Introduction
Achilles tendon, as the most powerful tendon in the
body, is responsible for the plantar flexion of ankle joint and
important for people's daily walking and life (1). Achilles tendon rupture is a common
ankle injury. Statistics have shown that the annual incidence rate
of acute Achilles tendon rupture is ~1.8‰ which increases with age,
and the patients are mostly young and middle-aged male athletes or
actors (2). The disease is caused by
a number of factors, mainly the sudden acceleration or deceleration
of movement and inappropriate modes of exercise (3). Clinically, conservative and surgical
treatments are controversial therapeutic schemes for Achilles
tendon rupture (4,5). However, a mate analysis has shown that
the incidence rate of re-rupture after surgical treatment is
significantly lower than that after conservative treatment, which
indirectly indicates that the former is more effective than the
latter (6). Although the two schemes
are controversial, most scholars advocate surgical treatment for
the important role of Achilles tendon in human body motion
(7).
Currently, the Achilles tendon rupture after
treatment is mainly evaluated based on the doctors' clinical
experience and the efficacy evaluation criteria of the American
Orthopaedic Foot and Ankle Society (AOFAS), due to the lack of
effective observational indexes (8).
However, young clinicians are inexperienced and the AOFAS scoring
is subjective, although it is the most important criterion for
evaluating Achilles tendon rupture. Therefore, it is vital to find
a biomarker for this problem. Transforming growth factor-β1
(TGF-β1) is a multifunctional protein that regulates cell
proliferation, differentiation and wound healing (9). Studies have shown that injection of
different concentrations of TGF-β1 can promote tendon formation,
growth and repair, suggesting that TGF-β1 expression is closely
related to tendon recovery (10).
The reduction of blood supply is one of the reasons of poor healing
of Achilles tendon, therefore, it is of great significance to
promote blood vessel production during Achilles tendon healing
(11). As a signal protein, vascular
endothelial growth factor (VEGF) belongs to the platelet-derived
growth factor family of cystine knot growth factor (10), with the function of regulating
angiogenesis (12). A study showed
that TGF-β1 and VEGF were differentially expressed in a rabbit
model of Achilles tendon injury (13). However, there are few studies on
whether the expression of TGF-β1 and VEGF in the human body is the
same, and whether TGF-β1 and VEGF can be used as prognostic
indicators.
Thus, in the present study, the expression of TGF-β1
and VEGF in patients with Achilles tendon rupture were
investigated, before and after treatment, and their potential
predictive values were explored, in order to provide new references
for clinicians.
Subjects and methods
Information of the study subjects
Forty-two patients with Achilles tendon rupture,
treated in the First Affiliated Hospital of University of South
China (Hengyang, China) from August 2016 to September 2017, were
selected as the observation group, including 32 males and 10
females, with an average age of 34.5±6.7 years, and a course of
disease of 3.51±1.42 days. There were 22 cases caused by football,
10 by basketball and 10 by other factors. Also, 30 normal subjects
undergoing physical examination in the hospital were selected as
the normal group, including 20 males and 10 females, with an
average age of 35.1±7.20 years. The study was approved by the
Medical Ethics Committee of the First Affiliated Hospital of
University of South China, and the patients who participated in
this research signed an informed consent and had complete clinical
data.
Inclusion criteria: Patients with depression and
tenderness at Achilles tendon; patients with positive Thompson's
test; patients diagnosed with Achilles tendon rupture by nuclear
magnetic resonance; patients with closed wounds; patients who
cooperated with treatment; patients with complete clinical data.
Exclusion criteria: Patients with congenital cardiovascular
diseases; patients with immunodeficiency diseases; patients with
arthritis, gout, infectious diseases and malignant tumors; patients
unable to receive operation for their own reasons.
Therapeutic regimens and postoperative
treatment
Patients were treated according to the therapeutic
regimens described in the study by Ismail et al (14). After operation the affected limbs
were fixed with long leg casts, with the knee bent and the ankle
joint at a plantar flexion of 30°. After 6 weeks, the affected
limbs were fixed with short leg casts for active/passive flexion
and extension of the ankle joint. The patients received partial
weight-bearing exercises with crutches after 8 weeks, and normal
weight-bearing exercises after 12 weeks. Intense exercises were
avoided for 6 months.
Main kits
EasyPure Genomic DNA kit and TransScript Green
Two-Step qRT-PCR SuperMix (EE101-01 and AQ201-01, respectively;
both from TransGen Biotech Co., Ltd.) were used.
Expression of serum TGF-β1 and
VEGF
Fasting peripheral venous blood (5 ml) was collected
from subjects and patients, let to stand for 30 min, and
centrifuged at 1,500 × g, at 25°C for 10 min in order to collect
the supernatant for subsequent experiments. Total RNA was extracted
using the EasyPure Genomic DNA kit. One microliter of the extracted
Total RNA, 4 µl of 5X TransScript® Tip Green qPCR
SuperMix and 1 µ gDNA Remover (both from Thermo Fisher Scientific,
Inc.) were added. RNase-free water was also added to a final volume
of 20 µl. After mixing, and incubating at 42°C for 15 min, and then
heating to 85°C for 5 sec, an ultraviolet spectrophotometer (Thermo
Fisher Scientific, Inc.; GENESYS™ 140/150) was used and agarose gel
electrophoresis was performed for purity, concentration and
integrity detection. 5X TransScript® II All-in-One
SuperMix for qPCR and gDNA Remover kits (both from Thermo Fisher
Scientific, Inc.) were used for reverse transcription, in strict
accordance with the manufacturer's instructions. Then, PCR
amplification was performed. Upstream and downstream primers for
TGF-β1 were 5′-TGCGCCTGCAGAGATTCAAG-3′ and
5′-AGGTAACGCCAGGAATTGTTGCTA-3′, respectively. Those of VEGF were
5′-GCACGTTGGCTCACTTCCAG-3′ and 5′-AGGTAACGCCAGGAATTGTTGCTA-3′,
respectively. The reaction system was as follows: 1 µl of cDNA, 0.4
µl of upstream and downstream primers, respectively, 10 µl of 2X
TransScript® Tip Green qPCR SuperMix, 0.4 µl of Passive
Reference Dye (50X) (both from Thermo Fisher Scientific, Inc.), and
Nuclease-free water were added to a final volume of 20 µl. The
reaction conditions were as follows: Pre-denaturation at 94°C for
30 sec, denaturation at 94°C for 5 sec, and annealing and extension
at 60°C for 30 sec for a total of 40 cycles. Each sample was
provided with three identical wells, and the experiment was carried
out 3 times. β-actin was used as an internal reference, and its
upstream and downstream primers were 5′-CTCCATCCTGGCCTCGCTG-3′ and
5′-GCTGTCACCTTCACCGTTCC-3′, respectively. 2−ΔCq was used
to analyze the data (15).
Observational indexes
Main observational indexes
The expression of serum TGF-β1 and VEGF was compared
between the observation and normal group, and the TGF-β1 and VEGF
expression levels in the observation group were observed before
treatment, and at 3 and 6 months after treatment. The patients were
divided into the excellent efficacy group and the good/general
efficacy group according to the predictive efficacy at 6 months
after treatment, and the expression levels of TGF-β1 and VEGF
before treatment were compared between the two groups. Receiver
operating characteristic (ROC) curves were plotted to analyze the
predictive values of TGF-β1 and VEGF for the efficacy.
Secondary observational indexes
AOFAS scoring system with 100 points in total was
adopted to evaluate the efficacy at 6 months after treatment,
including pain, function and foot line (16). Grading: 90–100 points, excellent
efficacy; 75–89 points, good efficacy; 50–74 points, general
efficacy, and <50 points, poor efficacy. Patients were separated
into the excellent efficacy group, good efficacy group and general
efficacy group, according to the AOFAS scores after treatment, and
the expression of TGF-β1 and VEGF was compared between the three
groups. The correlation of TGF-β1 and VEGF with efficacy was
analyzed, and the clinical data were compared between the
observation and normal group.
Statistical analysis
SPSS 20.0 (Guangzhou Pomine Information Technology
Co., Ltd.) was used to statistically analyze the data, and GraphPad
Prism 7 (Cabit Information Technology Co., Ltd.) to create the
graphs. Enumeration data were expressed as ratio (%) and were
compared by Chi-square test. Measurement data were expressed as the
mean ± standard deviation. The data between groups were compared
using the independent-samples t-test, while comparisons within
groups, before and after treatment, were carried out using the
paired t-test. ROC curve analysis was adopted to analyze the
predictive values of TGF-β1 and VEGF expression in clinical
efficacy before treatment, and Spearman's correlation was used to
analyze the correlation of TGF-β1 and VEGF with efficacy. One-way
ANOVA was carried out for the comparisons between multiple groups
(F analysis), and LSD-t test was adopted for post hoc pairwise
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of clinical data
Comparison of clinical data between the normal and
observation group showed that there was no statistically
significant difference in age, sex, body mass index (BMI), medical
history, place of residence, level of education, history of smoking
or alcoholism (all P>0.05) (Table
I).
 | Table I.Comparison of clinical data. |
Table I.
Comparison of clinical data.
| Factors | Normal group
(n=30) | Observation group
(n=42) | t/χ2
value | P-value |
|---|
| Sex |
|
| 0.791 | 0.374 |
| Male | 20 (66.67) | 32 (76.19) |
|
|
|
Female | 10 (33.33) | 10 (23.81) |
|
|
| Age (years) |
35.1±7.20 |
34.5±6.70 | 0.363 | 0.718 |
| BMI
(kg/m2) | 22.88±1.74 | 22.51±1.82 | 0.866 | 0.389 |
| Medical history |
|
Hypertension | 4 (13.33) | 6 (14.29) | 0.013 | 0.908 |
|
Diabetes | 2 (6.67) | 2 (4.76) | 0.121 | 0.728 |
| COPD | 0 (0.00) | 2 (4.76) | 1.469 | 0.225 |
| Place of
residence |
|
| 0.159 | 0.690 |
| City | 15 (50.00) | 23 (54.76) |
|
|
|
Countryside | 15 (50.00) | 19 (45.24) |
|
|
| Level of
education |
|
| 0.411 | 0.521 |
| ≥
Senior high school | 12 (40.00) | 20 (47.62) |
|
|
| <
Senior high school | 18 (60.00) | 22 (52.38) |
|
|
| History of
smoking |
|
| 0.266 | 0.606 |
|
Yes | 22 (73.33) | 33 (78.57) |
|
|
| No | 8 (26.67) | 9 (21.43) |
|
|
| History of
alcoholism |
|
| 0.150 | 0.600 |
|
Yes | 4 (13.33) | 7 (16.67) |
|
|
| No | 26 (86.67) | 35 (83.33) |
|
|
| Pathogenesis |
|
Football |
| 22 (52.38) |
|
|
|
Basketball |
| 10 (23.81) |
|
|
|
Others |
| 10 (23.81) |
|
|
| Course of disease
(days) |
| 3.51±1.42 |
|
|
Expression of serum TGF-β1 and VEGF in
the normal and observation group
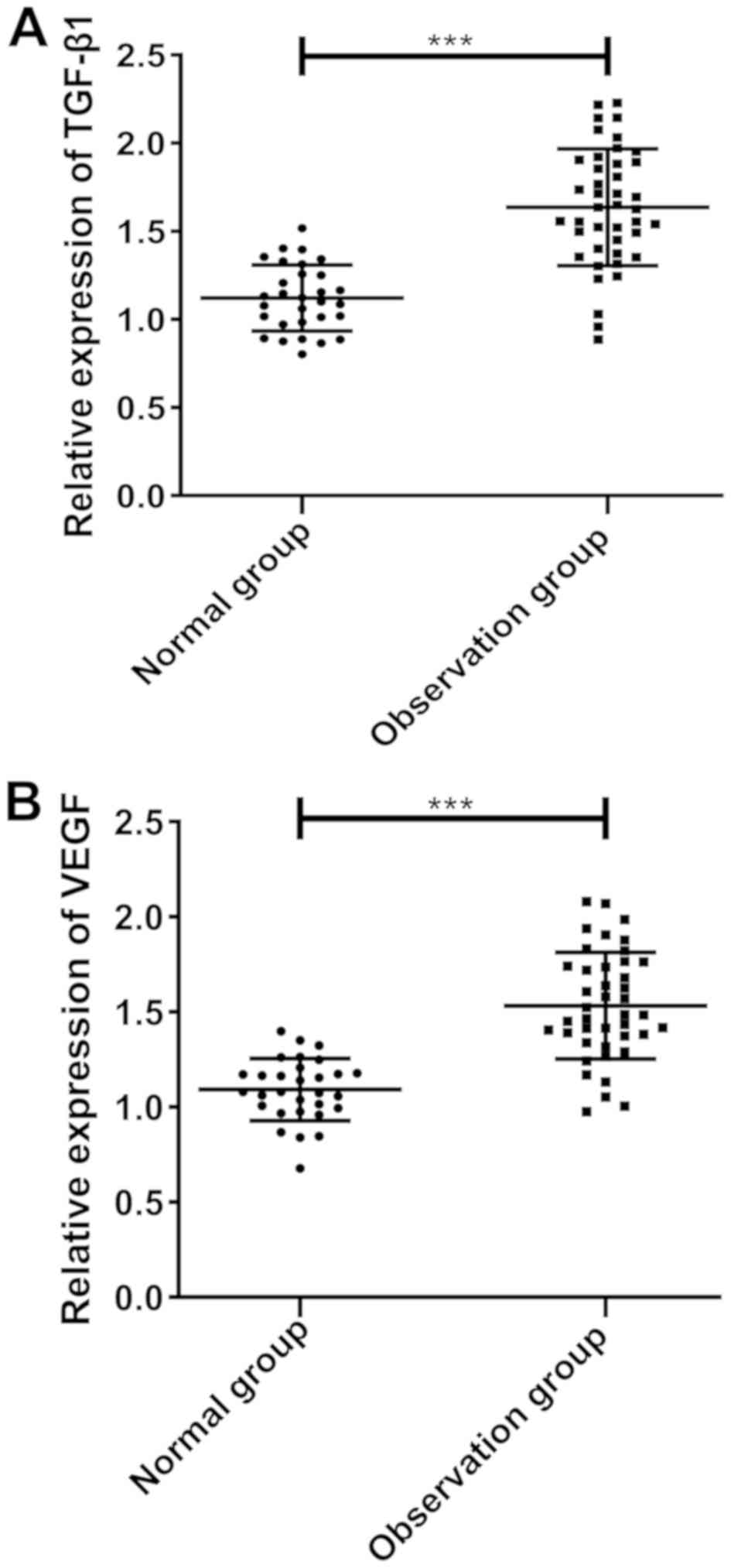
According to the results, the expression of TGF-β1
and VEGF in the normal group before treatment was 1.122±0.187 and
1.092±0.163, respectively, while that in the observation group was
1.636±0.331 and 1.533±0.281, respectively. The expression of TGF-β1
and VEGF in the observation group was significantly higher than
that in the normal group (both P<0.001) (Fig. 1).
Expression of TGF-β1 and VEGF in the
observation group before and after treatment
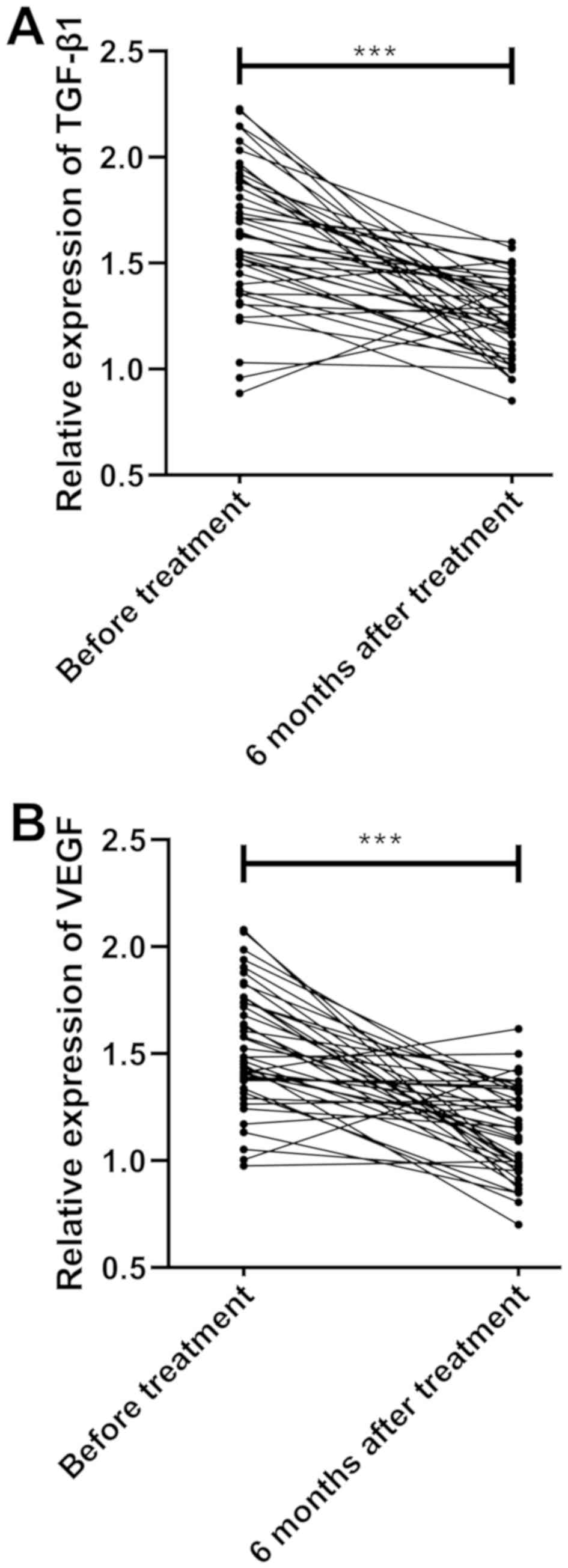
Comparisons of the expression of TGF-β1 and VEGF in
patients before treatment, and at 3 and 6 months after treatment
indicated that there was a significant difference in the expression
of TGF-β1 and VEGF before and after treatment (P<0.001). The
results showed significantly higher expression of TGF-β1 and VEGF
at 3 months after treatment and slightly decreased expression at 6
months after treatment, compared to the results before treatment
(both P<0.001) (Fig. 2 and
Table II).
 | Table II.Expression of TGF-β1 and VEGF before
and after treatment. |
Table II.
Expression of TGF-β1 and VEGF before
and after treatment.
| Time | TGF-β1 | VEGF |
|---|
| Before
treatment | 1.636±0.331 | 1.533±0.281 |
| At 3 months after
treatment |
2.225±0.340a |
2.013±0.262a |
| At 6 months after
treatment |
1.238±1.190a,b |
1.138±0.211a,b |
| F-value | 108.735 | 135.136 |
| P-value | <0.001 | <0.001 |
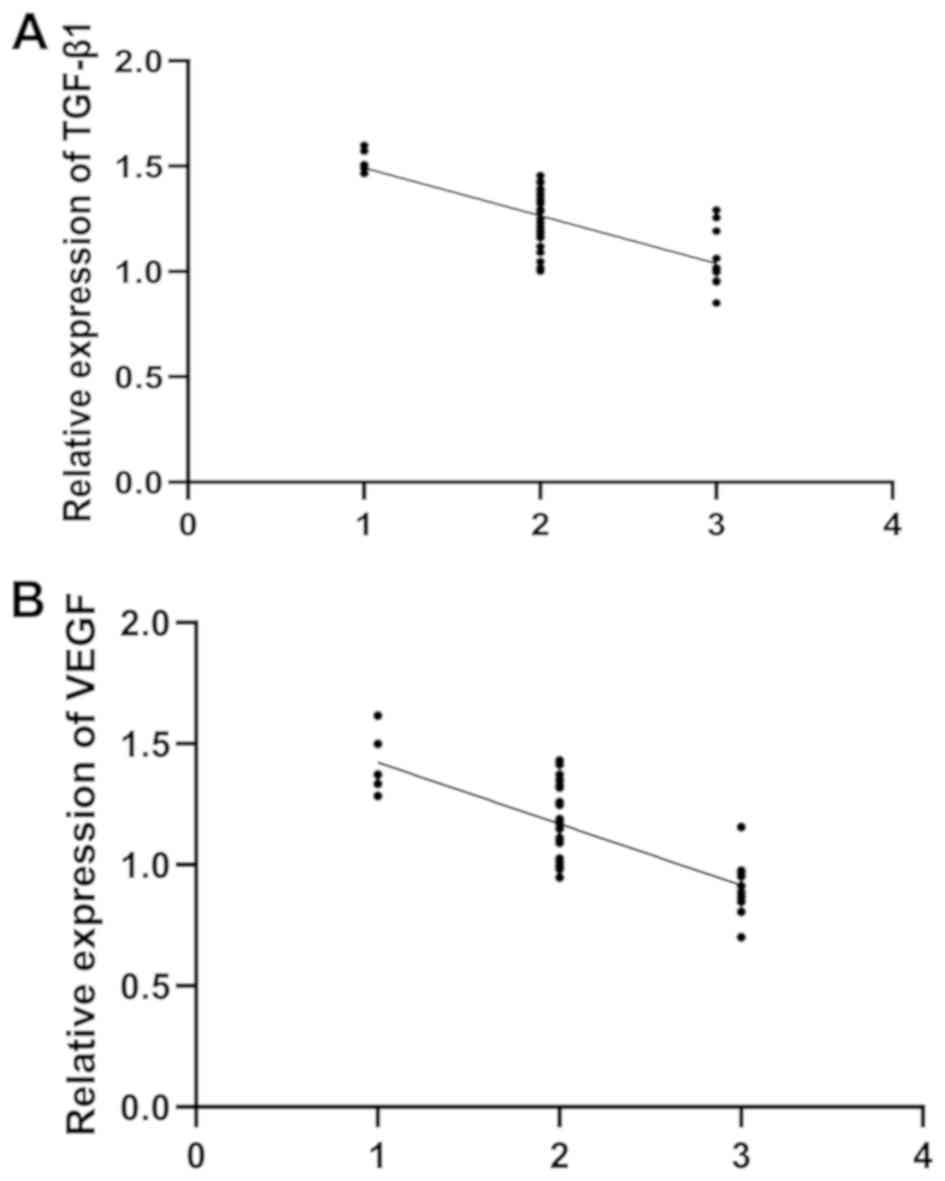
Correlation of TGF-β1 and VEGF
expression with the clinical efficacy after treatment
After treatment for 6 months, the AOFAS score in the
observation group was 84.29±7.91 points. There were 11 patients
with excellent efficacy, 25 patients with good efficacy and 6
patients with general efficacy. The comparison of the expression of
serum TGF-β1 and VEGF between the excellent efficacy, good efficacy
and general efficacy groups showed a significant difference (all
P<0.05), and the analysis with Spearman's correlation showed
that the expression of TGF-β1 and VEGF decreased with the
improvement of efficacy (rTGF-β1=−0.734,
PTGF-β1<0.001; rVEGF=−0.767,
PVEGF<0.001) (Fig. 3
and Table III).
 | Table III.Correlation of clinical efficacy with
the expression of TGF-β1 and VEGF. |
Table III.
Correlation of clinical efficacy with
the expression of TGF-β1 and VEGF.
| Efficacy | TGF-β1 | VEGF |
|---|
| Excellent
(n=11) | 1.055±0.137 | 0.902±0.116 |
| Good (n=25) |
1.250±0.132a |
1.180±0.151a |
| General (n=6) |
1.522±0.052a,b |
1.398±0.133a,b |
| F-value | 26.993 | 27.024 |
| P-value | <0.001 | <0.001 |
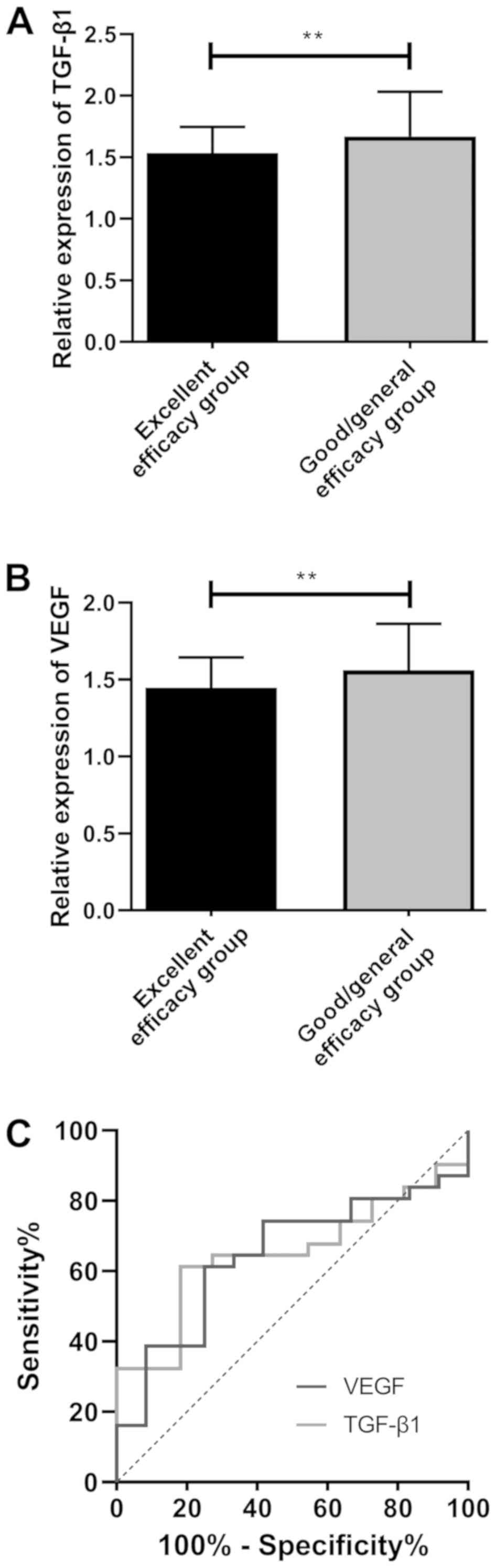
Predictive values of TGF-β1 and VEGF
in clinical efficacy before treatment
According to the predictive efficacy, the patients
were further divited into the excellent efficacy group and the
good/general efficacy group. The comparison of the expression of
TGF-β1 and VEGF between the two groups indicated that the excellent
efficacy group showed significantly lower expression of TGF-β1 and
VEGF than that of the good/general efficacy group (P<0.01).
According to ROC curves, the areas under the curves (AUCs) of
TGF-β1 and VEGF were 0.651 and 0.645, respectively (Fig. 4 and Table
IV).
 | Table IV.ROC parameters. |
Table IV.
ROC parameters.
| Parameters | TGF-β1 | VEGF |
|---|
| AUC | 0.651 | 0.645 |
| Standard error | 0.086 | 0.088 |
| 95% CI | 0.483–0.819 | 0.473–0.817 |
| Sensitivity | 61.29% | 61.29% |
| Specificity | 81.81% | 75.00% |
| Youden index | 43.11% | 36.29% |
| Cut-off value | >1.631 | >1.475 |
Discussion
Achilles tendon is the most common ruptured tendon
of lower limbs. According to Ganestam et al (17), a total of 33,160 patients suffered
from Achilles tendon rupture from 1994 to 2013 in Denmark, with
males (aged 40–50 years) accounting for >75%. The treatment of
Achilles tendon rupture is essential, as it affects the patients'
daily living and especially the careers of injured athletes
(18). At present, the treatment of
the disease is controversial (19).
Some people advocate conservative treatment, while others surgical
treatment, both of which have positive effects (20). However, a study has shown that
surgical treatment reduces the incidence of re-rupture of Achilles
tendon (21), therefore surgical
treatment is considered to be slightly superior to conservative
treatment.
Achilles tendon rupture is currently treated by
numerous surgical treatments, one of which is the minimally
invasive percutaneous treatment with rivet with thread (22). Also, Kessler suture (23), Krachow suture (24), and minimally invasive suture
(25) are adopted according to the
degree of rupture. A study has shown that the minimally invasive
Achilles tendon repair causes little damage to tissues and blood
vessels around the Achilles tendon rupture, and is widely used in
the treatment of the disease (26).
The suture with Achillon device, that was used in the present
study, is a therapeutic scheme originally proposed by Kakiuchi
(27) in 1995. With the advantages
of small incision and conveniental operation, it is more effective
than Kessler suture. Suture with Achillon device is markedly
effective in the treatment of Achilles tendon rupture, however, its
evaluation for postoperative efficacy is mainly based on AOFAS
score and the experience of clinicians, so it has limitations.
Therefore, the identification of biomarkers for observation is
particularly important.
TGF-β1 and VEGF are important growth factors, as
TGF-β1 promotes cell growth and development, wound healing, and
modulation of immune responses (28), and VEGF is a powerful angiogenesis
regulatory factor with an important influence on revascularization
(29). A relevant study has shown
that TGF-β1 and VEGF are highly expressed in an animal model of
Achilles tendon injury (30),
however, no clinical study has been carried out. Therefore, the
expression and significance of TGF-β1 and VEGF in the treatment of
Achilles tendon rupture were explored in this study. The results
showed that the expression of TGF-β1 and VEGF in the observation
group was significantly higher than that in the normal group,
indicating that the expression of TGF-β1 and VEGF increases after
injury. This is probably because after Achilles tendon injury,
patients' vascular tissues at the injured part are damaged, which
causes excessive secretion of TGF-β1 and VEGF in the body. A study
by Lyras et al (31) has
shown that the expression of VEGF in an animal model of Achilles
tendon injury decreases after surgical treatment. According to
another study (32), exogenous VEGF
for the treatment of rats with Achilles tendon injury improves the
tensile strength of Achilles tendon, and increases the expression
of TGF-β1. The expression of TGF-β1 and VEGF in the observation
group before treatment, and at 3 and 6 months after treatment was
compared and the findings showed that the patients had
significantly higher expression of TGF-β1 and VEGF after 3 months
of treatment, but slightly decreased expression after 6 months of
treatment. This indicates that the expression of TGF-β1 and VEGF in
patients after treatment increases in a certain period of time. It
may be due to the fact that the body releases a large number of
inflammatory factors after Achilles tendon rupture, while TGF-β1
and VEGF are not only angiogenesis and growth factors, but also
important inflammatory factors, thus, TGF-β1 and VEGF increase
after injury. In addition, as inflammatory factors, TGF-β1 and VEGF
can promote angiogenesis and cell repair in the injured area. When
the patient's inflammatory response is alleviated, the expression
of TGF-β1 and VEGF decreases, and the Achilles tendon is healed. In
this study, the expression of TGF-β1 and VEGF at 6 months after
treatment was significantly lower than that before treatment.
Additionally, according to correlation analysis, the expression of
TGF-β1 and VEGF decreased with the improvement of efficacy,
indicating that TGF-β1 and VEGF can be used as potential indicators
for the clinical observation of efficacy after treatment.
Differences in individuals lead to differences in
postoperative recovery, so it is particularly important to predict
the clinical efficacy by observing serological indicators before
treatment, in order to promote the patients' recovery. In the
present study, the patients were grouped based on the predictive
efficacy after treatment to observe the expression of TGF-β1 and
VEGF before treatment. The results showed that the expression in
the excellent efficacy group was lower than that in the
good/general efficacy group, indicating that TGF-β1 and VEGF may be
potential predictors of clinical efficacy. According to the results
of the ROC curve analysis, the AUCs of TGF-β1 and VEGF were
>0.5, suggesting that the two indicators could be potential
predictors of the efficacy in Achilles tendon rupture.
This study was focused on efficacy prediction, and
did not confirm that the two indexes can be adopted as observation
indexes for Achilles tendon rupture. However, it is undeniable that
the results of this study confirmed through the relevant research
that the two indexes do have certain clinical value. In the present
study, there are still some limitations. The AUCs of TGF-β1 and
VEGF were only just >0.5, suggesting that their clinical
significance is not high, and PCR detection is expensive, so it may
increase the economic burden of patients.
In conclusion, TGF-β1 and VEGF can be used as
observational indexes and predictors for clinical efficacy in
patients with Achilles tendon rupture before and after
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC and ZC conceived and designed the study. JC
acquired the patients' data. ZC and WW analyzed and interpreted the
data regarding the Achilles tendon rupture. JC wrote the article.
WW reviewed the article. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of University of South China
(Hengyang, China). Patients who participated in this research
signed an informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Demirel M, Turhan E, Dereboy F and Yazar
T: Augmented repair of acute tendo Achilles ruptures with
gastrosoleus turn down flap. Indian J Orthop. 45:45–52. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frankewycz B, Krutsch W, Weber J,
Ernstberger A, Nerlich M and Pfeifer CG: Rehabilitation of Achilles
tendon ruptures: Is early functional rehabilitation daily routine?
Arch Orthop Trauma Surg. 137:333–340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aisaiding A, Wang J, Maimaiti R, Jialihasi
A, Aibek R, Qianman B, Shawutali N, Badelihan A, Bahetiya W, Kubai
A, et al: A novel minimally invasive surgery combined with early
exercise therapy promoting tendon regeneration in the treatment of
spontaneous Achilles tendon rupture. Injury. 49:712–719. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang X, Meng H, Quan Q, Peng J, Lu S and
Wang A: Management of acute Achilles tendon ruptures: A review.
Bone Joint Res. 7:561–569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lantto I, Heikkinen J, Flinkkila T,
Ohtonen P, Siira P, Laine V and Leppilahti J: A prospective
randomized trial comparing surgical and nonsurgical treatments of
acute Achilles tendon ruptures. Am J Sports Med. 44:2406–2414.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weatherall JM, Mroczek K and Tejwani N:
Acute achilles tendon ruptures. Orthopedics. 33:758–764. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alcelik I, Diana G, Craig A, Loster N and
Budgen A: Minimally invasive versus open surgery for acute Achilles
tendon ruptures a systematic review and meta-analysis. Acta Orthop
Belg. 83:387–395. 2017.PubMed/NCBI
|
|
8
|
Macaulay A, Nandyala SV, Miller CP,
Ghorbanhoseini M, Walley KC and Kwon JY: Potential for Bias and the
American Orthopaedic Foot and Ankle Society Ankle-Hindfoot Scoring
System. Foot Ankle Spec. 11:416–419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hinz B: The extracellular matrix and
transforming growth factor-β1: Tale of a strained relationship.
Matrix Biol. 47:54–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou Y, Mao Z, Wei X, Lin L, Chen L, Wang
H, Fu X, Zhang J and Yu C: The roles of TGF-beta1 gene transfer on
collagen formation during Achilles tendon healing. Biochem Biophys
Res Commun. 383:235–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Filardo G, Presti ML, Kon E and Marcacci
M: Nonoperative biological treatment approach for partial Achilles
tendon lesion. Orthopedics. 33:120–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferrara N and Adamis AP: Ten years of
anti-vascular endothelial growth factor therapy. Nat Rev Drug
Discov. 15:385–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pedowitz D and Kirwan G: Achilles tendon
ruptures. Curr Rev Musculoskelet Med. 6:285–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ismail M, Karim A, Shulman R, Amis A and
Calder J: The Achillon achilles tendon repair: Is it strong enough?
Foot Ankle Int. 29:808–813. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sayyed-Hosseinian SH, Hassankhani GG,
Bagheri F, Alavi N, Shojaie B and Mousavian A: Validation of the
persian version of the American Orthopedic Foot and Ankle Society
Score (AOFAS) questionnaire. Arch Bone Jt Surg. 6:233–239.
2018.PubMed/NCBI
|
|
17
|
Ganestam A, Kallemose T, Troelsen A and
Barfod KW: Increasing incidence of acute Achilles tendon rupture
and a noticeable decline in surgical treatment from 1994 to 2013. A
nationwide registry study of 33,160 patients. Knee Surg Sports
Traumatol Arthrosc. 24:3730–3737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mai HT, Alvarez AP, Freshman RD, Chun DS,
Minhas SV, Patel AA, Nuber GW and Hsu WK: The NFL Orthopaedic
Surgery Outcomes Database (NO-SOD): The Effect of common
orthopaedic procedures on football careers. Am J Sports Med.
44:2255–2262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Wang C, Ma X, Wang X, Zhang C and
Chen L: Rehabilitation regimen after surgical treatment of acute
Achilles tendon ruptures: A systematic review with meta-analysis.
Am J Sports Med. 43:1008–1016. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng S, Sun Z, Zhang C, Chen G and Li J:
Surgical treatment versus conservative management for acute
Achilles tendon rupture: A systematic review and meta-analysis of
randomized controlled trials. J Foot Ankle Surg. 56:1236–1243.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maffulli G, Buono AD, Richards P, Oliva F
and Maffulli N: Conservative, minimally invasive and open surgical
repair for management of acute ruptures of the Achilles tendon: A
clinical and functional retrospective study. Muscles Ligaments
Tendons J. 7:46–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gulati V, Jaggard M, Al-Nammari SS,
Uzoigwe C, Gulati P, Ismail N, Gibbons C and Gupte C: Management of
achilles tendon injury: A current concepts systematic review. World
J Orthop. 6:380–386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Walden G, Liao X, Donell S, Raxworthy MJ,
Riley GP and Saeed A: A clinical, biological, and biomaterials
perspective into tendon injuries and regeneration. Tissue Eng Part
B Rev. 23:44–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mait JE, Hayes WT, Blum CL, Pivec R, Zaino
CJ, Jauregui JJ, Saha S, Uribe JA and Urban WP: A biomechanical
comparison of different tendon repair techniques. J Long Term Eff
Med Implants. 26:167–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carmont MR: Achilles tendon rupture: The
evaluation and outcome of percutaneous and minimally invasive
repair. Br J Sports Med. 52:1281–1282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Braunstein M, Baumbach SF, Boecker W,
Carmont MR and Polzer H: Development of an accelerated functional
rehabilitation protocol following minimal invasive Achilles tendon
repair. Knee Surg Sports Traumatol Arthrosc. 26:846–853. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kakiuchi M: A combined open and
percutaneous technique for repair of tendo Achillis. Comparison
with open repair. J Bone Joint Surg Br. 77:60–63. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Inoue M, Nakajima M, Oi Y, Hojo T, Itoi M
and Kitakoji H: The effect of electroacupuncture on tendon repair
in a rat Achilles tendon rupture model. Acupunct Med. 33:58–64.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tempfer H, Kaser-Eichberger A, Lehner C,
Gehwolf R, Korntner S, Kunkel N, Wagner A, Gruetz M, Heindl LM,
Schroedl F, et al: Bevacizumab improves Achilles tendon repair in a
rat model. Cell Physiol Biochem. 46:1148–1158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuksel S, Guleç MA, Gultekin MZ, Adanır O,
Caglar A, Beytemur O, Onur Küçükyıldırım B, Avcı A, Subaşı C, İnci
Ç, et al: Comparison of the early period effects of bone
marrow-derived mesenchymal stem cells and platelet-rich plasma on
the Achilles tendon ruptures in rats. Connect Tissue Res.
57:360–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lyras DN, Kazakos K, Verettas D,
Polychronidis A, Tryfonidis M, Botaitis S, Agrogiannis G,
Simopoulos C, Kokka A and Patsouris E: The influence of
platelet-rich plasma on angiogenesis during the early phase of
tendon healing. Foot Ankle Int. 30:1101–1106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang F, Liu H, Stile F, Lei MP, Pang Y,
Oswald TM, Beck J, Dorsett-Martin W and Lineaweaver WC: Effect of
vascular endothelial growth factor on rat Achilles tendon healing.
Plast Reconstr Surg. 112:1613–1619. 2003. View Article : Google Scholar : PubMed/NCBI
|