Introduction
A hypertrophic scar is a clinically common skin
fibroplasia disease that is caused by an excessive healing response
of the human body to trauma (1). It
is characterized by the abnormal proliferation of fibroblasts,
abundant collagen synthesis and excessive collagen deposition
(2). It was recently reported that
the incidence of hypertrophic scars in burn patients in China
reached 90% (3). Scar formation
affects a patient's appearance, and results in contracture that may
lead to different degrees of dysfunctions including tendon
contracture, joint dislocation and dyskinesia (4,5). At
present, the pathogenesis of hypertrophic scar has not been fully
elucidated. It is widely accepted that fibroblasts are the major
effector cells in hypertrophic scars, and abnormal cell
proliferation and extracellular matrix deposition are the main
characteristics of hypertrophic scars (6). The transforming growth factor
(TGF)-GFmothers against decapentaplegic homolog (SMAD) signaling
pathway is closely associated with a variety of physiological and
pathological processes including collagen metabolism and the
proliferation, differentiation, migration and apoptosis of
fibroblasts (7,8). Therefore, studies on the TGF-β/SMAD
signaling pathway in fibroblasts are important to elucidating the
mechanisms of formation and development in hypertrophic scars.
microRNAs (miRNAs or miRs) form a class of
non-encoding small RNA molecules with 18–24 nucleotides. miRNA
molecules bind with the 3-untranslated region (UTR) of mRNA to
inhibit its translation into proteins, and are associated with the
regulation of pathophysiological processes including cell
proliferation, migration, apoptosis, autophagy, differentiation and
tumor formation (9,10). It has been reported that miRNAs
regulate the formation and repair of skin and its accessory organs,
suggesting that miRNAs serve important roles in hypertrophic scar
(11). In addition, the abnormal
expression of miRNA molecules was identified in hypertrophic scar
tissues, suggesting that miRNA molecules are associated with the
formation of scar tissues (12).
Notably, miR-145 was revealed to be closely associated with
tumorigenesis, myocardial injury and angiogenesis (13). For example, Yuan et al
(14) demonstrated that miR-145
regulates myocardial ischemic injury by inhibiting the expression
of cluster of differentiation (CD)40. Additionally, reduced miR-145
expression in peripheral blood has clinical diagnostic value in
patients with coronary heart disease (15). miR-145 inhibits the proliferation and
migration of lung cancer cells by regulating the expression of
cadherin-2 (16). The aforementioned
study also demonstrated that miR-145 is associated with the
occurrence and development of hypertrophic scars (16). Zhu et al (17) reported that a peroxisome
proliferator-activated receptor-agonist upregulates the expression
of miR-145, and inhibits the TGF-β/SMAD3 signaling pathway and
hypertrophic scar formation. However, the target genes of miR-145
associated with the regulation of hypertrophic scars and the
expression of miR-145 in hypertrophic scar tissues remain unclear.
In the present study, the expression of miR-145 in hypertrophic
scar tissues was measured and its mechanism of action was
investigated.
Materials and methods
Patients
A total of 36 patients (21 males and 15 females) who
were diagnosed with hypertrophic scars and received dermoplasty
between May 2013 and June 2016 at The First Affiliated Hospital
(Hangzhou, China) were included in the present study. The age range
of the patients was 23–43 years and hyperplasia duration was
between 3 months and 2 years. Skin damage sites exhibited hyperemia
redness and lesions that did not exceed wound surface. The
inclusion criteria were: i) Patients with hypertrophic scar tissues
identified by clinicians; ii) patients without pituitary or adrenal
diseases, infectious diseases, skin diseases, immune diseases,
local infection or ulcer; and iii) patients not currently taking
prescribed treatments. Patients with other tumors, chronic basic
disease or a long history of drug intake were excluded.
Hypertrophic scar tissues collected from all patients were used as
the experimental group. For the control group, normal skin tissues
from the hypogastrium of respective patients were used. The tissues
were frozen and stored in liquid nitrogen. All procedures were
approved by the Ethics Committee of Zhejiang University (Hangzhou,
China). Written informed consent was obtained from all patients or
their families.
Cells
Collected hypertrophic scar and control tissue
samples were washed with PBS containing 100 µ/ml penicillin and
streptomycin 3 times prior to removing epidermal and adipose
tissues. The samples were cut into 1 mm3 sections, and
washed with PBS containing 100 µ/ml penicillin and streptomycin 2
times. Subsequently, the samples were placed into culture flasks,
and Dulbecco's modified Eagle medium (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was added prior to incubation
at 37°C and 5% CO2. After 2 h, 2 ml DMEM containing 10%
fetal bovine serum (Thermo Fisher Scientific, Inc.) was added into
the flasks. One week later, spindle shape fibroblasts were observed
around the tissue samples in the flasks under a light microscope
(magnification, ×200). Next, the tissues and culture medium were
discarded, and fresh DMEM with 10% fetal bovine serum was added to
the remaining fibroblasts in the culture flask. Once the cells
reached 80% confluence, they were passaged and cells in 3–5
passages were used for subsequent experiments. A strain of
hypertrophic scar fibroblasts (HSFB) and a strain of normal colonic
fibroblasts (NCFB) were obtained.
Prior to transfection, fibroblasts in log-phase
growth (2×105) were seeded onto 24-well plates
containing antibiotic-free DMEM supplemented with 10% fetal bovine
serum and cultured at 37°C. Once the cells reached 70% confluence,
the cells were collected for transfection.
Transfection
In the first vial, 1.5 µl miR-negative control (NC
group; 20 pmol/µl; Hanbio Biotechnology Co., Ltd., Shanghai,
China), miR-145 inhibitors (20 pmol/µl; the inhibitor group;
5′-GGAUUCCTGGAAATACTGTTCT-3′; Hanbio Biotechnology Co., Ltd.) or
mimics (20 pmol/µl; the mimics group;
5′-GTCCAGTTTTCCCAGGAATCCCT-3′; Hanbio Biotechnology Co., Ltd.) was
mixed with 50 µl Opti-MEM medium (Thermo Fisher Scientific, Inc.).
In another vial, 1 µl Lipofectamine® 3000 Transfection
Reagent (Thermo Fisher Scientific, Inc.) was mixed with 50 µl
Opti-MEM medium. The aforementioned two vials were incubated at
room temperature for 5 min, and then combined prior to incubation
for 20 min at room temperature. The mixtures were subsequently
added to the cells in respective groups at room temperature. A
total of 6 h later, the medium was replaced with DMEM containing
10% fetal bovine serum. After culturing at 37°C for 48 h, the cells
were collected for further assays.
For rescue experiments, fibroblasts
(1×105 cells) in the miR-145 mimics and inhibitors
groups were seeded onto 24-well plates containing antibiotic-free
DMEM supplemented with 10% fetal bovine serum at 37°C. Once the
cells reached 60% confluence, fibroblasts in the miR-145 mimics and
inhibitors groups were transfected with 0.5 µl pLKO.1-sh-sox9 and
pcDNA3.1-SOX-9 plasmids (Hanbio Biotechnology Co., Ltd.),
respectively, using Lipofectamine® 3000, and named the
miR-145 mimics + pLKO.1-sh-sox9 group and the miR-145 inhibitor +
pcDNA3.1-SOX-9 group. After incubation at 37°C and under 5%
CO2 for 6 h, the medium was changed to fresh DMEM
containing 10% fetal bovine serum prior to continued cultivation
for 72 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Hypertrophic scar and control tissues (100 mg) were
ground into powder using liquid nitrogen and lysed with 1 ml
TRIzol™ Reagent (Thermo Fisher Scientific, Inc.). Then, total RNA
was extracted using the phenol chloroform method (18). The purity of RNA was determined by
the A260/A280 ratio using ultraviolet spectrophotometry (NanoDrop™
2000; Thermo Fisher Scientific, Inc.). cDNA was obtained by reverse
transcription from 1 µD RNA and stored at −20°C. Reverse
transcription was performed using miScript II RT kit (Qiagen GmbH,
Hilden, Germany) following the manufacturer's protocol.
The expression of miRNA was determined by miScript
SYBR® Green PCR kit (Qiagen GmbH) using U6 (forward,
5-CTCGCTTCGGCAGCACA-3; reverse, 5-AACGCTTCACGAATTTGCGT-3) as an
internal reference. The reaction mixture (20 µ 20 contained 10 µl
RT-qPCR-Mix, 0.5 µM upstream primer (miR-145,
5-GTCCAGTTCCCAGGAATCCCT-3), 0.5 µ, downstream universal primer
(provided in PCR kit), 2 µk cDNA and 7 µc double-distilled
H2O, and was performed using an iQ5 thermocycler
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The reaction
protocol was as follows: Initial denaturation at 95°C for 10 min,
and 40 cycles of 95°C for 1 min and 60°C for 30 sec. The
2−ΔΔCq method (19) was
used to calculate the relative expression of miR-145 by comparing
it against the expression of the internal reference. Each sample
was tested in triplicate.
Cell counting Kit (CCK)-8 assay
Cells in each group were seeded at a density of
2,000 cells/well in 96-well plates at 37°C. At 0, 24, 48 and 72 h,
20 µl CCK-8 (5 g/l; Beyotime Institute of Biotechnology, Shanghai,
China) was added to the cells and incubated at 37°C for 2 h. The
absorbance of each well was measured at 490 nm and cell
proliferation curves were plotted. Each group was tested in 3
replicate wells and the mean value was calculated.
Flow cytometry
Cells in each group were seeded onto 12-well plates
in triplicate at a density of 2×105 cells/well at 37°C.
A total of 24 h later, the cells were harvested and the cell cycle
was determined using Cycletest™ Plus DNA Reagent kit (BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocol. Briefly, the cells were incubated with 150
µi liquid A at room temperature for 10 min, and 150 µl liquid B for
a further 10 min. Cells were subsequently incubated with 120 µl
liquid C in the dark for 10 min before analysis with a FACSCanto II
Flow Cytometer (BD Biosciences). The result was analyzed using
ModFit software (version 3.2; Verity Software House, Inc., Topsham,
ME, USA).
To detect cell apoptosis, FITC Annexin V Apoptosis
Detection Kit I (BD Biosciences) was used. Briefly, the cells were
trypsinized and centrifuged at 500 × g at room temperature for 3
min. Then, the cells were washed with precooled PBS twice and
centrifuged at 500 × g at room temperature for 3 min. Following the
removal of the supernatant, 5 µl Annexin V-fluorescein
isothiocyanate and 5 µn propidine iodide staining solutions were
added to the cells, and the samples were incubated in dark at room
temperature for 15 min prior to flow cytometry. The result was
analyzed using ModFit software.
Matrigel assay
Matrigel was thawed at 4°C overnight and diluted
with serum-free DMEM medium (dilution 1:2). The mixture (50 µ o was
evenly smeared into the upper chamber of Transwell plates (Merck
KGaA, Darmstadt, Germany) and incubated at 37°C for 1 h. Following
the solidification of the gel, 1×105 cells from each
group were seeded into the upper chamber containing 200 µl
serum-free DMEM medium. In addition, 500 µl DMEM medium
supplemented with 10 % fetal bovine serum was added into the lower
chamber. After 24 h, the chamber was removed and the cells in the
upper chamber were discarded. Following fixation at room
temperature with 4% formaldehyde for 10 min, the membrane was
stained using the Giemsa method (20) for light microscopic observation of 5
random fields (magnification, 200×). The number of Transwell cells
was calculated for the evaluation of cell invasion ability. All
procedures were carried out on ice with pipetting tips being
precooled at 4°C.
Western blotting
Cells in each group were trypsinized and collected.
Then, precooled Radio-Immunoprecipitation Assay (RIPA) lysis buffer
(600 µl; 50 mM Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1% sodium
dodecyl sulfate, 1% TritonX-100, 1% sodium deoxycholate; Beyotime
Institute of Biotechnology) was added to the samples. Following
lysis for 30 min on ice, the mixture was centrifuged at 12,000 × g
and 4°C for 10 min. The supernatant was used to determine protein
concentration by bicinchoninic acid protein concentration
determination kit (cat. no. RTP7102; Real-Times Biotechnology Co.,
Ltd., Beijing, China). Protein samples (6 µl/lane) were then mixed
with 5X SDS loading buffer prior to denaturation in a boiling water
bath for 10 min. The samples were then separated by 10% SDS-PAGE
(100V). The resolved proteins were transferred to polyvinylidene
difluoride membranes on ice (250 mA, 1 h) and blocked with 5%
skimmed milk at room temperature for 1 h. Then, the membranes were
incubated with rabbit anti-human SOX-9 polyclonal (1:1,000; cat.
no. sc-20095) and GAPDH (1:4,000; cat. no. sc-293335; both Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) primary antibodies at
4°C overnight. Following extensive washing with Tween 20 in PBS 5
times (5 min each), the membranes were incubated with goat
anti-rabbit (cat. no. sc-2007) and goat anti-mouse (cat. no.
sc-2039) horseradish peroxidase-conjugated secondary antibodies
(1:4,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature prior to washing with Tween 20 in PBS 5 times (5 min
each). Subsequently, the membrane was developed with the enhanced
chemiluminescence detection kit (Sigma-Aldrich; Merck KGaA) for
imaging. Image lab software (version 3.0; Bio-Rad Laboratories,
Inc.) was used to analyze imaging signals. The relative content of
SOX-9 protein was expressed as a SOX-9/GAPDH ratio.
Dual luciferase reporter assay
According to bioinformatics results (www.targetscan.com), wild-type (WT) and mutant seed
regions of miR-145 in the 3′-UTR of SOX-9 gene were chemically
synthesized in vitro. The seed regions were cut with
Spe−1 and HindIII restriction sites, and then cloned
into pMIR-REPORT luciferase reporter plasmids (Thermo Fisher
Scientific, Inc.). Plasmids (0.5 µg) with WT or mutant 3′-UTR DNA
sequences were co-transfected with miR-145 mimics into 293T cells
(Type Culture Collection of the Chinese Academy of Sciences,
Shanghai, China) using Lipofectamine 3000 (Thermo Fisher
Scientific, Inc.). After cultivating at 37°C for 24 h, the cells
were lysed using dual luciferase reporter assay kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol, and fluorescence intensity was measured using a GloMax
20/20 luminometer (Promega Corporation). Using Renilla
luciferase activity as an internal reference, the luciferase
activity of each group of cells was measured.
Statistical analysis
Results were analyzed using SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Data are presented as mean
± standard deviation. The differences between datasets containing
multiple groups were analyzed using one-way analysis of variance
followed by Tukey's test. Differences between datasets containing
two groups were analyzed using a Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
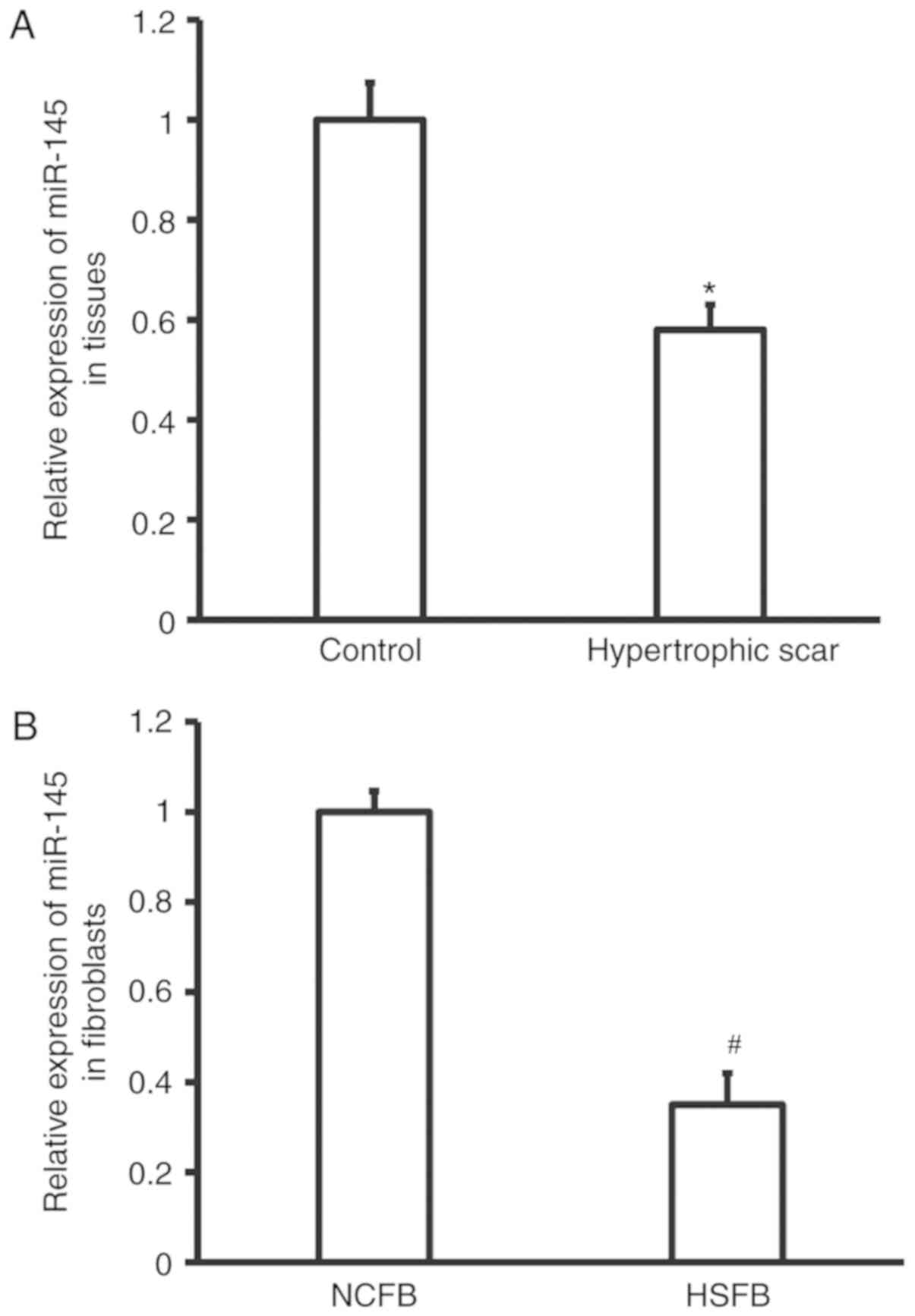
Expression of miR-145 is reduced in
hypertrophic tissues and fibroblasts
To measure the expression of miR-145 in hypertrophic
scar tissues and fibroblasts, RT-qPCR analyses were performed. The
data demonstrated that the expression of miR-145 in hypertrophic
scar tissues was significantly reduced compared with that of the
control tissues (P<0.05; Fig.
1A). Similarly, the expression of miR-145 in HSFB was
significantly lower than that in NCFB (P<0.05; Fig. 1B). These results demonstrate that
expression of miR-145 is reduced in hypertrophic tissues and
fibroblasts.
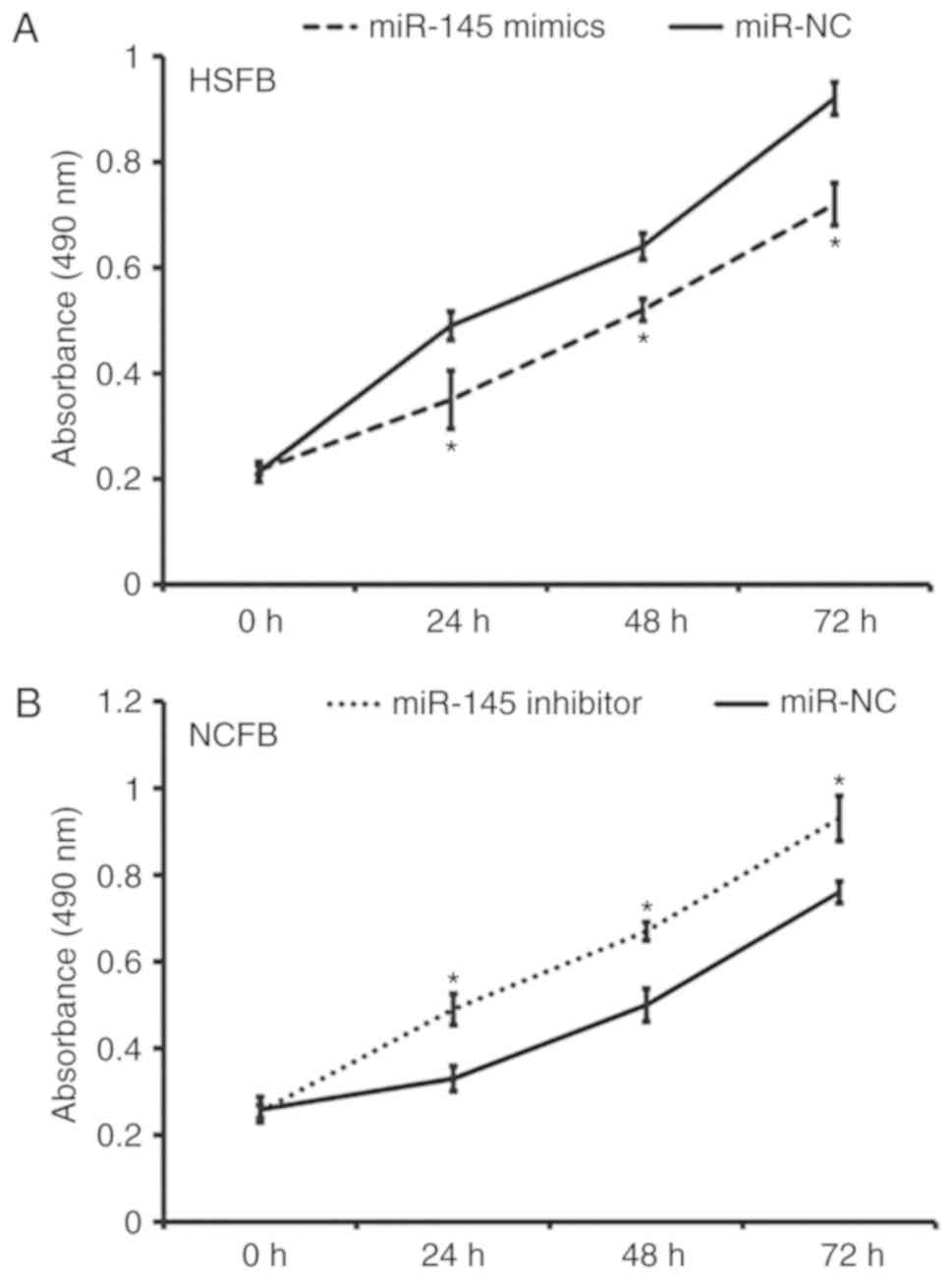
Overexpression of miR-145 inhibits the
proliferation of fibroblasts in vitro
To determine fibroblast proliferation, a CCK-8 assay
was performed. The data revealed that the absorbance of HSFB
transfected with miR-145 mimics was significantly lower than that
of HSFB transfected with miR-NC at 24, 48 and 72 h (all P<0.05;
Fig. 2A). In addition, the
absorbance of NCFB transfected with miR-145 inhibitor was
significantly higher than that of NCFB transfected with miR-NC at
24, 48 and 72 h (all P<0.05; Fig.
2B). These results indicate that miR-145 overexpression
inhibits the proliferation of fibroblasts in vitro.
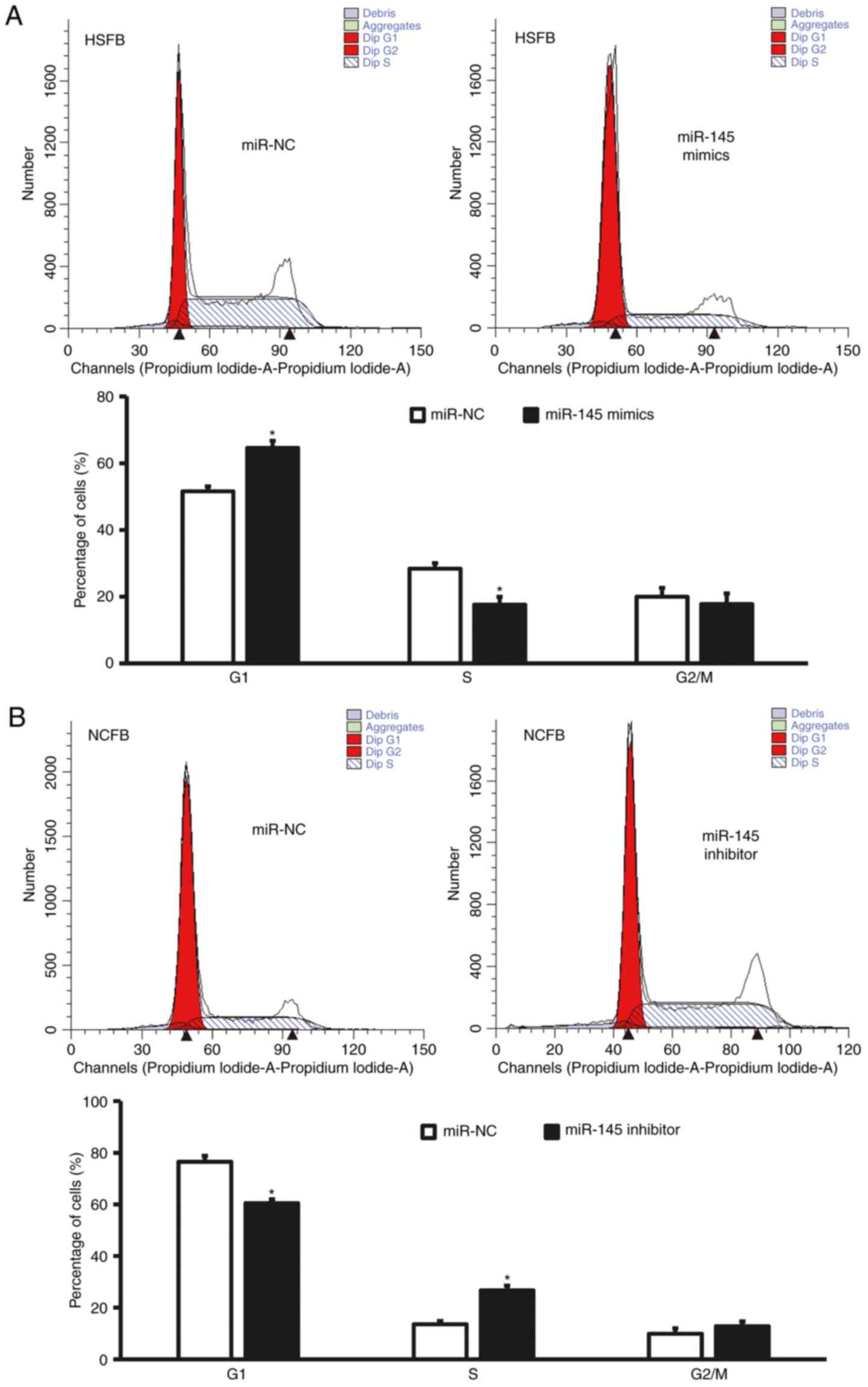
Overexpression of miR-145 delays G1/S
phase transition of fibroblasts
For cell cycle determination, flow cytometry was
performed. The data revealed that G1/S phase transition of HSFB
transfected with miR-145 mimics was reduced (P<0.05; Fig. 3A), whereas G1/S phase transition of
NCFB transfected with miR-145 inhibitors was promoted (P<0.05;
Fig. 3B). These result suggest that
overexpression of miR-145 delays G1/S phase transition of
fibroblasts.
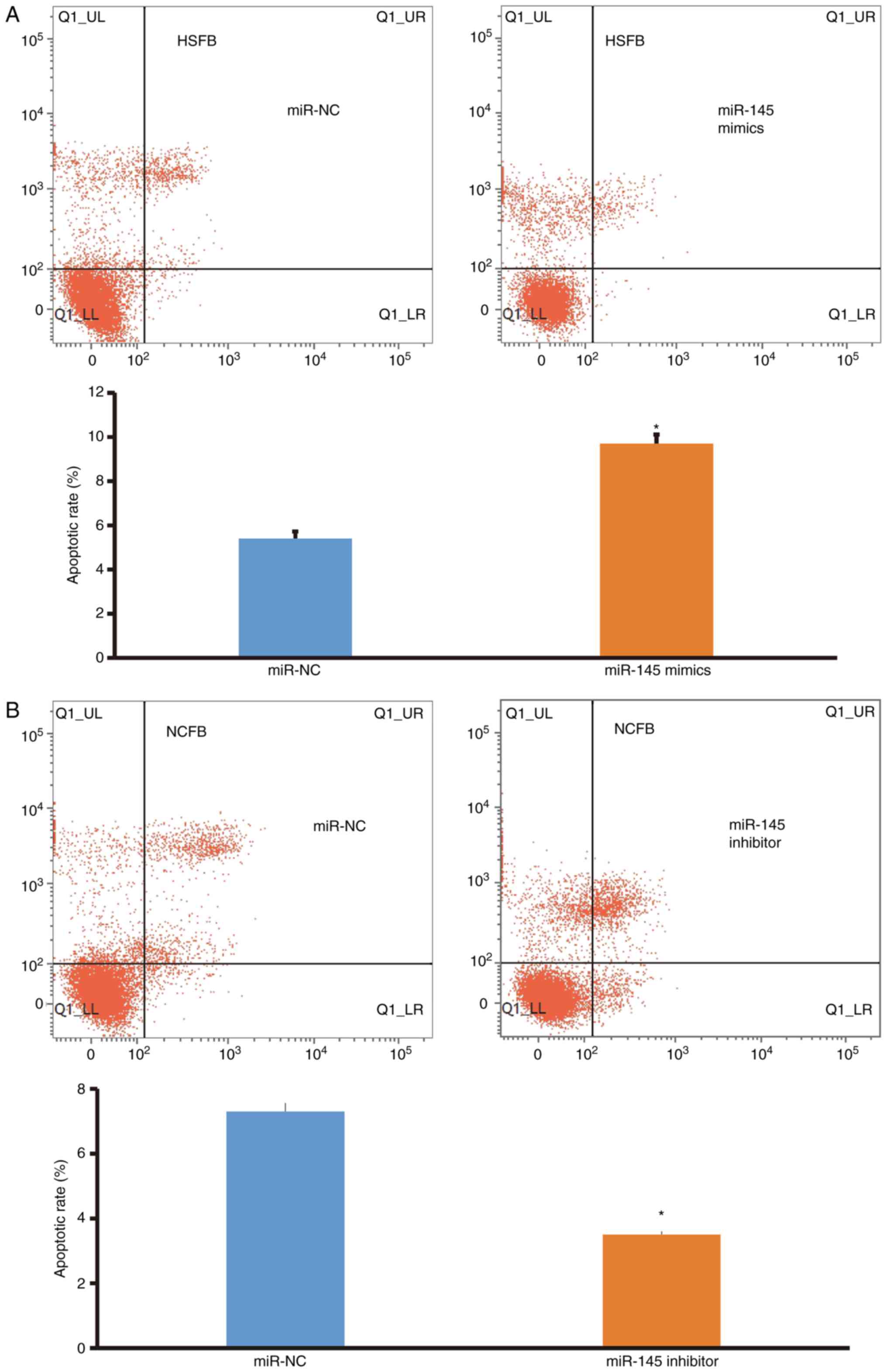
Overexpression of miR-145 promotes
apoptosis of fibroblasts
To examine cell apoptosis, flow cytometry was
performed. The data demonstrated that the apoptotic rate of HSFB
transfected with miR-145 mimics was significantly higher than that
in the miR-NC group (P<0.05; Fig.
4A), whereas the apoptotic rate of NCFB transfected with
miR-145 inhibitors was significantly lower than that in the miR-NC
group (P<0.05; Fig. 4B). These
results indicate that overexpression of miR-145 promotes the
apoptosis of fibroblasts.
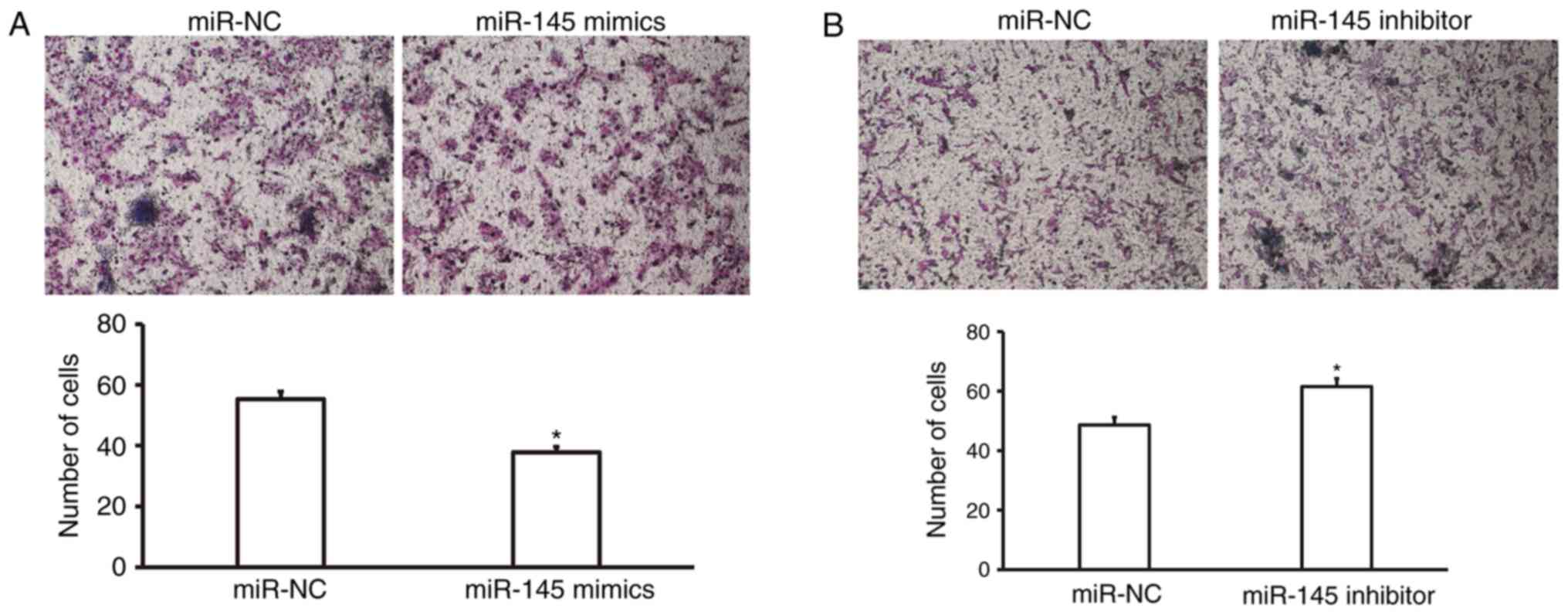
Overexpression of miR-145 suppresses
invasion of fibroblasts
To determine the invasion ability of fibroblasts, a
Matrigel assay was employed. The data revealed that the number of
HSFB that crossed the membrane in the miR-145 mimics group was
significantly lower than that in the miR-NC group (P<0.05;
Fig. 5A). In addition, the number of
NCFB that crossed the membrane in the miR-145 inhibitor group was
significantly higher than that in the miR-NC group (P<0.05;
Fig. 5B). These results suggest that
overexpression of miR-145 suppresses invasion of fibroblasts.
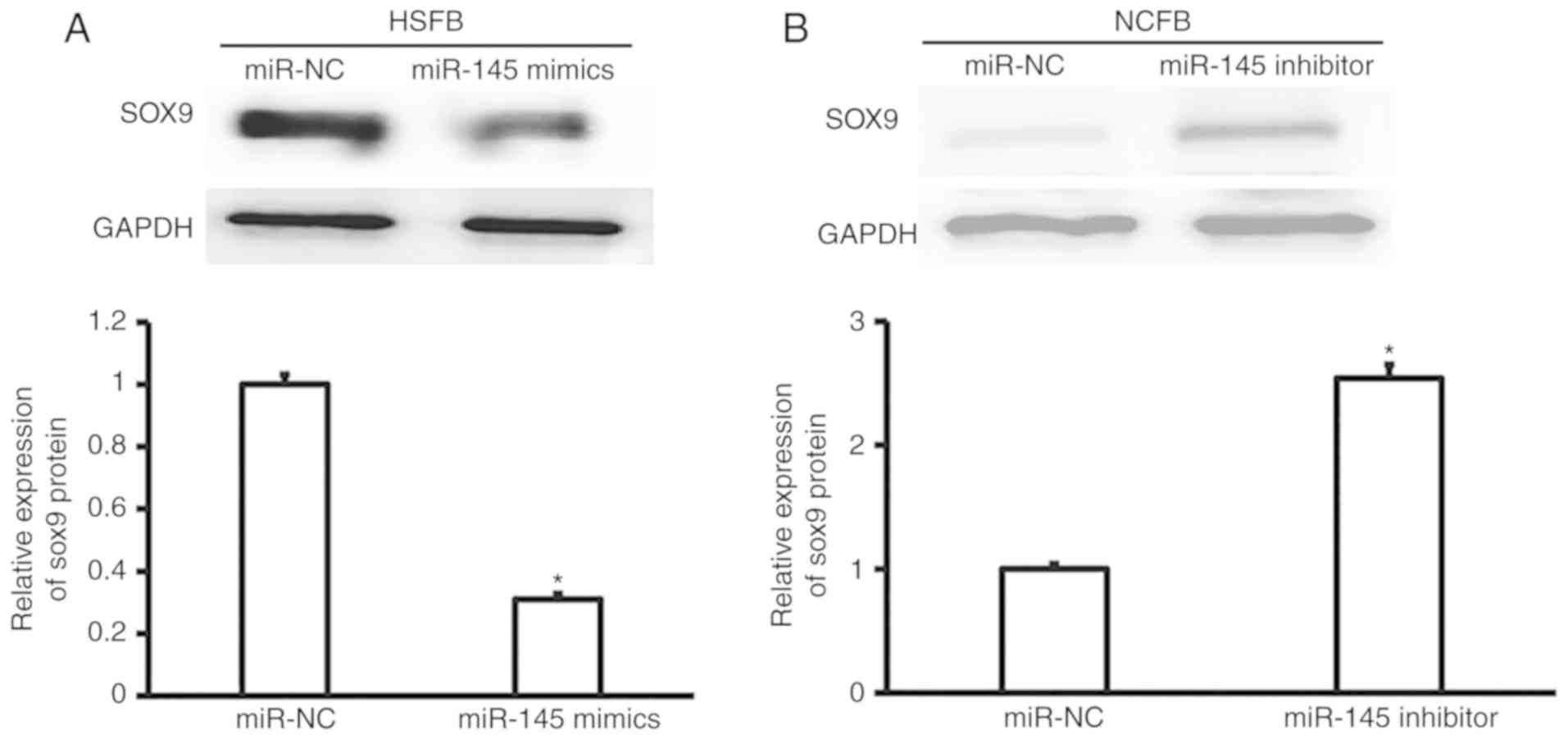
Overexpression of miR-145 may inhibit
SOX-9 protein expression
To measure the expression of SOX-9 protein, western
blotting was performed. The data demonstrated that the expression
of SOX-9 in hypertrophic fibroblasts transfected with miR-145
mimics was significantly reduced compared with that in hypertrophic
fibroblasts transfected with miR-NC (P<0.05; Fig. 6A). By contrast, the expression of
SOX-9 in normal fibroblasts transfected with miR-145 inhibitors was
significantly enhanced compared with that in normal fibroblasts
transfected with miR-NC (P<0.05; Fig.
6B). The results indicate that overexpression of miR-145
inhibits SOX-9 protein expression.
Expression of SOX-9 can reverse the
effects of miR-145 on the proliferation, cell cycle, apoptosis and
invasion of fibroblasts
To test whether miR-145 affects the biological
functions of fibroblasts by regulating the expression of SOX-9
protein, CCK-8, flow cytometry and Matrigel assay were performed.
These assays analyzed the proliferation, cell cycle determination,
apoptosis rate and invasion of fibroblasts transfected with miR-145
mimics or inhibitors following the overexpression of the SOX-9
protein by a plasmid containing the SOX-9 gene or the silencing of
the SOX-9 protein by a plasmid containing a SOX-9 shRNA,
respectively. The CCK-8 assay revealed that the downregulation of
SOX-9 expression by the SOX-9 shRNA in miR-145
inhibitor-transfected NCFB significantly decreased cell
proliferation compared with NCFB transfected with miR-145
inhibitors alone and miR-NC (P<0.05; Fig. 7A), whereas the overexpression of
SOX-9 by the plasmid containing the SOX-9 gene in miR-145
mimic-transfected HSFB significantly decreased cell proliferation
compared with HSFB transfected with miR-145 mimics alone and miR-NC
(P<0.05; Fig. 7B). Flow cytometry
demonstrated that the overexpression of SOX-9 significantly
enhanced the G1/S phase transition of HSFB transfected with miR-145
mimics compared with HSFB transfected with miR-145 mimics alone and
miR-NC (P<0.05; Fig. 7C), whereas
downregulation of SOX-9 expression significantly decreased the G1/S
phase transition of NCFB transfected with miR-145 inhibitors
compared with NCFB transfected with miR-145 inhibitors alone and
miR-NC (P<0.05; Fig. 7D).
Overexpression of SOX-9 significantly decreased the apoptotic rate
of HSFB transfected with miR-145 mimics compared with HSFB
transfected with miR-145 mimics alone and miR-NC (P<0.05;
Fig. 7E) and the downregulation of
SOX-9 expression significantly decreased the apoptotic rate of NCFB
transfected with miR-145 inhibitors compared with NCFB transfected
with miR-145 inhibitors alone and miR-NC (P<0.05; Fig. 7F). The Matrigel assay demonstrated
that the overexpression of SOX-9 significantly increased the number
of transmembrane HSFB transfected with miR-145 mimics compared with
HSFB transfected with miR-145 mimics alone and miR-NC (P<0.05;
Fig. 7G) and the down-regulation of
SOX-9 expression significantly decreased the number of
transmembrane NCFB transfected with miR-145 inhibitors compared
with NCFB transfected with miR-145 inhibitors alone and miR-NC
(P<0.05; Fig. 7H). These results
demonstrate that the expression of SOX-9 reverses the effects of
miR-145 on the proliferation, cell cycle determination, apoptosis
and invasion of fibroblasts.
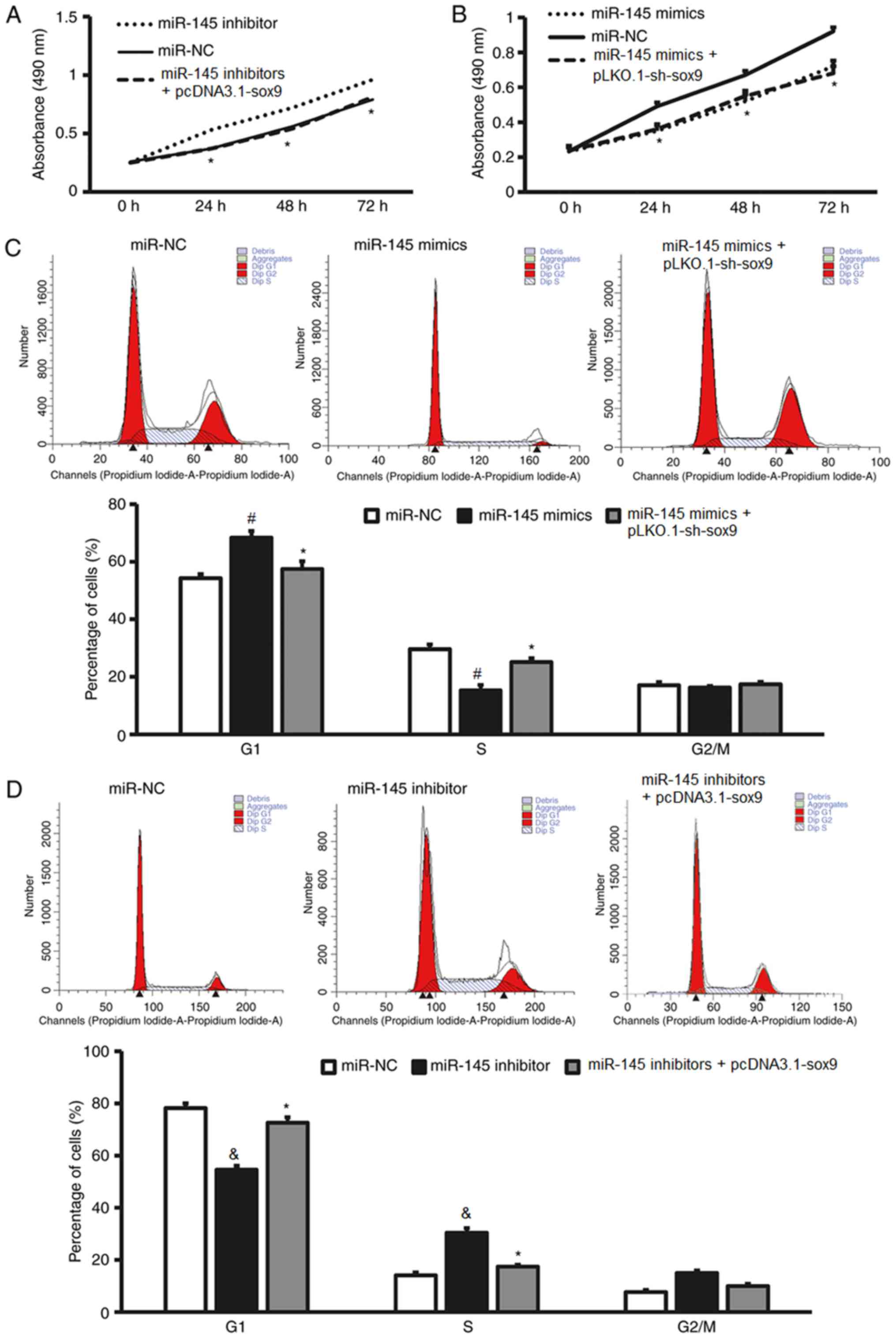 | Figure 7.Increased SOX-9 expression reverses
effect of miR-145 mimics on the proliferation, cell cycle,
apoptosis and invasion of fibroblasts. (A) The proliferation of
NCFB transfected with miR-145 inhibitors or miR-NC, as determined
by a CCK-8 assay. (B) The proliferation of HSFB transfected with
miR-145 mimics or miR-NC, as determined by a CCK-8 assay. (C) Flow
cytomery results and the percentages of HSFB transfected with
miR-NC, miR-145 mimics or miR-145 mimics + pLKO.1-sh-sox9 in each
cell cycle phase. (D) Flow cytomery results and the percentages of
NCFB transfected with miR-NC, miR-145 inhibitor or miR-145
inhibitors + pcDNA3.1-sox9 in each cell cycle phase. *P<0.05 vs.
miR-NC; #P<0.05 vs. miR-145 mimics + pLKO.1-sh-sox9
group; &P<0.05 vs. miR-145 inhibitors +
pcDNA3.1-sox9 group. Increased SOX-9 expression reverses effect of
miR-145 mimics on the proliferation, cell cycle, apoptosis and
invasion of fibroblasts. (E) The apoptotic rate of HSFB transfected
with miR-NC, miR-145 mimics or miR-145 mimics + pLKO.1-sh-sox9, as
determined by flow cytometry. (F) The apoptotic rate of NCFB
transfected with miR-NC, miR-145 inhibitor or miR-145 inhibitors +
pcDNA3.1-sox9, as determined by flow cytometry. (G) Matrigel
invasion assay images and the number of transmembrane HSFB
transfected with miR-NC, miR-145 mimics or miR-145 mimics +
pLKO.1-sh-sox9. (H) Matrigel invasion assay images and the number
of transmembrane NCFB transfected with miR-NC, miR-145 inhibitor or
miR-145 inhibitors + pcDNA3.1-sox9. *P<0.05 vs. miR-NC;
#P<0.05 vs. miR-145 mimics + pLKO.1-sh-sox9 group;
&P<0.05 vs. miR-145 inhibitors + pcDNA3.1-sox9
group. CCK-8, Cell Counting Kit-8; miR, microRNA; NC, negative
control; HSFB, hypertrophic scar fibroblasts; NCFB, normal colonic
fibroblasts. |
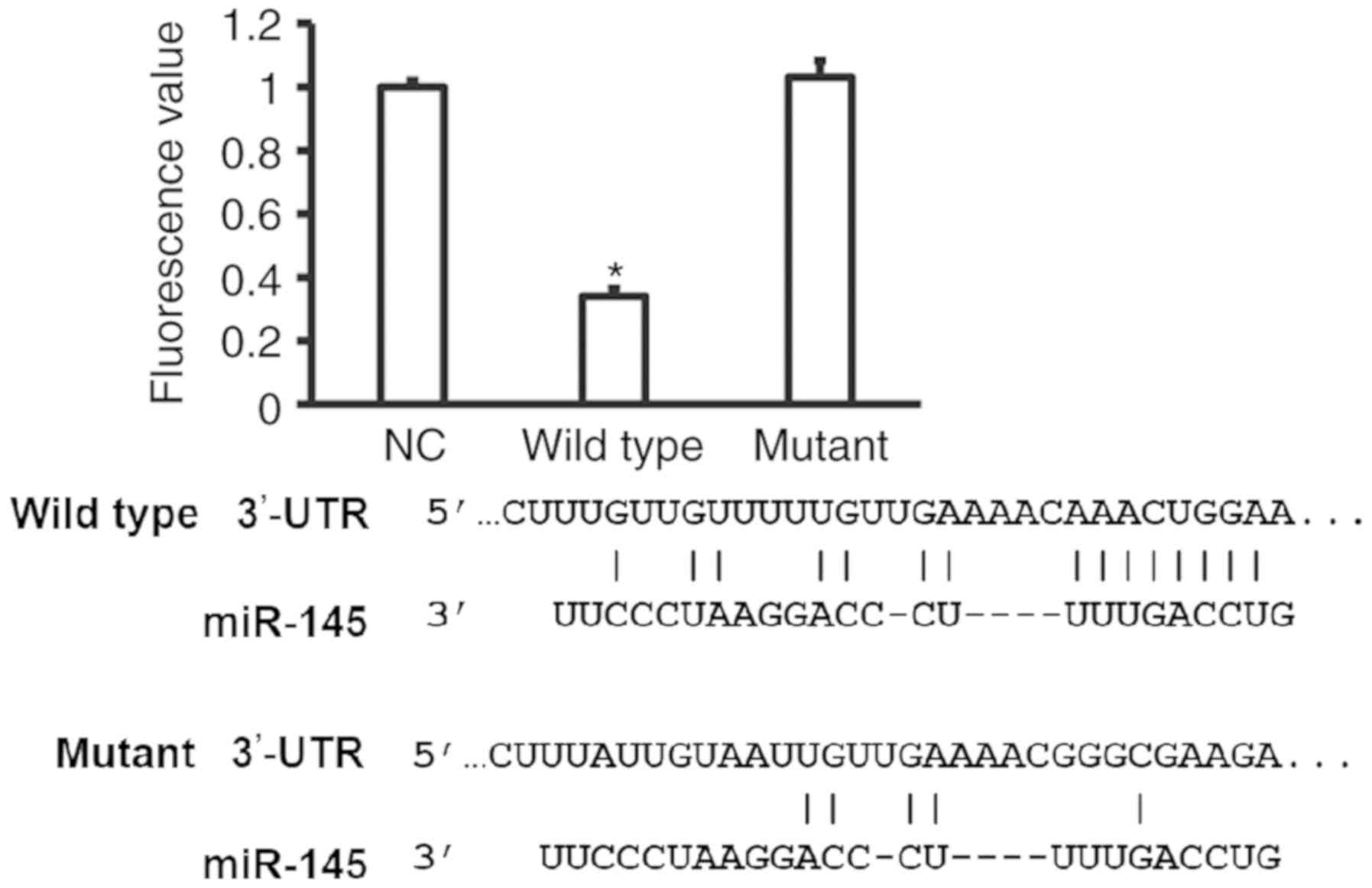
miR-145 binds with the 3′-UTR of the
SOX-9 mRNA to regulate SOX-9 protein expression
To identify the interaction between the seed region
of miR-145 and the 3′-UTR of SOX-9 mRNA, a dual luciferase reporter
assay was performed. The fluorescence value of cells co-transfected
with miR-145 mimics and pMIR-REPORT-WT luciferase reporter plasmids
was significantly lower than that in the negative control group
(P<0.05; Fig. 8). By contrast, no
significant differences were identified between the fluorescence
value of cells co-transfected with miR-145 mimics and
pMIR-REPORT-mutant luciferase reporter plasmids, and that of the NC
group. The result indicates that the miR-145 seed region can bind
with the 3′-UTR of the SOX-9 mRNA to regulate SOX-9 protein
expression.
Discussion
Fibroblasts are the major effector cells in wound
healing (21). In the process of
hypertrophic scar formation, fibroblasts typically exhibit
excessive proliferation, enhanced migration and inhibited
apoptosis, and secrete abundant extracellular matrix (22,23). It
was previously identified that miRNA molecules serve important
roles in the biological functions of hypertrophic scar fibroblasts,
but their mechanisms of action requires further elucidation
(24). In the present study, it was
identified that miR-145 expression is significantly downregulated
in hypertrophic scar tissues, suggesting that miR-145 may be
associated with the occurrence and development of hypertrophic
scar. In vitro experiments revealed that miR-145 inhibits
the proliferation, invasion and G1/S phase transition of
fibroblasts, and promotes the apoptosis of fibroblasts.
Bioinformatics and molecular biology experiments demonstrated that
miR-145 exerts these effects by regulating SOX-9 gene expression.
Therefore, miR-145 may be a potential therapeutic target in the
treatment of hypertrophic scar.
As an important class of post-transcriptional
regulators, miRNA molecules are widely associated with the
proliferation, aging, apoptosis and migration of skin fibroblasts
(25). For example, Xie et al
(26) revealed that miR-377
facilitates the aging of skin fibroblasts by targeting the DNA
(cytosine-5)-methyltransferase 1 mRNA. Zeng et al (27) demonstrated that miR-27b inhibits the
activation of fibroblasts by regulating the TGF-β signaling
pathway. Li et al (28)
reported that miR-19 inhibits the release of cytokines from
fibroblasts by targeting Toll-like receptor 2 mRNA. In vitro
experiments demonstrated that miR-145 expression in HSFB is
significantly lower than that in NCFB, suggesting that miR-145 has
regulatory roles in biological functions of fibroblasts. It was
reported that miR-145 regulates the TGF-βGF-rted that miR-145
regulates is significantly lower than that in NCFB, subcomponents
by fibroblasts and the formation of hypertrophic scar (17). The aforementioned study is consistent
with the results of the current study.
An miRNA may regulate multiple target genes
(29). A recent study revealed that
miR-145 targets SMAD3 expression and exerts its biological
functions in fibroblasts (14).
However, it remains unclear whether miR-145 regulates hypertrophic
scar formation via other target mRNA. SOX-9 is a member of the SOX
gene family, which is closely associated with the proliferation,
apoptosis and differentiation of cells (30). For example, SOX-9 gene transcription
regulates the activity of the wnt signaling pathway in intestinal
epithelial stem cells (31). In
addition, SOX-9 promotes the proliferation, migration and
differentiation of multiple tumor cells, including lung cancer,
thyroid carcinoma and gastric cancer (32). Furthermore, SOX-9 mRNA is regulated
by several miRNA molecules. It was recently demonstrated that
miR-105 inhibits the occurrence and development of glioma by
targeting SOX-9 (33). miR-592 also
suppresses the proliferation and metastasis of non-small cell lung
cancer by downregulating the expression of SOX-9 (34). It was also reported that miR-124,
miR-30a and miR-494 directly regulate the expression of the SOX-9
gene (35–37). In the present study, bioinformatics
revealed that SOX-9 is a potential target gene of miR-145, and that
miR-145 expression is negatively associated with SOX-9 expression.
Notably, rescue experiments demonstrate that miR-145 exerts its
biological functions in fibroblasts by regulating SOX-9.
Importantly, dual luciferase reporter assay identified that SOX-9
is a direct target gene of miR-145.
In conclusion, the present study demonstrated that
miR-145 expression is downregulated in hypertrophic scar tissues.
In addition, miR-145 inhibited fibroblast proliferation and
invasion, and promoted the apoptosis of fibroblasts by targeting
SOX-9 expression. Therefore, it is suggested that miR-145 regulates
the occurrence and development of hypertrophic scars. The results
of the current study may provide a potential target for the
clinical diagnosis and treatment of hypertrophic scars.
Acknowledgements
The authors would like to thank Dr Weifang Zhu from
Department of Dermatology, The First Affiliated Hospital, Zhejiang
University.
Funding
The present study was supported by Zhejiang
University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW and JQ designed the current study. SW, CL and YY
performed the experiments. SW, CL and JQ analyzed the data. SW and
JQ interpreted results and prepared the manuscript. The final
version of the manuscript has been read and approved by all
authors.
Ethics and consent to participate
All procedures performed in the current study were
approved by the Ethics Committee of Zhejiang University. Written
informed consent was obtained from all patients or their families
prior to enrollment.
Patient consent for publication
Written informed consent for the publication of
associated data and accompanying images were obtained from all
patients or their parents, guardians or next of kin.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Füller J and Müller-Goymann CC:
Anti-proliferative and anti-migratory effects of hyperforin in 2D
and 3D artificial constructs of human dermal fibroblasts-A new
option for hypertrophic scar treatment? Eur J Pharm Biopharm.
126:108–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu DQ, Li XJ and Wenj XJ: Effect of BTXA
on inhibiting hypertrophic scar formation in a rabbit ear model.
Aesthetic Plast Surg. 41:721–728. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo L, Xu K, Yan H, Feng H, Wang T, Chai L
and Xu G: MicroRNA expression signature and the therapeutic effect
of the microRNA21 antagomir in hypertrophic scarring. Mol Med Rep.
15:1211–1221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ai JW, Liu JT, Pei SD, Liu Y, Li DS, Lin
HM and Pei B: The effectiveness of pressure therapy (15–25 mmHg)
for hypertrophic burn scars: A systematic review and meta-analysis.
Sci Rep. 7:401852017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen YY, Lu YH, Ma CH, Tao WW, Zhu JJ and
Zhang X: A novel elastic liposome for skin delivery of papain and
its application on hypertrophic scar. Biomed Pharmacother.
87:82–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carney BC, Liu Z, Alkhalil A, Travis TE,
Ramella-Roman J, Moffatt LT and Shupp JW: Elastin is differentially
regulated by pressure therapy in a porcine model of hypertrophic
scar. J Burn Care Res. 38:28–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Yang L, Zhang Y and Gao Z:
Kaempferol inhibits fibroblast collagen synthesis, proliferation
and activation in hypertrophic scar via targeting TGF-β receptor
type I. Biomed Pharmacother. 83:967–974. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong JY, Song F, Liu YK and Wang XQ:
Effects of severe hypoxia and low concentration of serum protein on
the function of human hypertrophic scar fibroblasts. Zhonghua Shao
Shang Za Zhi. 32:594–598. 2016.(In Chinese). PubMed/NCBI
|
|
9
|
Liu J, Xiao X, Shen Y, Chen L, Xu C, Zhao
H, Wu Y, Zhang Q, Zhong J, Tang Z, et al: MicroRNA-32 promotes
calcification in vascular smooth muscle cells: Implications as a
novel marker for coronary artery calcification. PLoS One.
12:e01741382017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng Y, Wang Z and Zhou Z: miRNAs: Novel
regulators of autoimmunity-mediated pancreatic β-cell destruction
in type 1 diabetes. Cell Mol Immunol. 14:488–496. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herter EK and Xu Landén N: Non-coding
RNAs: New players in skin wound healing. Adv Wound Care (New
Rochelle). 6:93–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mu S, Kang B, Zeng W, Sun Y and Yang F:
MicroRNA-143-3p inhibits hyperplastic scar formation by targeting
connective tissue growth factor CTGF/CCN2 via the Akt/mTOR pathway.
Mol Cell Biochem. 416:99–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim H, Banerjee N, Sirven MA, Minamoto Y,
Markel ME, Suchodolski JS, Talcott ST and Mertens-Talcott SU:
Pomegranate polyphenolics reduce inflammation and ulceration in
intestinal colitis-involvement of the miR-145/p70S6K1/HIF1a axis in
vivo and in vitro. J Nutr Biochem. 43:107–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan M, Zhang L, You F, Zhou J, Ma Y, Yang
F and Tao L: MiR-145-5p regulates hypoxia-induced inflammatory
response and apoptosis in cardiomyocytes by targeting CD40. Mol
Cell Biochem. 431:123–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faccini J, Ruidavets JB, Cordelier P,
Martins F, Maoret JJ, Bongard V, Ferrières J, Roncalli J, Elbaz M
and Vindis C: Circulating miR-155, miR-145 and let-7c as diagnostic
biomarkers of the coronary artery disease. Sci Rep. 7:429162017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mo D, Yang D, Xiao X, Sun R, Huang L and
Xu J: MiRNA-145 suppresses lung adenocarcinoma cell invasion and
migration by targeting N-cadherin. Biotechnol Lett. 39:701–710.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu HY, Li C, Zheng Z, Zhou Q, Guan H, Su
LL, Han JT, Zhu XX, Wang SY, Li J and Hu DH: Peroxisome
proliferator-activated receptor-γ (PPAR-γ) agonist inhibits
collagen synthesis in human hypertrophic scar fibroblasts by
targeting Smad3 via miR-145. Biochem Biophys Res Commun. 459:49–53.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmad J, Baig MA, Ali AA, Al-Huqail A,
Ibrahim MM and Qureshi MI: Comparative assessment of four RNA
extraction methods and modification to obtain high-quality RNA from
Parthenium hysterophorus leaf. 3 Biotech. 7:3732017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu J, Zhang D, Li J, Deng X, Liang G, Long
Y, He X, Dai T and Ren D: MACC1 induces autophagy to regulate
proliferation, apoptosis, migration and invasion of squamous cell
carcinoma. Oncol Rep. 38:2369–2377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seo GY, Lim Y, Koh D, Huh JS, Hyun C, Kim
YM and Cho M: TMF and glycitin act synergistically on keratinocytes
and fibroblasts to promote wound healing and anti-scarring
activity. Exp Mol Med. 49:e3022017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Kuang F, Liu CL, Chen B, Tang WB
and Li XJ: Effects of silencing Smad ubiquitination regulatory
factor 2 on the function of human hypertrophic scar-derived
fibroblasts. Zhonghua Shao Shang Za Zhi. 33:145–151. 2017.(In
Chinese). PubMed/NCBI
|
|
23
|
Shen C, Jiang L, Shao H, You C, Zhang G,
Ding S, Bian T, Han C and Meng Q: Targeted killing of
myofibroblasts by biosurfactant di-rhamnolipid suggests a therapy
against scar formation. Sci Rep. 6:375532016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pfaff N, Liebhaber S, Möbus S, Beh-Pajooh
A, Fiedler J, Pfanne A, Schambach A, Thum T, Cantz T and Moritz T:
Inhibition of miRNA-212/132 improves the reprogramming of
fibroblasts into induced pluripotent stem cells by de-repressing
important epigenetic remodelling factors. Stem Cell Res. 20:70–75.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Do DN, Li R, Dudemaine PL and
Ibeagha-Awemu EM: MicroRNA roles in signalling during lactation: An
insight from differential expression, time course and pathway
analyses of deep sequence data. Sci Rep. 7:446052017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie HF, Liu YZ, Du R, Wang B, Chen MT,
Zhang YY, Deng ZL and Li J: miR-377 induces senescence in human
skin fibroblasts by targeting DNA methyltransferase 1. Cell Death
Dis. 8:e26632017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng X, Huang C, Senavirathna L, Wang P
and Liu L: miR-27b inhibits fibroblast activation via targeting
TGFβ signaling pathway. BMC Cell Biol. 18:92017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Cai J and Cao X: MiR-19 suppresses
fibroblast-like synoviocytes cytokine release by targeting toll
like receptor 2 in rheumatoid arthritis. Am J Transl Res.
8:5512–5518. 2016.PubMed/NCBI
|
|
29
|
Wang T, O'Brien EC, Rogers JG, Jacoby DL,
Chen ME, Testani JM, Bowles DE, Milano CA, Felker GM, Patel CB, et
al: Plasma levels of MicroRNA-155 Are Upregulated with long-term
left ventricular assist device support. ASAIO J. 63:536–541. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohnesorg T, van den Bergen JA, Belluoccio
D, Shankara-Narayana N, Kean AM, Vasilaras A, Ewans L, Ayers KL and
Sinclair AH: A duplication in a patient with 46,XX ovo-testicular
disorder of sex development refines the SOX9 testis-specific
regulatory region to 24 kb. Clin Genet. 92:347–349. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang CZ, Xu JH, Zhong W, Xia ZS, Wang SY,
Cheng D, Li JY, Wu TF, Chen QK and Yu T: Sox9 transcriptionally
regulates Wnt signaling in intestinal epithelial stem cells in
hypomethylated crypts in the diabetic state. Stem Cell Res Ther.
8:602017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bremmer F, Behnes CL, Schildhaus HU, Gaisa
NT, Reis H, Jarry H, Radzun HJ, Stroebel P and Schweyer S: The role
of beta-catenin mutation and SOX9 expression in sex cord-stromal
tumours of the testis. Virchows Arch. 470:421–428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Wang H, Zhu Z, Ye Y, Mao H and
Zhang S: MicroRNA-105 targets SOX9 and inhibits human glioma cell
progression. FEBS Lett. 590:4329–4342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Z, Li B, Niu L and Ge L: miR-592
functions as a tumor suppressor in human non-small cell lung cancer
by targeting SOX9. Oncol Rep. 37:297–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X, Liu Y, Liu X, Yang J, Teng G,
Zhang L and Zhou C: MiR-124 inhibits cell proliferation, migration
and invasion by directly targeting SOX9 in lung adenocarcinoma.
Oncol Rep. 35:3115–3121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang T, Xie J, Li H, Li D, Liu P and Hu
Y: MicroRNA-30a promotes extracellular matrix degradation in
articular cartilage via downregulation of Sox9. Cell Prolif.
49:207–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Wang L, Liu Z, Zu C, Xing F, Yang P,
Yang Y, Dang X and Wang K: MicroRNA-494 inhibits cell proliferation
and invasion of chondrosarcoma cells in vivo and in vitro by
directly targeting SOX9. Oncotarget. 6:26216–26229. 2015.PubMed/NCBI
|