Introduction
Hypertensive disorder complicating pregnancy (HDCP),
a common condition with a high incidence rate in the Obstetrics and
Gynecology Department, usually occurs after 20 weeks of gestation
(1,2). With the changes of the social
environment in recent years, the incidence rate of the disease has
been increasing year by year due to unhealthy living habits and
dietary structure (3,4). Severe HDCP poses a threat to maternal
and child health which can lead to massive intra-abdominal
hemorrhage and patient death (4).
As an anticonvulsant, magnesium sulfate is currently
the first choice for the prevention and treatment of HDCP (5). It inhibits the dilatation of peripheral
vessels through central inhibition, and indirectly reduces blood
pressure by relieving vasospasm (6,7).
However, although magnesium sulfate is clinically effective in the
treatment of HDCP, it has a slow effect and its therapeutic dose
greatly influences the patients' blood concentration (8,9).
According to recent studies, nifedipine, a long-acting calcium
antagonist, inhibits angiotensin converting enzymes and
significantly lowers blood pressure, and its safety is better than
that of antihypertensive drugs of the same type (10,11).
Phentolamine, a blocker that is mainly used for treating peripheral
vascular diseases (12), blocks
norepinephrine, increases myocardial contractility and reduces the
related resistance of peripheral vessels, effectively dilating
blood vessels (13).
Antihypertensive drugs are effective on HDCP patients, but there is
no conclusion as to which therapeutic regimen is the most
effective. Therefore, the efficacy and safety of the combination of
magnesium sulfate, phentolamine and nifedipine in the treatment of
HDCP patients and its effect on hemodynamics and urinary protein
level were investigated in this study.
Patients and methods
General information
One hundred and six patients with HDCP diagnosed at
the Affiliated Hospital of Beihua University (Jilin, China) from
February 5, 2016 to May 9, 2017 were retrospectively analyzed, and
divided into the magnesium sulfate group (n=53) and the combination
group (n=53) according to the therapeutic schemes. Patients in the
combination group, were treated with magnesium sulfate,
phentolamine and nifedipine, and were 22–40 years of age with an
average age of 26.48±6.93 years. Patients in the magnesium sulfate
group were treated with magnesium sulfate alone, and were 22–38
years of age with an average age of 26.53±7.03 years. Inclusion
criteria: patients with HDCP only treated in the Affiliated
Hospital of Beihua University; those who had no abortion caused by
abnormal chromosome; with no endocrine abnormality, any
reproductive system infection or autoimmune disease (14). Exclusion criteria: patients with
hypertension, hepatitis B virus, gallstones, AIDS and blood
diseases were excluded, as well as pregnant women with abnormal
pregnancy history. Patients included had complete clinical data.
The study was approved by the Ethics Committee of the Affiliated
Hospital of Beihua University. Patients and their families signed
an informed consent form in advance.
Therapeutic regimens
Patients in the magnesium sulfate group were treated
with magnesium sulfate alone (Hebei Tiancheng Pharmaceutical Co.,
Ltd.; SFDA approval no. H20033861). The patients received an
intravenous drip with 100 ml of 5% glucose solution (Newland
Pharmaceutical Co., Ltd.; SFDA approval no. H20065564) rapidly for
30 min, and then 40 ml of 25% magnesium sulfate for 6–8 h, that was
dissolved in 500 ml of 5% glucose. Patients in the combination
group were treated with phentolamine (Shanghai Xudong Haipu
Pharmaceutical Co., Ltd.; SFDA approval no. H31020589) on the basis
of the magnesium sulfate group. The patients received an
intravenous drip with 20 mg of phentolamine, that was dissolved in
200 ml of 5% glucose, and then 20 mg of nifedipine (Guangdong
Xinfeng Pharmaceutical Co., Ltd.; SFDA approval no. H44021999) were
orally administered once daily. Both groups of patients were
treated for 1 week.
Observational indexes
The general clinical data of patients in the two
groups were recorded, and data were acquired with respect to
hemodynamic indexes before and after treatment [changes of S/D
ratio of umbilical artery flow, cardiac index and total peripheral
resistance (TPR)], the 24-h urinary protein level, clinical
efficacy and safety [adverse drug reactions (ADR) and maternal and
neonatal outcomes]. Markedly effective: patients with HDCP that had
normal clinical symptoms and signs, and significantly reduced blood
pressure, without HDCP-related complications; systolic blood
pressure was reduced by ≥30 mmHg, diastolic blood pressure by ≥20
mmHg, urinary protein by ≥20 mg. Effective: the clinical symptoms
and signs were significantly improved with less complications;
blood pressure was reduced by ≤10 mmHg, urine protein by ≤20 mg.
Invalid: the clinical symptoms and signs were unchanged and blood
pressure was not reduced, with more complications (4). Total effective rate = (markedly
effective + effective)/total cases ×100%.
Statistical analysis
SPSS 19.0 software [Bizinsight (Beijing) Information
Technology Co., Ltd.] was used for statistical analysis. Count data
were expressed as the number of cases and percentage [n (%)], and
were tested by χ2 test. Measurement data were expressed
as the mean ± standard deviation (mean ± SD), and t-test was used
for comparisons between two groups, while one-way ANOVA, with Least
Significant Difference post hoc test, for comparisons of multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
General clinical data
There was no statistically significant difference
between the two groups in general data (P>0.05). The two groups
of patients were comparable. Details are shown in Table I.
 | Table I.General clinical data [n (%)]. |
Table I.
General clinical data [n (%)].
| Groups | Combination group
(n=53) | Magnesium sulfate
group (n=53) | t/χ2
value | P-value |
|---|
| Age (years) | 26.48±6.93 | 26.53±7.03 | 0.037 | 0.971 |
| Body mass index
(kg/m2) | 18.47±3.14 | 18.68±2.41 | 0.386 | 0.700 |
| Gestational age
(weeks) | 34.56±4.68 | 34.32±4.59 | 0.267 | 0.790 |
| Pregnancy
history |
|
| 0.050 | 0.824 |
|
Primipara | 40 (75.47) | 39 (73.58) |
|
|
|
Multipara | 13 (24.53) | 14 (26.42) |
|
|
| Excessive nutritional
supplement |
|
| 0.632 | 0.230 |
| Yes | 41 (77.36) | 43 (81.13) |
|
|
| No | 12 (22.64) | 10 (18.86) |
|
|
| History of
preeclampsia |
|
| 0.376 | 0.540 |
| Yes | 5 (9.43) | 7
(13.21) |
|
|
| No | 48 (90.57) | 46 (86.79) |
|
|
| History of chronic
nephritis |
|
| 0.050 | 0.824 |
| Yes | 13 (24.53) | 14 (26.42) |
|
|
| No | 40 (75.47) | 39 (73.58) |
|
|
| Hypertension |
|
| 0.099 | 0.952 |
| Mild | 23 (43.40) | 23 (43.40) |
|
|
|
Moderate | 15 (28.30) | 16 (30.19) |
|
|
|
Severe | 15 (28.30) | 14 (26.42) |
|
|
Changes of hemodynamic indexes
i) Changes of S/D ratio of umbilical
artery flow
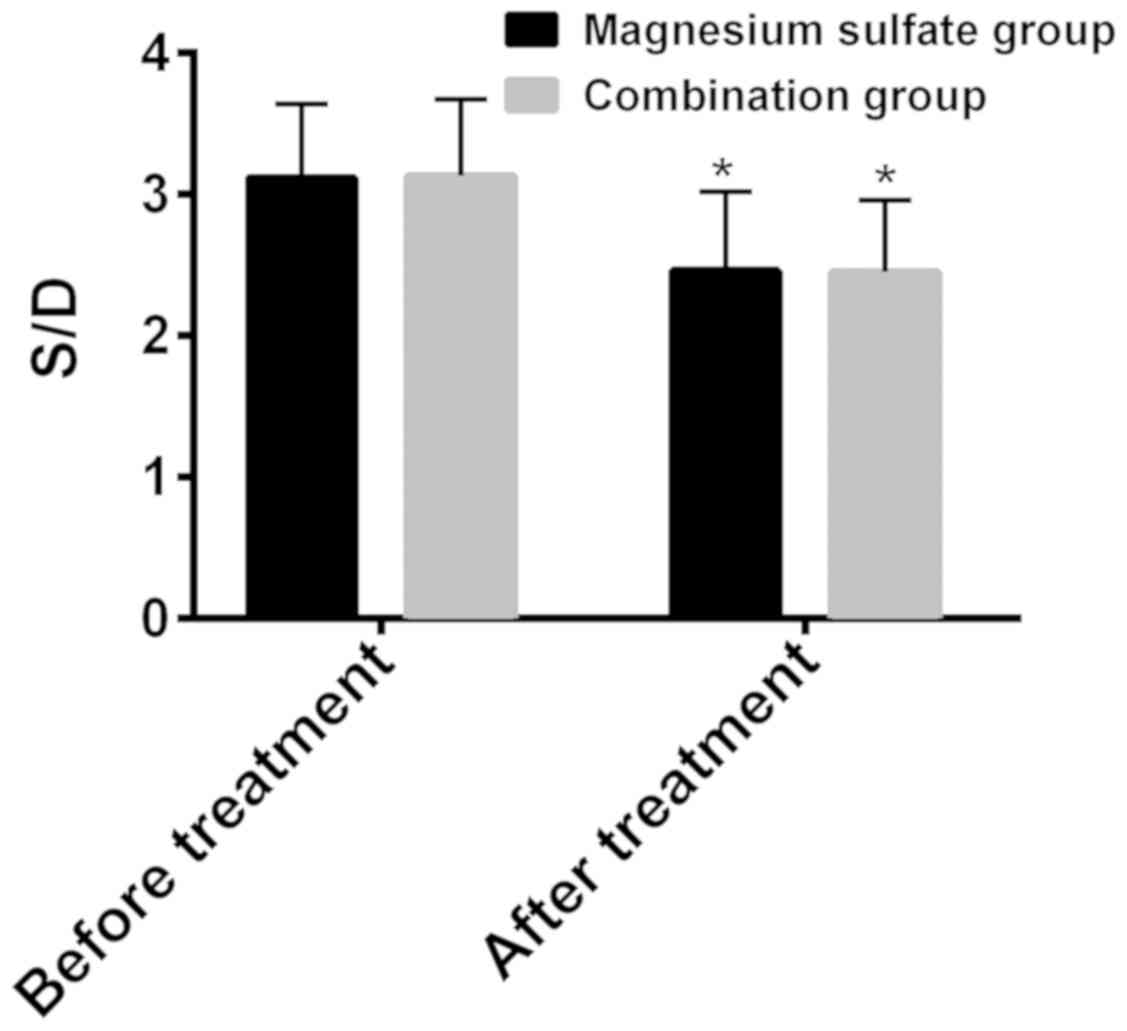
The S/D ratios of umbilical artery flow before and
after treatment were respectively 3.12±0.52 and 2.46±0.56 in the
magnesium sulfate group, 3.13±0.54 and 2.45±0.51 in the combination
group (Fig. 1). Before treatment,
there was no statistically significant difference between the two
groups in terms of S/D ratio of umbilical artery flow (P>0.05).
After treatment the S/D ratio was significantly lower than that
before treatment in both groups (P<0.05), and there was no
statistically significant difference between the two groups
(P>0.05) (Fig. 1).
ii) Changes of cardiac index
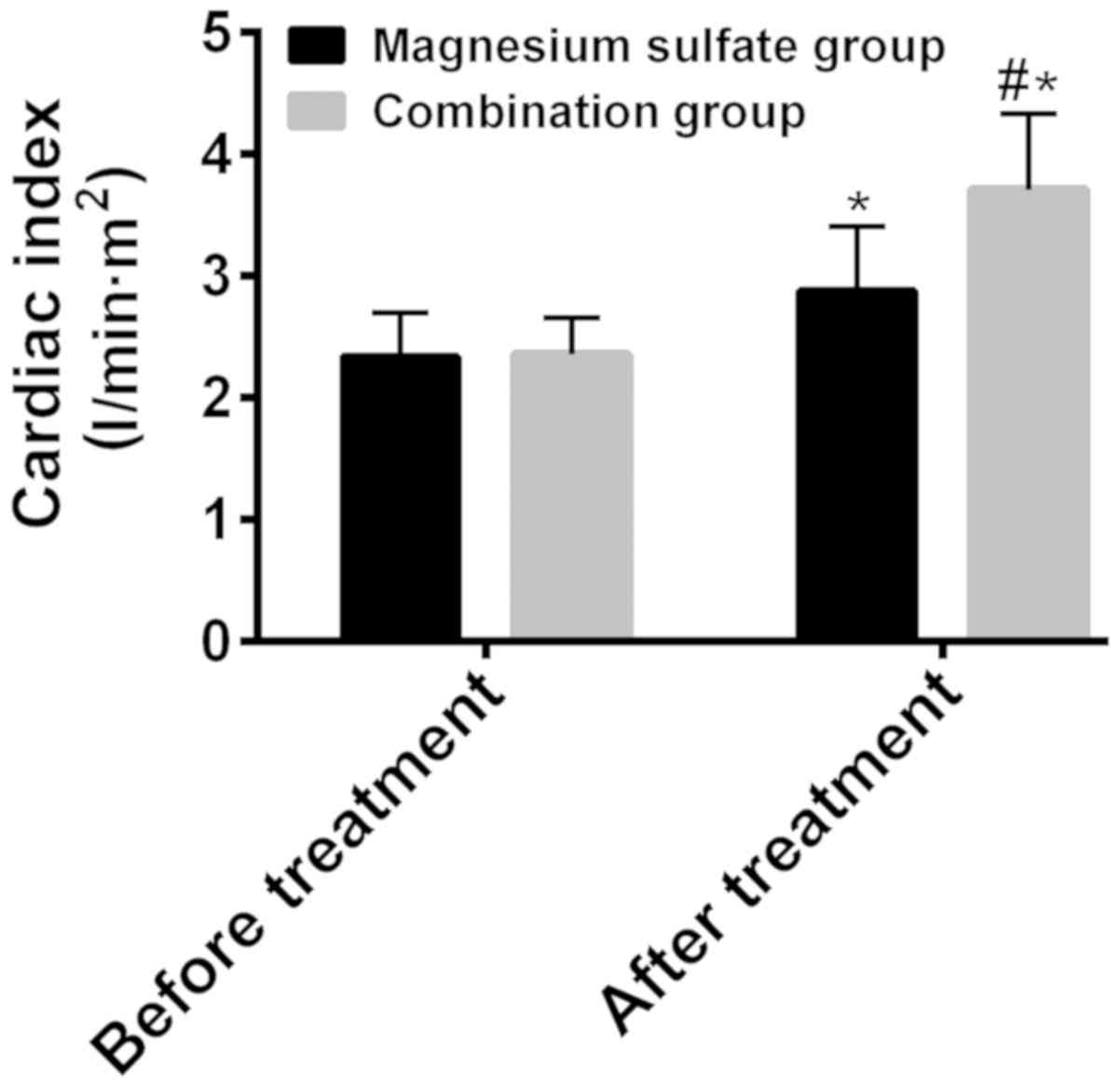
The cardiac indexes before and after treatment were
respectively 2.34±0.36 and 2.88±0.53 l/min•m2 in the
magnesium sulfate group, 2.36±0.30 and 3.71±0.62
l/min•m2 in the combination group (Fig. 2). Before treatment, there was no
statistically significant difference between the two groups in
terms of cardiac index (P>0.05). After treatment the cardiac
index was significantly higher than that before treatment in both
groups (P<0.05), and it was significantly higher in the
combination group than that in the magnesium sulfate group
(P<0.05) (Fig. 2).
iii) Changes of TPR
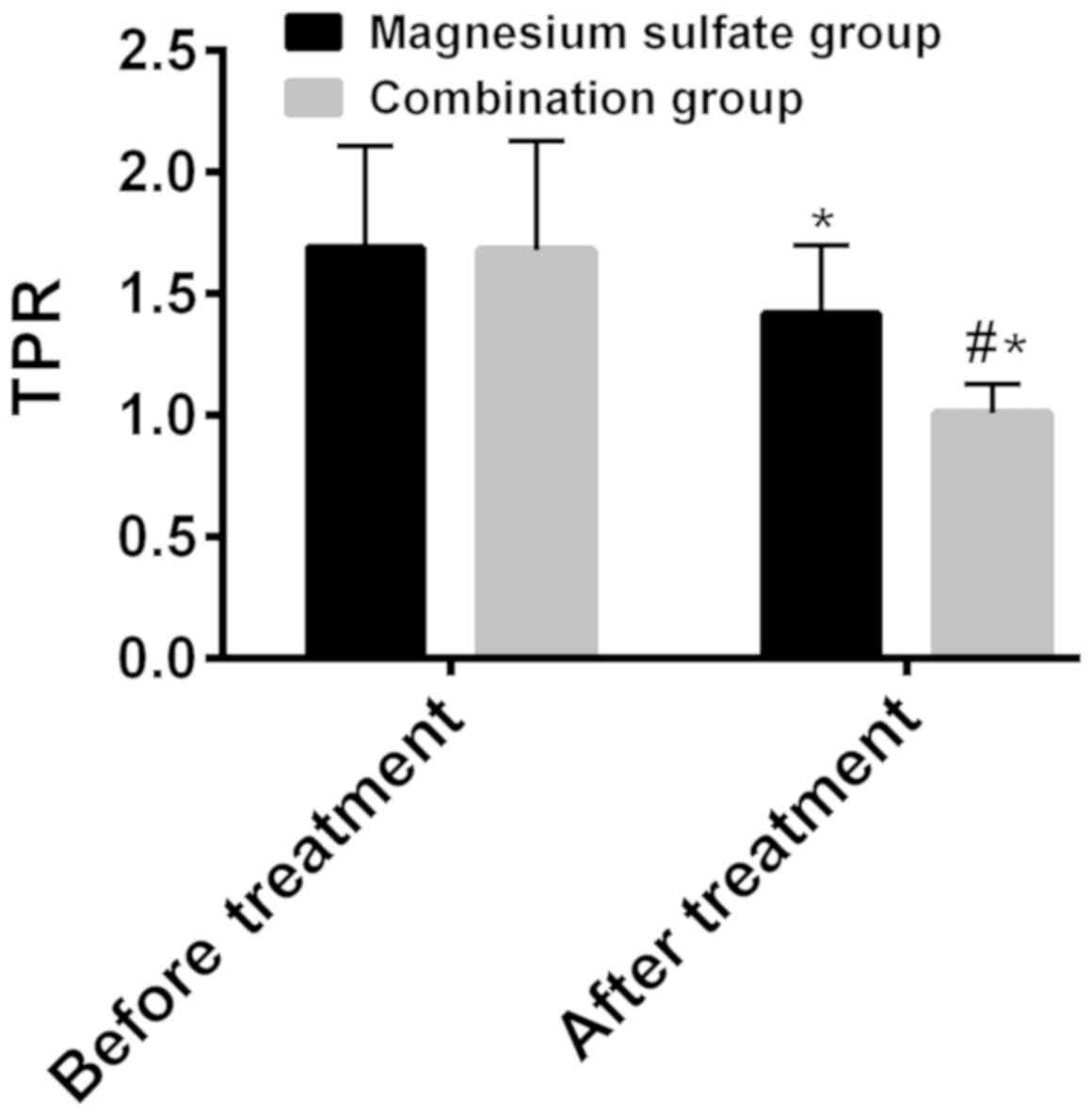
TPR before and after treatment was respectively
1.69±0.42 and 1.42±0.28 in the magnesium sulfate group, 1.68±0.45
and 1.01±0.12 in the combination group (Fig. 3). Before treatment, there was no
statistically significant difference between the two groups in
terms of TPR (P>0.05). After treatment TPR was significantly
lower than that before treatment in both groups (P<0.001), and
it was significantly lower in the combination group than that in
the magnesium sulfate group (P<0.001) (Fig. 3).
Changes of urinary protein level
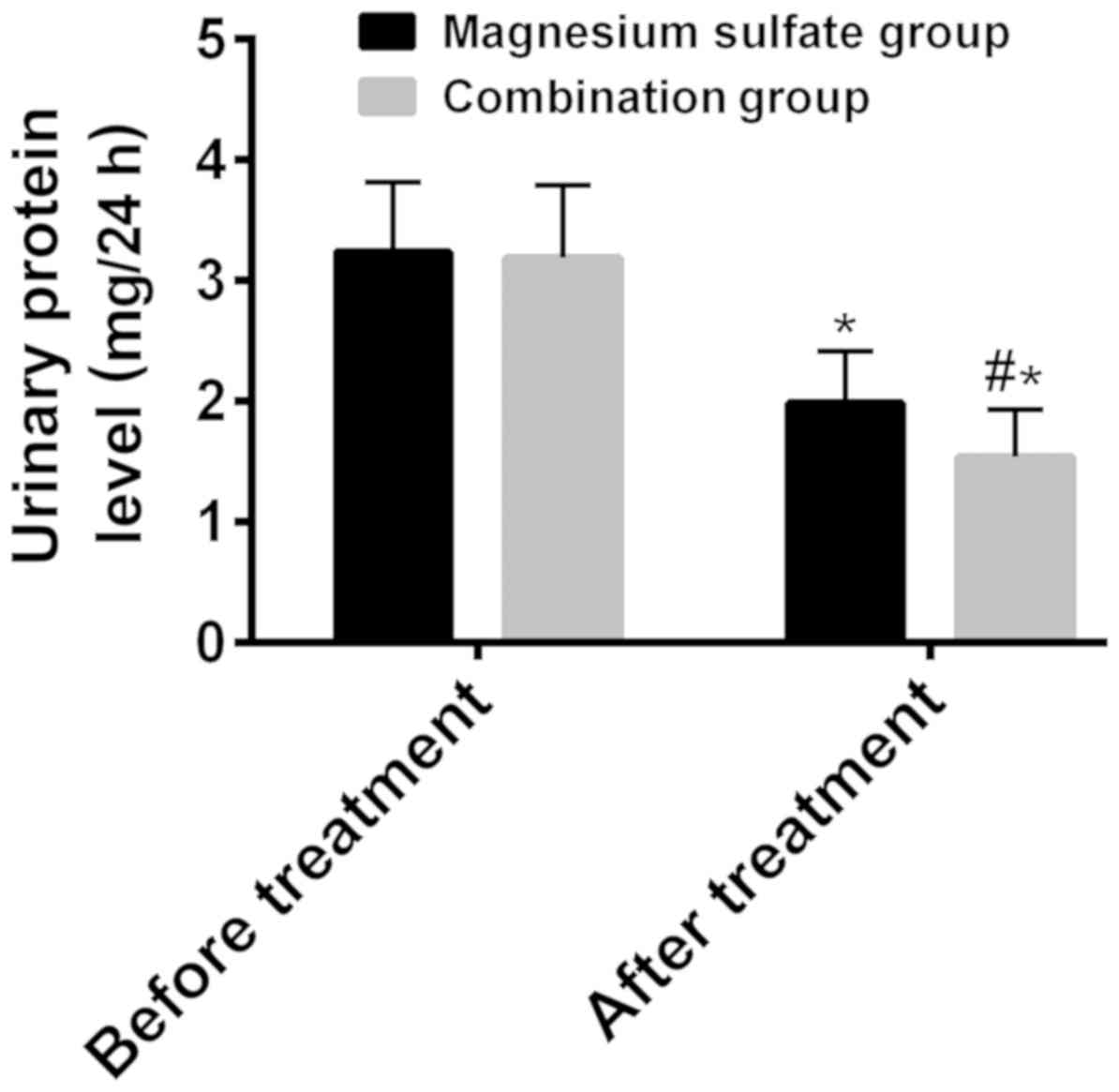
The 24-h urinary protein levels before and after
treatment were respectively 3.24±0.58 and 1.99±0.43 mg/24 h in the
magnesium sulfate group, 3.19±0.60 and 1.54±0.39 mg/24 h in the
combination group (Fig. 4). Before
treatment, there was no statistically significant difference
between the two groups in terms of 24-h urinary protein level
(P>0.05). After treatment the urinary protein level was
significantly lower than that before treatment in both groups
(P<0.001), and it was significantly lower in the combination
group than that in the magnesium sulfate group (P<0.001)
(Fig. 4).
Efficacy observation
In the magnesium sulfate group, the treatment was
markedly effective in 24 patients, effective in 20 patients, and
invalid in 9 patients, with a total effective rate of 83.02%. In
the combination group, the treatment was markedly effective in 31
patients, effective in 20 patients, and invalid in 2 patients, with
a total effective rate of 96.23%. The total effective rate in the
combination group was significantly higher than that in the
magnesium sulfate group (P<0.05) (Table II).
 | Table II.Efficacy observation [n (%)]. |
Table II.
Efficacy observation [n (%)].
| Groups | n | Markedly
effective | Effective | Invalid | Total effective
rate |
|---|
| Magnesium sulfate
group | 53 | 24 (45.28) | 20 (37.74) | 9
(16.98) | 44 (83.02) |
| Combination
group | 53 | 31 (58.49) | 20 (37.74) | 2 (3.77) | 51 (96.23) |
| χ2
value | – | – | – | – | 4.970 |
| P-value | – | – | – | – | 0.026 |
Safety observation
i) Comparison of ADR
The total number of patients with vomiting,
diarrhea, fever, weakness, headache and rash was 37 in the
magnesium sulfate group and 19 in the combination group. The
incidence rate of ADR in the combination group was significantly
lower than that in the magnesium sulfate group (P<0.001)
(Table III).
 | Table III.Comparison of ADR [n (%)]. |
Table III.
Comparison of ADR [n (%)].
| Groups | Vomiting | Diarrhea | Fever | Weakness | Headache | Rash | Total |
|---|
| Magnesium sulfate
group | 9 (16.98) | 3 (5.66) | 6 (11.32) | 10 (18.87) | 7 (13.21) | 2 (3.77) | 37 (69.81) |
| Combination
group | 1 (1.89) | 1 (1.89) | 3 (5.66) | 9 (16.98) | 5 (9.43) | 0 (0.00) | 19 (35.85) |
| χ2
value | – | – | – | – | – | – | 12.270 |
| P-value | – | – | – | – | – | – | <0.001 |
ii) Comparison of maternal and
neonatal outcomes
The total number of patients with premature
delivery, caesarean delivery, postpartum hemorrhage, neonatal
asphyxia and perinatal death was 35 in the magnesium sulfate group
and 15 in the combination group. The incidence rate of adverse
maternal and neonatal outcomes in the combination group was
significantly lower than that in the magnesium sulfate group
(P<0.001) (Table IV).
 | Table IV.Comparison of maternal and neonatal
outcomes [n (%)]. |
Table IV.
Comparison of maternal and neonatal
outcomes [n (%)].
| Groups | Premature
delivery | Caesarean
delivery | Postpartum
hemorrhage | Neonatal
asphyxia | Perinatal
death | Total |
|---|
| Magnesium sulfate
group | 8 (15.09) | 12 (22.64) | 7 (13.21) | 7 (13.21) | 1 (1.89) | 35 (66.04) |
| Combination
group | 1 (1.89) | 10 (18.87) | 2 (3.77) | 2 (3.77) | 0 (0.00) | 15 (28.30) |
| χ2
value | – | – | – | – | – | 15.140 |
| P-value | – | – | – | – | – | <0.001 |
Discussion
The general clinical data of patients in this study
revealed that the two groups of patients were comparable.
Hemodynamic indexes before and after treatment, the 24-h urinary
protein level, clinical efficacy and safety of therapeutic regimens
were observed. The S/D ratio of umbilical artery flow after
treatment was significantly lower than that before treatment in the
both groups, whereas there was no statistically significant
difference between the two groups after treatment. According to
relevant studies, the S/D ratio of umbilical artery flow is helpful
to determine the intrauterine growth of fetus, and reflects whether
a pregnant woman has HDCP or whether the fetus has the tendency of
intrauterine hypoxia and developmental retardation (15,16). It
gradually stabilizes with the increase of gestational age, and its
increase caused by HDCP leads to fetal anoxia and even brain tissue
injury (17). Therefore, it is
believed that the two therapeutic schemes in this study reduce the
S/D ratio of umbilical artery flow, with similar effects on the
ratio. In this study, TPR after treatment was significantly lower
than that before treatment in the two groups, which after treatment
was significantly lower in the combination group than that in the
magnesium sulfate group. Cardiac index after treatment was
significantly higher than that before treatment in both groups, and
after treatment it was significantly higher in the combination
group than that in the magnesium sulfate group. Changes of TPR and
cardiac index are common hemodynamic indexes for observing patients
with HDCP (18). The pathological
changes of blood vessels caused by HDCP result in small blood
vessel spasm, vascular stenosis and increased peripheral
resistance, as well as decreased cardiac index in pregnant women
(19). Therefore, it is believed
that the combination of magnesium sulfate, phentolamine and
nifedipine is better than magnesium sulfate alone in improving the
hemodynamic indexes of patients with HDCP. In a study by Adamo
et al, magnesium sulfate combined with other
antihypertensive drugs was shown to be better than magnesium
sulfate alone in this improvement (20). In this study, the 24-h urinary
protein level after treatment was significantly lower than that
before treatment in the two groups, and after treatment was
significantly lower in the combination group than that in the
magnesium sulfate group. Also, the total effective rate in the
combination group was significantly higher than that in the
magnesium sulfate group. High blood pressure and increased urine
protein are the most direct clinical features of HDCP in pregnant
women, which are caused by kidney damage as a result of
hypertension. According to a relevant study, the 24-h urine protein
level is a monitoring index for patients with HDCP (21), the effective downregulation of which
indicates improvement of the condition (22). In this study, the incidence rate of
adverse maternal and neonatal outcomes (premature delivery,
caesarean delivery, postpartum hemorrhage, neonatal asphyxia and
perinatal death) in the combination group were significantly lower
than that in the magnesium sulfate group. A large number of studies
on the treatment of HDCP have confirmed that magnesium sulfate
combined with other antihypertensive drugs is more effective in
preventing ADR of patients with HDCP and improving maternal and
neonatal outcomes (23–25).
The regional limitations of the patients included
and the lack of rich monitoring indexes may have affected the
statistical results. Therefore, further research on the patients
included and valuable monitoring indexes for HDCP will be
appropriately added in our future studies.
In summary, the combination of magnesium sulfate,
phentolamine and nifedipine can significantly improve the
hemodynamic indexes, the 24-h urinary protein level, clinical
efficacy, ADR and maternal and neonatal outcomes of patients with
HDCP.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ interpreted the data, drafted the manuscript,
conceived and designed the study. JL collected and analyzed the
data, and finally revised the manuscript. Both authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Beihua University (Jilin, China).
Patients who participated in this research had complete clinical
data. The patients and their families signed an informed consent
form in advance.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barakat R, Pelaez M, Cordero Y, Perales M,
Lopez C, Coteron J and Mottola MF; Randomized Clinical Trial, :
Exercise during pregnancy protects against hypertension and
macrosomia: Randomized clinical trial. Am J Obstet Gynecol.
214:649.e1–649.e8. 2016. View Article : Google Scholar
|
|
2
|
Liu FM, Zhao M, Wang M, Yang HL and Li L:
Effect of regular oral intake of aspirin during pregnancy on
pregnancy outcome of high-risk pregnancy-induced hypertension
syndrome patients. Eur Rev Med Pharmacol Sci. 20:5013–5016.
2016.PubMed/NCBI
|
|
3
|
Madsen C, Håberg SE, Aamodt G, Stigum H,
Magnus P, London SJ, Nystad W and Nafstad P: Preeclampsia and
hypertension during pregnancy in areas with relatively low levels
of traffic air pollution. Matern Child Health J. 22:512–519. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson P, Montgomery M and Ewell P:
Elevated blood pressure in low-income, rural preschool children is
associated with maternal hypertension during pregnancy. J Community
Health Nurs. 35:12–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maged AM, Hashem AM, Gad Allah SH, Mahy
ME, Mostafa WA and Kotb A: The effect of loading dose of magnesium
sulfate on uterine, umbilical, and fetal middle cerebral arteries
Doppler in women with severe preeclampsia: A case control study.
Hypertens Pregnancy. 35:91–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niwa K: Adult congenital heart disease
with pregnancy. Korean Circ J. 48:251–276. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brookfield KF, Su F, Elkomy MH, Drover DR,
Lyell DJ and Carvalho B: Pharmacokinetics and placental transfer of
magnesium sulfate in pregnant women. Am J Obstet Gynecol.
214:737.e1–737.e9. 2016. View Article : Google Scholar
|
|
8
|
Cho CK, Sung TY, Choi SJ, Choi HR, Kim YB,
Lee JU and Yang HS: The effect of magnesium sulfate concentration
on the effective concentration of rocuronium, and
sugammadex-mediated reversal, in isolated left phrenic nerve
hemi-diaphragm preparations from the rat. Korean J Anesthesiol.
71:401–406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarma AK, Khandker N, Kurczewski L and
Brophy GM: Medical management of epileptic seizures: Challenges and
solutions. Neuropsychiatr Dis Treat. 12:467–485. 2016.PubMed/NCBI
|
|
10
|
Park JB, Shin JH, Kim DS, Youn HJ, Park
SW, Shim WJ, Park CG, Kim DW, Lee HY, Choi DJ, et al FOCUS
Investigators, : Safety of the up-titration of nifedipine GITS and
valsartan or low-dose combination in uncontrolled hypertension: The
FOCUS Study. Clin Ther. 38:832–842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Webster LM, Myers JE, Nelson-Piercy C,
Harding K, Cruickshank JK, Watt-Coote I, Khalil A, Wiesender C,
Seed PT and Chappell LC: Labetalol versus Nifedipine as
antihypertensive treatment for chronic hypertension in pregnancy: A
randomized controlled trial. Hypertension. 70:915–922. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin J, Shen X, Tai Y, Li S, Liu M, Zhen C,
Xuan X, Zhang X, Hu N, Zhang X, et al: Arterial relaxation is
coupled to inhibition of mitochondrial fission in arterial smooth
muscle cells: Comparison of vasorelaxant effects of verapamil and
phentolamine. Acta Pharm Sin B. 7:319–325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan Z, Shang Y, Li F, Xie F, Qian H, Zhang
Y and Yue B: Therapeutic efficacy of phentolamine in the management
of severe hand, foot and mouth disease combined with pulmonary
edema. Exp Ther Med. 13:1403–1407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schausberger CE, Jacobs VR, Bogner G,
Wolfrum-Ristau P and Fischer T: Hypertensive disorders of pregnancy
- a life-long risk?! Geburtshilfe Frauenheilkd. 73:47–52. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hromadnikova I, Kotlabova K, Ivankova K,
Vedmetskaya Y and Krofta L: Profiling of cardiovascular and
cerebrovascular disease associated microRNA expression in umbilical
cord blood in gestational hypertension, preeclampsia and fetal
growth restriction. Int J Cardiol. 249:402–409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Petrakos G, Andriopoulos P and Tsironi M:
Pregnancy in women with thalassemia: Challenges and solutions. Int
J Womens Health. 8:441–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Flo K, Blix ES, Husebekk A, Thommessen A,
Uhre AT, Wilsgaard T, Vårtun Å and Acharya G: A longitudinal study
of maternal endothelial function, inflammatory response and uterine
artery blood flow during the second half of pregnancy. Acta Obstet
Gynecol Scand. 95:225–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guy GP, Ling HZ, Garcia P, Poon LC and
Nicolaides KH: Maternal cardiac function at 35–37 weeks' gestation:
Prediction of pre-eclampsia and gestational hypertension.
Ultrasound Obstet Gynecol. 49:61–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tiralongo GM, Lo Presti D, Pisani I,
Gagliardi G, Scala RL, Novelli GP, Vasapollo B, Andreoli A and
Valensise H: Assessment of total vascular resistance and total body
water in normotensive women during the first trimester of
pregnancy. A key for the prevention of preeclampsia. Pregnancy
Hypertens. 5:193–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adamo KB, Ferraro ZM and Brett KE: Can we
modify the intrauterine environment to halt the intergenerational
cycle of obesity? Int J Environ Res Public Health. 9:1263–1307.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel AM, Tita AT, Machemehl H, Biggio JR
and Harper LM: Evaluation of institute of medicine guidelines for
gestational weight gain in women with chronic hypertension. AJP
Rep. 7:e145–e150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Demirci O, Kumru P, Arınkan A, Ardıç C,
Arısoy R, Tozkır E, Tandoğan B, Ayvacı H and Tuğrul AS: Spot
protein/creatinine ratio in preeclampsia as an alternative for
24-hour urine protein. Balkan Med J. 32:51–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stepan H, Kuse-Föhl S, Klockenbusch W,
Rath W, Schauf B, Walther T and Schlembach D: Diagnosis and
treatment of hypertensive pregnancy disorders. Guideline of DGGG
(S1-level, AWMF registry no. 015/018, December 2013). Geburtshilfe
Frauenheilkd. 75:900–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kongwattanakul K, Saksiriwuttho P,
Chaiyarach S and Thepsuthammarat K: Incidence, characteristics,
maternal complications, and perinatal outcomes associated with
preeclampsia with severe features and HELLP syndrome. Int J Womens
Health. 10:371–377. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asgharnia M, Mirblouk F, Kazemi S,
Pourmarzi D, Mahdipour Keivani M and Dalil Heirati SF: Maternal
serum uric acid level and maternal and neonatal complications in
preeclamptic women: A cross-sectional study. Int J Reprod Biomed
(Yazd). 15:583–588. 2017. View Article : Google Scholar : PubMed/NCBI
|