Introduction
Psoriasis represents a chronic immune-mediated
systemic disease with multifactorial etiopathogenesis. It has a
great impact on the patient's quality of life, affecting the skin
and joints and being usually diagnosed according to clinical
appearance (1). In ambiguous cases,
a biopsy for histopathological examination is required. Taking into
account that psoriasis is a chronic disease, more effective in
vivo techniques are needed, in order to monitor plaque
psoriasis progression. Currently, it can be monitored following
topical or systemic treatment, using imaging techniques such as
dermoscopy, videodermoscopy, conventional ultrasound and high
frequency ultrasonography (HFUS), videocapillaroscopy (VC),
reflectance confocal microscopy (RCM), laser Doppler imaging (LDI),
optical coherence tomography (OCT), optical microangiography (OMAG)
or multiphoton tomography (MPT). The aim was to collect and analyze
data concerning types, indications, advantages and disadvantages of
modern imaging techniques for in vivo psoriasis assessment.
This review focuses on two main methods, videodermoscopy and HFUS,
which can be included in daily dermatologists' practice and which
may assist in establishing diagnoses, as well as in monitoring
response to topical and/or systemic therapy of psoriasis.
As reported, classical clinical evaluation should be
accompanied by imaging evaluation; videodermoscopy provides
important data and magnifies the vascular pattern, which may
persist in spite of clinical resolution. HFUS allows measurements
of skin plaque, thickness being the first parameter to decrease in
response to therapy (2).
Dermoscopy and videodermoscopy as
non-invasive techniques in the diagnosis of psoriasis vulgaris
Dermoscopy offers a horizontal view/section and
visualized structures have superficial vascular pattern. Psoriatic
lesions assessed by dermoscopy technique offer details on typical
feature of vessels, which are uniformly distributed as ‘dotted’,
‘pinpoint’ capillaries and coiled (or glomerular) vessels on a
light red background accompanied by white diffuse scales. The same
dermoscopic feature can be described using red dots (with diameters
up to 0.1 mm) or ‘red globules’ terms (3,4). The
vessels are correspondent to capillaries from elongated dermal
papillae (5). The identification of
other vessel patterns should give clues for other diagnoses apart
from plaques psoriasis (6). In other
inflammatory diseases, such as lichen planus, porokeratosis,
pityriasis rubra pilaris, this capillary pattern can be
encountered, although not evenly distributed.
Analyzed skin lesions of vulgar psoriasis called
‘target lesions’ can be recorded and stored in a computer archive
under standardized conditions using videodermoscopy. Psoriasis
plaque analysis can be performed with or without immersion fluid
(oil or ultrasound gel). The use of videodermoscopy with 15- to
120-fold magnification enables a detailed evaluation of the size
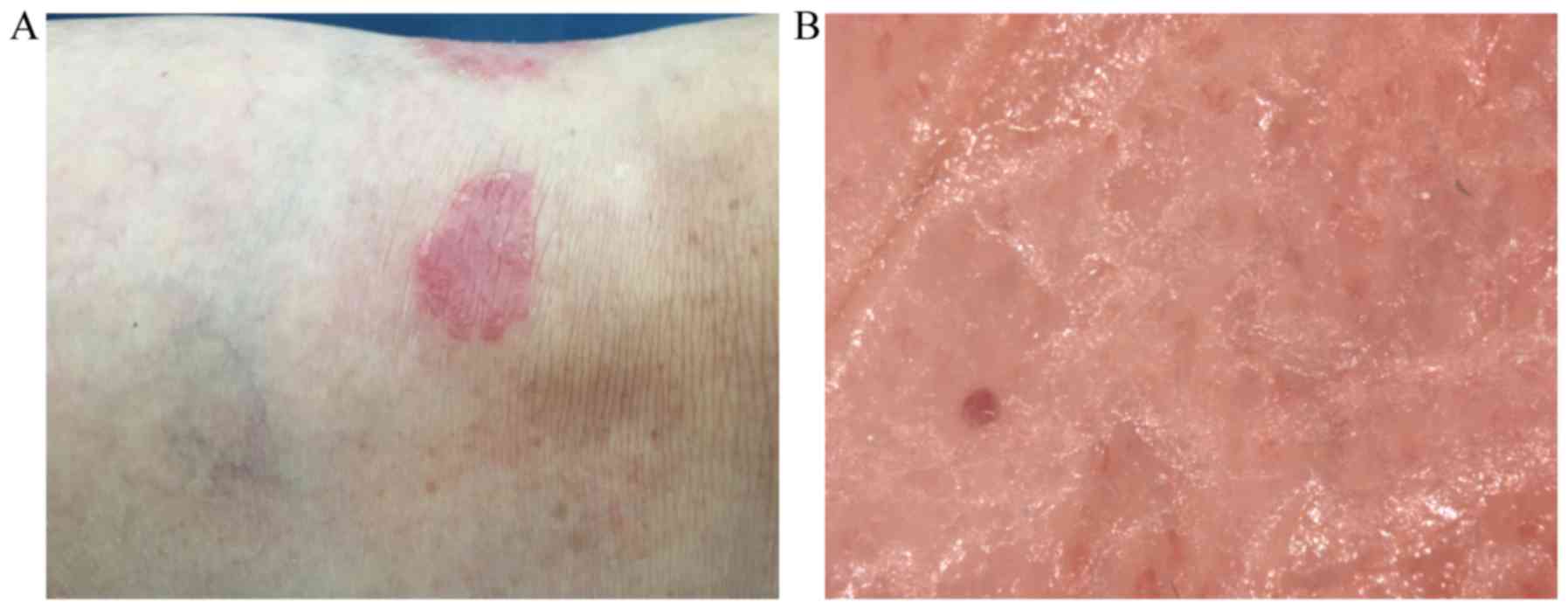
and the structure of vessels (Fig.
1). Red dots/globules visualized by over 50-fold magnification
are called ‘bushy capillaries’, ‘capillary bushes’, ‘convoluted
basket-like capillaries’ or ‘basket-weave capillaries’. Other
vascular patterns such as hairpin vessels, radial capillaries,
globular rings, red lines, comma and lacunar vessels may also be
observed. In palmoplantar psoriasis, pinpoint-like capillaries are
linearly arranged along the furrows at a 50-fold magnification.
Using high magnifications (×100-x400), psoriatic vessels have
elongated, dilated and convoluted capillaries pattern (7). Scale removal in hyperkeratotic plaques
may reveal typical capillaries pattern and may similarly identify
small red blood drops associated with Auspitz's sign (3).
Other two dermoscopic plaque psoriasis criteria are
represented by light red background and white superficial scales
(3). Scale color is valuable for
differentiating plaque psoriasis from other dermatoses with
erythema and scales. Yellow scales are suggesting dermatitis as
diagnosis (8). Dermoscopic pattern
in specific localizations such as scalp, palmoplantar or inverse
psoriasis is similar to plaque psoriasis with variations of
scaling. Thick hyperkeratotic lesions on palmoplantar areas need
scale removal, while in lesions lacking scaling, such as genital or
flexural areas, red dots are regularly identified (3).
Dermoscopy may show evolution of psoriatic lesions
during therapy and, similarly, it may identify disease recurrence
early or side effects of topical steroids such as linear vessels in
skin atrophy before they are clinically visualized (9,10).
Micali et al (2) evaluated the therapeutical response in a
study conducted on 42 patients with moderate to severe plaque
psoriasis treated with biological agents including adalimumab,
etanercept and ustekinumab. ‘Target’ psoriasis plaques at baseline
and after 15, 30 and 60 days were monitored by clinical
observation, videodermoscopy and HFUS. Specific target lesion
ranged from 0 to 8 and included a 4-point scale for quantification
of erythema, scaling and infiltration. Results showed that skin
thickness improved first, followed by clinical and respectively
vascular response. Clinical response, vascular pattern and skin
thickness of ‘target’ psoriasis plaques were reduced by 83.9, 73.5
and 90% in adalimumab-treated patients, by 67.9, 49.7 and 79.3% in
etanercept-treated patients; and reduced by 80.9, 66.4 and 80.1% in
ustekinumab-treated patients. After 60 days of treatment, skin
thickness was the first parameter that improved. Vascular
improvement was slower than clinical and ultrasound response. A
complete normalization of vascularization (capillaries with
diameters ≤25 µm) was absent in all patients despite their complete
clinical response and the fact that clinical regression did not
correlate with vascular changes. Further investigations are needed
to evaluate if persistent ‘bushy’ capillaries, despite
ultrasonography and clinical remission, represent a criterion for
disease progression or may be correlated with recurrences rates
(2).
In the study by Lacarrubba et al (11), it was noted that 87% of the examined
patients had scalp psoriasis with typical vascular pattern on
erythematous background accompanied by scales; this allowed a
correct diagnosis of psoriasis. The registered specificity and
sensitivity in this study was 88 and 84.9%, respectively, whereas
seborrheic dermatitis was excluded. The most common terminology of
vessel pattern distribution observed in psoriatic plaques in 77.4%
cases (388 out of 501 patients with psoriasis vulgaris) were
‘regular’, with ‘diffuse’ and ‘homogeneous’ erythema. In addition,
regularly distributed red dots and ‘bushy’ glomerular vessels on a
reddish background with white scales were described (6,12).
Likewise, the color of the scale was evaluated in a
study (10) with a total of 228
psoriatic plaques. Most frequently, the scale was described as
‘white’, ‘whitish’ or ‘silvery white’ and it was observed in a
total of 214/228 (94%) psoriatic plaques at specific body sites
(scalp, genitalia,-folds, palmo-plantar area).
High frequency ultrasonography in monitoring
therapeutic response in plaques psoriasis
HFUS is a non-invasive method of morpho-functional
evaluation for the epidermal and dermal structures, subcutaneous
fat and skin appendages. It constitutes a useful imaging method in
in vivo studies of psoriatic lesions; the indications of
this imaging technique are multiple, such as the evaluation of
inflammatory diseases including psoriasis, scleroderma or
pathologies such as contact dermatitis or acne. It allows
high-resolution imaging and direct measurements regarding the
thickness and acoustic density of the epidermis, dermis and
subcutaneous fat. It generally uses variable frequency transducers
(5–20 MHz) that are able to focus on different tissue layers. The
higher the resolution is, the better the details of the epidermis,
dermis, subcutaneous fat and fascia. HFUS offers the classical
two-dimensional, B-mode, vertical view/section, which allows in
vivo cross-sectional images. The lateral resolution is 120 µm
with a 22-MHz system and 32 µm with a 100-MHz probe. The
penetration depth is 1–12 mm and both the epidermis and the dermis
are visualized structures (7). HFUS
has several advantages. It represents a real time and non-invasive
imaging technique, which is well tolerated by patients. It has no
exposure to X-rays and similarly, it has no special restrictions.
They are portable and miniaturized medical devices largely
accessible for doctors. Ultrasound may provide parameters such as
depth of inflammatory lesions, which cannot be physically
appreciated. The relevance of assessing the inflammation depth
(superficial, intermediate or deep) is important in therapy
management, including topical treatment, systemic/topical and
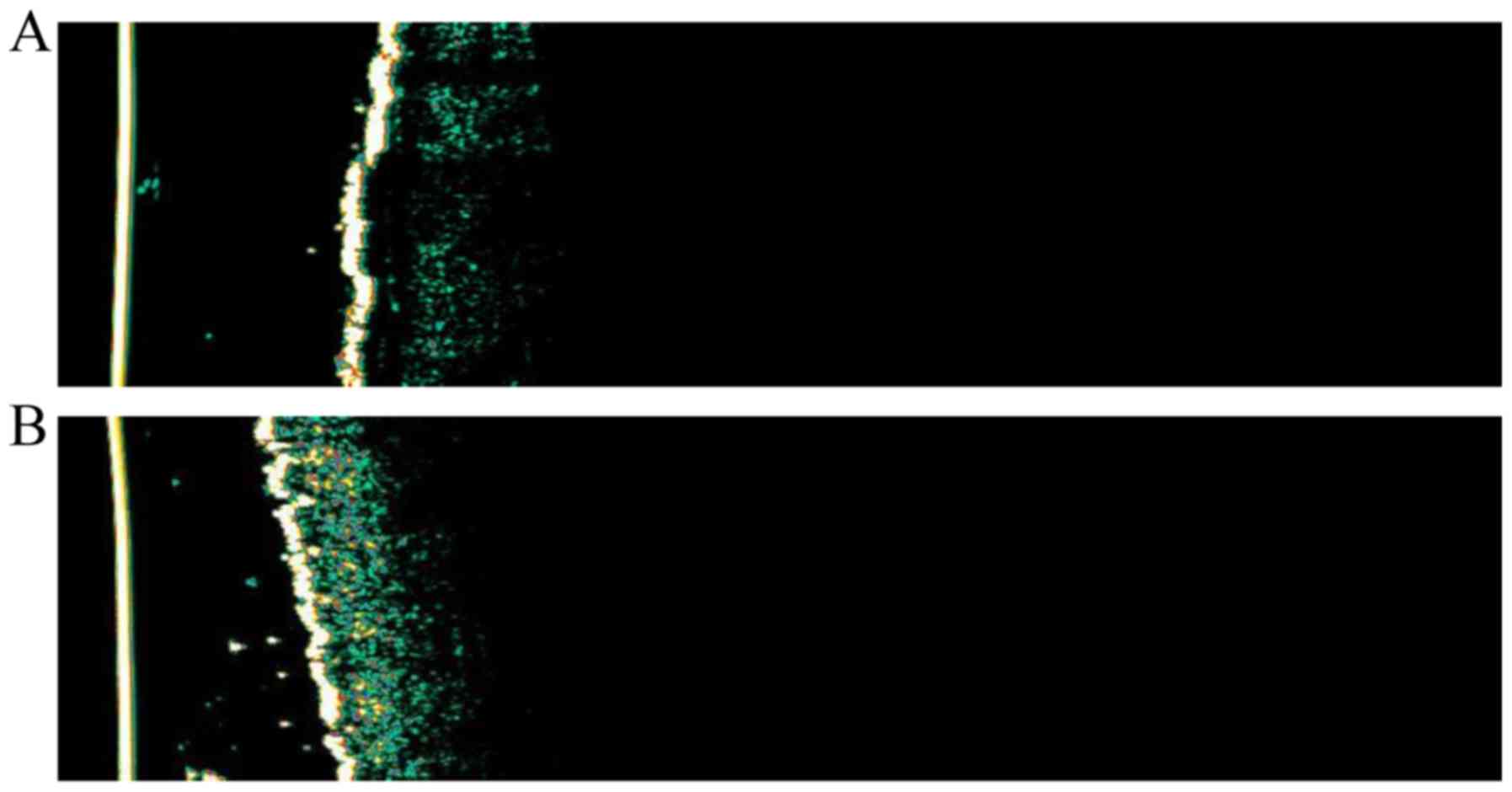
systemic treatment, respectively (Fig.
2).
The main utility of HFUS in plaque psoriasis
consists of the evaluation of the diseases under treatment. Plaques
psoriasis examination using ultrasound shows intermediate
inflammation; the main sonographic features are epidermal and
dermal thickening and variable vascularization. The examination of
psoriasis plaque lesions with HFUS indicates the following:
hyperechogenic band representing the epidermis with hyperkeratosis
and parakeratosis; echogenic or hypoechogenic band corresponding to
the elongation of the dermal papillae as one of the most common
features. This hypoechoic band in the upper dermis may be
particularly detectable in the most active stages of active
disease; hyperechogenic band given by the reticular dermis;
subcutaneous hypoechogenic layer; increased dermal blood flow
(vasodilatation) within the lesion, due to the local inflammatory
process in Doppler examination (13).
Elastography is an ultrasound-based technique used
in measuring tissue hardness with two modalities: strain
(qualitative) and shear wave (quantitative). Using elastography,
increased tissue stiffness induced by the inflammatory reaction can
be compared to the surrounding healthy skin. The response of
stiffness, which decreases after topical or oral therapies, can be
of great value for the assessment of plaque progression in
psoriasis (13).
Indicators of effective therapy are represented by
the reduction of epidermal and dermal thickness and particularly
the disappearance of the hypoechogenic band from the superficial
dermis (13–15). Nail assessment under therapy shows
thickness variation of the nail plate and nail bed with objective
reduced thickness. In a study with 19 psoriasis patients assessed
using HFUS, the average increase in skin thickness in 31 plaques
compared with apparently normal skin was an estimated 67% in the
whole skin and 200% in the epidermis (14). In a study of 30 patients using 0.05%
clobetasol propionate foam 20-MHz HFUS indicated a reduction in
lesion thickness with values equal to those of unaffected skin
(16). Musumeci et al
(17) also reported that skin
thickness was the first improved parameter during therapy in 20
psoriasis patients treated with cyclosporine prior to clinical
improvement.
In nail psoriasis, the involvement may appear
without cutaneous plaques. Psoriasis onychopathy can be detected
early by loss of bimorph aspect, presence of pitting and
irregularities of the nail plate surface. Ultrasonographic
morphologic changes include thickening of the nail bed, focal
hyperechoic areas and thickening (significant values >2 mm) of
both dorsal and ventral plates with a convex nail appearance
(13,18).
Power Doppler sonography represents a promising tool
in the detection and semi-quantitative evaluation of superficial
soft tissue perfusion. In the subclinical forms of the disease, the
active status may be detected by Doppler examination identifying
the pattern of microcirculation. The therapeutic response can be
appreciated by the lesions vascular changes. Early psoriatic
arthritis can be distinguished from seronegative rheumatoid
arthritis by identifying dotted vessels in periungual tissue at
ultrasonographic examination, increasing diagnostic specificity. In
a study conducted on 30 patients with psoriasis and 15 healthy
participants, 7–14 MHz power Doppler sonography permitted the
detection of an increased blood flow signal in active psoriatic
plaques (18). Another study showed
that 12 patients with plaque psoriasis were evaluated using power
Doppler sonography in comparison with clinical appearance,
Psoriasis Area and Severity Index (PASI) scores and histologic
findings before and after treatment with biologicals such as
etanercept and data provided a significant correlation between
these examinations. The results showed a significant positive
correlation between power Doppler sonography examination, PASI
scores and histologic degree of vascularization (19). Psoriasis onychopathy shows
hypervascularisation in proximal and distal parts of the nail bed
in the active phase of the disease, whereas in chronic phases
hypovascularisation is remarked in distal parts. Other parameters
of interest are the index of pulsatility, the index of resistivity,
the speed and thickness of the nail bed and nail plate (13). The index of pulsatility is the first
parameter changing in case of local inflammation and values >1
indicate a subclinical inflammatory process, while values >2 are
suggestive of interphalangeal articular changes. An increased index
of resistance towards 1, is present in psoriasis onychopathy
(13).
The limitations and artifacts of HFUS include the
presence of air or irregular surfaces of the skin. In addition, it
is a time-consuming procedure and it depends on the operator's
experience.
Other imaging techniques
RCM is a non-invasive imaging technique that allows
in vivo imaging of dermatologic conditions, such as
pigmented skin lesions, non-melanoma skin cancer (20,21) and
several inflammatory diseases, including acute contact dermatitis,
discoid lupus erythematous (22),
lichen planus (23) or plaque
psoriasis (24–27). It helps to obtain a microscopic
morphometric evaluation of plaque psoriasis with a resolution close
to conventional histopathology (28,29).
Real skin view/section is horizontal and the depth of penetration
is 200–300 µm. Visualized structures are represented by epidermis
and superficial dermis with a 0.5–1 µm lateral resolution and a 3–5
µm axial resolution (11). RCM
identifies hyperkeratosis, parakeratosis, reduced or absent
granular layer and papillomatosis. Stratum corneum shows refractile
nucleated structures corresponding to parakeratotic keratinocytes
(22). Stratum spinosum shows
honeycomb pattern of the epithelium, whereas stratum granulosum
shows granular layer reduction with presence of cells with bright
granules in the center of the nucleus. Dermal papillae correspond
to dark holes with small bright dots according to inflammatory
infiltrates and papillomatosis (22).
Ardigo et al (22) obtained in more than 90% of 36
psoriasis vulgaris cases features of psoriasis vulgaris that were
correlated with histopathological examination. Similarly, normal
skin from 12 healthy control patients was compared with
histopathological evaluation of psoriasis skin samples. In
psoriasis cases, acanthosis with 75 to 300 µm thickness compared to
normal skin (60–90 µm) was observed. Dermal papillae diameter was
more than 100 µm and it was compared (bigger than 80 µm) with those
from similar topographic areas of normal skin in the control group
(22).
OMAG is an imaging technique that reproduces dynamic
tissue bed microcirculation and blood perfusion using 2 mm imaging
depth (29). 3D microcirculation
assessing may be used in the diagnosis, treatment and management of
human skin diseases, such as psoriasis vulgaris, skin cancer or
hemangiomas. Ultra-high sensitive OMAG may reveal dense network of
microvessels in psoriatic lesions and blood vessel elongation in
comparison with normal skin (29).
Using fast scan B with a 1310 nm system, the
capillary flow may be achieved with a imaging rate of 300
frames/sec and 3D imaging needs 5 sec to complete. Very slow blood
flows at ~4 µm/sec may be also revealed using this sensitive
technique (30).
Laser Doppler perfusion imaging system is intended
for visualization of skin blood perfusion and captures images based
on Doppler effects, obtaining 2D cutaneous blood flow maps
(31,32). Studies in psoriatic skin show that,
in cutaneous psoriatic lesions, the blood flow is 9–13 times
greater than the normal skin blood flow. Similarly, perilesional
skin has a greater blood flow between 2.5 and 4.5 times in
comparison with healthy skin (33).
Murray et al (34) studied the cutaneous blood flow in 23
plaques on the forearms of 20 patients with chronic plaque
psoriasis using dual wavelength laser Doppler perfusion. Perfusion
was determined within the plaque, in uninvolved skin adjacent to
the plaque and in nonadjacent skin. Results showed that perfusion
in psoriatic plaques was increased as imaged by 633 nm (red
wavelength) or 532 nm (green wavelength) in comparison with
adjacent and nonadjacent uninvolved skin for both deeper (large)
and superficial (small) vessels (34).
The effect of calcipotriol in 13 patients with
plaque-type psoriasis who started twice a week PUVA was studied
using LDI (35). In each patient, 2
plaques on symmetrical body sites were assessed. Calcipotriol
therapy was used twice daily for one site, and placebo to the
other; response was evaluated weekly for 6 weeks. Blood flux was
measured using a scanning laser-Doppler velocimeter. In nine
calcipotriol-treated plaques of 11 patients who completed the study
it either cleared before the placebo-treated plaque (n=7), or was
consistently judged to be better (n=2). Mean blood flux was
identified significantly lower in the calcipotriol-treated plaques
than in those treated with placebo from the third week of study.
There was a median reduction in the UVA dose of 26.5% for
calcipotriol compared with placebo in the 7 patients whose
psoriatic lesions were cleared at the end of the study (35). Therefore, this technique may be used
in assessing pathophysiology and treatment response in psoriasis
(34,35).
MPT provides in vivo non-invasive virtual
skin histologic examination with ultra-high subcellular resolution.
Horizontal and vertical optical 3D images (0.36×0.36×0.001
mm3) of the region of interest with resolution down to
200 µm tissue depth are obtained using this imaging technique. It
is based on fluorescent emission of excited molecules of endogenous
fluorophores such as melanin, elastin, collagen, keratin and
flavoprotein, which are detected with photomultipliers. This
technique indicated pigmented lesions, psoriasis and skin aging in
the evaluation (36).
Koehler et al (37) evaluated comparatively MPT and
confocal laser scanning microscopy (CLSM) in different
dermatological entities. Both methods were used for 47 patients (31
male, 16 female, aged between 24 and 88 years) with dermatological
disorders including psoriasis, actinic keratosis, seborrheic
dermatitis, angiomas, melanocytic nevi, malignant melanoma,
pemphigus vulgaris and scarring. The results revealed that both
methods are suitable for in vivo imaging of superficial skin
layers and may therefore be useful in dermatological practice for
the diagnosis of skin diseases. Synergies of the combination of MPT
and CLSM may be obtained in order to benefit from the fast overview
given by CLSM and the detailed imaging of skin structures provided
by MPT.
OCT represents a non-invasive technique for
morphological investigation of tissue based on the principle of
Michelson interferometry. The light sources used are low coherent
superluminescent diodes operating at a wavelength of ~1300 nm and
OCT offers two-dimensional images with a scan length of a few
millimeters, a resolution of ~15 microns and a maximum detection
depth of 1.5 mm in near real-time (32). High-definition OCT offers high
lateral resolution of 1–3 µm (38–40). The
measurement is non-invasive and has no side effects (36). Inflammatory skin diseases show
thickening of the epidermis and reduction of the light attenuation
in the dermis. The evaluation of therapy, such as swelling of the
horny layer due to application of a moisturizer and the monitoring
of changes over time are possible. Due to its high resolution, this
technique is an interesting addition to other morphological
techniques (36,41). It may offer visualization of stratum
corneum, papillary dermis and appendages in various dermatologic
conditions. In psoriasis, it may reveal imaging of hyperkeratosis,
acanthosis and the entrance signal is higher than in normal skin;
likewise, it may evaluate the thickness of the epidermis (39). Dilated signal free cavities that
correspond to dilated blood vessels of elongated dermal papillae
may be identified (40).
In a study of 23 patients with psoriasis vulgaris
treated with topical corticosteroids, cyclosporine and
phototherapy, the mean epidermal thickness of psoriatic lesions was
evaluated (41). The authors
reported that epidermal thickness was 30–40 µm greater than that of
normal skin, and these values decreased from initial registered
values of 157.1 µm to a mean value of 128.84 µm after therapy.
These measurements correlated with other parameters of disease,
such as psoriasis area and severity index (PASI) (42).
Conclusions
The need for more reliable non-invasive assessment
techniques in dermatology is increasing. Available imaging
techniques vary in their sections, depth of penetration, lateral
and axial resolution and in visualized structures. Besides clinical
evaluation, high definition imaging techniques, such as dermoscopy
and HFUS, may help in better monitoring psoriasis vulgaris.
Dermoscopy is useful in diagnosing and assessing
psoriatic lesions in special locations, such as the scalp, nails,
palms, soles and genital regions. The most common vascular feature
of skin psoriatic lesions identified with dermoscopy using a
10-fold magnification is the presence of red dots/globules in a
regular distribution. A detailed visualization of the cutaneous
vessels is facilitated by videodermoscopy with a >50-fold
magnification and, most commonly, it reveals the presence of bushy
(glomerular) vessels. The vascular changes are accompanied by a
light red or pink background.
HFUS is an effective method that can be utilized
alongside the physical examination for the diagnosis of
inflammatory diseases, such as psoriasis vulgaris, providing
accurate information on skin and nail anatomy; it is especially
indicated for the assessment of therapeutic response in long-term
follow-up (12,42,43).
RCM and OCT may compare to virtual histopathology
and may provide high resolution microscopic imaging of cutaneous
psoriatic lesions. Other imaging techniques including LDI, OMAG or
MPT represent advanced high-definition promising imaging techniques
and require further study.
Acknowledgements
Professional editing, linguistic and technical
assistance performed by Individual Service Provider Irina Radu,
certified translator in Medicine and Pharmacy.
Funding
No funding was received.
Availability of data and materials
Imaging data were provided using
MicroDermVisiomed® system analysis and Dermascan C USB
® (20 MHz B-mode) equipments. The datasets used and/or
analyzed during the current study are available from the
corresponding author on reasonable request.
Authors' contributions
All authors contributed to the acquisition of the
data and critical revision of manuscript for important intellectual
content. IAG and LGS performed videodermoscopy and HFUS techniques
and wrote the manuscript. LS, IAP, DV and MC wrote review sections
about dermoscopy and ultrasound. EAP, AIP and TT searched data
about the other imagistic techniques. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Clinical Research
Ethics Committee of The Clinical Emergency County Hospital ‘Sf.
Spiridon’ (Iasi, Romania) and by the Research Ethics Committee of
The ‘Grigore T. Popa’ University of Medicine and Pharmacy (Iasi,
Romania). Written informed consent was obtained from all the
patients prior to publication.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Olteanu R, Constantin M-M, Zota A,
Dorobanțu DM, Constantin T, Șerban E-D, Bălănescu P, Mihele D and
Gheucă Solovăstru L: Original clinical experience and approach to
treatment study with interleukine 12/23 inhibitor in
moderate-to-severe psoriasis patients. Farmacia. 64:918–921.
2016.
|
|
2
|
Micali G, Lacarrubba F, Santagati C, Egan
CG, Nasca MR and Musumeci ML: Clinical, ultrasound, and
videodermatoscopy monitoring of psoriatic patients following
biological treatment. Skin Res Technol. 22:341–348. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lallas A, Zalaudek I, Argenziano G, Longo
C, Moscarella E, Di Lernia V, Al Jalbout S and Apalla Z: Dermoscopy
in general dermatology. Dermatol Clin. 31:679–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vázquez-López F, Manjón-Haces JA,
Maldonado-Seral C, Raya-Aguado C, Pérez-Oliva N and Marghoob AA:
Dermoscopic features of plaque psoriasis and lichen planus: New
observations. Dermatology. 207:151–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vázquez López F, González-Lara L, Martin
JS and Argenziano G: Dr K. Holubar (1936–2013). Teaching with
dermoscopy: Revealing the subsurface morphology of Auspitz's sign
and psoriasis. Int J Dermatol. 53:e322–e324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stinco G, Buligan C, Maione V, Valent F
and Patrone P: Videocapillaroscopic findings in the
microcirculation of the psoriatic plaque during etanercept therapy.
Clin Exp Dermatol. 38:633–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Angelis R, Bugatti L, Del Medico P,
Nicolini M and Filosa G: Videocapillaroscopic findings in the
microcirculation of the psoriatic plaque. Dermatology. 204:236–239.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lallas A, Kyrgidis A, Tzellos TG, Apalla
Z, Karakyriou E, Karatolias A, Lefaki I, Sotiriou E, Ioannides D,
Argenziano G, et al: Accuracy of dermoscopic criteria for the
diagnosis of psoriasis, dermatitis, lichen planus and pityriasis
rosea. Br J Dermatol. 166:1198–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gniadecki R, Kragballe K, Dam TN and Skov
L: Comparison of drug survival rates for adalimumab, etanercept and
infliximab in patients with psoriasis vulgaris. Br J Dermatol.
164:1091–1096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vázquez-López F and Marghoob AA:
Dermoscopic assessment of long-term topical therapies with potent
steroids in chronic psoriasis. J Am Acad Dermatol. 51:811–813.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lacarrubba F, Pellacani G, Gurgone S,
Verzì AE and Micali G: Advances in non-invasive techniques as aids
to the diagnosis and monitoring of therapeutic response in plaque
psoriasis: A review. Int J Dermatol. 54:626–634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Penmetcha Lakshmi C, Praneet A and Madhavi
K: A cross-sectional analysis of dermoscopic patterns
distinguishing between psoriasis and lichen planus: A study of 80
patients. J Evol Med Dent Sci. 4:17017–17022. 2015. View Article : Google Scholar
|
|
13
|
Cucoş M, Crişan M, Lenghel M, Dudea M,
Croitoru R and Dudea SM: Conventional ultrasonography and
sonoelastography in the assessment of plaque psoriasis under
topical corticosteroid treatment - work in progress. Med Ultrason.
16:107–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vaillant L, Berson M, Machet L, Callens A,
Pourcelot L and Lorette G: Ultrasound imaging of psoriatic skin: A
noninvasive technique to evaluate treatment of psoriasis. Int J
Dermatol. 33:786–790. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Serup J: Non-invasive quantification of
psoriasis plaques - measurement of skin thickness with 15 mHz
pulsed ultrasound. Clin Exp Dermatol. 9:502–508. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lacarrubba F, Nardone B, Musumeci ML and
Micali G: Ultrasound evaluation of clobetasol propionate 0.05% foam
application in psoriatic and healthy skin: A pilot study. Dermatol
Ther. 22 (Suppl 1):S19–S21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Musumeci ML, Lacarrubba F, Verzì AE and
Micali G: Evaluation of the vascular pattern in psoriatic plaques
in children using videodermatoscopy: An open comparative study.
Pediatr Dermatol. 31:570–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gutierrez M, Wortsman X, Filippucci E, De
Angelis R, Filosa G and Grassi W: High-frequency sonography in the
evaluation of psoriasis: Nail and skin involvement. J Ultrasound
Med. 28:1569–1574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gutierrez M, De Angelis R, Bernardini ML,
Filippucci E, Goteri G, Brandozzi G, Lemme G, Campanati A, Grassi W
and Offidani A: Clinical, power Doppler sonography and histological
assessment of the psoriatic plaque: Short-term monitoring in
patients treated with etanercept. Br J Dermatol. 164:33–37. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
González S and Gilaberte-Calzada Y: In
vivo reflectance-mode confocal microscopy in clinical dermatology
and cosmetology. Int J Cosmet Sci. 30:1–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calzavara-Pinton P, Longo C, Venturini M,
Sala R and Pellacani G: Reflectance confocal microscopy for in vivo
skin imaging. Photochem Photobiol. 84:1421–1430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ardigo M, Cota C, Berardesca E and
González S: Concordance between in vivo reflectance confocal
microscopy and histology in the evaluation of plaque psoriasis. J
Eur Acad Dermatol Venereol. 23:660–667. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ianoși SL, Forsea AM, Lupu M, Ilie MA,
Zurac S, Boda D, Ianosi G, Neagoe D, Tutunaru C, Popa CM, et al:
Role of modern imaging techniques for the in vivo diagnosis
of lichen planus. Exp Ther Med. 17:1052–1060. 2019.PubMed/NCBI
|
|
24
|
Başaran YK, Gürel MS, Erdemir AT, Turan E,
Yurt N and Bağci IS: Evaluation of the response to treatment of
psoriasis vulgaris with reflectance confocal microscopy. Skin Res
Technol. 21:18–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ilie MA, Caruntu C, Lupu M, Lixandru D,
Tampa M, Georgescu SR, Bastian A, Constantin C, Neagu M, Zurac SA,
et al: Current and future applications of confocal laser scanning
microscopy imaging in skin oncology. Oncol Lett. 17:4102–4111.
2019.PubMed/NCBI
|
|
26
|
Caruntu C, Boda D, Caruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morpholembryo. 55:1191–1196. 2014.
|
|
27
|
Ilie MA, Caruntu C, Lixandru D, Tampa M,
Georgescu SR, Constantin MM, Constantin C, Neagu M, Zurac SA and
Boda D: In vivo confocal laser scanning microscopy imaging
of skin inflammation: Clinical applications and research
directions. Exp Ther Med. 17:1004–1011. 2019.PubMed/NCBI
|
|
28
|
Longo C, Zalaudek I, Argenziano G and
Pellacani G: New directions in dermatopathology: In vivo confocal
microscopy in clinical practice. Dermatol Clin. 30799–814.
(viii)2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin J, Jiang J, An L, Gareau D and Wang
RK: In vivo volumetric imaging of microcirculation within human
skin under psoriatic conditions using optical microangiography.
Lasers Surg Med. 43:122–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
An L, Qin J and Wang RK: Ultrahigh
sensitive optical microangiography for in vivo imaging of
microcirculations within human skin tissue beds. Opt Express.
18:8220–8228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fullerton A, Stücker M, Wilhelm KP,
Wårdell K, Anderson C, Fischer T, Nilsson GE and Serup J; European
Society of Contact Dermatitis Standardization Group, : Guidelines
for visualization of cutaneous blood flow by laser Doppler
perfusion imaging. A report from the Standardization Group of the
European Society of Contact Dermatitis based upon the HIRELADO
European community project. Contact Dermat. 46:129–140. 2002.
View Article : Google Scholar
|
|
32
|
Choi CM and Bennett RG: Laser Dopplers to
determine cutaneous blood flow. Dermatol Surg. 29:272–280. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stinco G, Lautieri S, Valent F and Patrone
P: Cutaneous vascular alterations in psoriatic patients treated
with cyclosporine. Acta Derm Venereol. 87:152–154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murray AK, Herrick AL, Moore TL, King TA
and Griffiths CE: Dual wavelength (532 and 633 nm) laser Doppler
imaging of plaque psoriasis. Br J Dermatol. 152:1182–1186. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Speight EL and Farr PM: Calcipotriol
improves the response of psoriasis to PUVA. Br J Dermatol.
130:79–82. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
König K, Speicher M, Köhler MJ,
Scharenberg R and Kaatz M: Clinical application of multiphoton
tomography in combination with high-frequency ultrasound for
evaluation of skin diseases. J Biophotonics. 3:759–773. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koehler MJ, Speicher M, Lange-Asschenfeldt
S, Stockfleth E, Metz S, Elsner P, Kaatz M and König K: Clinical
application of multiphoton tomography in combination with confocal
laser scanning microscopy for in vivo evaluation of skin diseases.
Exp Dermatol. 20:589–594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Welzel J: Optical coherence tomography in
dermatology: A review. Skin Res Technol. 7:1–9. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morsy H, Kamp S, Thrane L, Behrendt N,
Saunder B, Zayan H, Elmagid EA and Jemec GB: Optical coherence
tomography imaging of psoriasis vulgaris: Correlation with
histology and disease severity. Arch Dermatol Res. 302:105–111.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pagnoni A, Knuettel A, Welker P, Rist M,
Stoudemayer T, Kolbe L, Sadiq I and Kligman AM: Optical coherence
tomography in dermatology. Skin Res Technol. 5:83–87. 1999.
View Article : Google Scholar
|
|
41
|
Gambichler T, Valavanis K, Plura I,
Georgas D, Kampilafkos P and Stücker M: In vivo determination of
epidermal thickness using high-definition optical coherence
tomography. Br J Dermatol. 170:737–739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kleinerman R, Whang TB, Bard RL and Marmur
ES: Ultrasound in dermatology: Principles and applications. J Am
Acad Dermatol. 67:478–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jasaitiene D, Valiukeviciene S,
Linkeviciute G, Raisutis R, Jasiuniene E and Kazys R: Principles of
high-frequency ultrasonography for investigation of skin pathology.
J Eur Acad Dermatol Venereol. 25:375–382. 2011. View Article : Google Scholar : PubMed/NCBI
|