Introduction
Cerebral hemorrhage is a type of human
cardiovascular system disease, which serves an important adjunct
role in primary brain injury and often leads to vasospasm (1). A previous systematic review and
meta-analysis has indicated that intracranial hemorrhage increases
the risk of cerebral microbleeds in patients with acute stroke
(2). Cerebrovascular injury is
frequently caused by ischemia/reperfusion, which may lead to
cerebral infarct and neuronal apoptosis (3). Additionally, the inhibition of neuronal
apoptosis is beneficial for the recovery of cognitive disorders in
cerebral hemorrhage induced by ischemia/reperfusion (4). Therefore, understanding the apoptosis
of neurons is essential for the treatment of cerebral
hemorrhage.
Resveratrol is a multifunctional biological
polyphenol that serves therapeutic effects in certain types of
human disease (5). A previous study
has indicated that the neuroprotective effects of resveratrol in
ischemic injury are mediated by the improvement of brain energy
metabolism and the alleviation of oxidative stress in rats
(6). Zou et al (7) have revealed that the continuous
infusion of resveratrol over a 4-week period protected neurons from
cannulae implantation injury. In addition, pre-treatment with
resveratrol attenuated traumatic brain injury in rats by
suppressing NACHT, LRR and PYD domains-containing protein 3
inflammasome activation via sirtuin 1 (7). Furthermore, resveratrol also
ameliorated hypoxia/ischemia-induced behavioral deficits and brain
injury in the neonatal rat brain (8). A previous study has also demonstrated
that the decreased expression of the transforming growth factor-β
(TGF-β) gene superfamily is associated with adult neurogenic
regions following brain injury (9).
However, associations between resveratrol and TGF-β have not yet
been reported in neurons.
The present study assessed the therapeutic role of
resveratrol in a rat model of cerebral hemorrhage. Cerebral infarct
volume, hippocampal cell apoptosis and neuron viability were
analyzed following a 21-day experimental period. Notably, the
present study assessed the association between resveratrol and the
TGF-β-mediated extracellular signal-regulated kinase (ERK)
signaling pathway in neurons isolated from a rat model of cerebral
hemorrhage.
Materials and methods
Rat model of cerebral hemorrhage
A total of 16 male Sprague-Dawley rats (weight,
300–330 g; age, 8 weeks) were purchased from Harbin Veterinary
Research Institute (Harbin, China). All rats were housed at a
temperature of 23±1°C and humidity of 50±5%, with a 12-h light/dark
cycle and free access to food and water. The rat model of cerebral
hemorrhage was established using the modified ischemia/reperfusion
method as previously described (10). Rats received right middle cerebral
artery occlusion for 90 min and reperfusion by withdrawal of the
filament at 37°C Immediately, rats with
ischemia/reperfusion-induced cerebrovascular injury were randomly
divided into two groups (each, n=8) and were administered an
intravenous injection of resveratrol (10 mg/kg/day; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) or the same volume of PBS (serving
as the control) as described previously (11). The treatments were continued for 20
days. Rats were sacrificed using cervical dislocation following
intraperitoneal pentobarbital injection (35 mg/kg body weight) on
day 21 for further analysis.
Behavioral tests
Behavioral function (locomotor activity) was
examined in experimental rats using open-field tests on day 21.
These were used to analyze the efficacy of resveratrol on
ischemia/reperfusion injury and were performed as previously
described (12).
Cell culture
Rats were sacrificed via cervical dislocation.
Neuronal cells were isolated from rats with cerebral hemorrhage
prior to treatment as described previously (13). Cells were cultured in RPMI 1640
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.) for 24 h at 37°C. Cells were then
treated with a TGF-β inhibitor (TGF-βIR; 2 mg/ml; Sigma-Aldrich;
Merck KGaA) or TGF-β and/or resveratrol (2 mg/ml; Sigma-Aldrich;
Merck KGaA) for 24 h at 37°C.
Analysis of cerebral water content
(CWC)
On day 21, CWC was evaluated in the resveratrol and
PBS group as described in a previous study (14). Rat brains were isolated as detailed
previously (15). Brains were dried
in an electric oven at 100°C for 24 h to analyze CWC in the rat
model of intracerebral hemorrhage. The CWC was calculated using the
following formula: (wet weight-dry weight/wet weight) ×100 (%).
Quantitative analysis of blood-brain
barrier (BBB) permeability
BBB leakage was examined as previously described
(16). Briefly, experimental rats
received 100 µl 5% Evan's blue with resveratrol or PBS administered
intravenously 10 days following ischemia/reperfusion-induced
injury. Following the injection of Evan's blue (for 2 h), cardiac
perfusion was performed under deep anesthesia with 200 ml of saline
to clear the cerebral circulation of Evan's blue. Brains were
subsequently isolated and sliced. The two hemispheres were
homogenized in 750 µl of N,N-dimethylformamide (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Quantitative analysis of
blood-brain barrier permeability was performed using Evan's blue
and absorbance was measured at 689 nm using a microplate
reader.
Cell viability assay
Neuron cell viability was determined using an MTT
assay kit (cat. no. M6494; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Neuronal cells
(1×103 cells/well) were seeded in 96-well plates and
cultured in RPMI 1640 medium supplemented with 10% FBS at 37°C for
22 h. Following incubation, 15 µl MTT reagent was added to each
well and incubated for a further 4 h. Following incubation, DMSO
(150 µl) was added to each well to dissolve the purple formazan.
Cell viability was determined by measuring the absorbance was
measured at a wavelength of 570 nm using a microplate reader.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from neuronal cells using an
RNAeasy Mini kit (Qiagen Sciences, Inc., Gaithersburg, MD, USA)
according to the manufacturer's protocol. RNA was reverse
transcribed into cDNA at 42°C for 2 h using the High Capacity cDNA
Reverse Transcription kit (Thermo Fisher Scientific, Inc.). PCR
amplification involved preliminary denaturation at 95°C for 1 min,
followed by 45 cycles of 95°C for 30 sec. The annealing temperature
was reduced to 57°C for 30 sec and 72°C for 10 min. The reaction
volume was a total of 20 µl containing 40 ng genomic cDNA, 100 µM
dNTPs, 200 µM primers and Taq DNA polymerase and SYBR-Green (each,
2.5 U; Thermo Fisher Scientific, Inc.). The forward and reverse
primers were synthesized by Invitrogen (Thermo Fisher Scientific,
Inc.) and are listed in Table I.
Relative mRNA expression levels were calculated using the
2−ΔΔCq method (17).
 | Table I.Primers used in the current study. |
Table I.
Primers used in the current study.
| Gene | Primer sequence
(5′-3′) |
|---|
| Bcl-2 | F:
5′-CACAAGAGGCCAAGGCTACC-3′ |
|
| R:
5′-CAGGAAAGCAGGAAGTCTCAA-3′ |
| Bcl-xl | F:
5′-CCAAAATCCCTGCTCTTCATG-3′ |
|
| R:
5′-GCATTCTTGGCATCGTTATTCA-3′ |
| Bax | F:
5′-ATTGAGAAACGATTTGCCTAC-3′ |
|
| R:
5′-GGAAATGGCTTATTCTCCTTTGCTT-3′ |
| Bad | F:
5′-GGAAACCCGGTGGGGCCAC-3′ |
|
| R:
5′-ACCAGTAGCGGGTGGTC-3′ |
| Iba-1 | F:
5′-CACCATTAGCTGGGCGTCT-3′ |
|
| R:
5′-GATGCGGAAGTAGCAAAAGC-3′ |
| MPO | F:
5′-ATTCTCCACACCCTGTTTCG-3′ |
|
| R:
5′-ATGCAGCAGTGTGTCATTCC-3′ |
| TGF-β | F:
5′-GGCCAGATCCTGTCCAAGC-3′ |
|
| R:
5′-GTGGGTTTCCACCATTAGCAC-3′ |
| ERK | F:
5′-ACCTAGCCGTGGAGCTTGG-3′ |
|
| R:
5′-GCCCTTGGTTGTTTACCTGG-3′ |
| β-actin | F:
5′-AAGGACCTGTATGCCAACACA-3′ |
|
| R:
5′-ATCCACACAGAATACTTGCGTT-3′ |
Western blotting
On day 21, TGF-β, TGF-βIR and/or resveratrol-treated
neurons were lysed using radioimmunoprecipitation buffer containing
protease inhibitors (Thermo Fisher Scientific, Inc.) at 4°C for 10
min. Protein concentration was measured using a bicinchoninic acid
assay kit (Thermo Fisher Scientific, Inc.). Total protein (30
µg/lane) was separated via SDS-PAGE on 15% gels. The separated
proteins were then transferred to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MD, USA) and incubated in
blocking buffer (5% bovine serum albumin; Sigma-Aldrich; Merck
KGaA) for 2 h at 37°C. Membranes were incubated with the primary
antibodies against TGF-β (1:1,200; cat. no. ab31013), ERK (1:1,200;
cat. no. ab17942), phosphorylated (p-) ERK (1:1,200; cat. no.
ab184699), B cell lymphoma-2 (Bcl-2; 1:1,000; cat. no. ab692), B
cell lymphoma-extra large (Bcl-xl; 1:1,200; cat. no. ab32370),
Bcl-2-associated death promoter (Bad; 1:1,200; cat. no. ab32455),
Bcl-2-assocaited X protein (Bax; 1:1,200; cat. no. ab32503),
ionized calcium binding adaptor molecule 1 (Iba-1; 1:1,200; cat.
no. ab108539), myeloperoxidase (MPO; 1:1,200; cat. no. ab208670)
and β-actin (1:2,000; cat. no. ab8226; all Abcam, Cambridge, UK)
for 12 h at 4°C. Following incubation, membranes were washed in
triplicate with PBS and incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin G (1:2,000; cat.
no. PV-6001; OriGene Technologies, Inc., Rockville, MD, USA) for 1
h at 37°C. The membranes were washed three times with PBS. Protein
bands were visualized using an enhanced chemiluminescence substrate
ECL Select™ (Roche Applied Science, Penzberg, Germany) and Kodak
exposure films (Kodak, Rochester, NY, USA). Protein expression was
quantified using Quantity-One software (version 1.2; Bio-Rad
Laboratories, Inc.).
Immunocytochemistry
Neuronal cells (1×106) were fixed in 10%
formaldehyde for 30 min at 37°C. Cells were permeabilized with 0.5%
Triton X-100 in PBS for 5 min at 25°C. Endogenous peroxidase
activity was blocked using 3% hydrogen peroxide for 10 min at room
temperature as previously described (18). Subsequently, cells were incubated
with specific primary antibodies against Iba-1 (1:1,200; cat. no.
ab108539; Abcam) or MPO (1:1,200; cat. no. ab208670; Abcam) for 12
h at 4°C. Cells were washed with PBS and subsequently incubated
with anti-rabbit HRP-conjugated secondary antibody (1:2,000; cat.
no. ab191866; Abcam) for 12 h at 4°C. Cells were subsequently
washed with PBS three times and incubated with 5% DAPI
(Sigma-Aldrich; Merck KGaA) for 15 min at 37°C. Cells were observed
under a fluorescent microscope (magnification, ×40; Olympus
Corporation, Tokyo, Japan). The percentage of positively stained
cells was analyzed using Quantity One software (version 4.62;
Bio-Rad Laboratories, Inc.).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
Cells obtained from the rat model of cerebral
hemorrhage were analyzed using a TUNEL assay (DeadEnd™ Colorimetric
Tunel System; Promega Corporation, Madison, WI, USA) according to
the manufacturer's protocol. Cells were then incubated with 5% DAPI
(Sigma-Aldrich; Merck KGaA) for 15 min at 37°C and washed with PBS
three times and mounted onto slides using Aqueous Mounting Medium
(Sigma-Aldrich; Merck KGaA). TUNEL-positive cells were observed in
three randomly-selected fields under a ZEISS LSM 510 confocal
microscope (Zeiss GmbH, Jena, Germany) at 488 nm.
Statistical analysis
Data are presented as the mean ± standard deviation
of triplicate data. All data were analyzed using SPSS 19.0 software
(SPSS, IBM Corp., Armonk, NY, USA). Significant differences between
two groups were analyzed using a two-tail unpaired Student's
t-test. Multiple group differences were analyzed using one-way
analysis of variance followed by a Tukey's HSD test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Resveratrol decreases cerebral infarct
volume and improves cognitive competence in the rat model of
cerebral hemorrhage
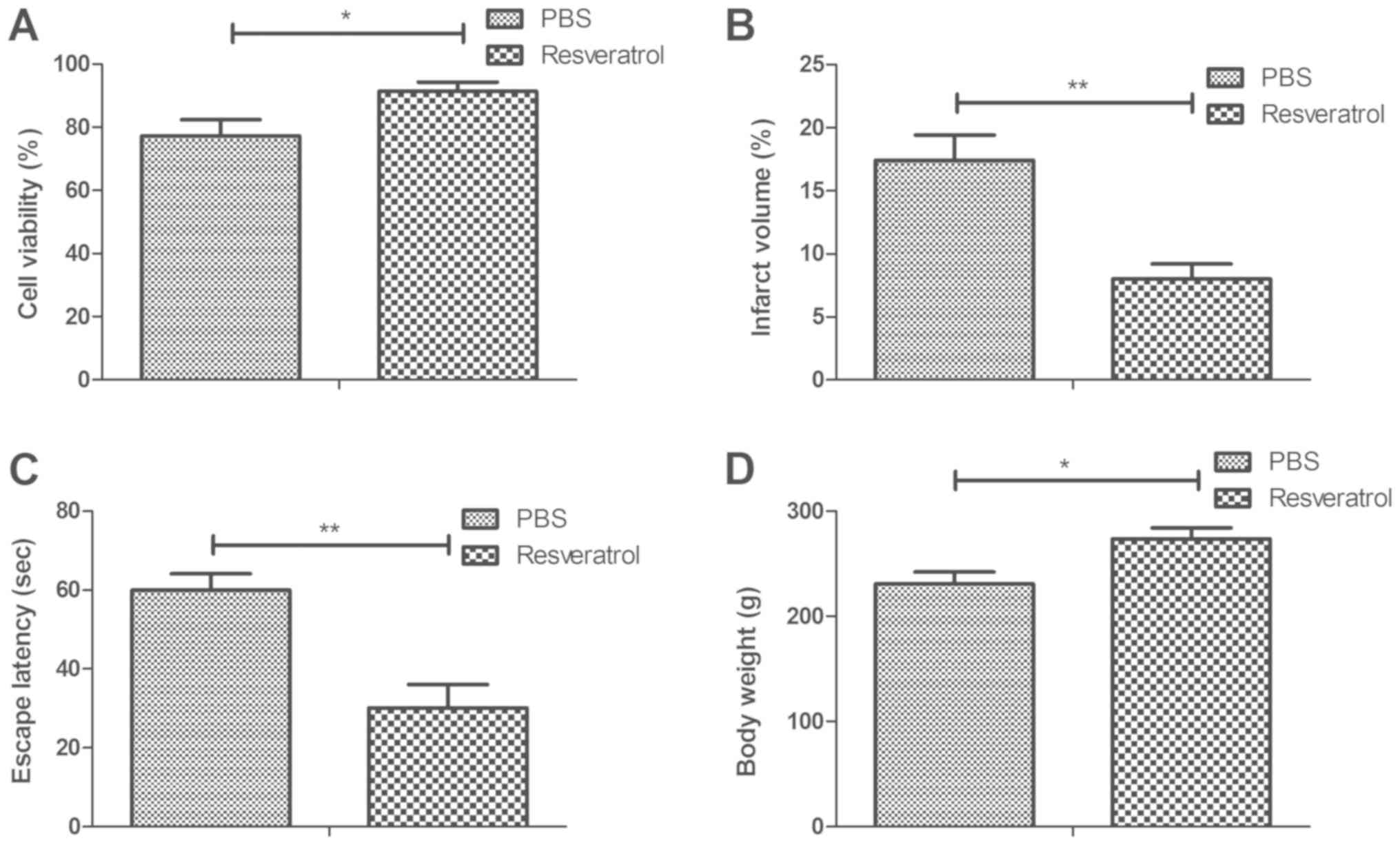
The therapeutic effects of resveratrol were analyzed
in a rat model of cerebral hemorrhage. The viability assay
demonstrated that treatment with resveratrol significantly
increased neuronal cell viability in hippocampus tissue compared
with the PBS group (Fig. 1A). It was
also revealed that resveratrol significantly decreased cerebral
infarct volume in the hippocampi of rats with cerebral hemorrhage
compared with the PBS group (Fig.
1B). Furthermore, treatment with resveratrol significantly
increased the escape latency of rats compared with the PBS group
(Fig. 1C). It was also demonstrated
that the body weight of resveratrol-treated rats increased
significantly compared with the PBS group (Fig. 1D). These results suggest that
resveratrol may be an efficient drug for the treatment of cerebral
hemorrhage.
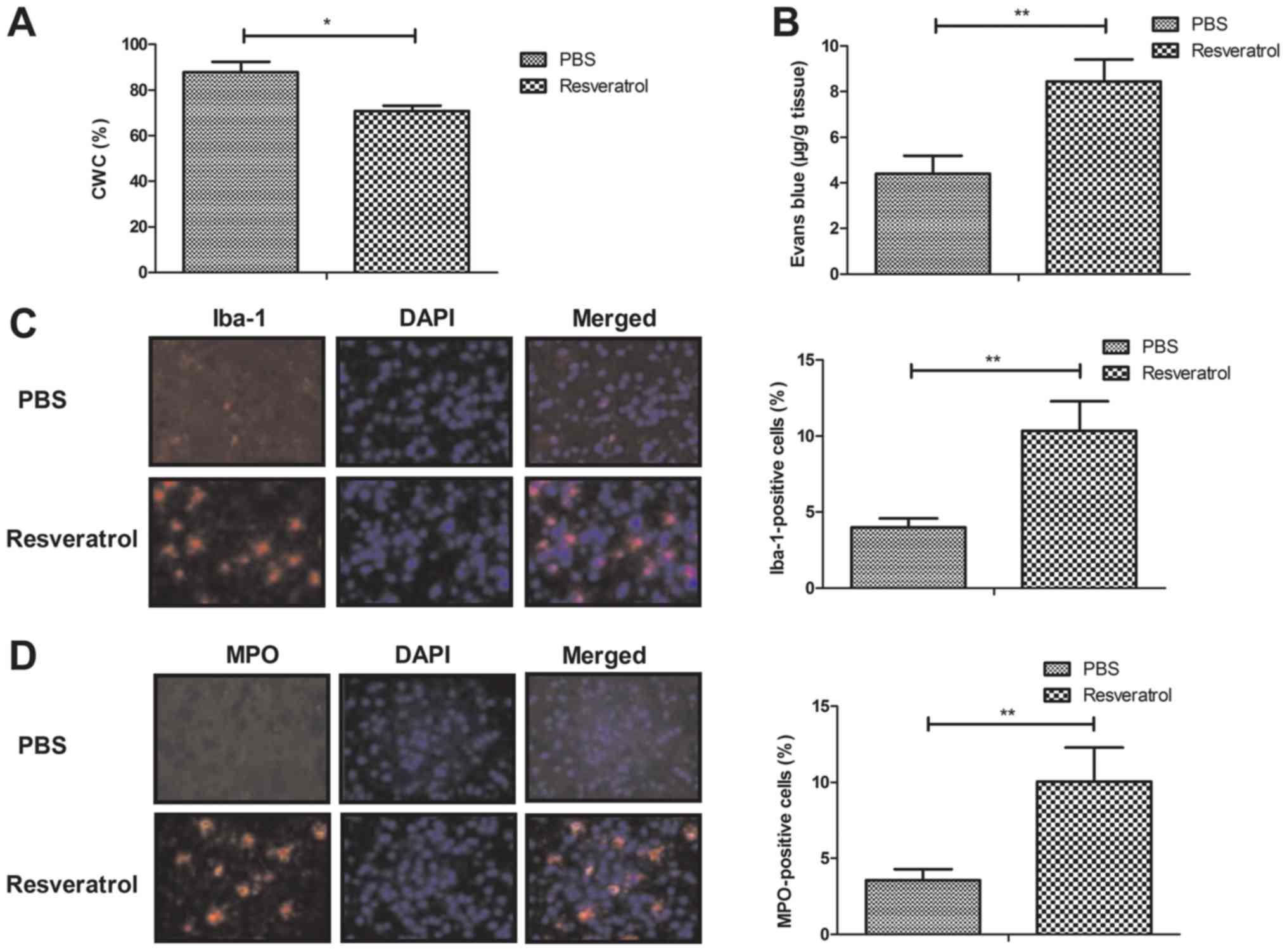
Resveratrol improves CWC and BBB
disruption in the rat model of cerebral hemorrhage
The results demonstrated that treatment with
resveratrol significantly decreased and improved CWC and BBB
disruption, respectively, when compared with the PBS group
(Fig. 2A-B). Furthermore, treatment
with resveratrol significantly increased microglial activation and
neutrophil infiltration as determined by Iba-1- and MPO-positive
cells, respectively (Fig. 2C-D).
These results indicate that resveratrol was beneficial for the
inhibition of the inflammatory response in the brain of mice with
cerebral hemorrhage.
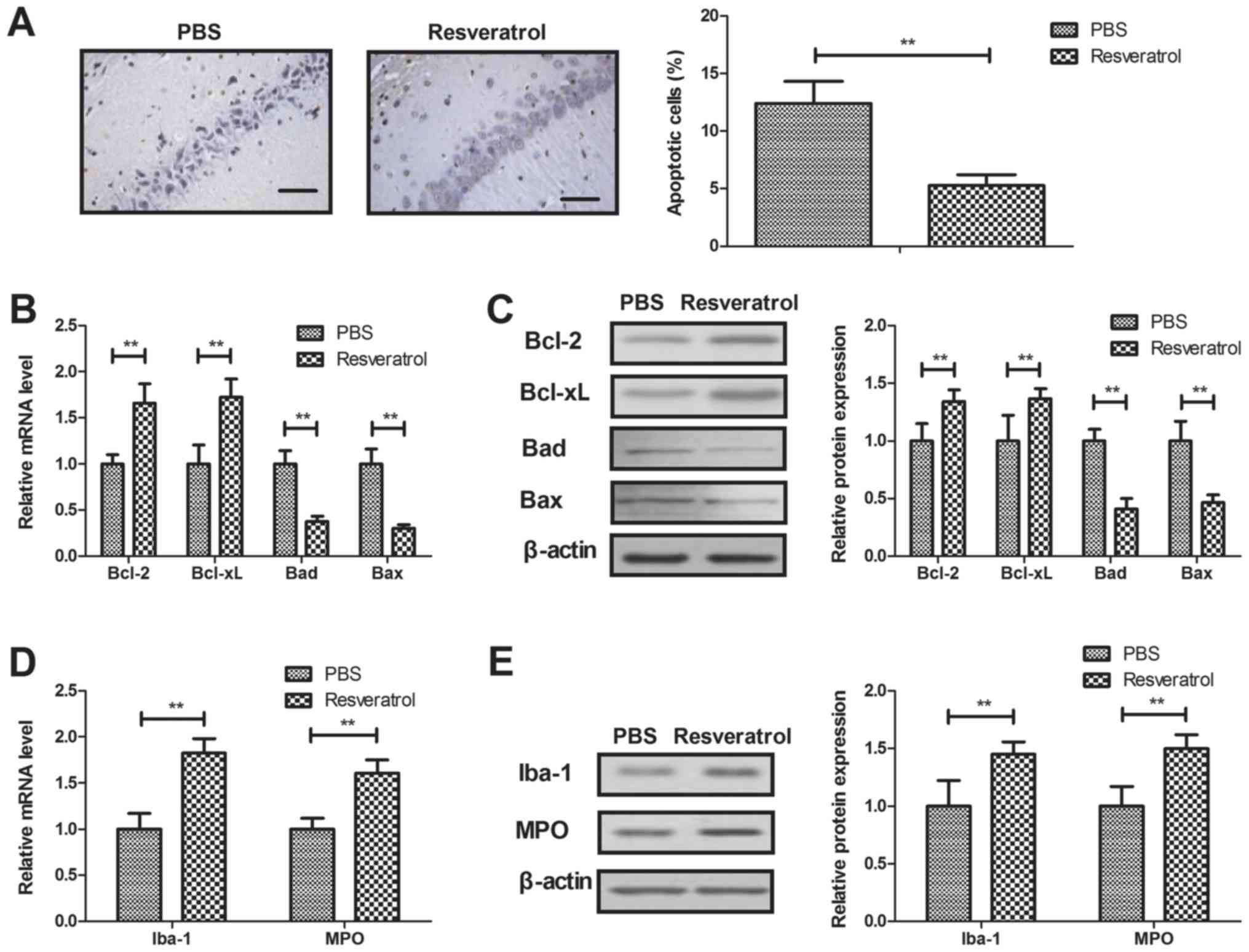
Treatment with resveratrol decreases
neuronal cell apoptosis and inflammation
The apoptosis of neurons was assessed following
treatment with resveratrol in the TUNEL assay. The results
indicated that resveratrol significantly decreased neuronal
apoptosis in rats with cerebral hemorrhage (Fig. 3A). Anti-apoptotic gene and protein
expression (Bcl-2 and Bcl-xl) was significantly upregulated, and
pro-apoptotic gene and protein levels (Bad and Bax) were
significantly downregulated by treatment with resveratrol, compared
with the control (Fig. 3B-C). It was
also revealed that treatment with resveratrol significantly
increased the expression levels of anti-inflammatory factors Iba-1
and MPO in neurons (Fig. 3D-E).
These results indicate that resveratrol decreased neuronal cell
apoptosis and inflammation in rats with cerebral hemorrhage.
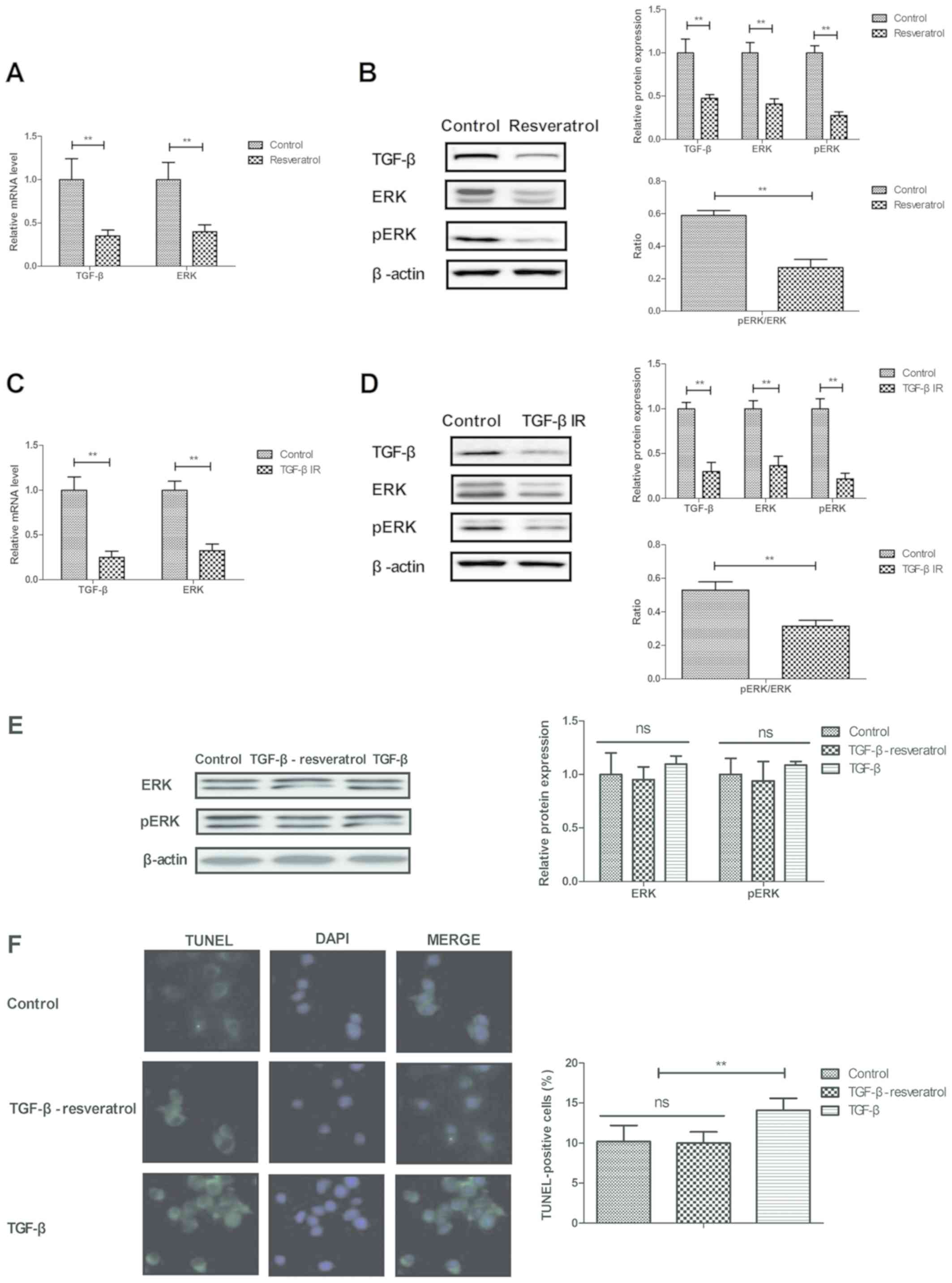
Resveratrol regulates neuronal cell
apoptosis by regulating the TGF-β-mediated ERK signaling
pathway
The current study analyzed the potential
resveratrol-mediated mechanism of apoptosis and inflammation in
neuron cells. It was demonstrated that resveratrol significantly
downregulated TGF-β and ERK gene and protein expression in neurons
isolated from rats with cerebral hemorrhage (Fig. 4A-B). Treatment with TGF-βIR
downregulated TGF-β and ERK gene and protein expression levels in
neurons (Fig. 4C-D).
Resveratrol-TGF-β treatment increased the protein expression levels
of ERK/pERK compared with TGF- β treatment alone (Fig. 4E). In addition, resveratrol-TGF-β
treatment significantly decreased the number of TUNEL positive
cells compared with TGF-β treatment (Fig. 4F) The results indicated that
treatment with TGF-β reversed the decrease in neuronal cell
apoptosis induced by resveratrol (Fig.
4F). These results suggest that resveratrol decreased apoptosis
by downregulating the TGF-β-mediated ERK signaling pathway.
Discussion
Neuronal apoptosis serves a crucial role in the
progression of brain injury (19). A
previous study has demonstrated that resveratrol prevents neuronal
apoptosis in an early brain injury model through the
phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signaling
pathway (20). The current study
assessed the therapeutic effects of resveratrol in a rat model of
cerebral hemorrhage induced by reperfusion injury and further
analyzed the potential mechanism of resveratrol in neuronal cells.
The results demonstrated that resveratrol decreased apoptosis and
inflammation in rats of the hippocampal reperfusion injury model.
The results also indicated that resveratrol inhibited hippocampal
cell apoptosis and inflammation by downregulating the of TGF-β-ERK
signaling pathway.
Evidence has indicated that the inhibition of neural
apoptosis attenuated early brain injury induced by subarachnoid
hemorrhage (21). A previous study
has demonstrated that resveratrol inhibited b-amyloid-induced
neuronal apoptosis by regulating the acetylation of cellular tumor
antigen p53 in PC12 cells (22). It
has also been indicated that increasing the activity of the Bak
signaling pathway may mediate apoptosis in cultured neural
progenitor cells (23). In the
current study, treatment with resveratrol inhibited hippocampal
cell apoptosis by decreasing the expression of Bad and Bax in
neurons. Additionally, treatment with resveratrol upregulated
anti-apoptotic Bcl-2 and Bcl-xl protein levels, which may be the
reason for the resveratrol-induced decrease of apoptosis in
neurons.
Previous studies have revealed that TGF-β promotes
recovery following intracerebral hemorrhage, indicating that TGF-β
may be a therapeutic target for the treatment of acute brain injury
(24). The current study
demonstrated that treatment with resveratrol decreased the
expression of TGF-β, which further led to the decrease of neuronal
cells apoptosis. A further study has revealed that ERK serves a
protective role in the apoptotic mechanisms involved in sympathetic
neuron survival (25). The results
of the present study demonstrated that treatment with resveratrol
inhibits hippocampal cell apoptosis by downregulating the
TGF-β-mediated ERK signaling pathway. Li et al (18) revealed that nerve growth factor
attenuates high glucose-induced endoplasmic reticulum stress, which
prevents Schwann cells from undergoing apoptosis by activating the
PI3K/Akt/glycogen synthase kinase-3 β and ERK1/2 signaling
pathways. Notably, the present study demonstrated that treatment
with resveratrol decreased cerebral infarct volume and CWC. It also
improved cognitive competence and BBB disruption in the rat model
of cerebral hemorrhage. However, the current study did not assess
the effects of an ERK inhibitor on resveratrol-treated brain
injury. Further study is therefore required to analyze the role of
an ERK inhibitor on the resveratrol-induced decrease of hippocampal
cell apoptosis in cerebral hemorrhage.
In conclusion, the current study assessed the
therapeutic effects of resveratrol in hippocampal cells of rats
with cerebral hemorrhage. The results revealed that treatment with
resveratrol inhibited neuronal cell apoptosis by downregulating the
TGF-β-mediated ERK signaling pathway in rats with cerebral
hemorrhage. These results indicate that resveratrol may be a
potential drug for the treatment of cerebral hemorrhage induced by
ischemic reperfusion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or generated during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
RZ performed the experiments. KZ, HS and PZ analyzed
all of the data generated in this study. NZ designed the study and
prepared this manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Baodi Hospital (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Al-Mufti F, Amuluru K, Changa A, Lander M,
Patel N, Wajswol E, Al-Marsoummi S, Alzubaidi B, Singh IP, Nuoman R
and Gandhi C: Traumatic brain injury and intracranial
hemorrhage-induced cerebral vasospasm: A systematic review.
Neurosurgical focus. 43:E142017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shoamanesh A, Kwok CS, Lim PA and
Benavente OR: Postthrombolysis intracranial hemorrhage risk of
cerebral microbleeds in acute stroke patients: A systematic review
and meta-analysis. Int J Stroke. 8:348–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bugeme M and Mukuku O: Neuropsychiatric
manifestations revealing cerebral subarachnoid hemorrhage caused by
electrification accident about a case and review of literature. Pan
AfrMed J. 18:2012014.(In French).
|
|
4
|
Wang X, Gao XD, Gao B, Jia LL, Yang MF,
Zhang YB and Sun BL: Cerebral lymphatic blockage aggravates
apoptosis of cortical neurons after subarachnoid hemorrhage in rats
in vivo. Sheng Li Xue Bao. 62:109–114. 2010.(In Chinese).
PubMed/NCBI
|
|
5
|
Jeong SI, Shin JA, Cho S, Kim HW, Lee JY,
Kang JL and Park EM: Resveratrol attenuates peripheral and brain
inflammation and reduces ischemic brain injury in aged female mice.
Neurobiol Aging. 44:74–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Yan Z, Zhu J, Yang J and He J:
Neuroprotective effects of resveratrol on ischemic injury mediated
by improving brain energy metabolism and alleviating oxidative
stress in rats. Neuropharmacology. 60:252–258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zou P, Liu X, Li G and Wang Y: Resveratrol
pretreatment attenuates traumatic brain injury in rats by
suppressing NLRP3 inflammasome activation via SIRT1. Mol Med Rep.
17:3212–3217. 2018.PubMed/NCBI
|
|
8
|
Karalis F, Soubasi V, Georgiou T, Nakas
CT, Simeonidou C, Guiba-Tziampiri O and Spandou E: Resveratrol
ameliorates hypoxia/ischemia-induced behavioral deficits and brain
injury in the neonatal rat brain. Brain Res. 1425:98–110. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Logan TT, Villapol S and Symes AJ: TGF-β
superfamily gene expression and induction of the Runx1
transcription factor in adult neurogenic regions after brain
injury. PLoS One. 8:e592502013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sugawara T, Jadhav V, Ayer R and Zhang J:
Simvastatin attenuates cerebral vasospasm and improves outcomes by
upregulation of PI3K/Akt pathway in a rat model of subarachnoid
hemorrhage. Acta Neurochir Suppl. 102:391–394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aguilar-Alonso P, Vera-Lopez O,
Brambila-Colombres E, Segura-Badilla O, Avalos-López R,
Lazcano-Hernández M and Navarro-Cruz AR: Evaluation of oxidative
stress in cardiomyocytes during the aging process in rats treated
with resveratrol. Oxid Med Cell Longev. 2018:13904832018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gamberini MT, Rodrigues DS, Rodrigues D
and Pontes VB: Effects of the aqueous extract of Pimpinella anisum
L. seeds on exploratory activity and emotional behavior in rats
using the open field and elevated plus maze tests. J
Ethnopharmacol. 168:45–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vatter H, Weidauer S, Konczalla J,
Dettmann E, Zimmermann M, Raabe A, Preibisch C, Zanella FE and
Seifert V: Time course in the development of cerebral vasospasm
after experimental subarachnoid hemorrhage: Clinical and
neuroradiological assessment of the rat double hemorrhage model.
Neurosurgery. 58:1190–1197; discussion 1190–1197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hijioka M, Matsushita H, Hisatsune A,
Isohama Y and Katsuki H: Therapeutic effect of nicotine in a mouse
model of intracerebral hemorrhage. J Pharmacol Exp Ther.
338:741–749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong H, Kim CJ, Kim JD and Seo JH:
β-glucan reduces exercise-induced stress through downregulation of
c-Fos and c-Jun expression in the brains of exhausted rats. Mol Med
Rep. 9:1660–1666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen H, Guan B, Chen X, Chen X, Li C, Qiu
J, Yang D, Liu KJ, Qi S and Shen J: Baicalin attenuates blood-brain
barrier disruption and hemorrhagic transformation and improves
neurological outcome in ischemic stroke rats with delayed t-PA
treatment: Involvement of ONOO(−)-MMP-9 pathway. Transl Stroke Res.
9:515–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li R, Wu Y, Zou S, Wang X, Li Y, Xu K,
Gong F, Liu Y, Wang J, Liao Y, et al: NGF attenuates high
glucose-induced ER stress, preventing schwann cell apoptosis by
activating the PI3K/Akt/GSK3β and ERK1/2 pathways. Neurochem Res.
42:3005–3018. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hausmann R, Biermann T, Wiest I, Tubel J
and Betz P: Neuronal apoptosis following human brain injury. Int J
Legal Med. 118:32–36. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou XM, Zhou ML, Zhang XS, Zhuang Z, Li
T, Shi JX and Zhang X: Resveratrol prevents neuronal apoptosis in
an early brain injury model. J Surg Res. 189:159–165. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song J, Cho KJ, Cheon SY, Kim SH, Park KA,
Lee WT and Lee JE: Apoptosis signal-regulating kinase 1 (ASK1) is
linked to neural stem cell differentiation after ischemic brain
injury. Exp Mol Med. 45:e692013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ai Z, Li C, Li L and He G: Resveratrol
inhibits beta-amyloid-induced neuronal apoptosis via regulation of
p53 acetylation in PC12 cells. Mol Med Rep. 11:2429–2434. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Liu J, Feng WK, Wu X and Chen SY:
MiR-125b protects against ethanol-induced apoptosis in neural crest
cells and mouse embryos by targeting Bak 1 and PUMA. Exp Neurol.
271:104–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taylor RA, Chang CF, Goods BA, Hammond MD,
Mac Grory B, Ai Y, Steinschneider AF, Renfroe SC, Askenase MH,
McCullough LD, et al: TGF-β1 modulates microglial phenotype and
promotes recovery after intracerebral hemorrhage. J Clin Invest.
127:280–292. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anderson CN and Tolkovsky AM: A role for
MAPK/ERK in sympathetic neuron survival: Protection against a
p53-dependent, JNK-independent induction of apoptosis by cytosine
arabinoside. J Neurosci. 19:664–673. 1999. View Article : Google Scholar : PubMed/NCBI
|