Introduction
Intrauterine adhesions (IUAs) or Asherman syndrome
(1) refers to the presence of
fibrous bands in the endometrium of the uterus that result from
damage due to physical or chemical factors, such as surgery or
inflammatory diseases that destroy the lining of the intima and its
normal self-repair capacity, causing the exudation and deposition
of large amounts of fibrinogen. Adhesions between the inner walls
of the uterus form where the walls abnormally adhere or stick to
each other. IUAs lead to alterations in the uterine cavity size and
shape, reductions in the normal endometrial area and changes in the
uterine microenvironment (2). In
addition, the endometrial receptivity is reduced, leading to
infertility and to adverse effects on women's physical and mental
health (3). Since the birth of a
baby resulting from in vitro fertilization (IVF) in 1978,
the rapid development of assisted reproductive technologies has
brought joy to numerous otherwise infertile couples. Hysteroscopic
surgery for the dissection of IUAs followed by reproductive
assistance technology to aid conception is the most effective
treatment for good pregnancy outcomes (4).
In this study, we examined cases of patients with
IUAs from January, 2011 to January, 2015 at the Fujian Province
Maternal and Child Health Hospital. These patients had been treated
with hysteroscopic operations followed by assisted reproductive
treatment for a total of 140 cycles, and the clinical factors
affecting their pregnancy outcomes were identified.
Materials and methods
Patients
This Institutional Review Board approved study,
analyzed cases from 140 cycles of assisted reproductive IVF-ET
treatment from patients with varying degrees of IUAs who had
undergone hysteroscopic surgery for the removal of IUAs at the
Fujian Maternal and Child Health Hospital from January, 2011 to
January, 2015. Informed written consent was obtained from all
patients for the surgical procedures. For the present study,
patient consent was waived as it was a retrospective analysis, with
no direct contact between the authors and the patients, and
personal privacy was protected through anonymization.
Data from 108 cycles of IVF and 32 cycles of
intracytoplasmic single sperm injections (ICSIs). We included data
from patient with ages ranging from 23 to 47 years with infertility
durations ranging from 1 to 15 years. The causes of infertility
varied (121 cases had secondary infertility and 19 had primary
infertility), but included tubal infertility, partners with
oligoasthenoteratozoospermia syndrome, repeated intrauterine
insemination (IUI) failure, pelvic endometriosis IV, follicular
rupture luteinization syndrome (LUFS), unexplained infertility and
others. We included data from women with basal endocrine levels
within the normal range and diagnosed as having varying degrees of
IUAs; we also excluded data from patients with premature ovarian
failure, space-occupying ovarian lesions, unilateral or bilateral
hydrosalpinx, or endometritis prior to embryo transfers or uterine
malformations.
Hysteroscopic surgery for the removal
of IUAs
All patients underwent diagnostic hysteroscopy and
treatment within 7 days following the clearance of their
menstruation cycles. The physicians used hysteroscopy to determine
the type and extent of the IUAs, and to place the micro-shear or
vaporization electrode to separate the adhesions, and restore the
shape and size of the uterine cavity to the greatest extent
possible. Following the procedure, an intrauterine device (IUD) was
placed into the uterus, followed by treatment with
Bujiale® (estradiol valerate tablets) and dydrogesterone
for 3 months. Finally, a hysteroscopy and IUD removal procedure
were performed, and if deemed necessary, the IUA removal was
repeated.
Classification of IUAs
Different classification systems for IUAs have been
proposed according to the American Fertility Society (AFS)
(5) as follows: i) According to the
uterine area involvement: <1/3 (1 point), between 1/3 and 2/3 (2
points), >2/3 (4 points). ii) According to adhesion types: Filmy
adhesion (1 point), filmy and dense (2 points), dense adhesion (4
points). iii) According to the menstrual pattern: Normal (0
points), hypomenorrhea (2 points) and amenorrhea (4 points). The
summation of the scores from the three systems yields a final
evaluation of the degree of IUAs (mild, 1–4 points; moderate, 5–8
points; and severe, 9–12 points).
IVF-ET method
Superovulation, egg retrieval, IVF, ICSI and embryo
cultures were carried out following the routine procedures for
assisted reproductive technology laboratories. On the third day of
embryo culture, 1–2 embryos were selected for transfer. Following
egg retrieval, the patients were injected progesterone to support
the progress of the corpus luteum. Blood tests for human chorionic
gonadotropin (hCG) were carried out 14 days after the embryo
transfers. Patients with positive detection underwent ultrasonic
tests after 5 weeks of implantation to observe the uterine cavity,
the existence of gestational sacs and the fetal heartbeat as
indicators of clinical pregnancy.
Embryo quality assessment
According to the evaluation standards presented in
the study by Van Royen et al (6), the best blastomeres according to their
morphology reach 8 cells on the third day following egg retrieval.
High-quality embryos were defined as those being regularly uniform
blastomeres with <20% fragmentation.
Grouping
Blood samples were obtained from patients for hCG
tests 14 days following the embryo transfers. Biochemical
pregnancies were identified by hCG levels >50 mIU/ml; ultrasound
monitoring of the gestational sac and fetal heartbeat were
performed 5 weeks following the embryo transfers to the uterine
cavity. The data from the 140 cycles in the study were divided into
a pregnancy and a non-pregnancy group according to the treatment
outcome. The cases with clinical pregnancy formed the pregnancy
group; while those without implantation, biochemical pregnancy, or
ectopic pregnancy formed part of the non-pregnancy group. The
following variables were compared between the two groups: Age,
infertility duration, infertility type, number of prior surgical
treatments for IUAs, IUA severity, number of prior uterine cavity
operations, number of spontaneous abortions, baseline
follicle-stimulating hormone/luteinizing hormone (FSH/LH) ratio,
baseline estradiol level, body mass index, time interval between
surgical treatment for IUAs and embryo transfer, endometrial
thickness on the day of human chorionic gonadotropin
administration, number of embryos transferred, and number of
high-quality embryos transferred.
Statistical analyses
Statistical analyses were performed using 24.0
software (IBM, Inc.). Measurement data are expressed as the means ±
standard deviation, and count data as numbers and percentages.
Comparisons were made between groups using independent sample
t-tests, and comparisons within groups using the Chi-squared test
of listed data in tabulated row and column formats. Multivariate
logistic regression and Pearson's correlation analyses were also
conducted for the variables with significant differences following
the t-test and the Chi-squared test. Values of P<0.05 were
considered to indicate statistically significant differences.
Results
Association between clinical
indicators and pregnancy outcomes in the two groups
Among the 140 cycles studied, we found 60 cases of
clinical pregnancy (for a clinical pregnancy rate of 42.9%), and 80
cases of no implantation, biochemical pregnancy, or ectopic
pregnancy. No significant differences were found in terms of
infertility years, infertility type, the numbers of IUA procedures,
the degree of the IUAs, the numbers of intrauterine surgeries, the
number of spontaneous abortions, baseline estradiol values, body
mass indexes, the interval between the IUA procedure and embryo
transfer, and the numbers of transferred high-quality embryos (all
P>0.05). The average endometrial thickness of the pregnancy
group was higher than that of the non-pregnancy group, and the
difference was statistically significant (t=2.771; P=0.006). In
addition, the age difference (t=−2.397, P=0.018), the difference in
basal FSH/LH levels (t=−1.996; P=0.048) and the difference in the
number of transferred embryos (t=2.093, P=0.039<0.05) between
the two groups were all statistically significant (Table I).
 | Table I.Comparison of clinical indicators in
the groups with different pregnancy outcomes. |
Table I.
Comparison of clinical indicators in
the groups with different pregnancy outcomes.
| Clinical
indicator | Pregnancy group
(n=60) | Non-pregnancy group
(n=80) | t/χ2 | P-value |
|---|
| Age (years) | 31.68±4.35 | 33.65±5.12 | −2.397 | 0.018a |
| <30 | 58.97% (23/39) | 41.03% (16/39) | 10.598 | 0.014a |
| 30–35 | 40.82% (20/49) | 59.18% (29/49) |
|
|
| 35–40 | 39.02% (16/41) | 60.98% (25/41) |
|
|
| ≥0. | 9.09% (1/11) | 90.91% (10/11) |
|
|
| Infertility duration
(years) | 4.85±3.22 | 4.35±3.04 | 0.938 | 0.350 |
| Primary
infertility | 52.63% (10/19) | 47.37% (9/19) | 0.858 | 0.354 |
| Secondary
infertility | 41.32% (50/121) | 58.68% (71/121) |
|
|
| Numbers of surgeries
for intrauterine adhesion removal | 1.30±0.63 | 1.58±1.15 | −1.749 | 0.083 |
| Intrauterine
adhesions (mild) | 41.54% (27/65) | 58.46% (38/65) | 1.163 | 0.559 |
| Intrauterine
adhesions (moderate) | 43.14% (22/51) | 56.86% (29/51) |
|
|
| Intrauterine
adhesions (severe) | 54.17% (13/24) | 45.83% (11/24) |
|
|
| Number of
intrauterine surgeries | 1.16±1.19 | 1.27±1.15 | −0.529 | 0.598 |
| Number of spontaneous
abortions | 1.70±0.88 | 1.85±1.00 | −0.590 | 0.558 |
| Interval between
intrauterine adhesion procedure (months) | 9.89±5.68 | 12.18±7.84 | −1.591 | 0.115 |
| Basal FSH/LH | 1.79±1.03 | 2.35±1.99 | −1.996 | 0.048a |
| Basal estradiol
(pg/ml) | 69.86±114.67 | 55.15±58.25 | 0.909 | 0.366 |
| body mass index
(kg/m2) | 21.0±2.60 | 21.51±3.44 | −0.759 | 0.450 |
| Endometrial
thickness (mm) on hCG trigger day | 11.23±1.73 | 10.35±1.96 | 2.771 | 0.006b |
| Number of
transferred embryos (one) | 1.96±0.19 | 1.86±0.39 | 2.093 | 0.039a |
| Number of
high-quality transferred embryos (one) | 1.67±0.70 | 1.42±0.85 | 1.863 | 0.065 |
Multivariate logistic regression
analysis of age, transformation endometrial thickness, basal FSH/LH
levels and the number of transferred embryos
We performed multivariate logistic regression
analyses for age, transformation day endometrial thickness, basal
FSH/LH levels and the number of transferred embryos. The results
indicated that the age (OR, 0.637; 95% CI, 0.418–0.971; P<0.05)
and endometrial thickness on the day of endometrial secretory
transformation (OR, 2.125; 95% CI, 1.222–3.697; P<0.01)
significantly affected the pregnancy outcomes. We found that the
basal FSH/LH level and the number of transferred embryos did not
differ significantly (P>0.05) affect pregnancy outcomes
according to our multivariate logistic regression analysis
(Table II).
 | Table II.multivariate logistic regression
analysis of age, endometrial thickness on hCG the trigger day,
basal FSH/LH level and the number of transferred embryos. |
Table II.
multivariate logistic regression
analysis of age, endometrial thickness on hCG the trigger day,
basal FSH/LH level and the number of transferred embryos.
| Index | Age | Transformation
endometrial thickness | Basal FSH/LH | Number of
transplanted embryos |
|---|
| B | −0.451 | 0.754 | −0.262 | 0.962 |
| Wald | 4.404 | 7.121 | 0.811 | 1.765 |
| OR value | 0.637 (0.418,
0.971) | 2.125 (1.222,
3.697) | 0.770 (0.435,
1.361) | 2.617 (0.633,
10.822) |
| (95% confidence
interval) |
| P-value | 0.036a | 0.008b | 0.368 | 0.184 |
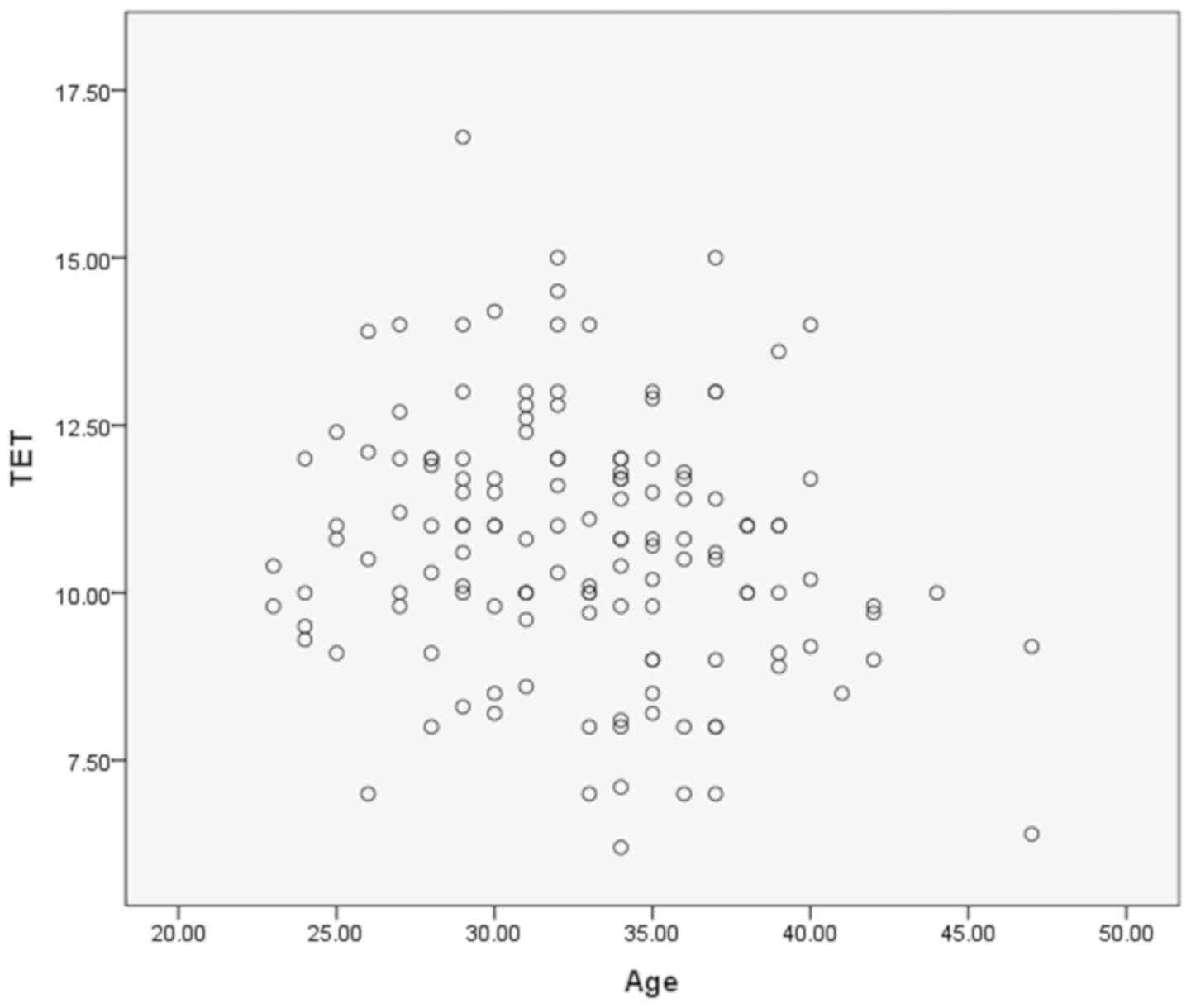
Correlation analysis of age and endometrial
thickness on the day of triple layer appearance. We assessed the
correlation between age and endometrial thickness on the day of
triple layer appearance using Pearson's correlation analysis. Our
analysis indicated no correlation between age and endometrial
thickness (r=−0.185, P=0.029) (Fig.
1).
Discussion
Occurrence, prevention and treatment of IUAs. IUAs
can be caused by trauma, infection, or low estrogen levels
(7). A history of dilation and
curettage (D&C) is an important risk factor for IUAs.
Intrauterine infections are also an important cause of IUAs
(8). IUAs can also occur in patients
with non-tuberculous endometritis and lacking a history of
intrauterine surgery; in fact, Lim et al demonstrated that
postpartum or post-surgical low estrogen levels affected the repair
of the endometrium, leading to IUAs (9). Thus, the endometrium post-pregnancy is
more vulnerable, accounting for approximately 90% of all IUAs, most
often following natural childbirth, cesarean section, or abortion.
In this study, we examined 121 cases of secondary infertility
(86.4%), and only 19 cases of primary infertility (13.6%),
suggesting that secondary infertility causes IUAs more often, in
agreement with the findings of the previous study mentioned
(9).
The treatment of IUAs involves the accurate and
thorough separation of adhesions, the prevention of post-operative
re-adhesions and the promotion of endometrial hyperplasia (10,11). The
current comprehensive treatment is mainly based on surgical
adhesion removal. Surgical treatment not only restores the normal
shape of the uterine cavity, but also improves the blood supply to
its tissues, preventing re-adhesions. In addition, the
comprehensive treatment utilizes estradiol valerate and
dydrogesterone to promote endometrial repair and restore its normal
function. Experts at home and abroad have recognized that
hysteroscopic separation of adhesions is the ‘golden’ method for
the treatment of IUAs (3). An
advantage of the treatment is that the remodeling of the uterine
cavity shape relieves the binding to the sub-endothelium muscle
layer, restores the normal peristaltic function, increases the
blood supply of the endometrial tissue, and facilitates the
regeneration of endometrial epithelial cells that accelerates
damage repairs. The formation of physical barriers helps prevent
re-adhesion, and drug-promoted endometrial hyperplasia promotes the
repair into a fully functional uterus. In this study, through
hysteroscopic surgery, the patients in all 140 cycles recovered
with a normal intrauterine morphology, and endometrial growth was
promoted by supplying the patients with post-operative estrogen
supplementation. Overall, we observed marked improvements in all
the patients.
Preventing unintended pregnancies to avoid
abortions, and if necessary, improving the D&C technique to
make it safer will be key to reducing the incidence of
complications, such as IUAs, and to improve successful pregnancy
rates. The accurate and complete treatment of IUAs is important in
order to re-establish normal menstruations and to improve pregnancy
outcomes. Thus far, no effective method for the prevention of IUAs
exists, at least to the best of our knowledge. The endometrium
lining is typically non-renewable, and if the basal layer is
severely traumatized, IUAs are unavoidable, and the probability of
re-adhesions following uterine adhesion removal is high. The key
for the fundamental prevention of IUAs is to minimize ‘avoidable’
uterine operations particularly abortions.
Effects of age and ovarian functional status on
pregnancy outcome following IVF-ET. A number of women tend to wait
a long time before attempting to have their first child, and with
the opening of the second child policy in China, older-aged women
are again having fertility needs. Age is associated with the female
reproductive capacity (12), and the
number of patients with diminished ovarian reserves (DORs) has
increased. The ovarian reserve reflects the reproductive potential
left within a woman's two ovaries; the ability of the ovarian
cortex to form fertile oocytes is mainly determined by the number
and quality of the antral follicles in that ovary (13). Due to the smaller number of oocytes
available to patients with DORs, the number of embryos that can be
obtained is lower, the cycle cancellation rate is higher, and the
clinical pregnancy rate is lower than that in patients without DORs
(14). This has been a difficult
problem to solve during IVF-ET treatment cycles (15). Fernandez et al (16) and others have demonstrated that the
post-operative pregnancy rates of patients with IUAs are 23.5% in
the >35-year-old group and 66.6% in the <35-year-old group;
in addition, the live birth rates have been shown to be 14.7 and
53.5%, respectively, with the difference being statistically
significant. A patient with a diminished ovarian reserve has an
increased basal serum FSH/LH ratio, and this ratio is a good
predictor of ovarian function (17).
In this study, the ratio of FSH/LH in the non-pregnancy group
(2.35±1.99) was higher than that in the pregnancy group
(1.79±1.03), and the difference was statistically significant
(t=−1.996; P=0.048); however, our multivariate logistic regression
analysis revealed that the difference was not statistically
significant (P>0.05), probably due to the small sample size in
this study.
We found that the mean age of the women in the
non-pregnancy group (33.65±5.12) was higher than that of women in
the pregnancy group (31.68±4.35), with a statistically significant
difference (t=−2.397; P=0.018). With increases in age, the clinical
pregnancy rate decreased significantly. Moreover, our multivariate
logistic regression analysis revealed that age had a significant
effect on pregnancy outcome, and our results suggest that age is
the main predictor of clinical pregnancy.
Improving the pregnancy outcome of patients with
DORs is a goal of many researchers. Weall et al have
demonstrated that the use of growth hormone (GH) during the
ovulation induction process can improve the ovarian response, the
clinical pregnancy rate and the live birth rate of patients with
DORs (18). IVF treatment together
with androgen administration or a modulator in patients with DORs
can improve the clinical pregnancy rate, reduce the total amount of
GH used, and improve the pregnancy outcome (19). However, due to the lack of
large-scale control studies, these results remain controversial.
Future large-scale randomized controlled trials are required to
obtain more convincing conclusions.
Effect of endometrial thickness on the outcome of
IVF-ET pregnancy. The ability of the endometrium to allow
blastocyst positioning, adhesion and implantation is known as
endometrial receptivity, and is one of the main factors influencing
the pregnancy outcome. The thickness of the intima must reach a
certain threshold to be receptive, that is, an endometrium above a
certain thickness is a pre-requisite condition for embryo
implantation, and thinning of the intima is an important cause of
low implantation rates. An appropriate uterine thickness can
increase the clinical pregnancy rate (20). The endometrial thickness during the
transfer window for the embryo is a predictor of the embryo
implantation outcome (21).
Clinically, the thickness of the endometrium on the hCG ‘trigger
day’ (or progesterone conversion ‘trigger day’) is regarded as an
appropriate evaluation of the endometrium embryo reception adequacy
(22). Clinical studies have shown
that if the endometrial thickness is ≤7 mm, the clinical pregnancy
probability is significantly reduced (20). In this study, the mean endometrial
thickness in the pregnancy group was 11.23±1.73 mm, higher than
that in the non-pregnancy group (10.35±1.96 mm), and the difference
between the two groups was statistically significant (t=2.771;
P=0.006). Our logistic regression analysis revealed that the
endometrial thickness on the hCG trigger day had a significant
effect on the pregnancy outcome (OR, 2.125; 95% CI, 1.222–3.697;
P<0.01). Our analysis of the correlation between age and
endometrial thickness on the hCG trigger day revealed no
significant correlation (r=−0.185; P=0.029). On the other hand,
Gonen et al have reported that the endometrial thickness on
the hCG trigger day correlates with age, and has predictive value
for pregnancy outcomes (23).
The recovery of uterine cavity morphology is a basic
requirement of the treatment of IUAs, and the improvement of
endometrial receptivity is equally important. The improvement of
endometrial thickness is the most direct and effective means with
which to improve clinical pregnancy rates. Measures to this effect
include traditional hormone therapy (24), vasoactive drug therapy (25), and regenerative medicine (26). However, a small number of patients
with thin endometria remain difficult to treat, and innovative and
effective treatment methods are still being actively explored.
Effect of number of transferred embryos on
pregnancy: Outcome of IVF-ET. A major challenge in the field of
assisted reproduction has been identifying methods with which to
reduce the rate of multiple pregnancies due to the high clinical
pregnancy rate of IVF-ET. Huang et al studied the IVF-ET
treatment cycles of women aged 35–36 years, comparing cycles after
transferring 3 embryos with those after transferring 2 embryos, and
found that the difference in the clinical pregnancy rate was not
statistically significant, although the multiple pregnancy
incidences increased significantly (27). Luo et al concluded that in
order to ensure a clinical pregnancy and reduce the incidence of
multiple pregnancies, transferring 3 embryos is optimal in women
>38 years of age or in those between 35 and 37 years of age
without high-quality embryos (28).
For women <38 years of age with high-quality embryos, the number
of transferred embryos should be reduced from 3 to 2, and the
remaining embryos can be cryopreserved, to avoid embryo waste and
reduce the multiple pregnancy incidence. In this study, the number
of embryos transferred in the pregnancy group (1.96±0.19) was
higher than that in the non-pregnancy group (1.86±0.39), and the
difference was statistically significant (t=2.093; P=0.039);
however, our multivariate logistic regression analysis revealed no
statistically significant differences (P>0.05).
In the presence of an appropriate endometrial
condition, the number of embryos to transfer can be determined
jointly by the doctor and the patient. The decision affects not
only the pregnancy outcome, but also the mother-child and
postpartum health. In recent years, single embryo transfers have
become the focus of many assisted reproductive centers worldwide to
ensure safe outcomes (29).
In conclusion, for patients undergoing IVF-ET
treatment following the surgical removal of IUAs, age and
endometrial thickness on the day of triple layer appearance are the
most important predictors of pregnancy outcomes. Future studies are
required however, to focus on identifying methods with which to
effectively improve endometrial thickness and ovarian response in
patients with a diminished ovarian reserve in order to improve
pregnancy rates and outcomes.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW was involved in the conception and design of the
study and in data collection, and in the writing of the manuscript.
JY was involved in the conception and design of the study and in
the editing of the manuscript. XX was involved in the data
interpretation and editing of the manuscript. SD and LL were
involved in data collection. XZ was involved in data collection.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Fujian Provincial
Maternity and Children's Hospital approved this retrospective
medical record review (approval no. 20192006). Informed written
consent was obtained from all patients for the surgical procedures.
For the present study, patient consent was waived as it was a
retrospective analysis, with no direct contact between the authors
and the patients, and personal privacy was protected through
anonymization.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu D, Wong YM, Cheong Y, Xia E and Li TC:
Asherman syndrome - one century later. Fertil Steril. 89:759–779.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kodaman PH and Arici A: Intra-uterine
adhesions and fertility outcome: How to optimize success? Curr Opin
Obstet Gynecol. 19:207–214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Conforti A, Alviggi C, Mollo A, De Placido
G and Magos A: The management of Asherman syndrome: A review of
literature. Reprod Biol Endocrinol. 11:1182013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo EJ, Chung JPW, Poon LCY and Li TC:
Reproductive outcomes after surgical treatment of asherman
syndrome: A systematic review. Best Pract Res Clin Obstet Gynaecol.
Jan 3–2019.(Epub ahead of print). doi:
10.1016/j.bpobgyn.2018.12.009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
The American Fertility Society
classifications of adnexal adhesions, distal tubal occlusion, tubal
occlusion secondary to tubal ligation, tubal pregnancies, müllerian
anomalies and intrauterine adhesions. Fertil Steril. 49:944–955.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Royen E, Mangelschots K, De Neubourg
D, Valkenburg M, Van de Meerssche M, Ryckaert G, Eestermans W and
Gerris J: Characterization of a top quality embryo, a step towards
single-embryo transfer. Hum Reprod. 14:2345–2349. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Inany H: Intrauterine adhesions. An
update. Acta Obstet Gynecol Scand. 80:986–993. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deans R and Abbott J: Review of
intrauterine adhesions. J Minim Invasive Gynecol. 17:555–569. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim DR, Hur H, Min BS, Baik SH and Kim NK:
Intrauterine contraceptive device-related actinomycosis infection
presenting as ovarian cancer with carcinomatosis. Surg Infect
(Larchmt). 15:826–828. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panayotidis C, Weyers S, Bosteels J and
van Herendael B: Intrauterine adhesions (IUA): Has there been
progress in understanding and treatment over the last 20 years?
Gynecol Surg. 6:197–211. 2009. View Article : Google Scholar
|
|
11
|
Di Spiezio Sardo A, Calagna G,
Scognamiglio M, O'Donovan P, Campo R and De Wilde RL: Prevention of
intrauterine post-surgical adhesions in hysteroscopy. A systematic
review. Eur J Obstet Gynecol Reprod Biol. 203:182–192. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shirasuna K and Iwata H: Effect of aging
on the female reproductive function. Contracept Reprod Med.
2:232017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galey-Fontaine J, Cédrin-Durnerin I,
Chaïbi R, Massin N and Hugues JN: Age and ovarian reserve are
distinct predictive factors of cycle outcome in low responders.
Reprod Biomed Online. 10:94–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jirge PR: Poor ovarian reserve. J Hum
Reprod Sci. 9:63–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karande VC: Managing and predicting low
response to standard in vitro fertilization therapy: A review of
the options. Treat Endocrinol. 2:257–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fernandez H, Al-Najjar F,
Chauveaud-Lambling A, Frydman R and Gervaise A: Fertility after
treatment of Asherman's syndrome stage 3 and 4. J Minim Invasive
Gynecol. 13:398–402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roudebush WE, Kivens WJ and Mattke JM:
Biomarkers of Ovarian Reserve. Biomark Insights. 3:259–268. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weall BM, Al-Samerria S, Conceicao J,
Yovich JL and Almahbobi G: A direct action for GH in improvement of
oocyte quality in poor-responder patients. Reproduction.
149:147–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keane KN, Hinchliffe PM, Rowlands PK,
Borude G, Srinivasan S, Dhaliwal SS and Yovich JL: DHEA
Supplementation Confers No Additional Benefit to that of Growth
Hormone on Pregnancy and Live Birth Rates in IVF Patients
Categorized as Poor Prognosis. Front Endocrinol (Lausanne).
9:142018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Y, Gao X, Lu X, Xi J, Jiang S, Sun Y
and Xi X: Endometrial thickness affects the outcome of in vitro
fertilization and embryo transfer in normal responders after GnRH
antagonist administration. Reprod Biol Endocrinol. 12:962014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao J, Zhang Q and Li Y: The effect of
endometrial thickness and pattern measured by ultrasonography on
pregnancy outcomes during IVF-ET cycles. Reprod Biol Endocrinol.
10:1002012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen SL, Wu FR, Luo C, Chen X, Shi XY,
Zheng HY and Ni YP: Combined analysis of endometrial thickness and
pattern in predicting outcome of in vitro fertilization and embryo
transfer: A retrospective cohort study. Reprod Biol Endocrinol.
8:302010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gonen Y and Casper RF: Prediction of
implantation by the sonographic appearance of the endometrium
during controlled ovarian stimulation for in vitro fertilization
(IVF). J In Vitro Fert Embryo Transf. 7:146–152. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen MS, Wang CW, Chen CH and Tzeng CR:
New horizon on successful management for a woman with repeated
implantation failure due to unresponsive thin endometrium: Use of
extended estrogen supplementation. J Obstet Gynaecol Res.
39:1092–1094. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Acharya S, Yasmin E and Balen AH: The use
of a combination of pentoxifylline and tocopherol in women with a
thin endometrium undergoing assisted conception therapies--a report
of 20 cases. Hum Fertil (Camb). 12:198–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barad DH, Kushnir VA, Shohat-Tal A,
Lazzaroni E, Lee HJ and Gleicher N: Prospective randomized study of
endometrial perfusion with granulocyte colony-stimulating factor
(G-CSF) in unselected IVF cycles: Impact on endometrial thickness
and clinical pregnancy rates. Fertil Steril. 100:S1442013.
View Article : Google Scholar
|
|
27
|
Huang Z, Li S, Ma Q, et al: The influence
of transferred embryo's number on pregnancy outcomes in aged
infertile patients undergoing IVF-ET*. Progr Mod Biomed. 9:835–837.
2009.
|
|
28
|
Luo Y, Liu F, Yi Y, et al: Effect of
number and quality of embryo transferred on clinical pregnancy rate
in women of different age. J Reprod Med. 23:361–366. 2014.
|
|
29
|
Lukassen HG, Braat DD, Wetzels AM,
Zielhuis GA, Adang EM, Scheenjes E and Kremer JA: Two cycles with
single embryo transfer versus one cycle with double embryo
transfer: A randomized controlled trial. Hum Reprod. 20:702–708.
2005. View Article : Google Scholar : PubMed/NCBI
|