 3 weeks, the suspension cells were collected
and passaged in accordance with the above method.
Construction and transfection of miR-124 expression
vector. The miR-124 mimic, miR-124 inhibitor and miR-control
expression vectors were designed and produced by Shanghai Gene
Pharmaceutical Technology Co., Ltd. The cells were digested by
trypsin for 24 h before transfection, and U87 stem cells were
transfected when the cells were fused to approximately 80%, the
specific steps referred to the specification of the kit. The cells
were cultured in 37°C, 5% CO2 incubator for 48 h, and
the medium was changed every 6 h. The transfection results were
detected by RT-qPCR. Lipofectamine™2000 transfection kit was
purchased from Shanghai Yanjin Biotechnology Co., Ltd.
RT-qPCR
The concentration of cell suspension was adjusted to
1×107, and the total RNA was extracted by adding
suspension and TRIzol lysate at 3:1. After extraction, 1.5% agarose
gel electrophoresis was used to analyze the integrity of RNA,
micro-amount of nucleic acid analyzer was used to detect the purity
of extracted RNA. A260/A280 value between 1.8 and 2.0 was
considered to meet the experimental requirements. RT-qPCR reaction
was performed after the completion of RNA extraction. Reverse
transcription system: oligo dt primer 1.0 µl, 5X PrimeScript Buffer
4.0 µl, 2.5 U/µl polyA polymerase 1 µl, dNTP mixture 1.0 µl, total
RNA 2 µg, RNase-free distilled water was added to 10 µl. Reaction
condition was: 40°C for 15 min, 85°C for 5 sec; PCR amplification
was carried out after the completion of reverse transcription
reaction. PCR amplification system was: cDNA template 2 µl,
SYBR-Green Mix 25 µl, upstream primer 0.5 µl, downstream primer 0.5
µl, double-steamed water was added to 50 µl. PCR reaction program
was: 30 cycles of predenaturation at 95°C for 3 min, denaturation
at 95°C for 30 sec, annealing at 55°C for 30 sec, elongation at
72°C for 60 sec, elongation at 72°C for 5 min after the cycle was
complete. GAPDH was used as the internal parameter of the
reaction. Samples were set in triplicate for each test, and
2−ΔCq was used to analyze the results (9). The primer sequence was designed and
produced by Hepeng (Shanghai) Biotechnology Co., Ltd. (Table I).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Forward primer | Reverse primer |
|---|
| miR-124 |
5′-GCTAAGGCACGCGGTG-3′ |
5′-GTGCAGGGTCCGAGGT-3′ |
| GAPDH |
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ |
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ |
Western blot analysis
The expression of Nogo-A and NgR protein was
detected by western blot analysis. The total protein was extracted
from the cell by RIPA total protein lysate (Wuhan Aspen Biological
Company) and the concentration of extracted protein was determined
by BCA, and the BCA protein quantitative detection kit was
purchased from Beyotime Institute of Biotechnology. Then, 40 µg
extracted protein solution was used for SDS-PAGE electrophoresis,
and buffer was added at a 1:4 dilution. β-actin protein was used as
an internal reference, the concentration gel electrophoresis was
performed at a constant 80 V for 40 min, and separation gel
electrophoresis was performed at a constant 120 V for 90 min.
Trans-membrane was conducted at a constant 100 V for 100 min and
blocked at 37°C for 60 min after the completion of electrophoresis.
After trans-membrane, the immunological reaction was carried out.
Incubation was performed with primary rabbit anti-human Nogo-A
polyclonal antibody (1:200; cat. no. 10740-1-AP; ProteinTech Group,
Inc.) at 4°C for 16 h. It was washed the next day in lukewarm water
3 times, 10 min each time with PBS. Then, incubation was performed
with secondary goat anti-rabbit polyclonal IgG (1:500; cat. no.
SA00001-2; ProteinTech Group, Inc.) at 37°C for 60 min. ECL
luminescent reagent was used for visualization and fixation.
QuantityOne software was used to analyze the strip after film
scanning. Protein relative expression level was calculated as: band
gray value/internal parameter gray value. Western blot detection
kit was purchased from Beyotime Institute of Biotechnology.
MTT proliferation in vitro
U87 brain glioma stem cells were prepared into a
single cell suspension of 4×106/ml, performed routine
vaccination, and cultured in 96-well cell culture plate. Then, 20
µl MTT solution (5 mg/ml) was added after 6 h, and cells were
cultured at 37°C for 4 h continuously. Supernatant containing
impurities was removed, dimethylsulfoxide was added, and the plate
was placed on a horizontal vibration table. The absorbance values
at wavelength 570 nm were measured at 12, 24, 48 and 72 h. The MTT
test kit was purchased from Shanghai LMAI Bioengineering Co.,
Ltd.
Detection of differentiation of U87
brain glioma stem cells by immunomagnetic beads
U87 brain glioma stem cells were prepared into a
single cell suspension of 1×106/ml. CD133+
cells were separated by immunomagnetic beads, washed, re-suspended,
counted in serum-free medium, and the cell morphology was observed.
The immunomagnetic beads kit was purchased from Shanghai Chem
Biotechnology Co., Ltd.
Statistical analysis
SPSS19.0 [AsiaAnalytics (formerly SPSS China)] was
used for statistical analysis. Measurement data were expressed as
mean ± standard deviation. ANOVA and the LSD post hoc test were
used for multigroup comparison. The t-test was used for comparison
between two groups and χ2 test was used for rate
comparison. The correlation between miR-124 and Nogo-A, and NgR
protein expression was analyzed by Spearman's correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
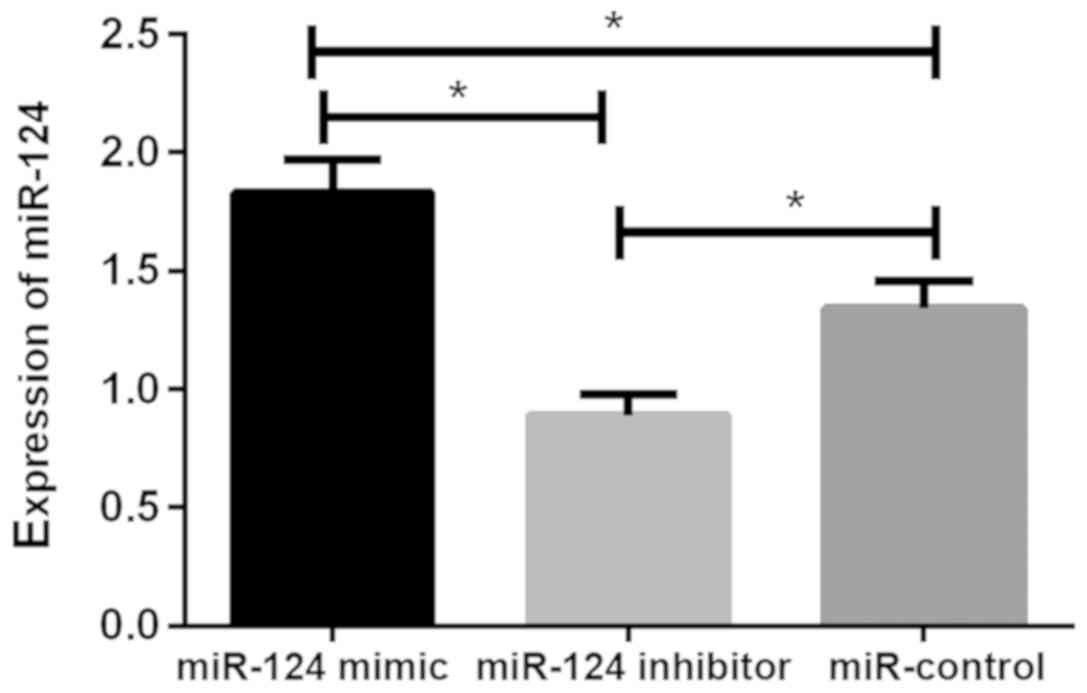
Relative expression of miR-124 in U87
brain glioma stem cells after transfection
The relative expression levels of miR-124 in the
miR-124 mimic, miR-124 inhibitor and miR-control groups were
1.83±0.14, 0.89±0.09 and 1.34±0.12, respectively. There was
statistical difference between the three groups (P<0.05). The
relative expression of miR-124 in cells of miR-124 mimic group was
significantly higher than that of miR-124 inhibitor and miR-control
groups (P<0.05), while the relative expression of miR-124 in
cells of miR-124 inhibitor group was lower than that of miR-control
group (P<0.05; Fig. 1).
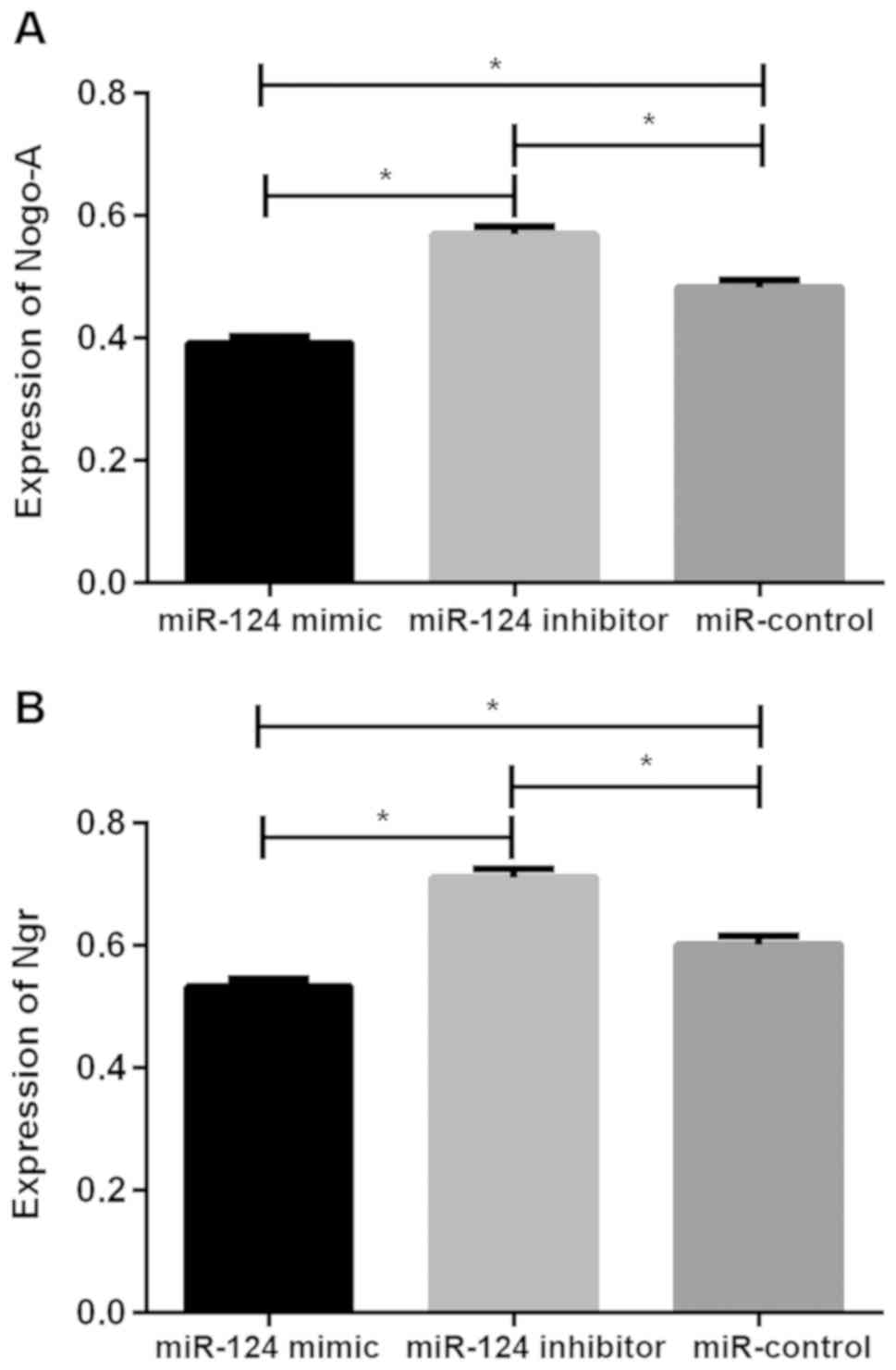
Expression of Nogo-A and NgR protein in U87 brain
glioma stem cells after transfection. The relative expression
levels of Nogo-A protein in the miR-124 mimic, miR-124 inhibitor
and miR-control groups were 0.392±0.011, 0.569±0.013 and
0.483±0.012, respectively, the relative expression levels of NgR
protein was 0.533±0.012, 0.711±0.014 and 0.601±0.014, respectively.
There was significant difference in Nogo-A and NgR protein between
the three groups (P<0.05), the relative expression of Nogo-A and
NgR protein in cells of miR-124 mimic group were significantly
lower than those of miR-124 inhibitor and miR-control groups
(P<0.05), while the relative expression of Nogo-A and NgR
protein in cells of miR-124 inhibitor group were higher than those
of miR-control group (P<0.05; Table
II and Fig. 2).
 | Table II.Expression of Nogo-A and NgR protein
in U87 brain glioma stem cells. |
Table II.
Expression of Nogo-A and NgR protein
in U87 brain glioma stem cells.
| Item | Nogo-A protein | NgR protein |
|---|
| miR-124 mimic | 0.392±0.011 | 0.533±0.012 |
| miR-124
inhibitor |
0.569±0.013a |
0.711±0.014a |
| miR-control |
0.483±0.012a,b |
0.601±0.014a,b |
| F | 162.463 | 135.470 |
| P-value |
<0.05 |
<0.05 |
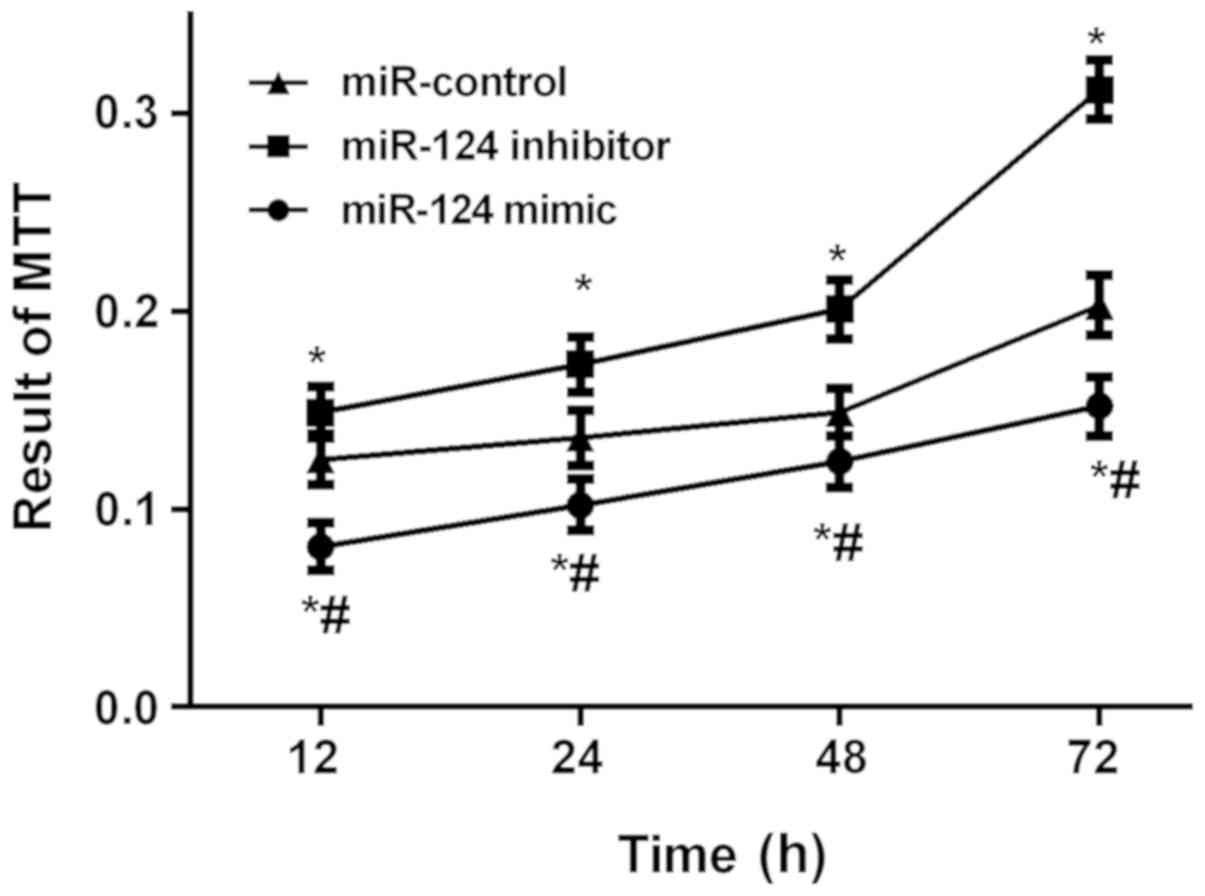
Detection results of U87 brain glioma
stem cells by MTT proliferation in vitro
The results of MTT proliferation in vitro
showed that the absorbance values in the three groups were
significantly different at each time point (P<0.05). The
absorbance values of the cells in the miR-124 mimic and miR-control
groups were significantly lower than those in the miR-124 inhibitor
group at each time point (P<0.05), while the values of the cells
in the miR-124 mimic group was significantly lower than that in
miR-control group (P<0.05; Fig.
3).
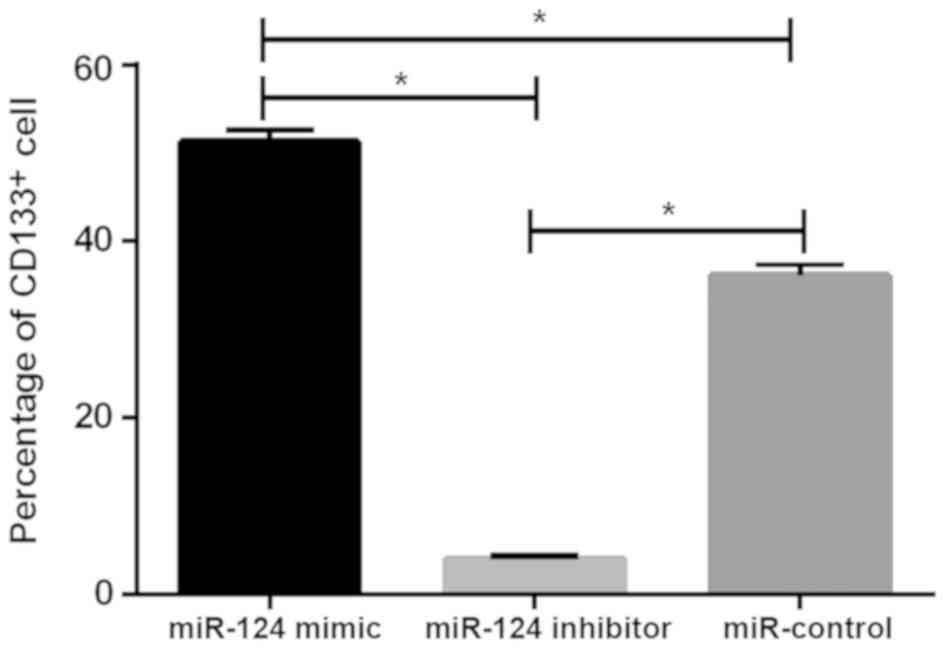
Detection results of CD133+
U87 brain glioma stem cells
The levels of CD133+ cells in the miR-124
mimic, miR-124 inhibitor and miR-control groups were 3.98±0.45,
36.12±1.34 and 51.25±1.48%, respectively. There was statistical
difference between the three groups (P<0.05), the level of
CD133+ cells in miR-124 mimic group was significantly
lower than that in miR-124 inhibitor and miR-control groups
(P<0.05), while the level of CD133+ cells in miR-124
inhibitor group was higher than that in miR-control group
(P<0.05; Fig. 4).
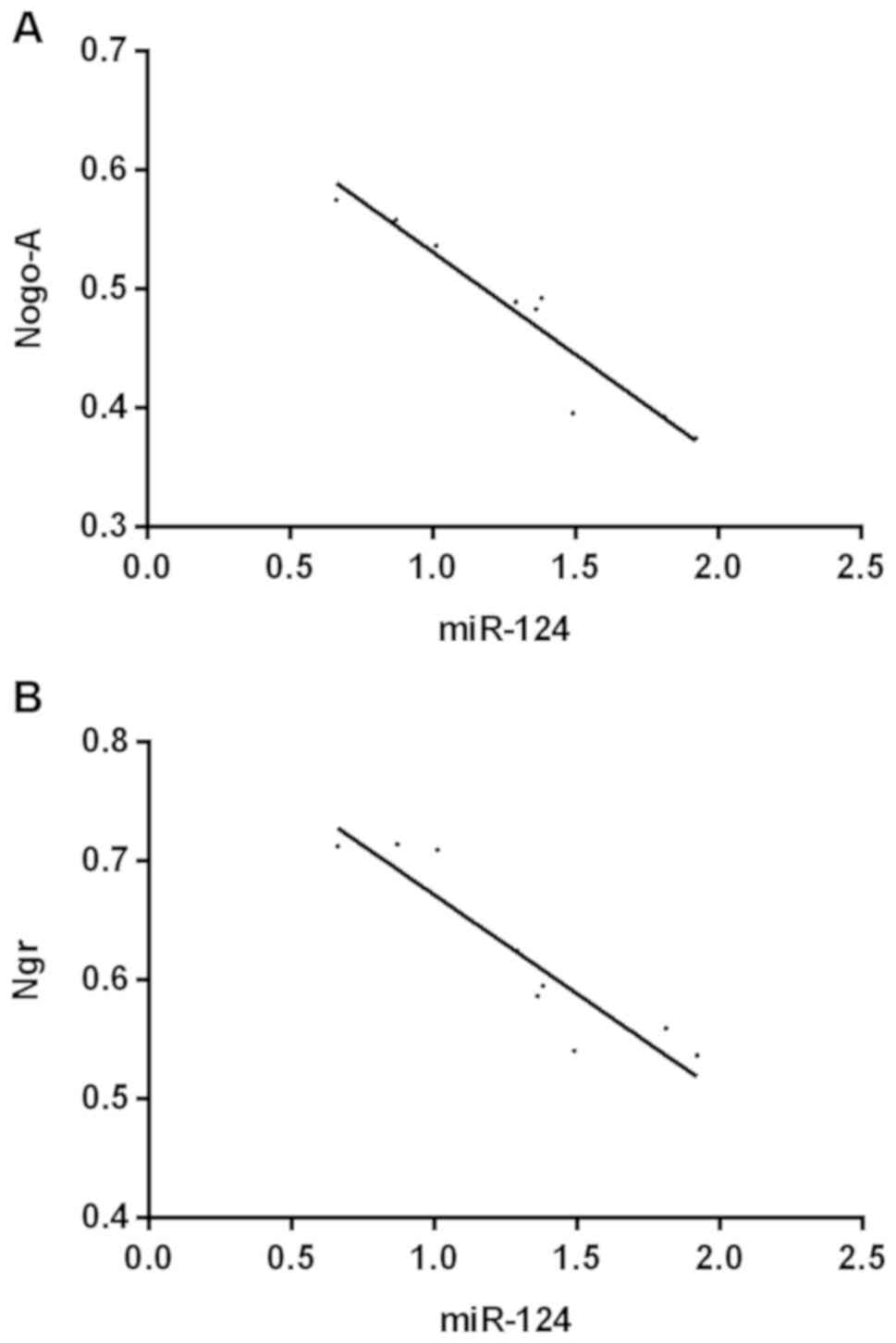
Correlation analysis
The results of correlation analysis showed that
there was a negative correlation between miR-124 and the expression
of Nogo-A (r=−0.855, 95% CI: −0.9908 to −0.7954, P<0.001) and
NgR (r=0825, 95% CI: −0.9845 to −0.6776, P<0.001; Fig. 5).
Discussion
With the development of molecular biology and
related treatments, an increasing number of scholars have paid
attention to the role of miRNA in tumors. miR-124 plays an
important role in neural development, but is rarely reported in
gliomas. Some studies have found that the downregulation and
upregulation of miR-124 expression in brain glioma can play the
role of tumor suppressor gene (10),
but the mechanism of miR-124 is not fully understood yet. There
have been few reports on the Nogo/NgR pathway, reporting that
reducing Nogo-A expression can protect brain damage and promote
neuronal regeneration (11,12), while brain gliomas develop from
carcinogenesis of spinal cord neurons (13). Therefore, whether the Nogo/NgR
pathway plays a role in brain glioma and whether it is a target for
miR-124 in the differentiation of brain glioma stem cells were
investigated in this study in order to provide a new direction for
clinical treatment.
U87 brain glioma cells were used for screening of
brain glioma stem cells because of their ability to grow into
spheres, which can be used to screen stem cells that meet the
experimental needs (14,15). Then the overexpression vector
(miR-124 mimic), underexpression vector (miR-124 inhibitor) and
blank vector (miR-control) of miR-124 were constructed to transfect
U87 brain glioma stem cells and to regulate the expression level of
miR-124. The RT-qPCR results showed that the relative expression of
miR-124 in cells of miR-124 mimic group was significantly higher
than that of miR-124 inhibitor and miR-control groups, while the
relative expression of miR-124 in cells of miR-124 inhibitor group
was lower than that of miR-control group, which indicated that the
transfection was successful. Then the expression levels of Nogo,
and NgR protein were detected and found that with the increase of
miR-124 expression level, the expression level of NgR protein
decreased, and correlation analysis also revealed that there was a
negative correlation between miR-124 and the expression level of
Nogo and NgR protein, which suggested that there might be some
regulatory relationship between miR-124 and Nogo/NgR pathway. CD133
is recognized as a surface marker of tumor stem cells, which is not
expressed on the surface of differentiated tumor cells (16). Therefore, expressing the quantitative
proportion of CD133 cells can reflect the differentiation of stem
cells to some extent. Analytic results of the proliferation of U87
brain glioma stem cells showed that cells with high miR-124
expression level had a low proliferative ability. The analytic
results of the differentiation of U87 brain glioma stem cells
showed that U87 brain glioma stem cells with high miR-124
expression had a low proportion of CD133+ cells,
indicating a high degree of the differentiation. These results are
similar to those reported in related studies: miR-124 can promote
the differentiation of brain glioma stem cells (6,10).
Brain glioma stem cells, a special subgroup of
cells, are the source of brain glioma formation with a potential to
differentiate into brain glioma cells (17). Differentiation is also an important
feature of brain glioma stem cells and brain glioma cells. Brain
glioma stem cells have no tumorigenic ability and their infinite
proliferative ability also becomes limited after differentiation
into brain glioma cells (18,19).
Therefore, promoting the differentiation of brain glioma stem cells
is also an important method for the treatment of brain glioma. On
the other hand, brain glioma stem cells are more tolerant than
brain glioma cells in the face of radiotherapy and chemotherapy,
and their DNA repair ability is also stronger than that of brain
glioma cells. Therefore, brain glioma stem cells are a new research
direction in the treatment of brain glioma (20). At present this kind of treatment is
still in the exploratory stage. miR-124 was first reported to
participate in the differentiation of glioma stem cells in 2008, it
was found that increased expression of miR-124 promoted the
differentiation of brain glioma stem cells (6), but there are few reports on miR-124 and
brain glioma stem cell differentiation since then. It has been
reported that miR-124 can regulate the differentiation of
astrocytes and other nerve cells (21). The finding verified the regulation
effect of miR-124 on the differentiation of brain glioma stem cells
to some extent. Nogo-A has been reported to inhibit the invasion
and migration of brain glioma cells (22). Consequently, there is a close
relationship between Nogo, NgR and tumor formation. The above
results also improve the credibility of our conclusions to some
extent.
However, this study also has some shortcomings.
Brain gliomas are diverse (23). In
addition, U87 brain glioma cells are only one of these subtypes;
thus, the establishment of a study in other gliomas is needed for
further verification. Furthermore, in vitro experiments
cannot simulate the complex tumor microenvironment in vivo.
Therefore, we hope that this study can promote further research in
this field.
In conclusion, miR-124 may participate in the
differentiation of brain glioma stem cells through the Nogo/NgR
pathway, which may bring a new direction for the clinical treatment
of brain glioma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM was responsible for construction and transfection
of miR-124 expression vector and PCR, as well as for drafting the
manuscript. FS and YZ helped with western blot and MTT assay. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Xiangyang Central Hospital, Affiliated Hospital of Hubei University
of Art and Science (Xiangyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eckel-Passow JE, Lachance DH, Molinaro AM,
Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML,
Smirnov IV, et al: Glioma groups based on 1p/19q, IDH, and TERT
promoter mutations in tumors. N Engl J Med. 372:2499–2508. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ceccarelli M, Barthel FP, Malta TM,
Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh
A, Pagnotta SM, et al TCGA Research Network, : Molecular profiling
reveals biologically discrete subsets and pathways of progression
in diffuse glioma. Cell. 164:550–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buckner JC, Shaw EG, Pugh SL, Chakravarti
A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, et
al: Radiation plus procarbazine, CCNU, and vincristine in low-grade
glioma. N Engl J Med. 374:1344–1355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson BE, Mazor T, Hong C, Barnes M,
Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et
al: Mutational analysis reveals the origin and therapy-driven
evolution of recurrent glioma. Science. 343:189–193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mantia C, Uhlmann EJ, Puligandla M, Weber
GM, Neuberg D and Zwicker JI: Predicting the higher rate of
intracranial hemorrhage in glioma patients receiving therapeutic
enoxaparin. Blood. 129:3379–3385. 2017.PubMed/NCBI
|
|
6
|
Silber J, Lim DA, Petritsch C, Persson AI,
Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello
JF, et al: miR-124 and miR-137 inhibit proliferation of
glioblastoma multiforme cells and induce differentiation of brain
tumor stem cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang Y, Yan J, Li C, Zhou X, Yao L, Pang
T, Yan M, Zhang L, Mao L and Liao H: The Nogo/Nogo receptor (NgR)
signal is involved in neuroinflammation through the regulation of
microglial inflammatory activation. J Biol Chem. 290:28901–28914.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang Y, Wang J, Yao L, Li C, Wang J, Liu
Y, Tao X, Sun H and Liao H: The adhesion and migration of microglia
to β-amyloid (Aβ) is decreased with aging and inhibited by Nogo/NgR
pathway. J Neuroinflammation. 15:2102018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Scmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia H, Cheung WK, Ng SS, Jiang X, Jiang S,
Sze J, Leung GK, Lu G, Chan DT, Bian XW, et al: Loss of
brain-enriched miR-124 microRNA enhances stem-like traits and
invasiveness of glioma cells. J Biol Chem. 287:9962–9971. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Q, Cheng Y, Bi MJ, Kang H, Qu Y, Lin H,
Guo Y and Zou Y: Effects of N-Butylphthalide on the expressions of
Nogo/NgR in rat brain tissue after carbon monoxide poisoning.
Environ Toxicol Pharmacol. 39:953–961. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buchli AD and Schwab ME: Inhibition of
Nogo: a key strategy to increase regeneration, plasticity and
functional recovery of the lesioned central nervous system. Ann
Med. 37:556–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hambardzumyan D, Gutmann DH and Kettenmann
H: The role of microglia and macrophages in glioma maintenance and
progression. Nat Neurosci. 19:20–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Zhang B, Wen C, Wen G, Zhou G,
Zhang J, He H, Wang N and Li W: Overexpressed miRNA-134b inhibits
proliferation and invasion of CD133+ U87 glioma stem
cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 33:637–642. 2017.(In
Chinese). PubMed/NCBI
|
|
15
|
Guo Y, Zhang P, Zhang H, Zhang P and Xu R:
RNAi for contactin 2 inhibits proliferation of U87-glioma stem
cells by downregulating AICD, EGFR, and HES1. OncoTargets Ther.
10:791–801. 2017. View Article : Google Scholar
|
|
16
|
Cervantes-Madrid D, Wettergren Y, Falk P,
Lundholm K and Asting AG: DNA alterations in Cd133+ and
Cd133− tumour cells enriched from intra-operative human
colon tumour biopsies. BMC Cancer. 17:2192017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SH, Ezhilarasan R, Phillips E,
Gallego-Perez D, Sparks A, Taylor D, Ladner K, Furuta T, Sabit H,
Chhipa R, et al: Serine/threonine kinase MLK4 determines
mesenchymal identity in glioma stem cells in an NF-κB-dependent
manner. Cancer Cell. 29:201–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SH, Joshi K, Ezhilarasan R, Myers TR,
Siu J, Gu C, Nakano-Okuno M, Taylor D, Minata M, Sulman EP, et al:
EZH2 protects glioma stem cells from radiation-induced cell death
in a MELK/FOXM1-dependent manner. Stem Cell Rep. 4:226–238. 2015.
View Article : Google Scholar
|
|
19
|
Miao H, Gale NW, Guo H, Qian J, Petty A,
Kaspar J, Murphy AJ, Valenzuela DM, Yancopoulos G, Hambardzumyan D,
et al: EphA2 promotes infiltrative invasion of glioma stem cells in
vivo through cross-talk with Akt and regulates stem cell
properties. Oncogene. 34:558–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neo WH, Yap K, Lee SH, Looi LS, Khandelia
P, Neo SX, Makeyev EV and Su IH: MicroRNA miR-124 controls the
choice between neuronal and astrocyte differentiation by
fine-tuning Ezh2 expression. J Biol Chem. 289:20788–20801. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Santos MC, Tegge AN, Correa BR, Mahesula
S, Kohnke LQ, Qiao M, Ferreira MA, Kokovay E and Penalva LO:
miR-124, −128, and −137 orchestrate neural differentiation by
acting on overlapping gene sets containing a highly connected
transcription factor network. Stem Cells. 34:220–232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cantalupo A, Zhang Y, Kothiya M, Galvani
S, Obinata H, Bucci M, Giordano FJ, Jiang XC, Hla T and Di Lorenzo
A: Nogo-B regulates endothelial sphingolipid homeostasis to control
vascular function and blood pressure. Nat Med. 21:1028–1037. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin SG, Ryu HH, Li SY, Li CH, Lim SH, Jang
WY and Jung S: Nogo-A inhibits the migration and invasion of human
malignant glioma U87MG cells. Oncol Rep. 35:3395–3402. 2016.
View Article : Google Scholar : PubMed/NCBI
|