Introduction
Osteoporosis affects >40% of postmenopausal
females (1) and is characterized by
damage to the bone microstructure, a decrease in bone mineral and
matrix composition, a decrease in bone density and an increase in
bone fragility and fracture risks (2). A survey performed in 2008 revealed that
~54.1 million Chinese females were diagnosed with osteoporosis,
while 113 million Chinese females exhibited a reduction in bone
mass (3). This has led to severe
health threats and a social and economic burden (3). Postmenopausal osteoporosis is caused by
imbalance of bone absorption and formation (4). Despite the availability and clinical
use of various drugs that effectively target bone resorption,
treatments that promote bone formation have yet to be identified
(5).
CD14+ peripheral blood mononuclear cells
(PBMCs) are precursors of osteoclasts and are associated with the
pathogenesis of osteoporosis (6,7). Under
the joint action of macrophage colony stimulating factor and
receptor activator of nuclear factor-κB (RANK) ligand (L),
CD14+ PBMCs that are differentiated from
CD14− PBMCs differentiate into osteoclasts during
cultivation, suggesting that CD14+ PBMCs are precursor
cells of osteoclasts (8).
CD14+ PBMCs express RANK, which is activated following
binding to RANKL and mediates osteoclast differentiation (9).
To date, a number of active bone morphogenetic
proteins (BMPs) have been discovered (10,11).
BMP-2 has been reported to be a member of transforming growth
factor-β supergene family (12), and
serves important roles in osteogenesis (13), fracture healing (14) and bone formation (15). BMP-2 further serves important roles
in the process of ossification, by stimulating the differentiation
of pluripotent stromal stem cells into osteoblasts and enhancing
the functions of osteoblasts (16–18).
There are various approaches to regulate BMP-2 expression,
including the use of microRNAs (miRs), which has been widely
studied. miR-98 (19), miR-203 and
miR-320 (20) have been reported to
regulate BMP-2 expression. Additional miRs may exert regulatory
effects on BMP-2. A previous study reported that miR-410 enhances
the stem cell characteristics of cells (21); however, whether miR-410 regulates
BMP-2 expression is currently unclear. Therefore, the aim of the
present study was to investigate the mechanisms underlying the
pathogenesis of postmenopausal osteoporosis, and to understand the
role of miR-410 and BMP-2 in the disease.
Materials and methods
Patients
A total of 26 female patients with postmenopausal
osteoporosis that received treatment at Changhai Hospital
(Shanghai, China) between October 2012 and March 2017 were included
in the present study. The age range was 50–59 years and the mean
age was 55.6±4.8 years. In addition, 29 aged-matched healthy female
subjects were recruited into the normal control group at the same
hospital within the same date range (age range, 50–59 years; mean
age, 55.1±4.6 years). Subjects in the control group and patients
with postmenopausal osteoporosis had similar serum levels of
estrogen, vitamin D and parathyroid hormone. Osteoporosis and
normal bone mass were determined according to the standards of the
World Health Organization (22–24). The
inclusion and exclusion criteria were the same as these standards.
Fasting peripheral blood was collected from all subjects in the
morning on the day of diagnosis and stored at −20°C. To obtain
serum samples, blood was centrifuged at 400 × g and 4°C for 10 min
and serum was transferred into fresh tubes (100 µl/tube). All
procedures were approved by the Ethics Committee of Changhai
Hospital. Written informed consent was obtained from all patients
or their families.
Animals
A total of 60 female C57BL/6 mice (age, 5 weeks)
were purchased from Chongqing TengXin Biotech Company (Chongqing,
China). The weight of the mice ranged between 18 and 22 g. Mice
were maintained in individual cages in a room with 50–65% humidity,
at 26°C with a 12-h light/dark cycle. One week prior to
experiments, mice had free access to food and water to acclimate to
the environment. Access to food and water was not changed during
the experiments. The Reduction, Replacement and Refinement animal
welfare principle was followed during the experiments (25). Mice were randomly divided into sham
operation group (sham; n=30) and ovariectomized model group (OVX;
n=30). Mice in the OVX group were anesthetized by intraperitoneal
injection of 5% chloral hydrate at a dosage of 400 mg/kg animal
body weight. Both sides of the ovaries were extirpated. Mice in the
sham group were treated using the same surgical protocols but
without ovarian extirpation. At 3 months following surgery, mice
were anesthetized by intraperitoneal injection of 5% chloral
hydrate at 400 mg/kg body weight and underwent distal femur
scanning using microcomputed tomography (80 kV, 500 µA; SkyScan;
Bruker Corporation, Billerica, MA, USA) along the long axis of
femur (360° scanning angle; 10.44 µm resolution) to test for
postmenopausal osteoporosis symptoms (data not shown). Peripheral
blood was collected from mice in the sham group (n=28) and OVX
group (n=25) during operation, and serum was obtained by
centrifugation at 400 × g and 4°C for 10 min. Mice that did not
develop postmenopausal osteoporosis were excluded from the study.
Serum was transferred into tubes (100 µl/tube). All animal
experiments were conducted according to the Ethical Guidelines of
Changhai Hospital. The present study was approved by the Ethics
Committee of Changhai Hospital.
Cells
CD14+ PBMCs were separated by gradient
centrifugation from both human and mice (26) and Ficoll-Paque according to the
manufacturer's instructions (cat. no. 17-1440-03; GE Healthcare,
Chicago, IL, USA). Cells were cultured in α-minimum essential
medium (MEM) supplemented with 10% fetal bovine serum, 100 U/ml
penicillin and 100 ng/ml streptomycin (all reagents from Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C and 5%
CO2. Following 2 h, non-adherent cells were removed and
adherent cells were resuspended in fresh complete α-MEM medium.
Using a monocyte isolation kit (cat. no., 130-117-337; Miltenyi
Biotec GmbH, Bergisch Gladbach, Germany), CD14+ PBMCs
were isolated from the cell suspension according to the
manufacturer's protocol. CD14+ PBMCs
(3×105/well) were cultured in 24-well plates according
to a previously published method (27).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Samples (200 µl serum or 3×106 PBMCs)
were lysed using 1 ml TRIzol reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Total RNA was
extracted using the phenol chloroform method. The concentration and
quality of RNA was measured spectrophotometrically (Nanodrop
ND2000; NanoDrop; Thermo Fisher Scientific, Inc., Pittsburgh, PA,
USA), and OD260/A280 and A260/A230 ratios were used for evaluation
of quality. cDNA was obtained by RT using 1 µg RNA, and stored at
−20°C. RT of the extracted RNA was achieved using the TIANScript II
cDNA First Strand Synthesis kit (Tiangen Biotech Co., Ltd.,
Beijing, China).
The SuperReal PreMix (SYBR Green) RT-qPCR kit
(Tiangen Biotech Co., Ltd.) was used to detect the expression of
human BMP-2 using GAPDH as internal standard, and mouse BMP-2
expression using β-actin as internal reference. The primer
sequences were as follows: Human BMP-2 forward,
5′-CCTATATGCTCGACCTGTAC-3′, and reverse,
5′-CCCACTCATTTCTGAAAGTTC-3′; GAPDH forward,
5′-GCACAGTCAAGGCTGAGAAT-3′, and reverse,
5′-TGAAGACGCCAGTAGACTCC-3′; mouse BMP-2 forward,
5′-TGTGAGGATTAGCAGGTCTT-3′, and reverse,
5′-GTTAGTGGAGTTCAGGTGGT-3′; β-actin forward,
5′-CTCTTTTCCAGCCTTCCTTCT-3′, and reverse,
5′-TGGAAGGTGGACAGTGAGG-3′. Reaction mixtures (20 µl) consisted of
qPCR-mix (10 µl), forward primer (0.5 µl; 10 µmol/µl), reverse
primer (0.5 µl; 10 µmol/µl), cDNA (2 µl) and ddH2O (7
µl). Thermocycling conditions were as follows: Initial denaturation
at 95°C for 30 sec followed by 39 cycles of denaturation at 95°C
for 5 sec and elongation at 60°C for 20 sec (iQ5; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The 2−ΔΔCq
method (28) was used to calculate
the relative expression of human or mouse BMP-2 mRNA vs. GAPDH or
β-actin, respectively. Each sample was analyzed in triplicate.
miR-410 expression was determined using the miRcute
miRNA qPCR detection kit (Tiangen Biotech Co., Ltd.) using U6 as an
internal reference. Primer sequences for human samples were as
follows: Human miR-410 forward, 5′-GTCAGCGCAATATAACACAG-3′; human
U6 forward, 5′-GTCAGCGCGTGCTCGCTTCG-3′, and the human universal
reverse primer, 5′-GTGCAGGGTCCGAGGT-3′ (provided in the kit). The
same aforementioned reaction mixtures were used. Thermocycling
conditions were as follows: Initial denaturation at 95°C for 5 min
followed by 40 cycles of denaturation at 95°C for 10 sec, annealing
at 60°C for 20 sec and extension at 72°C for 10 sec (iQ5; Bio-Rad
Laboratories, Inc.). Primer sequences for murine samples were as
follows: Mouse miR-410 forward, 5′-AGGTTGTCTGTGATGAGTTCG-3′; mouse
U6 forward, 5′-CTCGCTTCGGCAGCACATATACT-3′ and the mouse universal
reverse primer, 5′-ACGCTTCACGAATTTGCGTGTC-3′ (provided in the kit).
Reaction mixtures were prepared as described above. Thermocycling
conditions were as follows: Initial denaturation at 95°C for 5 min
followed by 40 cycles of denaturation at 95°C for 15 sec, annealing
at 60°C for 15 sec and extension at 72°C for 10 sec (iQ5; Bio-Rad
Laboratories, Inc.). The 2−ΔΔCq method was used to
calculate human or mouse miR-410 expression relative to U6. Each
sample was analyzed in triplicate.
Western blotting
PBMCs in each group were lysed using prechilled
radioimmunoprecipitation assay lysis buffer (600 µl; 50 mM
Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1% SDS, 1% TritonX-100, 1%
sodium deoxycholate; Beyotime Institute of Biotechnology, Haimen,
China). Following lysis for 30 min on ice, the mixture was
centrifuged at 12,000 × g for 10 min at 4°C. The protein
concentration of the supernatant was determined using a
bicinchoninic acid protein concentration determination kit (cat.
no., RTP7102; Real-Times (Beijing) Biotechnology Co., Ltd.,
Beijing, China). Protein samples (50 µg) were mixed with SDS
loading buffer (5X) and denatured in a boiling water bath for 10
min. Samples were then separated on 10% SDS-PAGE gels. Proteins
were transferred to polyvinylidene difluoride membranes (100 V, 1
h) in an ice box and blocked with 5% skimmed milk at room
temperature for 1 h. Membranes were incubated with rabbit
anti-human or rabbit anti-mouse BMP-2 polyclonal primary antibodies
(dilution, 1:1,000; cat. no. ab14933; Abcam, Cambridge, USA) and
rabbit anti-human or rabbit anti-mouse β-actin primary antibody
(dilution 1:5,000; cat. no. ab8227; Abcam) at 4°C overnight.
Following washing with PBS containing Tween 20 (concentration,
0.1%; five washes for 5 min each time), membranes were incubated
with goat anti-rabbit horseradish peroxidase-conjugated secondary
antibody (dilution, 1:3,000; cat. no. ab6721; Abcam) for 1 h at
room temperature prior to washing with PBS containing Tween 20 (5
washes for 5 min each time). Membranes were developed with an
enhanced chemiluminescence detection kit (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). Image lab 3.0 (Bio-Rad Laboratories,
Inc.) was used to analyze the results. BMP-2 protein levels were
quantified relative to β-actin.
ELISA
Human and mouse BMP-2 ELISA kits (ab119581 and
ab119582, respectively; Abcam) were used to determine
concentrations of human and mouse BMP-2 in serum samples. In
96-well plates, kit standards (50 µl) and samples (10 µl serum and
40 µl kit diluent) were added to the wells; empty wells served as
blanks. Horseradish peroxidase-labeled conjugate (100 µl) was added
to the wells prior to sealing and the plate was incubated at 37°C
for 1 h. The samples were washed five times using a washing reagent
supplied in the kit, substrates A (50 µl) and B (50 µl) were added
to each well and plates were incubated at 37°C for 15 min. Stop
solution (50 µl) was added to each well and the absorbance was
measured at 450 nm within 15 min of adding the stop solution.
Bioinformatics
To investigate the regulatory mechanisms of BMP-2,
miRanda (http://www.microrna.org/microrna/home.do), TargetScan
(http://www.targetscan.org), PITA
(http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
and PicTar (http://pictar.mdc-berlin.de/) were used to predict miR
targets that may regulate BMP-2.
Dual luciferase reporter assay
Wild-type (WT; UUAUAU) and mutant miR-410 seed
regions (AAUAUA) in the 3′-untranslated region (UTR) of BMP-2 were
synthesized in vitro. Spe-1 and HindIII restriction sites
were created at the ends and constructs were cloned into the
pMIR-REPORT luciferase reporter plasmid (Ambion; Thermo Fisher
Scientific, Inc.). Plasmids (0.8 µg) with WT or mutant 3′-UTR DNA
sequences were co-transfected with agomiR-410 (100 nM; Sangon
Biotech Co., Ltd., Shanghai, China) into 293T cells Cell Bank of
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China) using Lipofectamine® 2000 transfection reagent
(Thermo Fisher Scientific, Inc.). Negative control (NC) group was
transfected with agomiR-410 and empty plasmid. Following
cultivation at 37°C for 24 h, cells were lysed using the dual
luciferase reporter assay kit (Promega Corporation, Madison, WI,
USA) according to the manufacturer's protocol, and fluorescence
intensity was measured using a GloMax 20/20 luminometer (Promega
Corporation). Renilla fluorescence activity was used as
internal reference.
Statistical analysis
Results were analyzed using SPSS 18.0 (SPSS, Inc.,
Chicago, IL, USA). All data is presented as the mean ± standard
deviation. Data were tested for normality. Multigroup comparisons
were analyzed using one-way ANOVA. In case of homogeneity of
variance, the least significant difference and Student-Newman-Keuls
test were used; in case of heterogeneity of variance, Tamhane's T2
or Dunnett's test was used. Comparisons between two groups were
analyzed using a Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patients with postmenopausal
osteoporosis exhibit reduced BMP-2 and elevated miR-410
expression
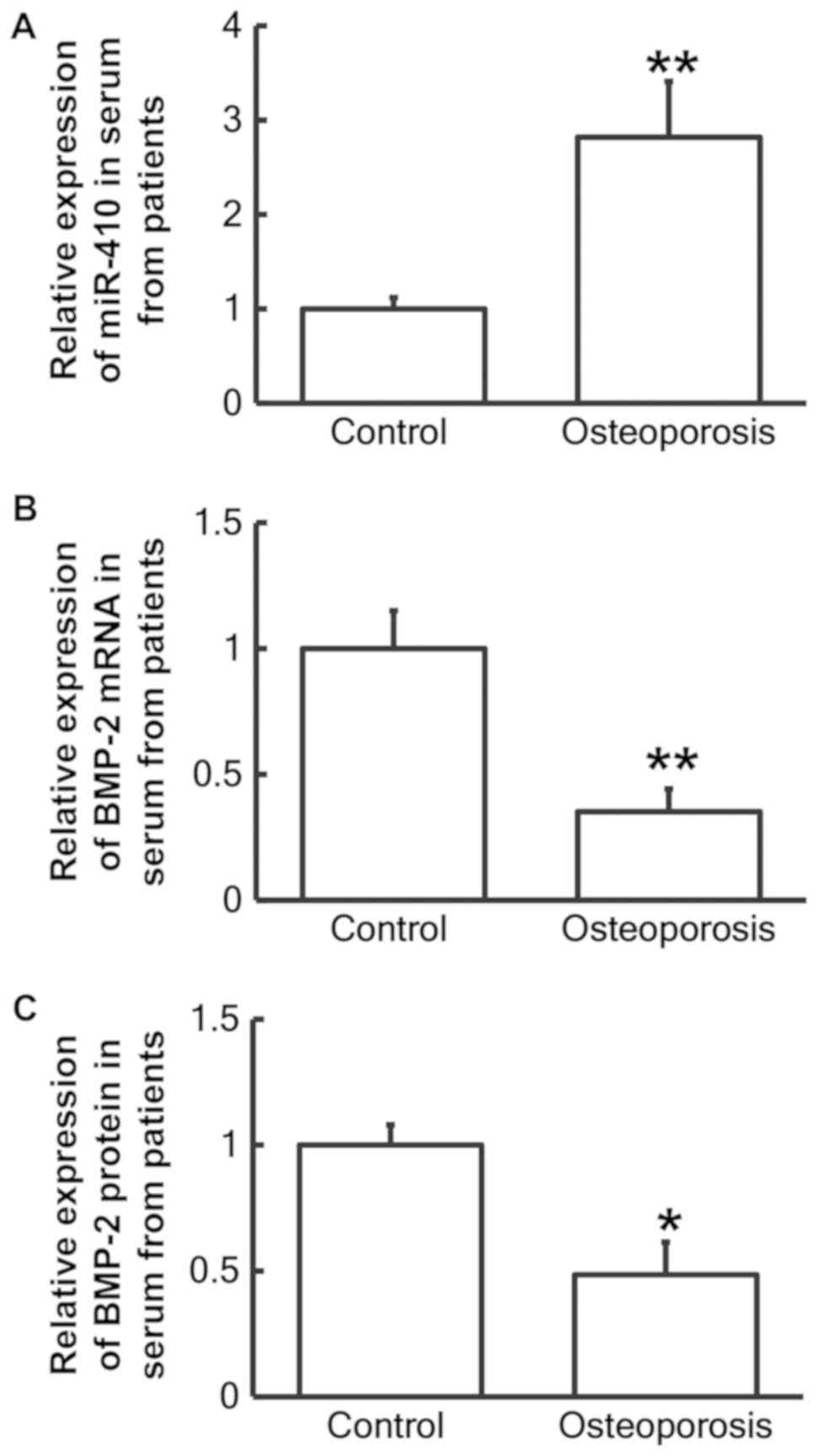
To measure miR-410 and BMP-2 mRNA and protein levels
in serum samples from patients with postmenopausal osteoporosis,
RT-qPCR and ELISA tests were employed, respectively. RT-qPCR
analysis revealed that miR-410 levels in the serum of patients with
postmenopausal osteoporosis were significantly increased when
compared with the healthy control group (P<0.01; Fig. 1A), while BMP-2 mRNA levels were
significantly decreased compared with the control group (P<0.01;
Fig. 1B). In addition, BMP-2 protein
levels in the serum were significantly decreased when compared with
the control group (P<0.05; Fig.
1C). These results suggest that reduced BMP-2 and elevated
miR-410 expression in serum may be associated with postmenopausal
osteoporosis.
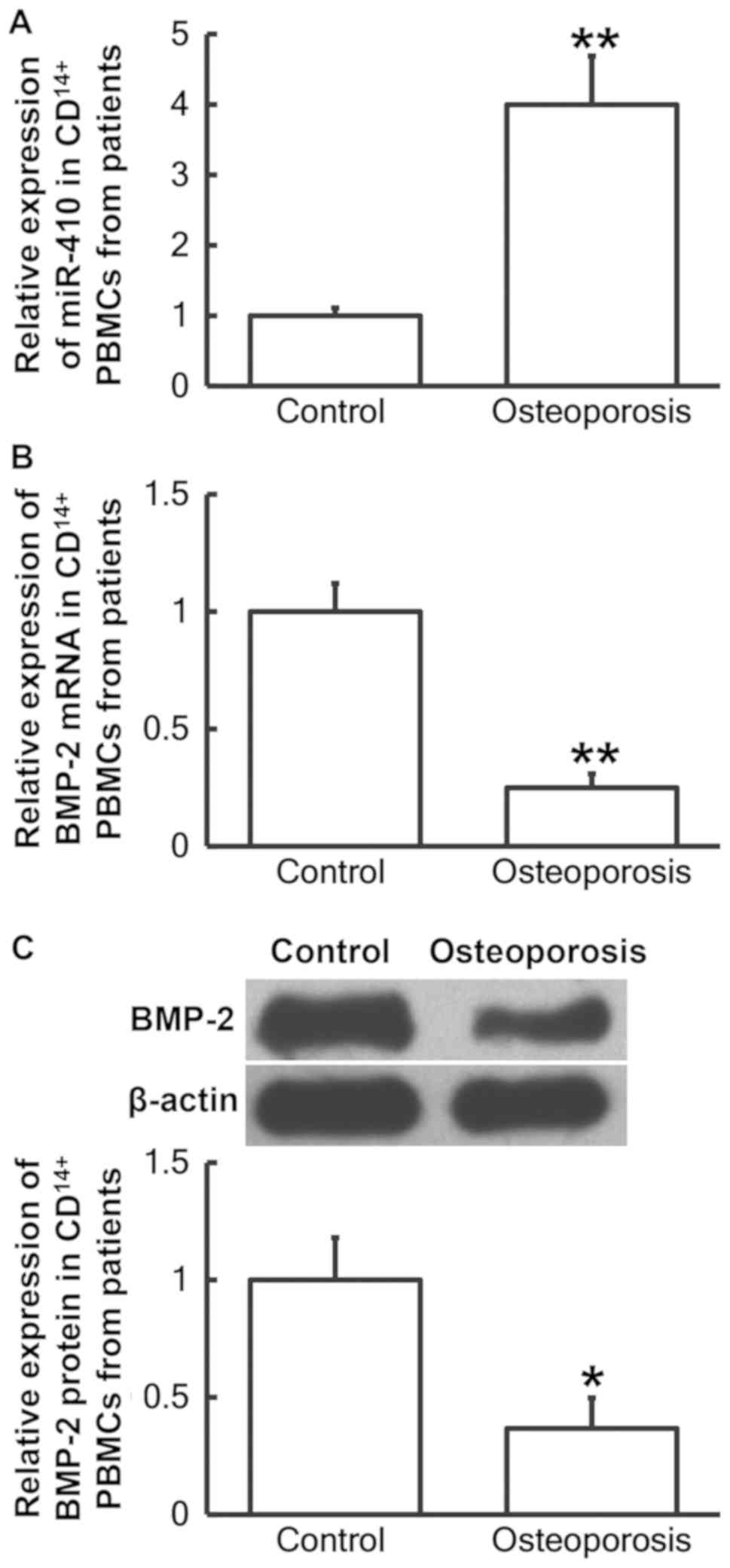
Patients with postmenopausal
osteoporosis exhibit decreased BMP-2 and increased miR-410
expression in CD14+ PBMCs
To determine miR-410 and BMP-2 mRNA and protein
levels in CD14+ PBMCs from patients with postmenopausal
osteoporosis, RT-qPCR and western blotting analyses were performed,
respectively. The RT-qPCR results demonstrated that miR-410 levels
in CD14+ PBMCs from patients with postmenopausal
osteoporosis were significantly increased when compared with the
control group (P<0.01; Fig. 2A),
while BMP-2 mRNA levels in CD14+ PBMCs were
significantly decreased compared with the control group (P<0.01;
Fig. 2B). In addition, BMP-2 protein
levels in CD14+ PBMCs from patients with postmenopausal
osteoporosis were significantly decreased compared with the control
group (P<0.05; Fig. 2C). The
results provide further evidence that decreased BMP-2 and increased
miR-410 expression in CD14+ PBMCs may be associated with
postmenopausal osteoporosis.
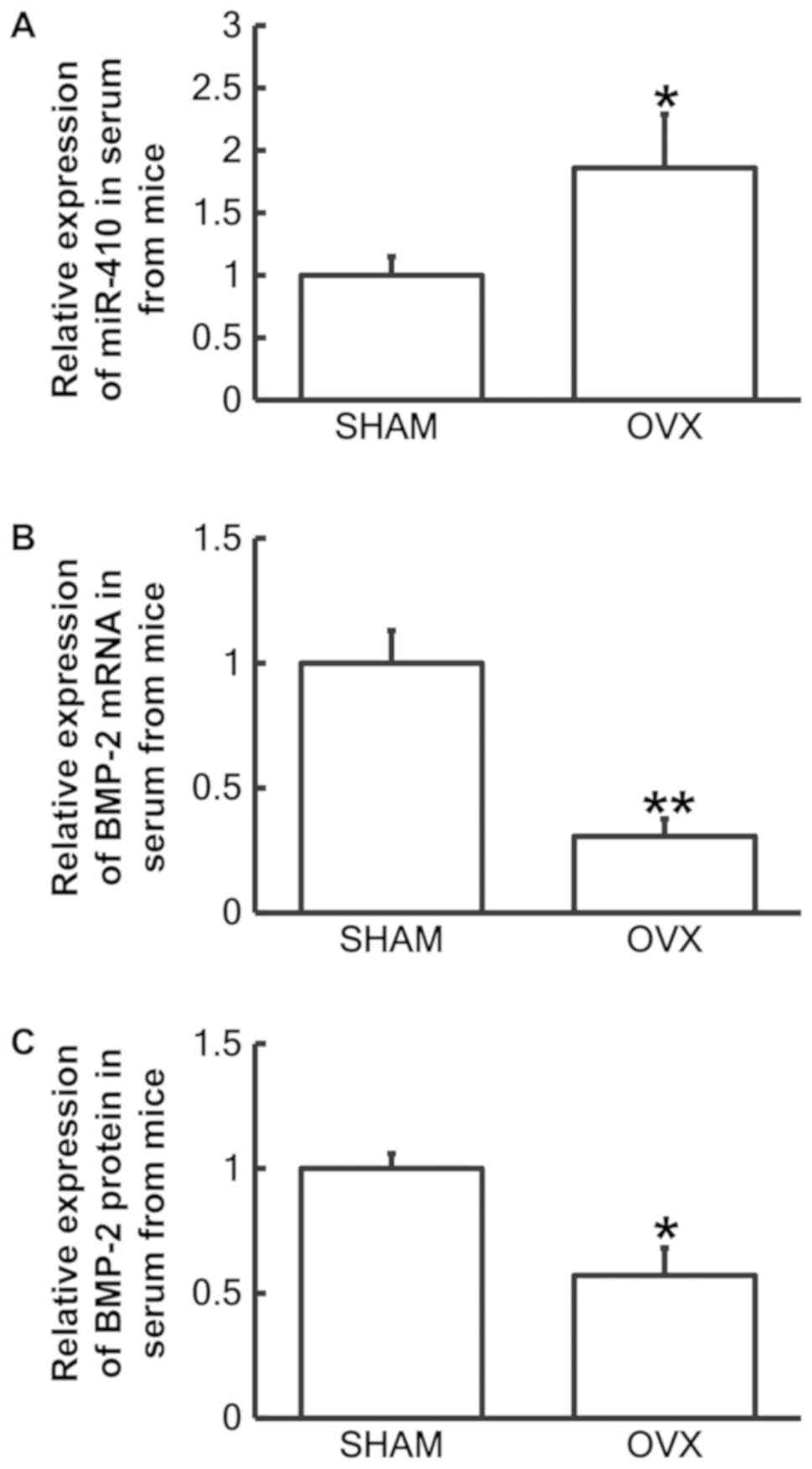
Reduced BMP-2 and increased miR-410
expression is observed in serum of mouse model with postmenopausal
osteoporosis
To examine miR-410 and BMP-2 mRNA and protein levels
in serum from mice in the sham (n=28) and OVX groups (n=25),
RT-qPCR and ELISA assays were performed, respectively. The RT-qPCR
results revealed that miR-410 levels in serum samples from the OVX
group were significantly increased when compared with the sham
group (P<0.05; Fig. 3A), while
BMP-2 mRNA levels in serum samples from the OVX group were
significantly decreased compared with the sham group (P<0.01;
Fig. 3B). In addition, BMP-2 protein
levels in serum from the OVX group were significantly decreased
compared with the sham group (P<0.05; Fig. 3C). These results confirmed that
reduced BMP-2 and increased miR-410 expression may be associated
with postmenopausal osteoporosis in vivo.
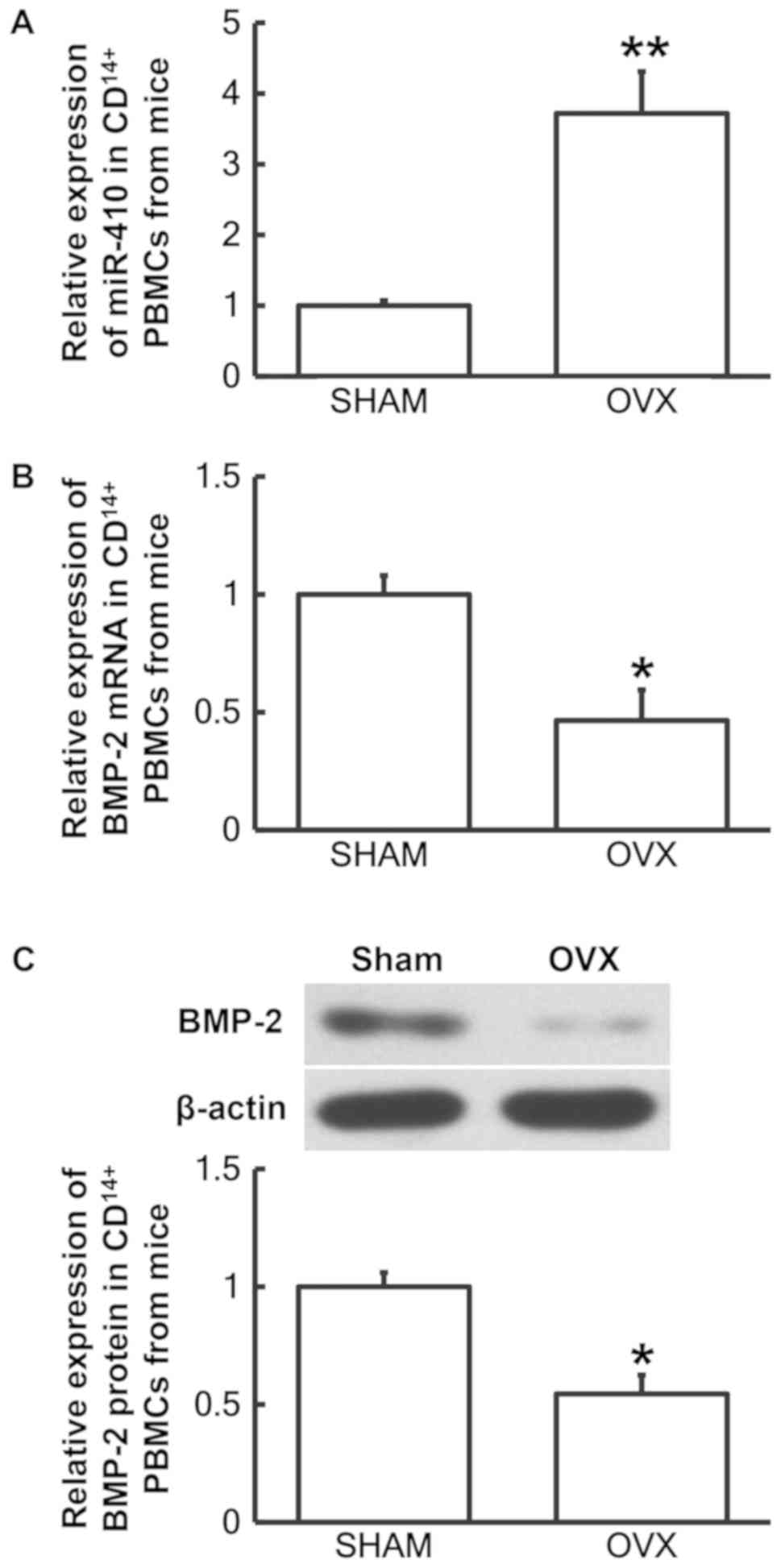
Decreased BMP-2 and increased miR-410
expression is observed in CD14+ PBMCs derived from a
mouse model of postmenopausal osteoporosis
To analyze miR-410 and BMP-2 mRNA and protein levels
in CD14+ PBMCs from mice, RT-qPCR and western blotting
analyses were performed, respectively. The RT-qPCR results
demonstrated that miR-410 levels in CD14+ PBMCs from the
OVX group were significantly increased when compared with the sham
group (P<0.01; Fig. 4A), while
BMP-2 mRNA levels in CD14+ PBMCs from OVX group were
significantly decreased compared with the sham group (P<0.05;
Fig. 4B). In addition, BMP-2 protein
levels in CD14+ PBMCs from the OVX group were
significantly decreased when compared with the sham group
(P<0.05; Fig. 4C). The results
indicated that CD14+ PBMCs derived from a mouse model of
postmenopausal osteoporosis exhibited decreased BMP-2 and elevated
miR-410 expression levels.
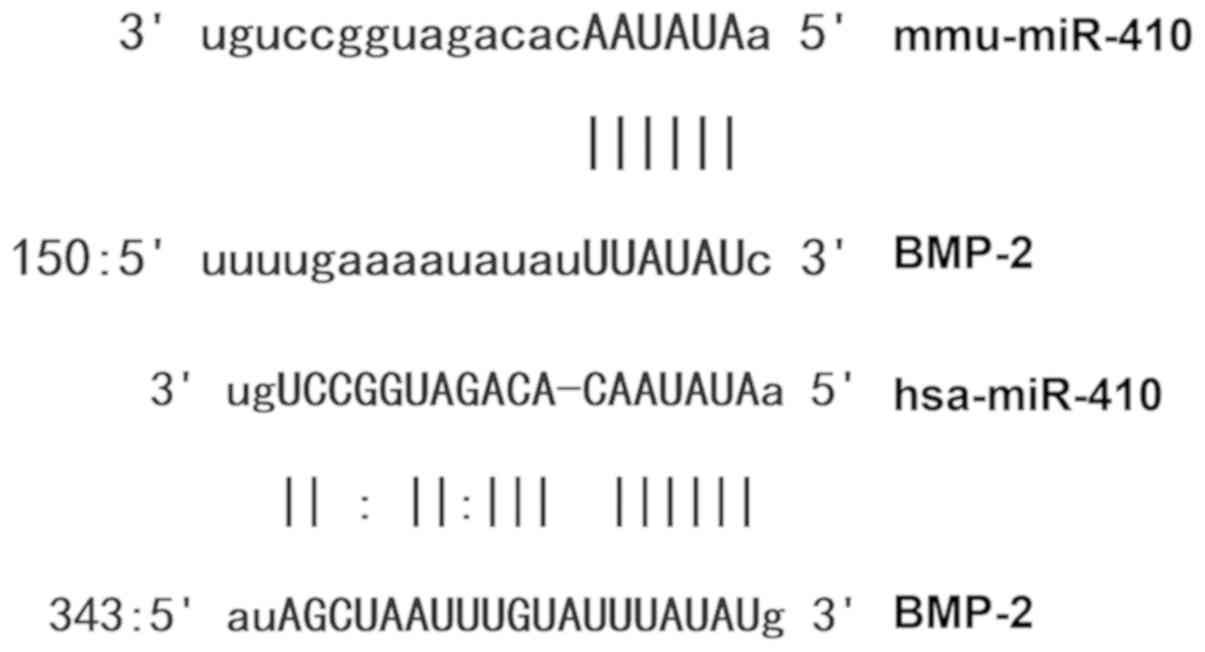
miR-410 binds to the 3′-UTR seed
region of BMP-2 and regulates its expression
Bioinformatics analysis revealed that miR-410 was a
potential regulator of BMP-2 (Fig.
5). To identify interactions between miR-410 and the 3′-UTR of
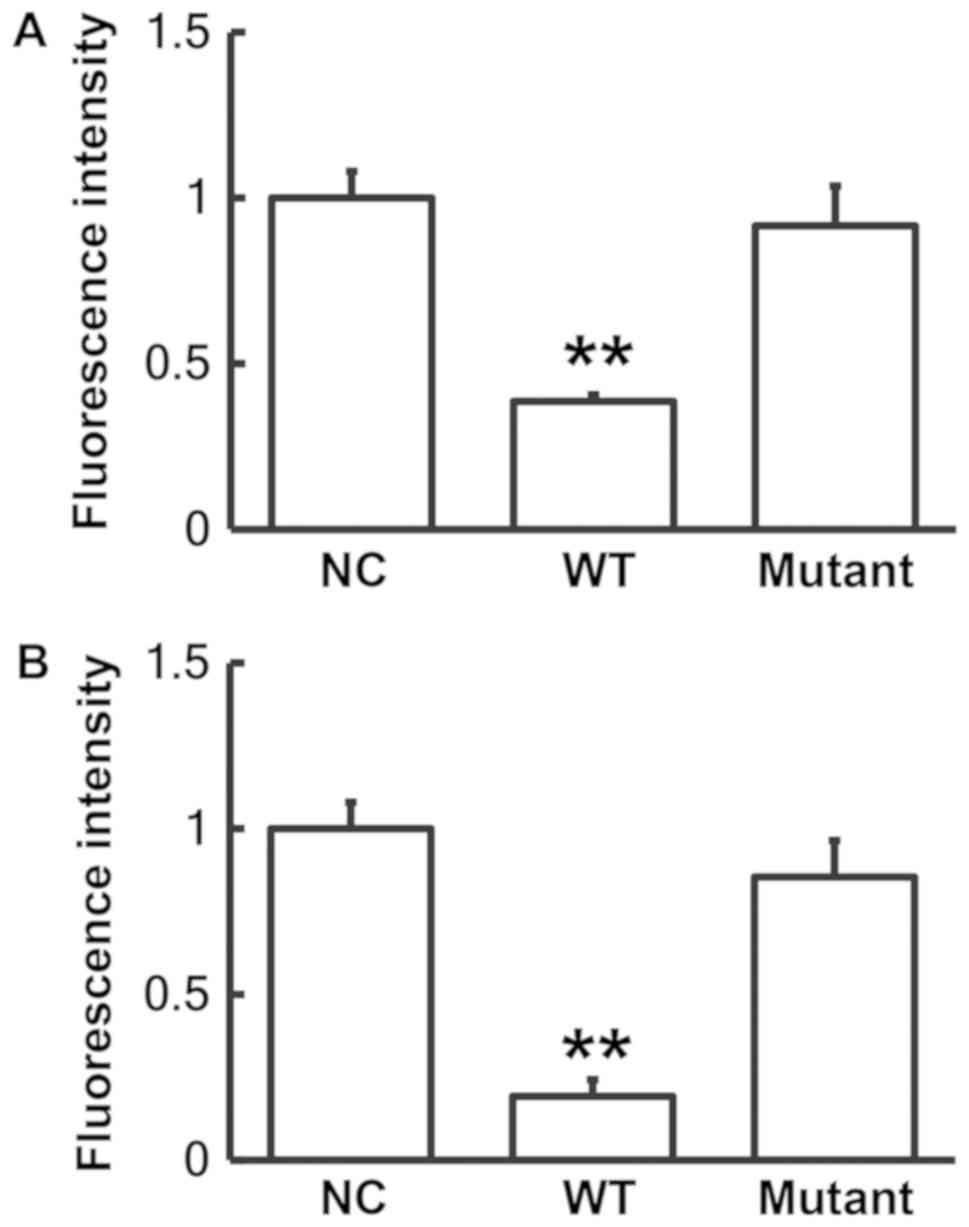
human and mouse BMP-2 mRNA, dual luciferase reporter assays were
performed. The level of fluorescence generated by cells
co-transfected with miR-410 mimics and pMIR-REPORT-WT luciferase
reporter plasmids was significantly decreased when compared with
the negative control group (P<0.01; Fig. 6). By contrast, the level of
fluorescence generated by cells co-transfected with miR-410 mimics
and pMIR-REPORT-mutant luciferase reporter plasmids was not
significantly altered when compared with the negative control group
(P>0.05; Fig. 6). These results
suggest that miR-410 binds to the 3′-UTR seed region of BMP-2 mRNA
and regulates its expression.
Discussion
It is generally accepted that the underlying cause
of postmenopausal osteoporosis is an imbalance between bone
formation and bone resorption induced by estrogen deficiency, which
leads to bone remodeling disorders (29). Treatment of postmenopausal
osteoporosis is focused on recovery and maintenance of a balance
between bone remodeling and bone resorption (30,31).
Understanding the molecular mechanisms underlying the disease is
beneficial for clinical prevention, diagnosis and treatment.
BMP, a factor that induces osteogenesis, promotes
the differentiation of mesenchymal cells into bone, cartilage,
ligament, tendon and nerve tissues (32). BMP-2 has been demonstrated to
transform murine myoblasts into osteoblast cells (33,34). It
is thought that BMP-2 levels in osteoblasts may reflect bone
formation ability (35,36). In the present study, BMP-2 levels in
serum samples and CD14+ PBMCs derived from patients with
postmenopausal osteoporosis were observed to be significantly lower
when compared with healthy individuals, suggesting that reduced
BMP-2 expression may be associated with postmenopausal
osteoporosis. Similarly, in a mouse model of postmenopausal
osteoporosis, BMP-2 expression in serum and CD14+ PBMCs
from mice in the OVX group was downregulated when compared with the
sham group. These results indicated that BMP-2 may be closely
associated with postmenopausal osteoporosis.
miRs are important post-transcriptional regulators.
It has been reported that miRs are widely associated with the
regulation of cartilage development, osteocyte proliferation and
osteoporosis (37,38). The authors of the present study
hypothesized an association between BMP-2 and postmenopausal
osteoporosis, and miRs that may regulate BMP-2 were investigated in
the present study. Previous studies have identified a number of
miRs as biomarkers for different diseases (39,40). In
the current study, bioinformatics tools were utilized to identify
upstream genes predicted to regulate BMP-2, which resulted in the
identification of miR-410 as a potential upstream regulator. To
date, there are a limited number of reports that have investigated
the functional role of miR-410 in human disease. Wheeler et
al (41) reported that miR-410
and miR-431 are expressed in the central nervous system. Goodarzi
et al (42) demonstrated that
miR-410 serves an important regulatory role in the pathological
process of male alopecia. Hennessy et al (43) discovered that miR-410 serves an
important role in the regulation of insulin secretion. A previous
study demonstrated that miR-410 expression was decreased in
endothelial cells with Hantaan virus-induced alterations in cell
permeability (44). In addition,
miR-410 has been demonstrated to serve important regulatory roles
in the occurrence and development of prostate, breast and colon
cancer (45–47). In the present study, miR-410
expression in serum and CD14+ PBMCs from patients with
postmenopausal osteoporosis was elevated when compared with normal
healthy controls. Considering that BMP-2 expression in serum and
CD14+ PBMCs was decreased, it was hypothesized that
upregulation of miR-410 may underlie the observed downregulation of
BMP-2 in patients with postmenopausal osteoporosis. Similar results
were observed in the mouse model of postmenopausal osteoporosis,
which indicates that an association between miR-410 and BMP-2 may
exists across different species. Dual luciferase reporter assays
revealed that miR-410 bound to the 3′-UTR of BMP-2 and regulated
its expression.
The present study was limited by the small number of
samples included and the lack of genetic diversity. Future studies
may include an increased number of samples from multiple ethnic
groups. In conclusion, the present study demonstrated that enhanced
miR-410 expression in serum and CD14+ PBMCs from
patients with postmenopausal osteoporosis targeted BMP-2 and may
downregulate its mRNA expression thus leading to decreased BMP-2
protein levels. The association between miR-410 and BMP-2 may
therefore serve a biological role in the occurrence and development
of postmenopausal osteoporosis. The present study provided a novel
insight into the mechanisms underlying postmenopausal osteoporosis,
and provided a theoretical basis for the diagnosis, prevention and
treatment of the disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272942).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The final version of the manuscript has been read
and approved by all authors, and each author believes that the
manuscript represents honest work. HZ, WD, FJ and DW collaborated
to design the study. HZ and WD were responsible for performing
experiments. HZ, WD, FJ and DW analyzed the data. All authors
collaborated to interpret results and develop the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Changhai Hospital. Written informed consent was
obtained from all patients or their families.
Patient consent for publication
Written informed consent for publication of any
associated data and accompanying images wsa obtained from all
patients or their parents, guardians or next of kin.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pietschmann P, Rauner M, Sipos W and
Kerschan-Schindl K: Osteoporosis: An age-related and
gender-specific disease-a mini-review. Gerontology. 55:3–12. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Committee for Chinese white paper on the
prevention and control of osteoporosis CHPF, . Chinese white paper
on osteoporosis. Chin J Health Manage. 3:148–154. 2009.(In
Chinese).
|
|
4
|
Zhang K, Li B, Chen Q, Zhang Z, Zhao X and
Hou H: Functional calcium binding peptides from pacific cod (Gadus
macrocephalus) bone: Calcium bioavailability enhancing activity and
anti-osteoporosis effects in the ovariectomy-induced osteoporosis
rat model. Nutrients. 10(pii): E13252018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanis JA, McCloskey EV, Johansson H and
Oden A: Approaches to the targeting of treatment for osteoporosis.
Nat Rev Rheumatol. 5:425–431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hemingway F, Cheng X, Knowles HJ, Estrada
FM, Gordon S and Athanasou NA: In vitro generation of mature human
osteoclasts. Calcif Tissue Int. 89:389–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sørensen MG, Henriksen K, Schaller S,
Henriksen DB, Nielsen FC, Dziegiel MH and Karsdal MA:
Characterization of osteoclasts derived from CD14+ monocytes
isolated from peripheral blood. J Bone Miner Metab. 25:36–45. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shalhoub V, Elliott G, Chiu L, Manoukian
R, Kelley M, Hawkins N, Davy E, Shimamoto G, Beck J, Kaufman SA, et
al: Characterization of osteoclast precursors in human blood. Br J
Haematol. 111:501–512. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu H, Lacey DL, Dunstan CR, Solovyev I,
Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, et
al: Tumor necrosis factor receptor family member RANK mediates
osteoclast differentiation and activation induced by
osteoprotegerin ligand. Proc Natl Acad Sci USA. 96:3540–3545. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sengle G, Ono RN, Lyons KM, Bächinger HP
and Sakai LY: A new model for growth factor activation: Type II
receptors compete with the prodomain for BMP-7. J Mol Biol.
381:1025–1039. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen D, Zhao M, Harris SE and Mi Z: Signal
transduction and biological functions of bone morphogenetic
proteins. Front Biosci. 9:349–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dahlin C, Linde A, Gottlow J and Nyman S:
Healing of bone defects by guided tissue regeneration. Plast
Reconstr Surg. 81:672–676. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yilgor P, Hasirci N and Hasirci V:
Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J
Biomed Mater Res A. 93:528–536. 2010.PubMed/NCBI
|
|
14
|
Murata M, Maki F, Sato D, Shibata T and
Arisue M: Bone augmentation by onlay implant using recombinant
human BMP-2 and collagen on adult rat skull without periosteum.
Clin Oral Implants Res. 11:289–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishibe T, Goto T, Kodama T, Miyazaki T,
Kobayashi S and Takahashi T: Bone formation on apatite-coated
titanium with incorporated BMP-2/heparin in vivo. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 108:867–875. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uchibe K, Son J, Larmour C, Pacifici M,
Enomoto-Iwamoto M and Iwamoto M: Genetic and pharmacological
inhibition of retinoic acid receptor γ function promotes
endochondral bone formation. J Orthop Res. 35:1096–1105. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cappato S, Tonachini L, Giacopelli F,
Tirone M, Galietta LJ, Sormani M, Giovenzana A, Spinelli AE,
Canciani B, Brunelli S, et al: High-throughput screening for
modulators of ACVR1 transcription: Discovery of potential
therapeutics for fibrodysplasia ossificans progressiva. Dis Model
Mech. 9:685–696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong J, Cui X, Jiang Z and Sun J:
MicroRNA-23a modulates tumor necrosis factor-alpha-induced
osteoblasts apoptosis by directly targeting Fas. J Cell Biochem.
114:2738–2745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang GP, Zhang J, Zhu CH, Lin L, Wang J,
Zhang HJ, Li J, Yu XG, Zhao ZS, Dong W and Liu GB: MicroRNA-98
regulates osteogenic differentiation of human bone mesenchymal
stromal cells by targeting BMP2. J Cell Mol Med. 21:254–264. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laxman N, Mallmin H, Nilsson O and
Kindmark A: miR-203 and miR-320 regulate bone morphogenetic
protein-2-induced osteoblast differentiation by targeting
distal-less homeobox 5 (Dlx5). Genes (Basel). 8(pii): E42016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ke X, Yuan Y, Guo C, Yang Y, Pu Q, Hu X,
Tang K, Luo X, Jiang Q, Su X, et al: MiR-410 induces stemness by
inhibiting Gsk3β but upregulating β-catenin in non-small cells lung
cancer. Oncotarget. 8:11356–11371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu XP, Liao EY, Zhang H, Shan PF, Cao XZ
and Liu SP: Establishment of BMD reference plots and determination
of peak BMD at multiple skeletal regions in mainland Chinese women
and the diagnosis of osteoporosis. Osteoporos Int. 15:71–79. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao EY, Wu XP, Luo XH, Zhang H, Dai RC,
Huang G and Wang WB: Establishment and evaluation of bone mineral
density reference databases appropriate for diagnosis and
evaluation of osteoporosis in Chinese women. J Bone Miner Metab.
21:184–192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanis JA, Melton LJ III, Christiansen C,
Johnston CC and Khaltaev N: The diagnosis of osteoporosis. J Bone
Miner Res. 9:1137–1141. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hovell R: Reduction, refinement and
replacement. Vet Rec. 172:6912013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bargalló ME, Guardo AC, Maleno MJ,
Miralles L, Egaña-Gorroño L, Escribà T, García F, Gatell JM, Arnedo
M and Plana M: Utility of systematic isolation of immune cell
subsets from HIV-infected individuals for miRNA profiling. J
Immunol Methods. 442:12–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bollen AM, Taguchi A, Hujoel PP and
Hollender LG: Case-control study on self-reported osteoporotic
fractures and mandibular cortical bone. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 90:518–524. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klein-Nulend J, van Oers RF, Bakker AD and
Bacabac RG: Bone cell mechanosensitivity, estrogen deficiency, and
osteoporosis. J Biomech. 48:855–865. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kehler T: Epidemiology of osteoporosis and
osteoporotic fractures. Reumatizam. 61:60–64. 2014.(In Croatian).
PubMed/NCBI
|
|
32
|
Sykaras N and Opperman LA: Bone
morphogenetic proteins (BMPs): How do they function and what can
they offer the clinician? J Oral Sci. 45:57–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gutierrez J, Osses N and Brandan E:
Changes in secreted and cell associated proteoglycan synthesis
during conversion of myoblasts to osteoblasts in response to bone
morphogenetic protein-2: Role of decorin in cell response to BMP-2.
J Cell Physiol. 206:58–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mundy G, Garrett R, Harris S, Chan J, Chen
D, Rossini G, Boyce B, Zhao M and Gutierrez G: Stimulation of bone
formation in vitro and in rodents by statins. Science.
286:1946–1949. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Su JL, Chiou J, Tang CH, Zhao M, Tsai CH,
Chen PS, Chang YW, Chien MH, Peng CY, Hsiao M, et al: CYR61
regulates BMP-2-dependent osteoblast differentiation through the
{alpha}v{beta}3 integrin/integrin-linked kinase/ERK pathway. J Biol
Chem. 285:31325–31336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang JK, Hsu YL, Teng IC and Kuo PL:
Piceatannol stimulates osteoblast differentiation that may be
mediated by increased bone morphogenetic protein-2 production. Eur
J Pharmacol. 551:1–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hao L, Fu J, Tian Y and Wu J: Systematic
analysis of lncRNAs, miRNAs and mRNAs for the identification of
biomarkers for osteoporosis in the mandible of ovariectomized mice.
Int J Mol Med. 40:689–702. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Y and Fang S: Small non-coding
RNAs-based bone regulation and targeting therapeutic strategies.
Mol Cell Endocrinol. 456:16–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi JL, Kao PF, Itriago E, Zhan Y,
Kozubek JA, Hoss AG, Banigan MG, Vanderburg CR, Rezvani AH,
Latourelle JC, et al: miR-149 and miR-29c as candidates for bipolar
disorder biomarkers. Am J Med Genet B Neuropsychiatr Genet.
174:315–323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Batistela MS, Josviak ND, Sulzbach CD and
de Souza RL: An overview of circulating cell-free microRNAs as
putative biomarkers in Alzheimer's and Parkinson's diseases. Int J
Neurosci. 127:547–558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wheeler G, Ntounia-Fousara S, Granda B,
Rathjen T and Dalmay T: Identification of new central nervous
system specific mouse microRNAs. FEBS Lett. 580:2195–2200. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goodarzi HR, Abbasi A, Saffari M, Tabei MB
and Noori Daloii MR: MicroRNAs take part in pathophysiology and
pathogenesis of male pattern baldness. Mol Biol Rep. 37:2959–2965.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hennessy E, Clynes M, Jeppesen PB and
O'Driscoll L: Identification of microRNAs with a role in glucose
stimulated insulin secretion by expression profiling of MIN6 cells.
Biochem Biophys Res Commun. 396:457–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pepini T, Gorbunova EE, Gavrilovskaya IN,
Mackow JE and Mackow ER: Andes virus regulation of cellular
microRNAs contributes to hantavirus-induced endothelial cell
permeability. J Virol. 84:11929–11936. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang YF, Yu Y, Song WZ, Zhang RM, Jin S,
Bai JW, Kang HB, Wang X and Cao XC: miR-410-3p suppresses breast
cancer progression by targeting Snail. Oncol Rep. 36:480–486. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu C, Zhang A, Cheng L and Gao Y: miR-410
regulates apoptosis by targeting Bak1 in human colorectal cancer
cells. Mol Med Rep. 14:467–473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang J, Ye H, Zhang D, Hu Y, Yu X, Wang L,
Zuo C, Yu Y, Xu G and Liu S: MicroRNA-410-5p as a potential serum
biomarker for the diagnosis of prostate cancer. Cancer Cell Int.
16:122016. View Article : Google Scholar : PubMed/NCBI
|