Introduction
Cervical cancer ranks third highest in incidence
rate and fourth in cancer-associated mortality among women
worldwide, with an estimated >500,000 new cases and 260,000
deaths caused by cervical cancer each year (1). In developing countries, the morbidity
and mortality of cervical cancer has gradually increased, primarily
due to the lack of screening programs, diagnosis methods and
effective therapeutic methods (2).
High-risk human papillomavirus (HPV) infection has been identified
as a key attribute among the numerous risk factors associated with
cervical cancer; however, HPV infection alone is inadequate to
cause carcinogenesis (3). Extensive
basic and clinical research has significantly facilitated the
development of novel diagnostic and therapeutic techniques for
patients with cervical cancer (4);
unfortunately, the prognosis remains dissatisfactory, with an
overall survival rate of ~30–50% for patients at an advanced stage
of disease (5). Thus, further
studies are critical in order to understand the molecular
mechanisms that underlie cervical cancer occurrence and development
in order to improve outcomes for patients with this malignancy.
MicroRNAs (miRNAs) are a large family of endogenous,
non-coding, short RNA molecules that are ~22 nucleotides in length
(6). miRNAs have been identified as
critical gene regulators via directly binding to complementary
sequences in the 3′-untranslated regions (3′-UTRs) of their target
genes, resulting in translation suppression and/or mRNA degradation
(7). It is well documented that one
single miRNA is able to modulate numerous target genes, and ~30% of
human protein-coding genes are predicted to be regulated by miRNAs
(8,9). Previous studies focusing on miRNA
expression revealed that a variety of miRNAs were dysregulated
during cervical carcinogenesis and progression, such as miR-152
(10), miR-224 (11), miR-302 (12) and miR-1297 (13). Aberrantly expressed miRNAs can affect
the aggressive behaviors of cervical cancer cells through acting as
oncogenes or tumor suppressors (14). Therefore, a complete understanding of
the abnormally expressed miRNAs in cervical cancer progression is
important for the identification of potential therapeutic targets
for treating patients with this disease.
Previous studies have indicated that miR-877 was
downregulated in hepatocellular carcinoma (15,16) and
renal cell carcinoma (17), and
functioned as a tumor-suppressive miRNA. However, it has rarely
been reported how miR-877 exerts an effect in cervical cancer
progression and its underlying molecular mechanisms. Hence, the
present study detected the expression levels of miR-877 in cervical
cancer and determined its clinical significance. In addition, the
effects of miR-877 in the malignant behaviors of human cervical
cancer cells and involved mechanisms were investigated.
Materials and methods
Tissue collection
The present study was approved by the Ethics
Committee of Jilin Cancer Hospital (Changchun, China). All patients
enrolled in the present study provided written informed consent
based on the principles of the Declaration of Helsinki. A total of
57 pairs of cervical cancer tissues and matched adjacent normal
tissues (2 cm away from tumor tissues) were collected from patients
(age range: 43–68 years old) in the Jilin Cancer Hospital between
March 2015 and August 2017, and were confirmed as cervical cancer
via analysis by a pathologist. Patients diagnosed as cervical
cancer and had not been treated with radiotherapy or chemotherapy
prior to surgery participated in the current study. All patients
were divided into two groups, a low miR-877 expression group and a
high miR-877 expression group, based on the median value of
miR-877. All tissues were frozen immediately in liquid nitrogen
following surgical resection and then stored at −80°C until further
use.
Cell lines and culture
A total of four human cervical cancer cell lines
(CaSki, HeLa, C-33A and SiHa) and a normal human cervix epithelial
cell line (Ect1/E6E7) were purchased from the American Type Culture
Collection. All cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1%
antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin; all
from Invitrogen; Thermo Fisher Scientific, Inc.). All cultures were
maintained at 37°C in an incubator containing 5% CO2
until further use.
Oligonucleotides, plasmids and
transfection
Cells were plated in six-well plates at a density of
5×106 cells per well. Cells were transfected with
miR-877 mimics (Shanghai GenePharma Co., Ltd.) to increase miR-877
expression. The small interfering RNA (siRNA) targeting MACC1
(MACC1 siRNA; Guangzhou RiboBio Co., Ltd.) was introduced into
cells to knockdown endogenous MACC1 expression. miRNA mimics
negative control (miR-NC) and scrambled negative control siRNA (NC
siRNA) were used as the control for miR-877 mimics and MACC1 siRNA
transfection, respectively. The miR-877 mimics sequence was
5′-GUAGAGGAGAUGGCGCAGGG-3′ and the miR-NC sequence was
5′-UAUGCACUCCUGAAGGGCUCGC-3′. The MACC1 siRNA sequence was
5′-AAGAUUGGACUUGUACACUGC-3′ and the NC siRNA sequence was
5′-UUCUCCGAACGUGUCACGUTT-3′. MACC1 overexpression plasmid
pCMV-MACC1 and empty pCMV plasmid were provided by the Chinese
Academy of Sciences. Cells were transfected with miRNA mimics (100
pmol), siRNA (100 pmol) or plasmid (4 µg) using Lipofectamine™ 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and a Transwell cell invasion
assay was performed at 24 h post-transfection. MTT assay and
western blot analysis was performed at 24 h and 72 h after
transfection, respectively.
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate total RNA from tissue
samples or cells. All-in-One™ miRNA RT-qPCR Detection kit
(GeneCopoeia, Inc.) was used to measure miR-877 expression. The
thermocycling conditions were as follows: 95°C for 10 min, 45
cycles of denaturation at 95°C for 15 sec and annealing/elongation
at 60°C for 15 sec. To quantify MACC1 mRNA expression, reverse
transcription was performed to produce cDNA from total RNA using a
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.). The
thermocycling conditions for reverse transcription was as follows:
37°C for 15 min and 85°C for 5 sec. The synthesized cDNA was then
subjected to MACC1 mRNA expression detection using a SYBR Premix Ex
Taq™ (Takara Biotechnology Co., Ltd.). The thermocycling conditions
for qPCR were performed as follows: 5 min at 95°C, followed by 40
cycles of 95°C for 30 sec and 65°C for 45 sec. U6 small nuclear RNA
and GAPDH served as the internal references for miR-877 and MACC1
mRNA levels, respectively. The relative gene expression was
analyzed and normalized using the 2−ΔΔCq method
(18). The primers were designed as
follows: miR-877 forward, ‘5-GTAGAGGAGATGGCGCAGGG-3′ and reverse,
5′-CAGTGCGTGTCGTGGAGT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′; MACC1 forward,
5′-CACAACTTGCGGAGGTCAC-3′ and reverse, 5′-AAGCTGTGGGGTTTTTCC-3′;
and GAPDH forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′.
MTT assay
Transfected cells were harvested and seeded into
96-well plates with a density of 3×103 cells/well. Cells
were incubated at 37°C in an incubator supplied with 5%
CO2 for 0, 24, 48 and 72 h. MTT assay was applied to
determine cellular proliferation at indicated time points by adding
20 µl MTT solution (5 mg/ml; Sigma-Aldrich; Merck KGaA) into each
well. After 4 h of incubation at 37°C, the culture medium was
carefully removed and 150 µl of DMSO (Sigma-Aldrich; Merck KGaA)
was added into each well in order to dissolve the formazan
crystals. Finally, the absorbance of each sample was detected at a
wavelength of 490 nm using a plate reader (Bio-Rad Laboratories,
Inc.).
Transwell cell invasion assay
The invasive ability was evaluated using 24-well
Transwell filters (8 micrometers pore size) that were pre-coated
with Matrigel (both from BD Biosciences). After 48 h of
transfection, cells were collected, suspended in FBS-free DMEM and
then inoculated into the upper compartments of each filter
(5×104 cells per filter). DMEM with 20% FBS was added
into the lower compartments as a chemoattractant. Following
incubation of the cells for 24 h at 37°C, non-invaded cells
remaining on the upper surface of the membranes were removed with a
cotton swab. The invasive cells were fixed in 4% paraformaldehyde
at room temperature for 30 min and stained with 0.5% crystal violet
at room temperature for 30 min. The number of invasive cells was
acquired from five randomly selected areas under a light microscope
(magnification, ×200).
Bioinformatics prediction of miR-877
targets
Target gene prediction software, including
microRNA.org (August 2010; Release Last Update:
2010-11-01; http://www.microrna.org/microrna/) and TargetScan
(Release 7.2: March 2018; http://www.targetscan.org/vert_71/), was used to
search for the potential targets of miR-877.
Luciferase reporter assay
The wild-type (wt) and mutant (mut) of MACC1 3′-UTR
was amplified by Shanghai GenePharma Co., Ltd., and cloned into the
pMIR-REPORT miRNA Expression Reporter vector (Ambion; Thermo Fisher
Scientific, Inc.) generating the pMIR-MACC1-3′-UTR wt and
pMIR-MACC1-3′-UTR mut, respectively. Cells were plated in 24-well
plates with a density of 1.0×105 cells per well and
co-transfected with wt or mut luciferase plasmid and miR-877 mimics
or miR-NC using Lipofectamine™ 2000. After a 48-h incubation,
transfected cells were harvested and assessed for luciferase
activity using a dual-luciferase reporter assay system (Promega
Corporation), according to the manufacturer's protocol. The firefly
luciferase activity was normalized to that of Renilla
luciferase activity.
Western blot analysis
Western blot analysis was applied to detect MACC1
protein expression. Total protein was isolated from cultured cells
or homogenized tissues using a cold radioimmunoprecipitation assay
buffer (Shanghai Qcbio Science & Technologies Co., Ltd.). Total
protein was quantified according to the protocol of a Bicinchoninic
Acid Protein Assay kit (Bio-Rad Laboratories, Inc.). An equal mass
of proteins (20 µg) were separated by SDS-PAGE (10% gel), blotted
onto PVDF membranes (EMD Millipore) and blocked at room temperature
in Tris-buffered saline containing 0.1% Tween-20 (TBST)
supplemented with 5% dried skimmed milk for 2 h. Subsequently, the
membranes were incubated with primary antibodies overnight at 4°C
followed by incubation with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibodies (1:5,000; catalog no. ab6721;
Abcam) at room temperature for 2 h. Following extensive washing
with TBST, an Enhanced Chemiluminescence (ECL) Western blotting kit
(Pierce; Thermo Fisher Scientific, Inc.) was used to visualize the
immune complex on the PVDF membranes. The primary antibodies used
in the present study were as follows: Rabbit anti-human MACC1
antibody (1:1,000; catalog no. ab106579) and rabbit anti-human
GAPDH antibody (1:1,000; catalog no. ab128915; both from Abcam).
GAPDH was used as an internal control. Quantity One software
(version 4.62; Bio-Rad Laboratories, Inc.) was utilized to analyze
the protein signals.
Statistical analysis
All assays were repeated at least three times. Data
are presented as the mean ± standard deviation and were analyzed
using SPSS software (version 17.0; SPSS Inc.). Differences between
groups were determined using Student's t-tests or one-way analysis
of variance (ANOVA). Student-Newman-Keuls (SNK) was used as the
post hoc analysis following ANOVA. The association between the
clinicopathological characteristics of the patients with cervical
cancer and miR-877 or MACC1 expression was assessed with
χ2 test. Spearman's correlation analysis was used to
evaluate the correlation between miR-877 and MACC1 mRNA expression
levels in cervical cancer tissues. P<0.05 was considered to
indicate a statistically significant result.
Results
miR-877 is downregulated in cervical
cancer tissues and cell lines
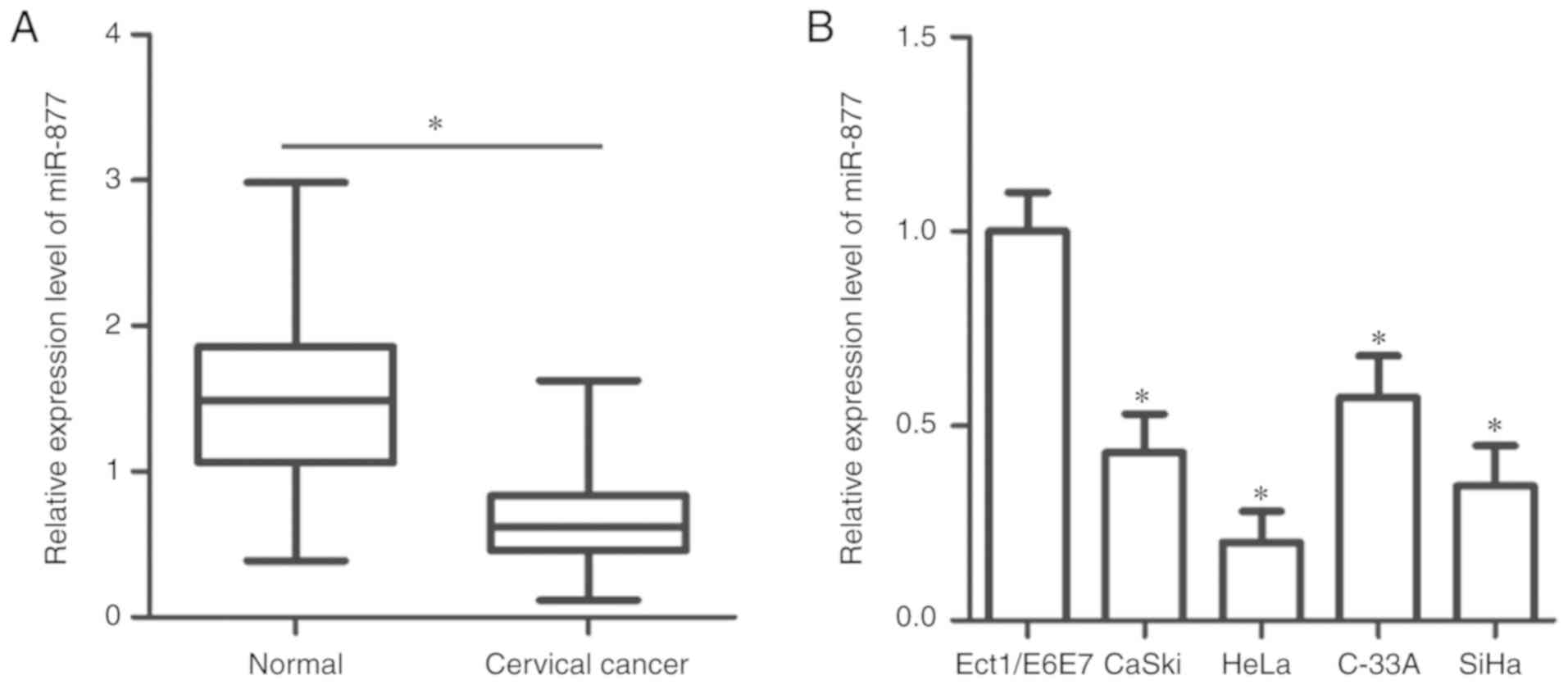
To determine the expression patterns of miR-877 in
cervical cancer, RT-qPCR was utilized to measure miR-877 expression
in 57 pairs of cervical cancer tissues and matched adjacent normal
tissues. The expression level of miR-877 was lower in cervical
cancer tissues when compared with the adjacent normal tissues
(P<0.05; Fig. 1A). In addition,
miR-877 expression was determined in a panel of human cervical
cancer cell lines, including CaSki, HeLa, C-33A and SiHa. A normal
human cervix epithelial cell line Ect1/E6E7 was used as the
control. The data obtained from RT-qPCR revealed that miR-877
expression was decreased in all four cervical cancer cell lines
compared with in Ect1/E6E7 (P<0.05; Fig. 1B).
To evaluate the clinical value of miR-877 in
cervical cancer, all patients enrolled in the present study were
divided into two groups, a low miR-877 expression group and a high
miR-877 expression group, based on the median value of miR-877
(0.62). As presented in Table I, low
miR-877 expression was significantly correlated with the
International Federation of Gynecology and Obstetric (FIGO) stage
(19) (P=0.017) and lymph node
metastasis (P=0.007), but not with age (P=0.395), tumor size
(P=0.417), HPV infection (P=0.514), vascular involvement (P=0.530)
or myometrium invasion (P=0.175). These results suggest that
downregulation of miR-877 may be involved in the malignant
progression of cervical cancer.
 | Table I.Association between miR-877 and
clinicopathological characteristics of patients with cervical
cancer. |
Table I.
Association between miR-877 and
clinicopathological characteristics of patients with cervical
cancer.
|
| miR-877 expression
level |
|
|---|
|
|
|
|
|---|
| Characteristic | Low | High | P-value |
|---|
| Age, years |
|
| 0.395 |
|
<45 | 11 | 7 |
|
|
≥45 | 18 | 21 |
|
| Tumor size, cm |
|
| 0.417 |
|
<4 | 16 | 19 |
|
| ≥4 | 13 | 9 |
|
| HPV |
|
| 0.514 |
|
Negative | 12 | 14 |
|
|
Positive | 17 | 14 |
|
| Vascular
involvement |
|
| 0.530 |
|
Yes | 8 | 5 |
|
| No | 21 | 23 |
|
| FIGO stage |
|
| 0.017 |
|
I–II | 9 | 18 |
|
|
III–IV | 20 | 10 |
|
| Myometrium
invasion |
|
| 0.175 |
|
<1/2 | 15 | 20 |
|
|
≥1/2 | 14 | 8 |
|
| Lymph node
metastasis |
|
| 0.007 |
| No | 11 | 21 |
|
|
Yes | 18 | 7 |
|
miR-877 has a suppressive effect on
the proliferation and invasion of cervical cancer cells
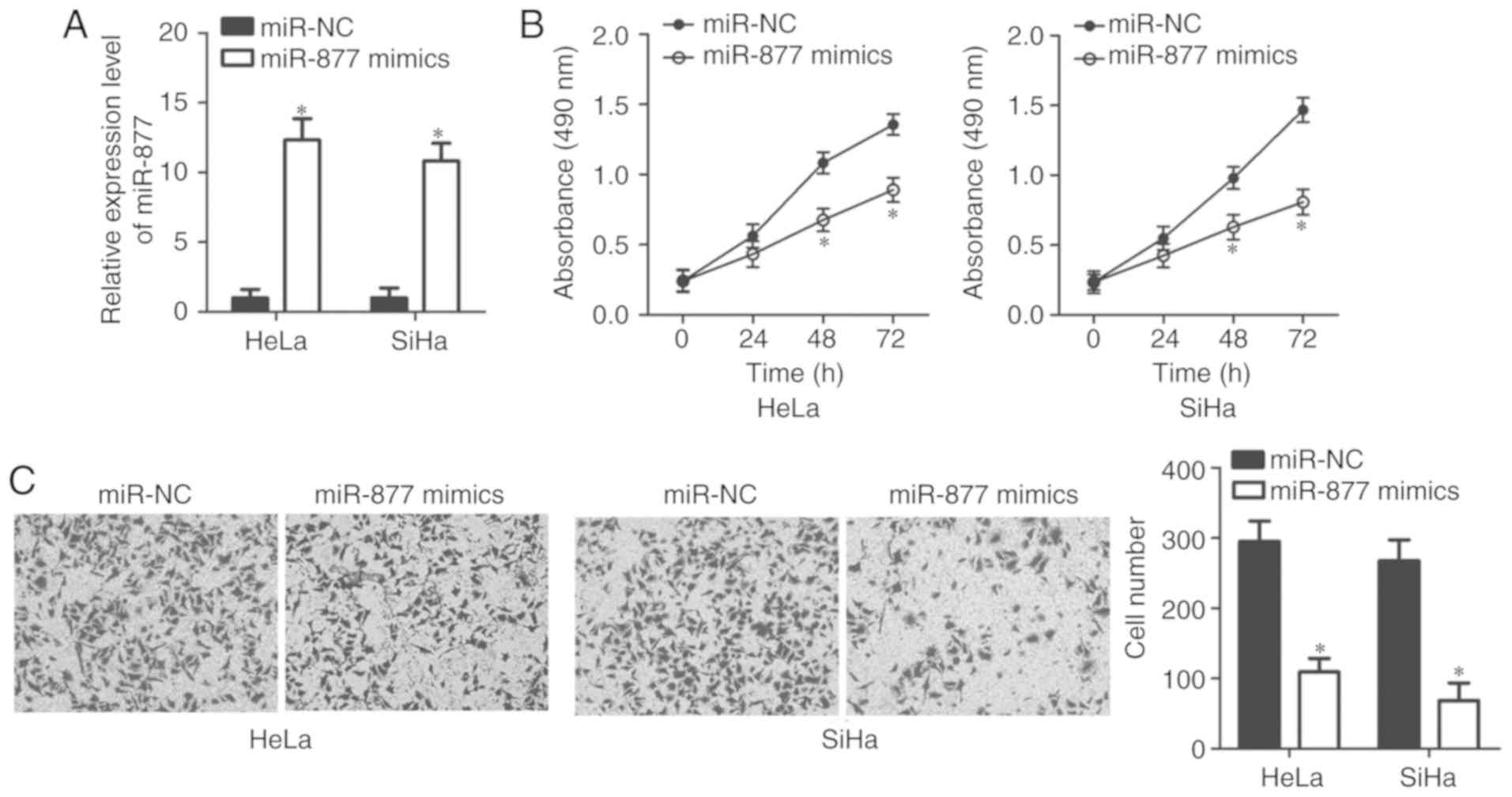
HeLa and SiHa cell lines exhibited relatively lower
miR-877 expression levels out of all the cervical cancer cell lines
(Fig. 1B), therefore the two cell
lines were selected for subsequent functional assays. In order to
investigate the potential functions of miR-877 in the malignant
phenotypes of cervical cancer, HeLa and SiHa cells were transfected
with miR-877 mimics or miR-NC. The results of the RT-qPCR confirmed
that miR-877 was notably upregulated in miR-877 mimic-transfected
HeLa and SiHa cells compared with the cells transfected with miR-NC
(P<0.05; Fig. 2A). The impact of
miR-877 upregulation on cell proliferation in cervical cancer was
examined using MTT assay. miR-877-overexpressing HeLa and SiHa
cells demonstrated a decreased proliferative ability when compared
with that in the miR-NC group (P<0.05; Fig. 2B). The role of miR-877 in the
regulation of cervical cancer cell invasion was then determined.
Data from the transwell cell invasion assay revealed that increased
expression levels of miR-877 markedly inhibited the invasion of
HeLa and SiHa cells (P<0.05; Fig.
2C). These results implied that miR-877 may play a tumor
suppressive role in the development of cervical cancer.
miR-877 inhibits MACC1 expression by
directly targeting its 3′-UTR in cervical cancer cells
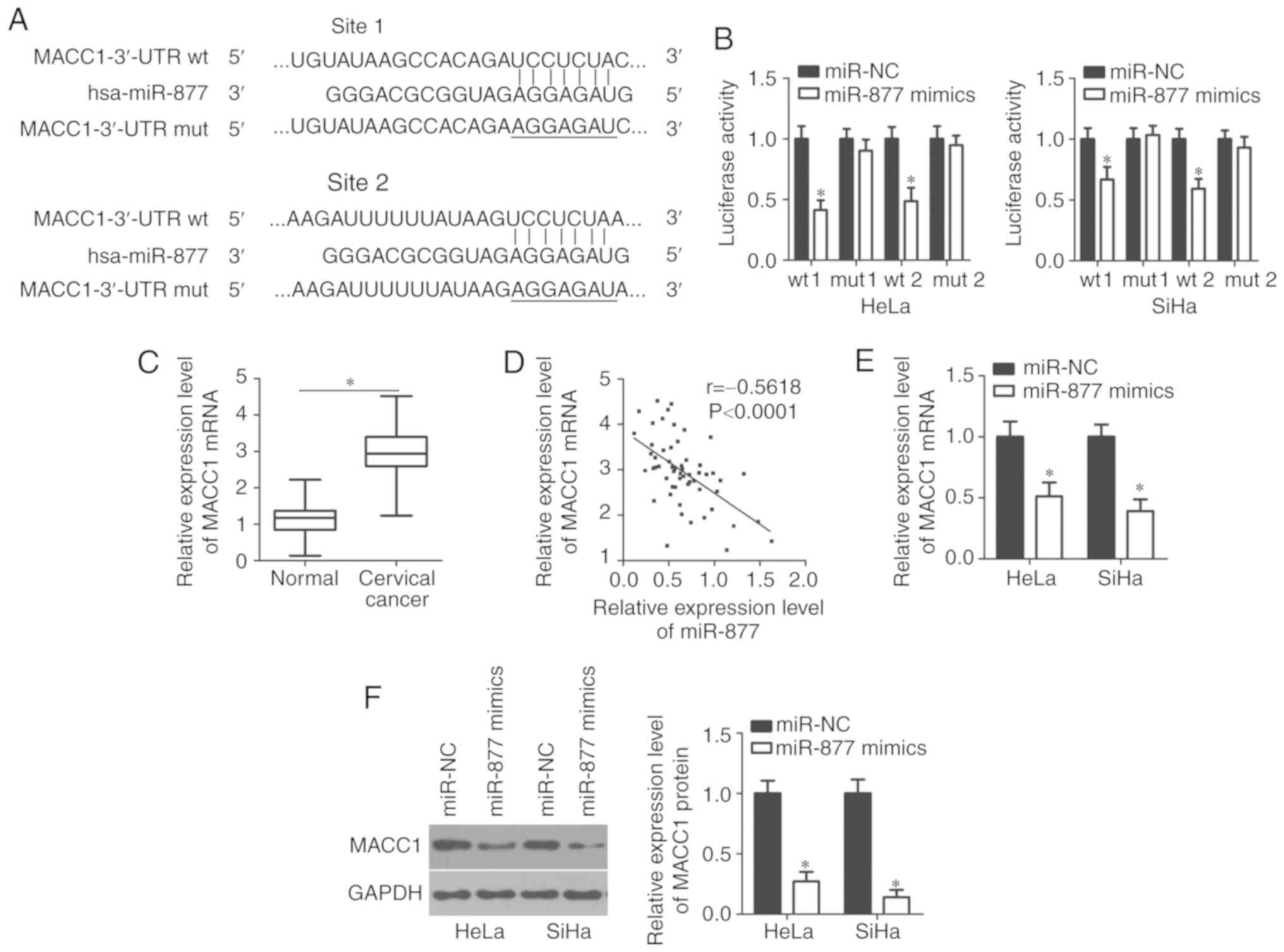
To decipher the mechanisms underlying how miR-877
affects cervical cancer progression, bioinformatics analyses were
performed using microRNA.org and TargetScan to search
for the potential target of miR-877. Two highly conserved putative
binding sites were observed in 3,470–3,476 bp and 5,492–5,499 bp of
MACC1 3′-UTR (Fig. 3A). MACC1 was
selected for further identification as this gene has been widely
reported as implicated in cervical cancer carcinogenesis and
progression (20–25). Luciferase reporter assay was applied
to confirm whether the 3′-UTR of MACC1 could be directly targeted
by miR-877 in cervical cancer cells. In HeLa and SiHa cells
co-transfected with pMIR-MACC1-3′-UTR wt (site 1 and 2) and miR-877
mimics, luciferase activity was significantly decreased compared
with that in cells co-transfected with miR-NC and pMIR-MACC1-3′-UTR
wt (P<0.05; site 1 and 2); however, no obvious influence was
indicated on the luciferase activity of the plasmid harboring
mutant miR-877 binding site (site 1 and 2; Fig. 3B).
The MACC1 mRNA expression levels in cervical cancer
tissues through RT-qPCR. The expression levels of MACC1 mRNA were
revealed to be markedly upregulated in cervical cancer tissues
compared with that in adjacent normal tissues (P<0.05; Fig. 3C). The association between the mRNA
level of MACC1 and the clinicopathological factors of patients with
cervical cancer was examined. As presented in Table II, an increased MACC1 expression
level was significantly associated with the increased FIGO stage
(P=0.047), increased myometrium invasion (P=0.002) and increased
lymph node metastasis (P=0.022). Notably, an inverse correlation
was validated between miR-877 and MACC1 mRNA levels in cervical
cancer tissues via Spearman's correlation analysis (P<0.0001;
r=−0.5618; Fig. 3D). Furthermore,
RT-qPCR and western blot analyses were performed in order to
investigate whether miR-877 could regulate endogenous MACC1
expression levels in cervical cancer cells. The data revealed that
ectopic miR-877 expression in HeLa and SiHa cells could decrease
MACC1 expression at both the mRNA (P<0.05; Fig. 3E) and protein (P<0.05; Fig. 3F) levels. The results demonstrated
that MACC1 was a direct target gene of miR-877 in cervical cancer
cells.
 | Table II.Association between MACC1 mRNA level
and clinicopathological characteristics of patients with cervical
cancer. |
Table II.
Association between MACC1 mRNA level
and clinicopathological characteristics of patients with cervical
cancer.
|
| MACC1 mRNA
level |
|
|---|
|
|
|
|
|---|
| Characteristic | High | Low | P-value |
|---|
| Age, years |
|
| 0.570 |
|
<45 | 8 | 10 |
|
|
≥45 | 21 | 19 |
|
| Tumor size, cm |
|
| 0.127 |
|
<4 | 15 | 20 |
|
| ≥4 | 14 | 8 |
|
| HPV |
|
| 0.470 |
|
Negative | 10 | 16 |
|
|
Positive | 19 | 12 |
|
| Vascular
involvement |
|
| 0.698 |
|
Yes | 6 | 7 |
|
| No | 23 | 21 |
|
| FIGO stage |
|
| 0.047 |
|
I–II | 10 | 17 |
|
|
III–IV | 19 | 11 |
|
| Myometrium
invasion |
|
| 0.002 |
|
<1/2 | 12 | 23 |
|
|
≥1/2 | 17 | 5 |
|
| Lymph node
metastasis |
|
| 0.022 |
| No | 12 | 20 |
|
|
Yes | 17 | 8 |
|
Knockdown of MACC1 restricts the
proliferation and invasion of cervical cancer cells
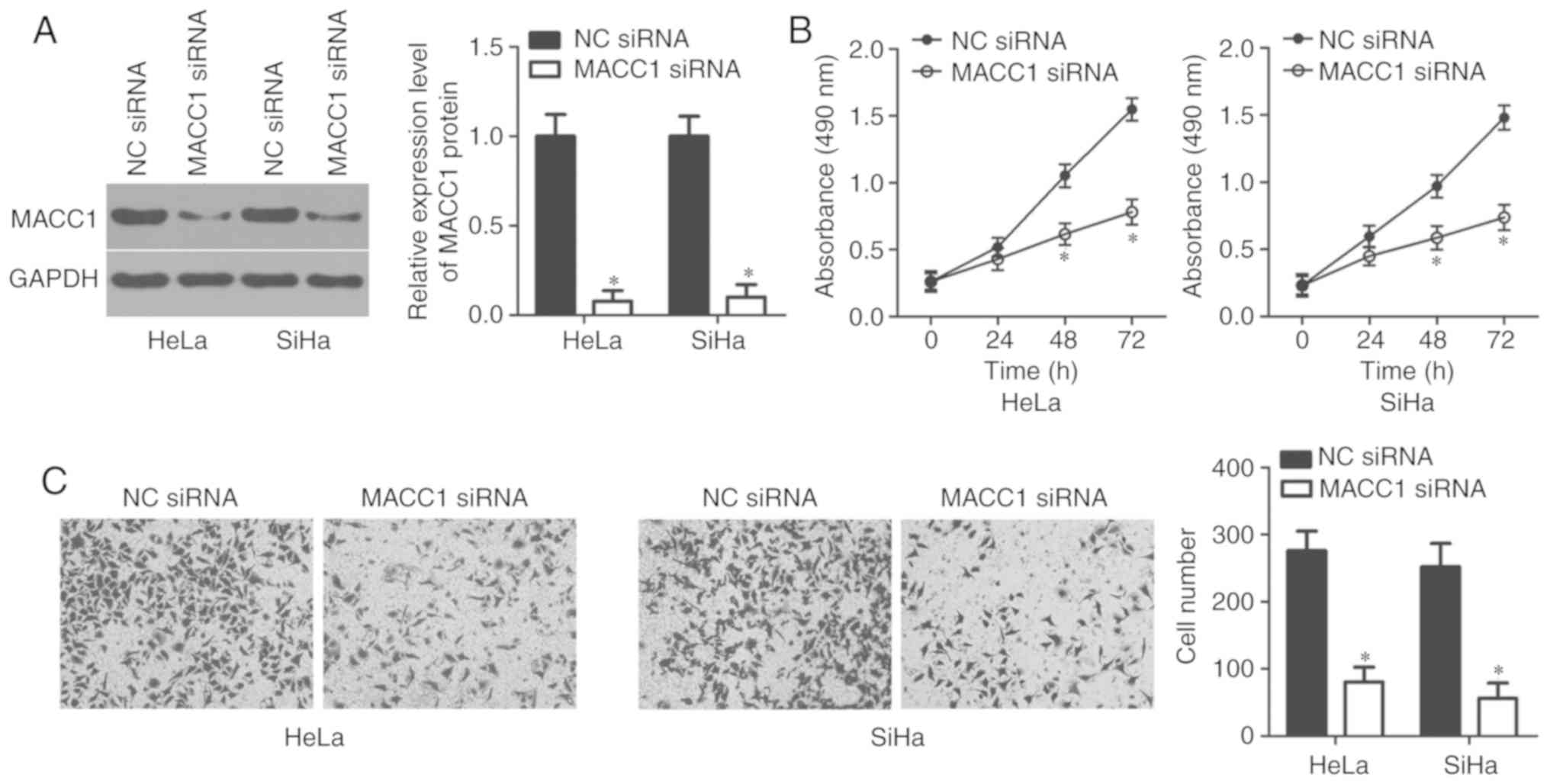
In order to investigate the specific roles of MACC1
in cervical cancer, HeLa and SiHa cells were transfected with MACC1
siRNA with the aim of knocking down endogenous MACC1 expression.
Transfection with NC siRNA served as the control group. Western
blot analysis confirmed that the protein level of MACC1 was notably
downregulated in HeLa and SiHa cells following MACC1 siRNA
transfection (P<0.05; Fig. 4A).
In addition, the results of the MTT and transwell cell invasion
assays revealed that inhibition of MACC1 led to the decreased
proliferative (P<0.05; Fig. 4B)
and invasive (P<0.05; Fig. 4C)
abilities of HeLa and SiHa cells, which was similar to the results
demonstrated by miR-877 upregulation. Therefore, these results
further suggest MACC1 as a functional downstream target of miR-877
in cervical cancer cells.
MACC1 restores miR-877
overexpression-mediated suppression of cervical cancer cell
proliferation and invasion
To verify whether MACC1 mediates the inhibitory
effects of miR-877 in cervical cancer cells, the
miR-877-overexpression HeLa and SiHa cells were further transfected
with MACC1 overexpression plasmid pCMV-MACC1 or empty pCMV plasmid.
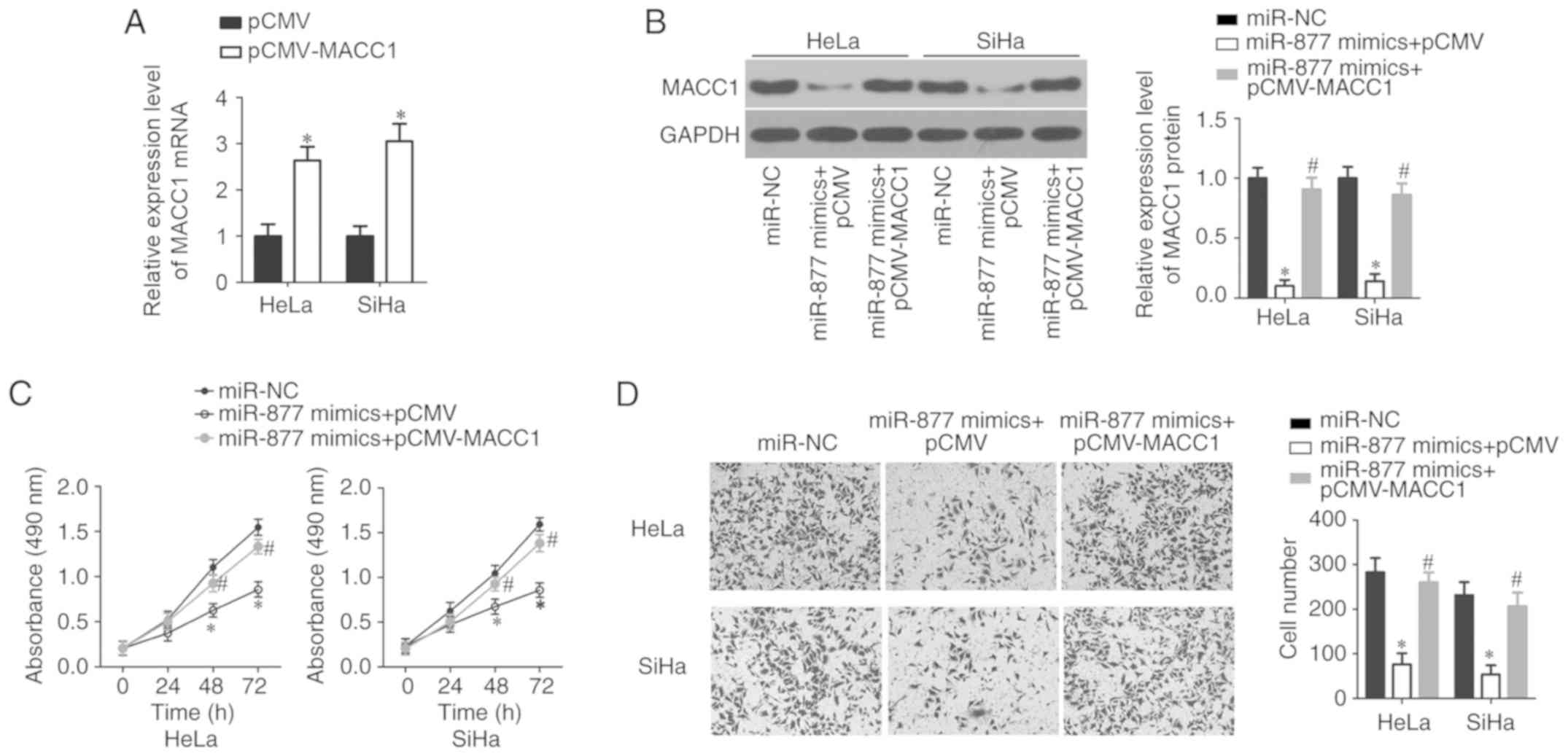
Firstly, RT-qPCR analysis confirmed that transfection with
pCMV-MACC1 significantly increased the expression level of MACC1 in
HeLa and SiHa cells (P<0.05; Fig.
5A). Following transfection, the western blot analysis
indicated that the expression levels of MACC1 protein were markedly
decreased in miR-877 overexpression-HeLa and SiHa cells; however,
the decreased MACC1 protein expression was almost recovered
following pCMV-MACC1 co-transfection (P<0.05; Fig. 5B). Furthermore, MTT and transwell
cell invasion assays demonstrated that MACC1 reintroduction
reversed the inhibitory effects of miR-877 overexpression on the
proliferation (P<0.05; Fig. 5C)
and invasion (P<0.05; Fig. 5D) of
HeLa and SiHa cells. These results suggest that MACC1 inhibition is
required for the suppressive effects of miR-877 on the malignant
phenotypes of cervical cancer cells.
Discussion
Dysregulation of miRNAs has been widely reported in
cervical cancer, and dysregulated miRNAs play crucial roles in
cervical cancer carcinogenesis and progression (26–29).
miRNAs are able to regulate all major cancer-associated biological
behaviors in cervical cancer, including differentiation,
proliferation, the cell cycle, apoptosis, migration and metastasis
(30–32). Hence, investigating the detailed
roles of miRNAs that are aberrantly expressed in cervical cancer
and the involved molecular mechanisms is essential for early
diagnosis and effective therapeutic approaches. To the best of our
knowledge, the present study detected the miR-877 expression levels
in cervical cancer and determined its clinical value for the first
time. More importantly, the functional roles and molecular
mechanisms responsible for the action of miR-877 in development of
cervical cancer were investigated.
A number of studies have reported that miR-877 was
downregulated in hepatocellular carcinoma (15,16).
Decreased miR-877 expression was significantly associated with the
histologic grade and the Tumor-Node-Metastasis stage of patients
with hepatocellular carcinoma (15).
Patients with hepatocellular carcinoma that exhibit low expression
levels of miR-877 had shorter overall survival and disease-free
survival rates (15). Furthermore,
miR-877 was validated as an independent biomarker for predicting
the poor prognosis of patients with hepatocellular carcinoma
(15). Low expression levels of
miR-877 expression were also revealed in the blood and tissues of
patients with renal cell carcinoma (17). However, the expression status of
miR-877 in cervical cancer remains unknown. In the present study,
RT-qPCR was used to measure miR-877 expression in both cervical
cancer tissues and cell lines. The data revealed that miR-877 was
downregulated in cervical cancer, and the downregulation of miR-877
was positively associated with the FIGO stage and lymph node
metastasis. These results suggest that miR-877 may emerge as a
potential biomarker for the diagnosis of patients with the
aforementioned human cancer types.
The dysregulation of miR-877 has been validated as
an important driver for human cancer progression. For example,
returning miR-877 expression levels to the norm inhibited
hepatocellular carcinoma cell proliferation, colony formation,
migration and invasion, as well as improving the chemosensitivity
to paclitaxel (15,16). In renal cell carcinoma, miR-877
restoration suppressed the cellular proliferative and migratory
capacities of renal cell carcinoma in vitro (17). However, the specific roles of miR-877
in the progression and development of cervical cancer remain
uncertain. To elucidate these roles, MTT and transwell cell
invasion assays were performed, which revealed that the
overexpression of miR-877 inhibited the proliferation and invasion
of cervical cancer cells. These observations suggest that miR-877
may be considered as a potential therapeutic target for treating
patients with these specific types of human cancer.
Several genes, including cyclin-dependent kinase 14
(15), Forkhead box protein M1
(16) and eukaryotic elongation
factor-2 kinase (17), have been
verified as direct targets of miR-877. The mechanisms underlying
the tumor-suppressive roles of miR-877 in cervical cancer were
investigated in the present study. MACC1 was identified to be a
direct target gene of miR-877 in cervical cancer cells.
MACC1, located on human chromosome 7 (7p21.1), was
previously reported as overexpressed in multiple types of human
cancer, such as glioma (33), breast
cancer (34), hepatocellular
carcinoma (35) and renal cell
carcinoma (36). MACC1 was also
observed as highly expressed in cervical cancer tissues and cell
lines. Increased MACC1 expression levels were significantly
correlated with the FIGO stage, pelvic lymph node metastasis and
recurrence (20,21). Patients with cervical cancer with
high MACC1 expression levels exhibited shorter overall survival
times than those patients with low MACC1 expression (21). Furthermore, multivariate analyses
validated MACC1 as an independent biomarker for predicting the
overall survival of patients with cervical cancer (21). MACC1 has a role in oncogenic activity
in the carcinogenesis and development of cervical cancer, and
regulated a variety of aggressive behaviors (22–25).
Herein, it was demonstrated that miR-877 was able to directly
target MACC1 and inhibit the malignant progression of cervical
cancer. Hence, the miR-877/MACC1 axis may represent a potential
therapeutic target for patients with cervical cancer.
In conclusion, the present study indicated that
miR-877 was downregulated in cervical cancer. Decreased miR-877
expression was significantly associated with the increased FIGO
stage and lymph node metastasis. miR-877 upregulation restricted
the proliferation and invasion of cervical cancer cells by directly
targeting MACC1. The observations of the present study suggest that
miR-877 may be a potential target for the therapy of cervical
cancer, and provide a new technique for the prevention and
treatment of patients with this disease. The present study was not
without limitations; the association between miR-877 and prognosis
of patients with cervical cancer was not investigated. Future
investigations should collect the prognosis data and examine the
association between miR-877 and prognosis of patients with cervical
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Youth
Foundation of Jilin Province Science and Technology Development
Plan (grant no. 20160520145JH).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
BS and FM designed the present study and performed
the statistical analyses. FM, JO, JL and XL performed the RT-qPCR,
the western blot analysis and the MTT assay. Transwell cell
invasion and luciferase reporter assays were performed by YM, LY
and PD. BS wrote the article. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jilin Cancer Hospital (Changchun, China). All patients
enrolled in the present study provided written informed consent
based on the principles of the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su SY, Huang JY, Ho CC and Liaw YP:
Evidence for cervical cancer mortality with screening program in
Taiwan, 1981–2010: Age-period-cohort model. BMC Public Health.
13:132013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hildesheim A and Wang SS: Host and viral
genetics and risk of cervical cancer: A review. Virus Res.
89:229–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakuragi N: Up-to-date management of lymph
node metastasis and the role of tailored lymphadenectomy in
cervical cancer. Int J Clin Oncol. 12:165–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mayr NA, Huang Z, Wang JZ, Lo SS, Fan JM,
Grecula JC, Sammet S, Sammet CL, Jia G, Zhang J, et al:
Characterizing tumor heterogeneity with functional imaging and
quantifying high-risk tumor volume for early prediction of
treatment outcome: Cervical cancer as a model. Int J Radiat Oncol
Biol Phys. 83:972–979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aigner A: MicroRNAs (miRNAs) in cancer
invasion and metastasis: Therapeutic approaches based on
metastasis-related miRNAs. J Mol Med (Berl). 89:445–457. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho WC: MicroRNAs: Potential biomarkers
for cancer diagnosis, prognosis and targets for therapy. Int J
Biochem Cell Biol. 42:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Lu Y, Wang S, Sheng X and Zhang
S: MicroRNA-152 acts as a tumor suppressor microRNA by inhibiting
Kruppel-like factor 5 in human cervical cancer. Oncol Res.
27:335–340. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu LM, Wang WW, Qi R, Leng TG and Zhang
XL: MicroRNA-224 inhibition prevents progression of cervical
carcinoma by targeting PTX3. J Cell Biochem. 119:10278–10290. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Y, Hou R, Li S, Li S and Dang G:
MicroRNA-302 inhibits cell migration and invasion in cervical
cancer by targeting DCUN1D1. Exp Ther Med. 16:1000–1008.
2018.PubMed/NCBI
|
|
13
|
Chen Z, Zhang M, Qiao Y, Yang J and Yin Q:
MicroRNA-1297 contributes to the progression of human cervical
carcinoma through PTEN. Artif Cells Nanomed Biotechnol 46 (Sup2).
S1120–S1126. 2018. View Article : Google Scholar
|
|
14
|
Srivastava SK, Ahmad A, Zubair H, Miree O,
Singh S, Rocconi RP, Scalici J and Singh AP: MicroRNAs in
gynecological cancers: Small molecules with big implications.
Cancer Lett. 407:123–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan TH, Qiu C, Sun J and Li WH: MiR-877-5p
suppresses cell growth, migration and invasion by targeting cyclin
dependent kinase 14 and predicts prognosis in hepatocellular
carcinoma. Eur Rev Med Pharmacol Sci. 22:3038–3046. 2018.PubMed/NCBI
|
|
16
|
Huang X, Qin J and Lu S: Up-regulation of
miR-877 induced by paclitaxel inhibits hepatocellular carcinoma
cell proliferation though targeting FOXM1. Int J Clin Exp Pathol.
8:1515–1524. 2015.PubMed/NCBI
|
|
17
|
Shi Q, Xu X, Liu Q, Luo F, Shi J and He X:
MicroRNA-877 acts as a tumor suppressor by directly targeting eEF2K
in renal cell carcinoma. Oncol Lett. 11:1474–1480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berek JS, Matsuo K, Grubbs BH, Gaffney DK,
Lee SI, Kilcoyne A, Cheon GJ, Yoo CW, Li L, Shao Y, et al:
Multidisciplinary perspectives on newly revised 2018 FIGO staging
of cancer of the cervix uteri. J Gynecol Oncol. 30:e402019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou X, Xu CJ, Wang JX, Dai T, Ye YP, Cui
YM, Liao WT, Wu XL and Ou JP: Metastasis-associated in colon
cancer-1 associates with poor prognosis and promotes cell invasion
and angiogenesis in human cervical Cancer. Int J Gynecol Cancer.
25:1353–1363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo L, Lu W, Zhang X, Luo D and Zhang H:
Metastasis-associated colon cancer-1 is a novel prognostic marker
for cervical cancer. Int J Clin Exp Pathol. 7:4150–4155.
2014.PubMed/NCBI
|
|
22
|
Chai H and Yang Y: Effects of MACC1 siRNA
on biological behaviors of HeLa. Arch Gynecol Obstet.
289:1271–1280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen XP, Ren XP, Lan JY, Chen YG and Shen
ZJ: Analysis of HGF, MACC1, C-met and apoptosis-related genes in
cervical carcinoma mice. Mol Biol Rep. 41:1247–1256. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hua F, Xia Y, Wang H, Chen R, Ren Y, Yang
J and Liang W: Effects of small interfering RNA silencing MACC-1
expression on cell proliferation, cell cycle and invasion ability
of cervical cancer SiHa cells. Zhonghua Zhong Liu Za Zhi.
36:496–500. 2014.(In Chinese). PubMed/NCBI
|
|
25
|
Hua FF, Liu SS, Zhu LH, Wang YH, Liang X,
Ma N and Shi HR: MiRNA-338-3p regulates cervical cancer cells
proliferation by targeting MACC1 through MAPK signaling pathway.
Eur Rev Med Pharmacol Sci. 21:5342–5352. 2017.PubMed/NCBI
|
|
26
|
Wang F, Li B and Xie X: The roles and
clinical significance of microRNAs in cervical cancer. Histol
Histopathol. 31:131–139. 2016.PubMed/NCBI
|
|
27
|
Servín-González LS, Granados-López AJ and
López JA: Families of microRNAs expressed in clusters regulate cell
signaling in cervical cancer. Int J Mol Sci. 16:12773–12790. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Díaz-González Sdel M, Deas J,
Benítez-Boijseauneau O, Gómez-Cerón C, Bermúdez-Morales VH,
Rodríguez-Dorantes M, Pérez-Plasencia C and Peralta-Zaragoza O:
Utility of microRNAs and siRNAs in cervical carcinogenesis. Biomed
Res Int. 2015:3749242015.PubMed/NCBI
|
|
29
|
Pedroza-Torres A, López-Urrutia E,
Garcia-Castillo V, Jacobo-Herrera N, Herrera LA, Peralta-Zaragoza
O, López-Camarillo C, De Leon DC, Fernández-Retana J, Cerna-Cortés
JF and Pérez-Plasencia C: MicroRNAs in cervical cancer: Evidences
for a miRNA profile deregulated by HPV and its impact on
radio-resistance. Molecules. 19:6263–6281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li JH, Zhang Z, Du MZ, Guan YC, Yao JN, Yu
HY, Wang BJ, Wang XL, Wu SL and Li Z: microRNA-141-3p fosters the
growth, invasion, and tumorigenesis of cervical cancer cells by
targeting FOXA2. Arch Biochem Biophys. 657:23–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shang A, Zhou C, Bian G, Chen W, Lu W,
Wang W and Li D: miR-381-3p restrains cervical cancer progression
by downregulating FGF7. J Cell Biochem. 120:778–789. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai N, Hu L, Xie Y, Gao JH, Zhai W, Wang
L, Jin QJ, Qin CY and Qiang R: MiR-17-5p promotes cervical cancer
cell proliferation and metastasis by targeting transforming growth
factor-β receptor 2. Eur Rev Med Pharmacol Sci. 22:1899–1906.
2018.PubMed/NCBI
|
|
33
|
Yang T, Kong B, Kuang YQ, Cheng L, Gu JW,
Zhang JH, Shu HF, Yu SX, He WQ, Xing XM and Huang HD:
Overexpression of MACC1 protein and its clinical implications in
patients with glioma. Tumour Biol. 35:815–819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Y, Zhang H, Cai J, Fang L, Wu J, Ye
C, Zhu X and Li M: Overexpression of MACC1 and Its significance in
human breast cancer progression. Cell Biosci. 3:162013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun DW, Zhang YY, Qi Y, Liu GQ, Chen YG,
Ma J and Lv GY: Prognostic and clinicopathological significance of
MACC1 expression in hepatocellular carcinoma patients: A
meta-analysis. Int J Clin Exp Med. 8:4769–4777. 2015.PubMed/NCBI
|
|
36
|
Jin Z, Xu N, Guo K, Xu P, Li P, Zhang Y,
Li X, Zheng S, Liu C, Xu A and Huang P: Increased expression of
metastasis-associated in colon cancer-1 in renal cell carcinoma is
associated with poor prognosis. Int J Clin Exp Pathol. 8:3857–3863.
2015.PubMed/NCBI
|