Introduction
Oral squamous cell carcinoma (OSCC) is the sixth
most common malignant tumor type worldwide, accounting for nearly
529,500 new cases and 292,300 deaths per year (1). Despite the advances in surgery,
radiation therapy and chemotherapy, the long-term survival rate for
OSCC has only improved by a small amount in the last three decades
(1–3). Most OSCC patients are diagnosed at an
advanced clinical stage, which is usually associated with poor
prognosis (4,5). Therefore, it is essential and urgent to
diagnose OSCC at an earlier stage and explore potential therapeutic
targets.
Increasing evidence has demonstrated that multiple
genetic and epigenetic alterations are responsible for the genesis
and progression of OSCC. A study has indicated that α-protein
kinase 1 (ALPK1) is associated with lymph node metastases and tumor
development in OSCC, probably via regulation of cell growth,
migration and invasion (6).
Similarly, tumor-associated calcium signal transducer 2 (TROP2) was
also determined to be involved in cell differentiation, TNM stage
and vascular invasion (7). Although
certain studies have reported on several genetic or epigenetic
alterations contributing to OSCC, the molecular mechanisms of OSCC
requires further elucidation.
Recent additions to the Cancer Genome Atlas (TCGA)
provide abundant expression profiles from patient samples, as well
as the associated clinicopathological data for >30 human cancer
types (8,9). With the bioinformatics methods
developed, integrated data analyses may be performed to further
assist oncology research in several ways, including primary
identification of dysregulated genes. In the present study, 2,013
differentially expressed (DE) mRNAs were identified from TCGA data.
To further clarify the potential association between dysregulated
mRNAs and clinical outcomes, 180 DEmRNAs were filtered according to
the clinical features of patients [pathological N stage, T stage
and pathological stage based on TNM staging system (10)], 26 of which were aberrantly expressed
regarding all 3 different types of subgroup. Finally, 6
survival-associated genes [DDB1 and CUL4 associated factor 4 like 2
(DCAF4L2), opiorphin prepropeptide (OPRPN), R3H domain containing
like (R3HDML), transmembrane phosphatase with tensin homology
(TPTE), actin like 8 (ACTL8) and protocadherin α 11 (PCDHA11)] were
identified from Kaplan-Meier survival curves with the log-rank
test.
Materials and methods
Patients and samples
Original The Cancer Genome Atlas (TCGA) sequencing
data and corresponding clinical information were obtained from TCGA
database (https://portal.gdc.cancer.gov/) in October 2018. After
excluding the patients without specific clinicopathological
features (Tx and Nx), a total of 307 patients and 30 normal
controls provided by TCGA dataset for OSCC were included. The
clinical and pathological characteristics of the OSCC patients
included are presented in Table I.
No paired test was used to check whether the groups were
age/sex-matched. Ethical approval was not required for the present
study, since the data were downloaded from a publically available
and open-ended TCGA database and did not involve primary data from
human subjects.
 | Table I.Clinicopathological characteristics
of 307 patients with oral squamous cell carcinoma. |
Table I.
Clinicopathological characteristics
of 307 patients with oral squamous cell carcinoma.
| Subtype | N (%) |
|---|
| Age (years) |
|
|
<60 | 132 (43) |
|
≥60 | 175 (57) |
| Sex |
|
|
Male | 207 (67.4) |
|
Female | 100 (32.6) |
| Ethnicity |
|
|
White | 266 (86.6) |
| Black
or African American | 21 (6.8) |
|
Asian | 9 (2.9) |
|
American Indian or Alaska
native | 1 (0.3) |
| Not
available | 10 (3.3) |
| Pathologic
stage |
|
| I | 19 (6.2) |
| II | 51 (16.6) |
|
III | 54 (17.6) |
| IV | 160 (52.1) |
| Not
available | 23 (7.5) |
| Pathologic
T-stage |
|
| T1 | 29 (9.4) |
| T2 | 94 (30.6) |
| T3 | 57 (18.6) |
| T4 | 109 (35.5) |
| TX | 9 (2.9) |
| Not
available | 9 (2.9) |
| Pathologic
N-stage |
|
| N0 | 115 (37.5) |
| N1 | 46 (15) |
| N2 | 100 (32.6) |
| N3 | 2 (0.7) |
| NX | 34 (11.1) |
| Not
available | 10 (3.3) |
| Pathologic
M-stage |
|
| M0 | 116 (37.8) |
| MX | 33 (10.7) |
| Not
available | 158 (51.5) |
| Survival
status |
|
|
Alive | 166 (54.1) |
|
Dead | 141 (45.9) |
Analysis of differential expression
profiles
DEmRNAs between OSCC and normal tissues were
identified using the edgeR package from Bioconductor analysis tools
(https://bioconductor.org/packages/release/bioc/html/edgeR.html).
For multiple testing corrections, a false discovery rate (FDR) was
applied to all P-values. The cut-off criteria were set as absolute
|log2fold change (FC)≥2| and FDR<0.01.
Associations between DEmRNA and
clinical features
The selected DEmRNAs were analyzed to determine
whether they were differentially expressed in different clinical
feature categories, including pathological T stage (T3 + T4 vs. T1
+ T2), pathological N stage (N2 + N3 vs. N0 + N1) and pathological
stage (stage III + IV vs. stage I + II). DEmRNAs with
|log2FC|≥1.5 and FDR<0.05 were regarded as being
significantly linked to the relevant clinical feature.
Functional enrichment analysis
Functional enrichment analysis was performed for
clinical feature-associated DEmRNAs (CFmRNAs) to reveal the
functional role of these mRNAs in the oncogenesis of OSCC. The
Database for Annotation Visualization and Integrated Discovery
(DAVID) online tool (https://david.ncifcrf.gov/) was employed to perform
such functional and pathway enrichment analyses. Gene Ontology (GO)
and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
enrichment analyses were applied to determine the potential
biological functions and pathways of the CFmRNAs (P<0.05).
Protein-protein interaction (PPI)
network construction
The Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING) online database (http://string-db.org) is a search tool for identifying
and describing PPIs via review of recorded interactions. This tool
was used to analyze the PPIs of CFmRNAs. In addition, Cytoscape
software (version 3.6.1; http://www.cytoscape.org/) was then utilized to
visualize the PPI network of the CFmRNAs.
Survival analysis
For each CFmRNA, the OSCC patients were classified
into either a high expression group or a low expression group. The
median expression value of the respective CFmRNA was used as the
cut-off value. The differences in survival time between the groups
were then evaluated by Kaplan-Meier survival curves and the
log-rank test to identify CFmRNAs that were significantly
associated with survival in OSCC. The threshold for statistical
significance was set as P<0.05.
Results
DEmRNAs in OSCC
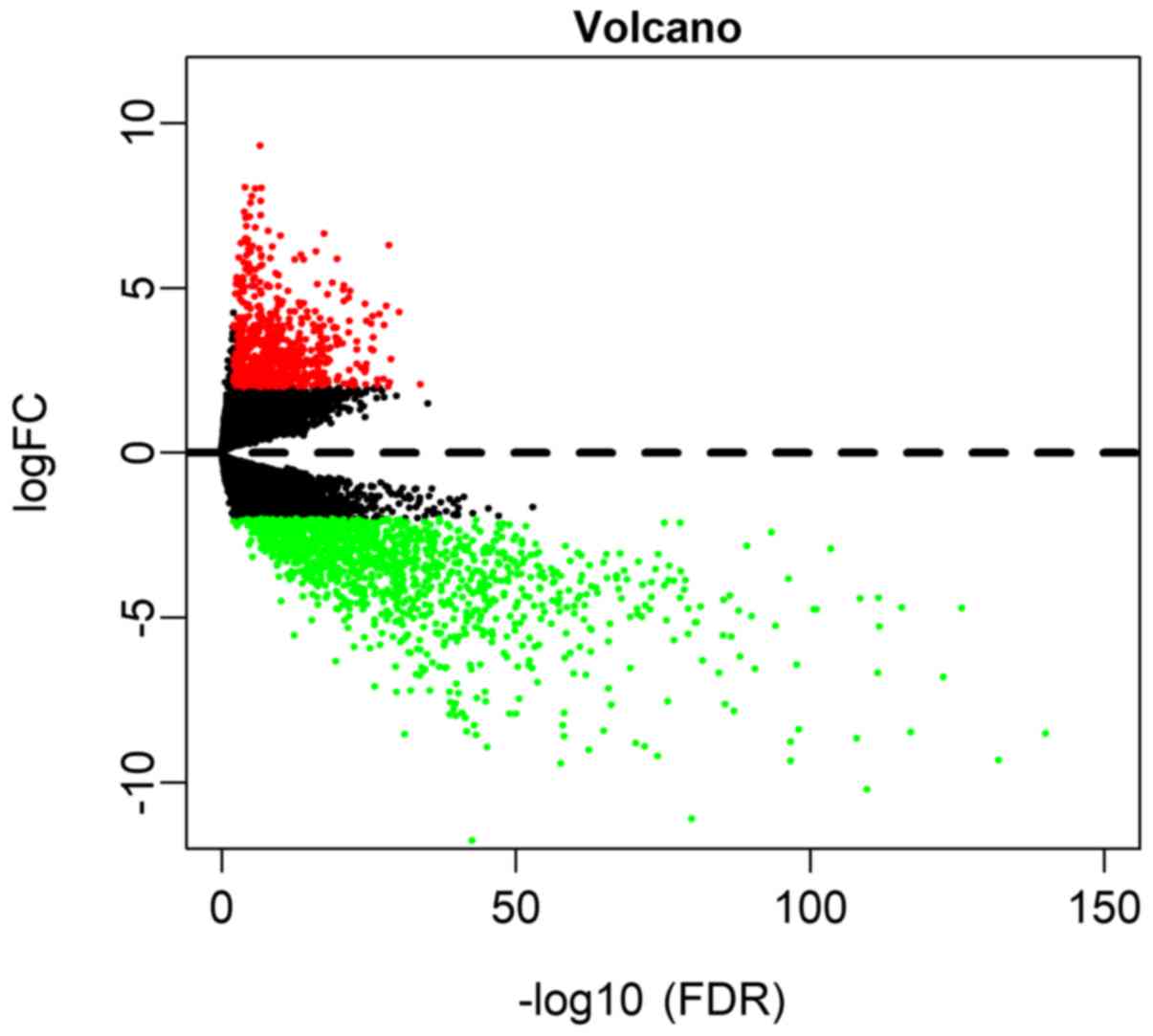
Based on the screening conditions as
|log2FC|≥2 and FDR<0.01, a total of 675 (33.53%)
upregulated and 1,338 (66.47%) downregulated genes were identified
in OSCC (Table SI). A volcano plot
was drawn to visualize the distribution of DEmRNAs between OSCC and
normal controls (Fig. 1).
DEmRNAs in association with clinical
parameters
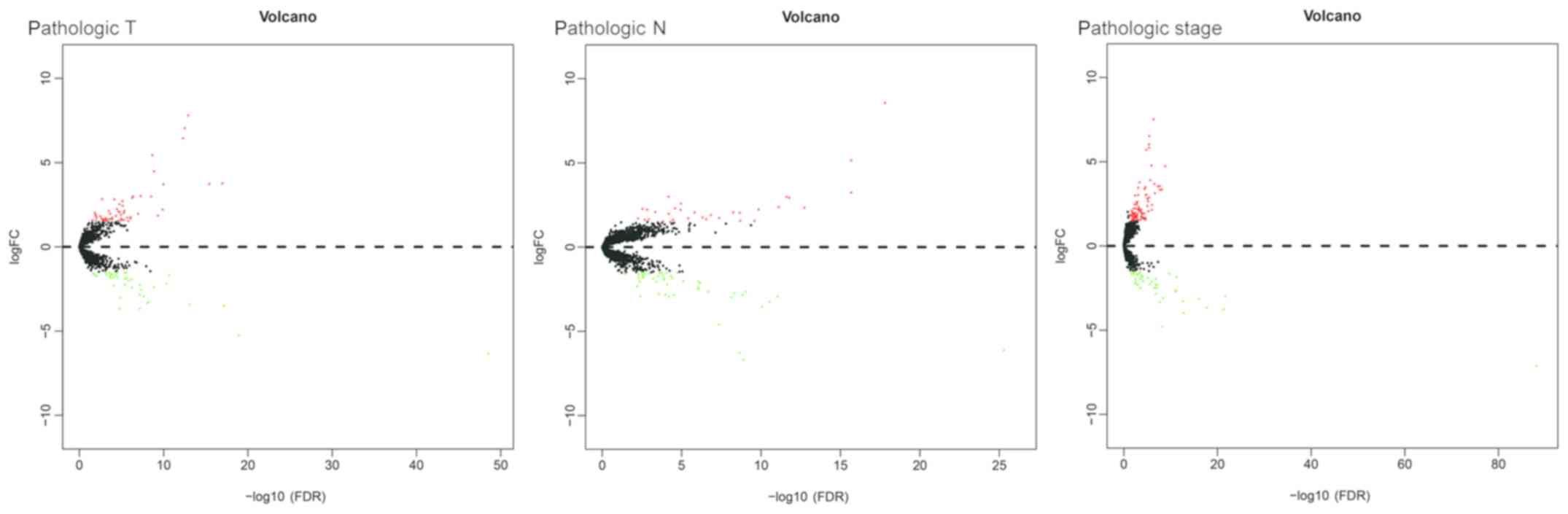
In order to further understand the association
between dysregulated mRNAs and patient outcomes, associations
between DEmRNAs and clinical characteristics, including pathologic
T, pathologic N and pathologic stage, were analyzed. The expression
levels of 180 DEmRNAs were significantly different in the clinical
feature comparisons (|log2FC|≥1.5 and FDR<0.05).
Among them, 95 mRNAs were differentially expressed between the
pathologic T subgroups, 79 mRNAs were differentially expressed
between the pathologic N subgroups and 120 mRNAs were
differentially expressed between the pathologic stage subgroups
(Table SII). The corresponding
volcano plots are provided in Fig.
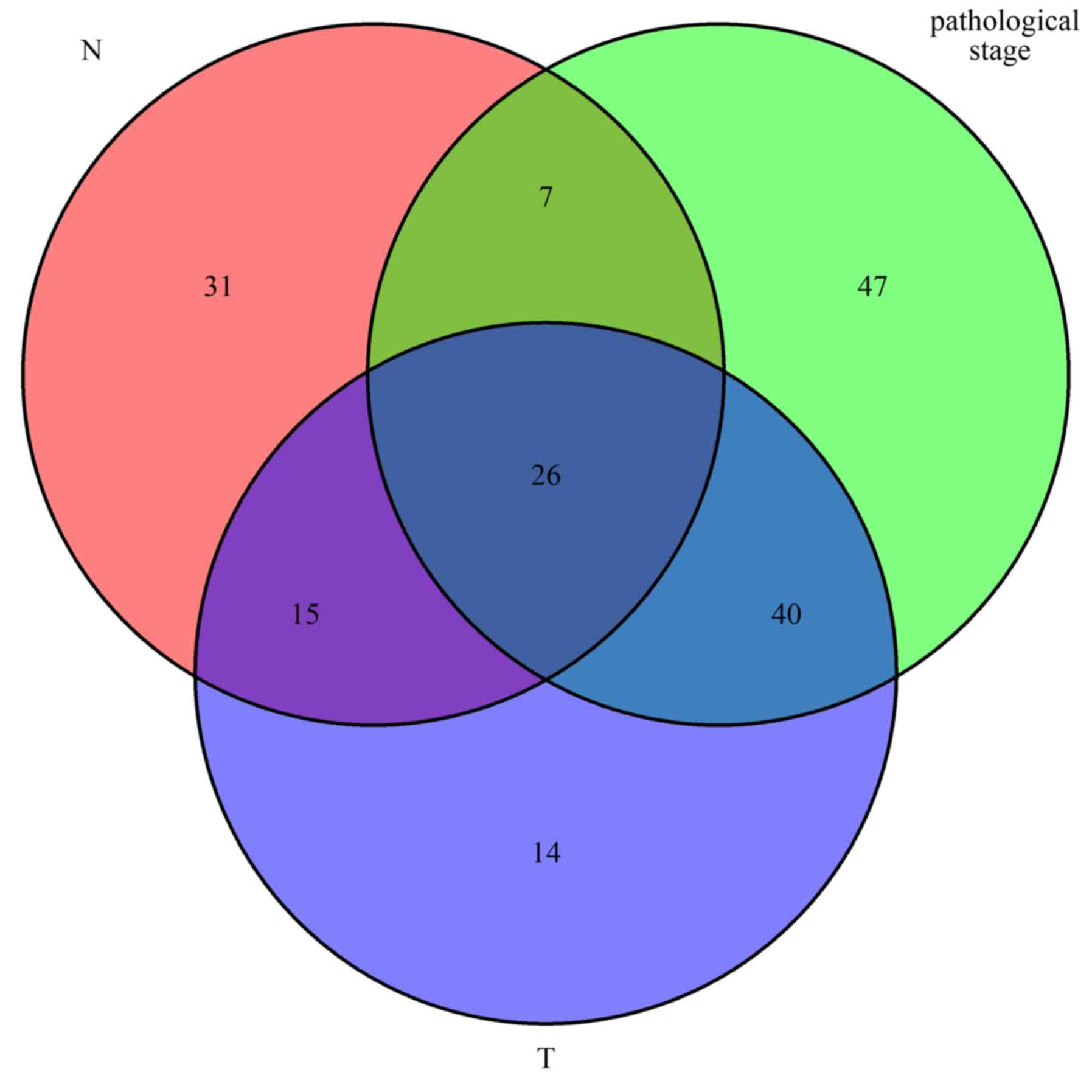
2. Finally, 26 mRNAs were screened out for all types of
subgroups (Fig. 3).
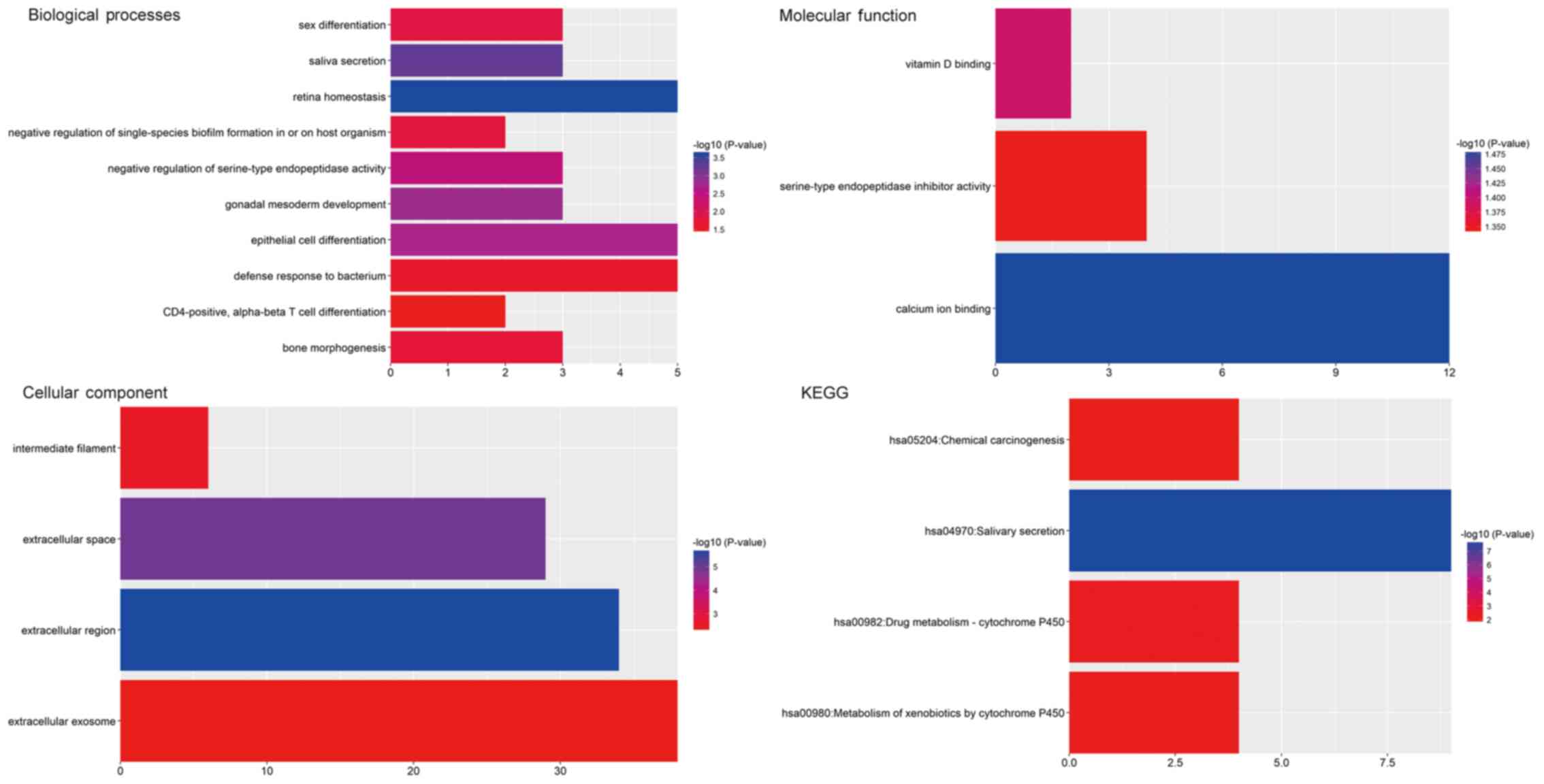
GO and pathway enrichment
analysis
GO and pathway enrichment analyses of the identified
CFmRNAs were performed using DAVID (Fig.
4). A total of 17 GO terms significantly enriched by the
CFmRNAs were identified (Table II).
In the GO category Biological Process, the dysregulated genes were
particularly enriched in terms including retina homeostasis, saliva
secretion, gonadal mesoderm development, epithelial cell
differentiation and negative regulation of serine-type
endopeptidase activity. In the Cellular Component category, the
genes were enriched in the terms extracellular region,
extracellular space, intermediate filament and extracellular
exosome. In the GO category Molecular Function, the CFmRNAs were
mainly enriched in calcium ion binding, vitamin D binding and
serine-type endopeptidase inhibitor activity.
 | Table II.Gene ontology analysis of
differentially expressed genes in association with clinical
parameters in oral squamous cell carcinoma. |
Table II.
Gene ontology analysis of
differentially expressed genes in association with clinical
parameters in oral squamous cell carcinoma.
| Category/term | N (%) | P-value | Genes |
|---|
|
GOTERM_CC_DIRECT |
|
GO:0005576~extracellular
region | 34 (19.1) |
2.12×10−6 | GC, DCD, C7,
BPIFB6, STATH, GAST, HTN3, TAC3, SPINK7, SPINK9, TTR, ALB, GP2,
PIP, LTF, FGF3, PRB2, CRISP3, BPIFA2, SCUBE3, BPIFA1, OPRPN,
R3HDML, DHRS7C, MUC6, IL22, PLG, NTS, C4ORF26, CST5, WIF1, SCGB3A2,
SMR3A, DMBT1 |
|
GO:0005615~extracellular
space | 29 (16.3) |
1.12×10−5 | GC, DCD, BPIFB2,
PRH2, MSMB, C6ORF58, SPINK1, GAST, OSTN, SCGB1A1, TAC3, ZG16B, TTR,
ALB, PIP, LTF, TFF1, FGFBP2, CRISP3, BPIFA1, OPRPN, IL22, PLG,
CST5, SCGB3A1, EPYC, MUC5B, DMBT1, SMR3B |
|
GO:0005882~intermediate
filament | 6 (3.4) | 0.002961 | KRT36, KRT27,
KRT40, KRTAP17-1, KRTAP19-5, KRTAP13-2 |
|
GO:0070062~extracellular
exosome | 38 (21.3) | 0.004723 | GC, DCD, C7,
BPIFB2, PKHD1, C6ORF58, ALDOB, AQP5, SPINK1, CRNN, CALB1, SCGB1A1,
TAC3, ZG16B, TTR, KRT27, ALB, UPK1A, GP2, PIP, LTF, TGM3, KRT84,
CRISP3, GSTA2, BPIFA2, VIL1, ARMC3, MUC7, PLG, LRRC26, KRT36, PYGM,
CST5, SCGB3A1, MUC5B, DMBT1, SMR3B |
|
GOTERM_BP_DIRECT |
|
GO:0001895~retina
homeostasis | 5 (2.8) |
2.38×10−4 | ZG16B, ALB, PIP,
LTF, OPRPN |
|
GO:0046541~saliva
secretion | 3 (1.7) |
5.68×10−4 | CHRM1, STATH,
AQP5 |
|
GO:0007506~gonadal mesoderm
development | 3 (1.7) | 0.001567 | TSPY2, TSPY1,
TSPY10 |
|
GO:0030855~epithelial cell
differentiation | 5 (2.8) | 0.002003 | GSTA2, UPK1A, VIL1,
DMBT1, ACTL8 |
|
GO:1900004~negative regulation
of serine-type endopeptidase activity | 3 (1.7) | 0.00362 | SPINK9, SPINK1,
SPINK7 |
|
GO:0007548~sex
differentiation | 3 (1.7) | 0.01426 | TSPY1, DMRT1,
TSPY10 |
|
GO:1900229~negative regulation
of single-species biofilm formation in or on host organism | 2 (1.1) | 0.015188 | BPIFA1, LTF |
|
GO:0060349~bone
morphogenesis | 3 (1.7) | 0.017868 | LTF, IFITM5,
PAX1 |
|
GO:0042742~defense response to
bacterium | 5 (2.8) | 0.02499 | DCD, BPIFA2, STATH,
HTN3, MUC5B |
|
GO:0043367~CD4-positive,
alpha-beta T cell differentiation | 2 (1.1) | 0.037541 | PAX1, NKX2-3 |
|
GOTERM_MF_DIRECT |
|
GO:0005509~calcium ion
binding | 12 (6.7) | 0.033232 | MYL7, SCUBE3,
ANXA10, MYL3, CAPSL, RPTN, VIL1, TGM3, PCDHA11, CRNN, CALB1,
NECAB2 |
|
GO:0005499~vitamin D
binding | 2 (1.1) | 0.040216 | GC, CALB1 |
|
GO:0004867~serine-type
endopeptidase inhibitor activity | 4 (2.2) | 0.045216 | SPINK9, SPINK1,
TFPI2, SPINK7 |
The results of the KEGG pathway enrichment analysis
are displayed in Fig. 4. The most
significant pathways associated with CFmRNAs were salivary
secretion, drug metabolism-cytochrome P450, metabolism of
xenobiotics by cytochrome P450 and chemical carcinogenesis.
Key candidate genes identified from
the CFmRNA PPI network
The STRING online database was used to further
investigate the potential links between abnormally expressed genes.
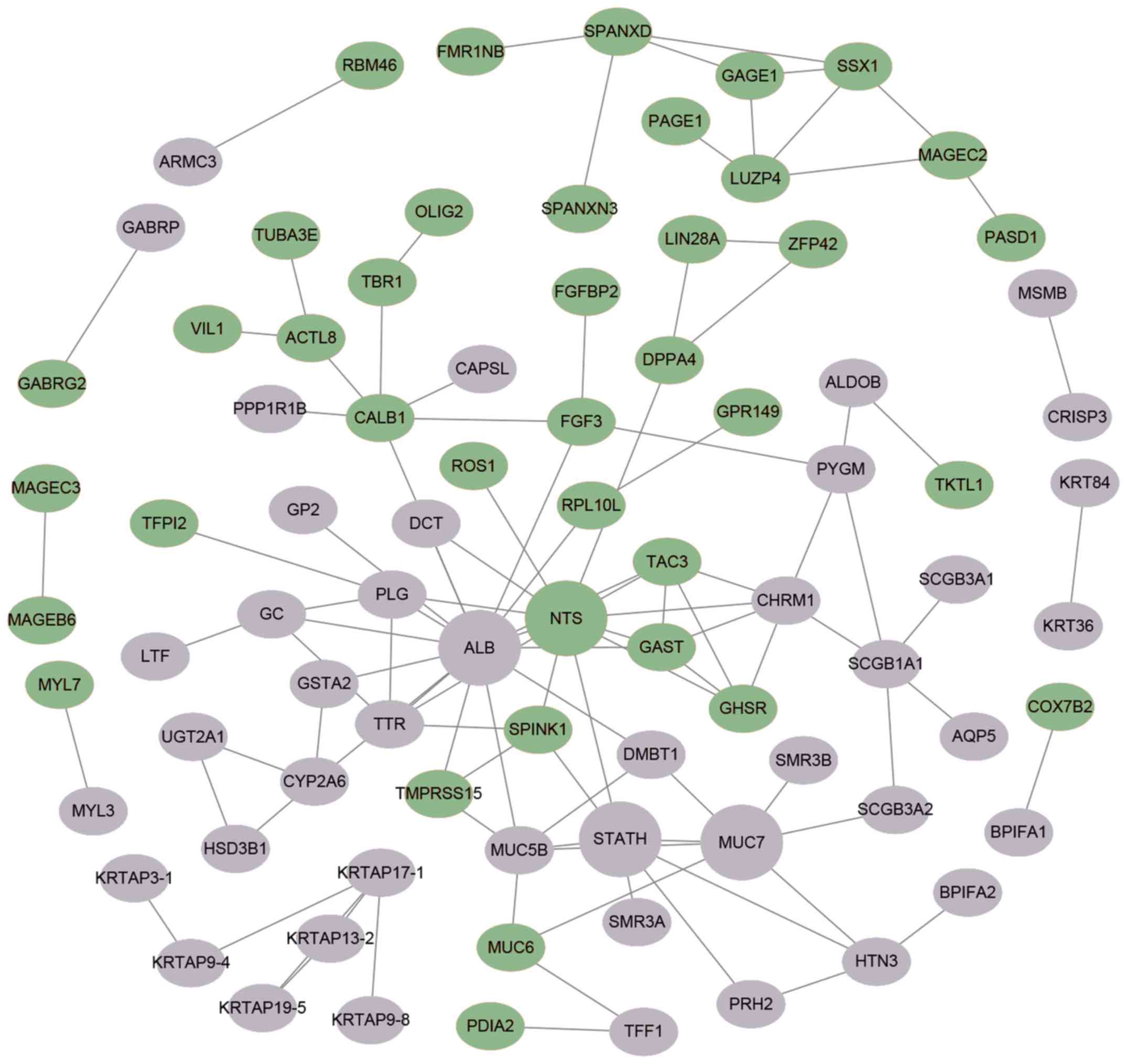
A PPI network was visualized by Cytoscape and is provided in
Fig. 5. A total of 4 central node
genes (>7 connections/interactions) were identified, including
albumin (ALB), statherin (STATH), neurotensin (NTS) and mucin 7,
secreted (MUC7).
CFmRNAs associated with survival
CFmRNAs associated with survival were detected by
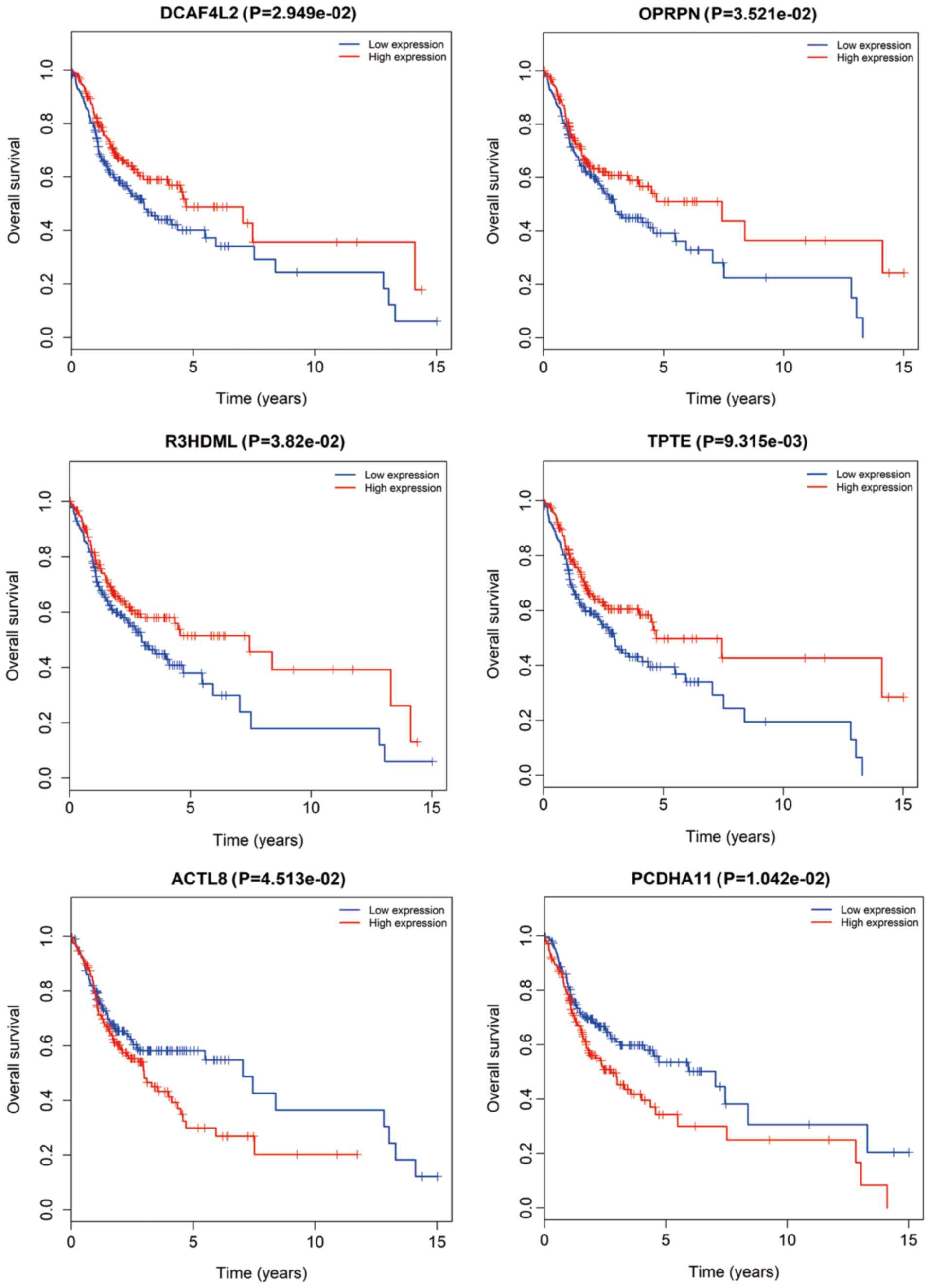
Kaplan-Meier survival curves and log-rank tests. A total of 6
CFmRNAs were identified to have a significant influence on the
overall survival rate (P<0.05). Of these, 4 (DCAF4L2, OPRPN,
R3HDML and TPTE) were associated with a favorable prognosis
regarding overall survival (Fig. 6).
Patients with higher expression of these mRNAs had a tendency for
longer survival, compared with patients with lower expression
(P<0.05). ACTL8 and PCDHA11 were associated with an elevated
risk, since their higher expression was associated with shorter
overall survival of OSCC patients.
Discussion
In spite of diagnostic and therapeutic advances in
the last decades, OSCC remains a huge threat to human health. With
the rapidly developed technology for gene analysis, in-depth
exploration of the molecular characteristics and identification of
potential valuable prognostic biomarkers for OSCC may be performed
(11). The multi-step progression of
carcinogenesis in the oral cavity, including gene amplification or
dysregulation, has an important role in OSCC (7). In the present study, 675 upregulated
and 1,338 downregulated mRNAs between OSCC and normal samples were
initially identified. Of these DEmRNAs, 180 were screened out
according to their association with clinical features. Furthermore,
6 survival-associated genes were identified (DCAF4L2, OPRPN,
R3HDML, TPTE, ACTL8 and PCDHA11).
In the GO and KEGG analyses, 17 enriched terms and 4
signaling pathways were identified. They were involved in several
relevant processes, particularly in saliva secretion and metabolism
by cytochrome P450. The epithelium of the oral cavity is
continuously immersed in saliva and its associated compositions,
and thus, changes in the saliva composition may particularly affect
the state of oral tissues (12).
The PPI network of the CFmRNAs comprised four hub
genes (ALB, STATH, NTS and MUC7). STATH is derived from a secretory
calcium-binding phosphoprotein gene cluster and encodes the protein
statherin. It was reported to be responsible for preventing calcium
phosphate precipitation in saliva, leading to persistent high
calcium and phosphate levels (13,14). The
levels of statherin in saliva are significantly reduced in
cancerous and pre-cancerous lesions compared with those in healthy
controls (15). Decreased expression
of STATH means less free calcium, perhaps resulting in initiation
of sustained proliferation and reduction of desmosomes, as well as
alterations in calcium-associated cellular functions. Dysfunction
of this gene may be a possible mechanism of carcinogenesis in
OSCC.
Overexpression of NTS and its affinity receptor
NTSR1 are frequently detected in cancerous tissues (16,17).
Numerous studies have reported that NTS is associated with tumor
progression in several solid tumor types, including breast cancer,
lung cancer and hepatocellular carcinoma (HCC) (18–20).
Recent studies on HCC demonstrated that NTS/NTSR1 co-expression
promotes tumor invasion and epithelial-mesenchymal transition via
Wnt/β-catenin signaling and the NTS/interleukin-8 pathway (21,22).
However, further evidence is necessary to prove the role of NTS in
the progression of OSCC.
Mucins are multifunctional glycoproteins, which may
be divided into membrane-bound and secreted protein (23). MUC7 is one of these secreted mucins
identified in the PPI network of the screened CFmRNAs. Higher
expression of MUC7 was previously reported to be associated with
poor overall survival in clear-cell renal cell carcinoma (24). In the oral cavity, MUC7 acts as an
innate immunity component, interacting with several oral
microorganisms (25). MUC7 is
typically distributed in normal salivary glands, while MUC7 is
rarely expressed in malignant transformations into mucoepidermoid
carcinoma (23,26). The secreted proteins MUC5B and MUC6
were identified to be dysregulated among the pathological T, N and
stage subgroups in the present study. A previous study suggested
that MUC5B may be associated with poor prognosis regarding survival
in lung cancer (27). The also
associated MUC1 belongs to the transmembrane mucins. It has been
indicated that MUC1 is a reliable biomarker to predict the
metastatic/invasive potential of OSCC (28,29).
However, further research is required to elucidate the detailed
function of MUC7 in OSCC.
From the 180 CFmRNAs, six mRNAs were identified that
were able to predict the survival probability. DCAF4L2, OPRPN,
R3HDML and TPTE were regarded as protective, as their expression
was associated with consistently increased survival rates. DCAF4L2
is a member of the WD-repeat proteins, which commonly mediates
protein-protein interlay (30), and
its expression is a potential cause of cleft lip with or without
cleft palate (31). In the present
study, DCAF4L2 expression was elevated in tumor tissues, but was
also associated with an increased survival rate. This may due to
increases in the expression of DCAF4L2, which promotes
polyubiquitination of protein phosphatase,
Mg2+/Mn2+-dependent 1B (PPM1B) and then
decreases in the totality of PPM1B (32). Reduced PPM1B in U2OS cells combined
with anti-cancer drugs suppresses cellular proliferation of these
cells and lowers the threshold of cell death (33). In colorectal cancer, upregulated
DCAF4L2 induces neoplastic cell invasion and metastasis (32). OPRPN (also known as PROL1) localizes
on chromosome 4q13.3, encoding basic proline-rich lacrimal protein
(34,35). In addition, dysregulated OPRPN was
reported to be associated with invasion in breast cancer (36).
TPTE and ACTL8 belong to the cancer-testis antigens
(CTAs). In the present study, elevated TPTE indicated longer
overall survival, while higher expression of ACTL8 predicted a
worse prognosis. Similarly, prolonged survival of TPTE-seropositive
lung cancer patients was reported in a previous study (37). CTAs consist of tumor-associated
antigens that are widely distributed in tumors, and are prospective
targets for cancer immunotherapy (38). Previous studies have already proved
that CT genes are more frequently expressed in invasive head and
neck squamous cell carcinoma (HNSCC) and are associated with an
unfavorable phenotype (39,40). Furthermore, the proliferation and
migration of HNSCC PCI-12 cells were significantly inhibited by
knockdown of ACTL8 (41). On the
contrary, co-expression of ACTL8, CCCTC-binding factor like, Opa
interacting protein 5 and X antigen family member 3 may be
associated with increased overall survival in glioblastoma patients
(42). Taken together, these results
suggest the prospect of CT genes as prognostic biomarkers that may
be useful in the development of vaccine treatments based on CTAs
(43).
At present, little is known regarding the role of
dysregulated R3HDML and PCDHA11 in various diseases, including
OSCC, and further research is required to investigate their
potential molecular mechanisms. Of note, the present study has
certain limitations. For instance, it lacks corresponding
experiments to validate the in silico results; therefore,
further experiments, including quantitative PCR analysis, are
required to confirm the association of the genes identified with
OSCC in future studies. Furthermore, a paired-test was not
performed to assess whether the groups were age/sex-matched in the
current study, which needs to be taken in to account in further
studies.
In conclusion, six differently expressed genes
linked to oncogenesis and development were identified to be
associated different clinical features in the present study. These
genes (DCAF4L2, OPRPN, R3HDML, TPTE, ACTL8 and PCDHA11) may serve
as potential prognostic markers for OSCC. Although further
experimental verification is imperative, these results may provide
a basis for in-depth study regarding the diagnosis, therapy and
prognosis of OSCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
QW, XX and RC designed the study and wrote the
manuscript. RC collected and analyzed the data. JC helped to
interpret the results. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shimomura H, Sasahira T, Nakashima C,
Shimomura-Kurihara M and Kirita T: Downregulation of DHRS9 is
associated with poor prognosis in oral squamous cell carcinoma.
Pathology. 50:642–647. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sandulache VC, Michikawa C, Kataria P,
Gleber-Netto FO, Bell D, Trivedi S, Rao X, Wang J, Zhao M, Jasser
S, et al: High-risk TP53 mutations are associated with extranodal
extension in oral cavity squamous cell carcinoma. Clin Cancer Res.
24:1727–1733. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng Q, Deng Z, Pan H, Gu L, Liu O and
Tang Z: Mitogen-activated protein kinase signaling pathway in oral
cancer (Review). Oncol Lett. 15:1379–1388. 2018.PubMed/NCBI
|
|
4
|
Sahingur SE and Yeudall WA: Chemokine
function in periodontal disease and oral cavity cancer. Front
Immunol. 6:2142015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao X, Sun S, Zeng X and Cui L:
Expression profiles analysis identifies a novel three-mRNA
signature to predict overall survival in oral squamous cell
carcinoma. Am J Cancer Res. 8:450–461. 2018.PubMed/NCBI
|
|
6
|
Chen PK, Hua CH, Hsu HT, Kuo TM, Chung CM,
Lee CP, Tsai MH, Yeh KT and Ko YC: ALPK1 expression is associated
with lymph node metastasis and tumor growth in oral squamous cell
carcinoma patients. Am J Pathol. 189:190–199. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang G, Tang Q, Jia L, Xia S, Li J, Chen
Y, Li H, Ding X, Wang F, Hou D, et al: High expression of TROP2 is
correlated with poor prognosis of oral squamous cell carcinoma.
Pathol Res Pract. 214:1606–1612. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Liu J, Fu X and Yang A:
Identification of key genes and pathways in tongue squamous cell
carcinoma using bioinformatics analysis. Med Sci Monit.
23:5924–5932. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Niu L, Jiang S, Zhai J, Wang P,
Kong F and Jin X: Comprehensive analysis of aberrantly expressed
profiles of lncRNAs and miRNAs with associated ceRNA network in
muscle-invasive bladder cancer. Oncotarget. 7:86174–86185. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lydiatt WM, Patel SG, O'Sullivan B,
Brandwein MS, Ridge JA, Migliacci JC, Loomis AM and Shah JP: Head
and Neck cancers-major changes in the American Joint Committee on
cancer eighth edition cancer staging manual. CA Cancer J Clin.
67:122–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao C, Zhuang J, Zhou C, Ma K, Zhao M, Liu
C, Liu L, Li H, Feng F and Sun C: Prognostic value of aberrantly
expressed methylation gene profiles in lung squamous cell
carcinoma: A study based on The cancer genome atlas. J Cell
Physiol. 234:6519–6528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarode SC, Sarode GS and Patil S: Role of
statherin in oral carcinogenesis. Oral Oncol. 50:e55–e56. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Sousa-Pereira P, Amado F, Abrantes J,
Ferreira R, Esteves PJ and Vitorino R: An evolutionary perspective
of mammal salivary peptide families: Cystatins, histatins,
statherin and PRPs. Arch Oral Biol. 58:451–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sabatini LM, Carlock LR, Johnson GW and
Azen EA: cDNA cloning and chromosomal localization (4q11-13) of a
gene for statherin, a regulator of calcium in saliva. Am J Hum
Genet. 41:1048–1060. 1987.PubMed/NCBI
|
|
15
|
Contucci AM, Inzitari R, Agostino S,
Vitali A, Fiorita A, Cabras T, Scarano E and Messana I: Statherin
levels in saliva of patients with precancerous and cancerous
lesions of the oral cavity: A preliminary report. Oral Dis.
11:95–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Souazé F, Dupouy S, Viardot-Foucault V,
Bruyneel E, Attoub S, Gespach C, Gompel A and Forgez P: Expression
of neurotensin and NT1 receptor in human breast cancer: A potential
role in tumor progression. Cancer Res. 66:6243–6249. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Z, Galmiche A, Liu J, Stadler N, Wendum
D, Segal-Bendirdjian E, Paradis V and Forgez P: Neurotensin
regulation induces overexpression and activation of EGFR in HCC and
restores response to erlotinib and sorafenib. Cancer Lett.
388:73–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morgat C, Mishra AK, Varshney R, Allard M,
Fernandez P and Hindié E: Targeting neuropeptide receptors for
cancer imaging and therapy: Perspectives with bombesin,
neurotensin, and neuropeptide-Y receptors. J Nucl Med.
55:1650–1657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dupouy S, Viardot-Foucault V, Alifano M,
Souazé F, Plu-Bureau G, Chaouat M, Lavaur A, Hugol D, Gespach C,
Gompel A and Forgez P: The neurotensin receptor-1 pathway
contributes to human ductal breast cancer progression. PLoS One.
4:e42232009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Younes M, Wu Z, Dupouy S, Lupo AM, Mourra
N, Takahashi T, Fléjou JF, Trédaniel J, Régnard JF, Damotte D, et
al: Neurotensin (NTS) and its receptor (NTSR1) causes EGFR, HER2
and HER3 over-expression and their autocrine/paracrine activation
in lung tumors, confirming responsiveness to erlotinib. Oncotarget.
5:8252–8269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye Y, Long X, Zhang L, Chen J, Liu P, Li
H, Wei F, Yu W, Ren X and Yu J: NTS/NTR1 co-expression enhances
epithelial-to-mesenchymal transition and promotes tumor metastasis
by activating the Wnt/β-catenin signaling pathway in hepatocellular
carcinoma. Oncotarget. 7:70303–70322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao P, Long X, Zhang L, Ye Y, Guo J, Liu
P, Zhang R, Ning J, Yu W, Wei F and Yu J: Neurotensin/IL-8 pathway
orchestrates local inflammatory response and tumor invasion by
inducing M2 polarization of Tumor-Associated macrophages and
epithelial-mesenchymal transition of hepatocellular carcinoma
cells. Oncoimmunology. 7:e14401662018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alos L, Lujan B, Castillo M, Nadal A,
Carreras M, Caballero M, de Bolos C and Cardesa A: Expression of
membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2,
MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of
salivary glands. Am J Surg Pathol. 29:806–813. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
NguyenHoang S, Liu Y, Xu L, Chang Y, Zhou
L, Liu Z, Lin Z and Xu J: High mucin-7 expression is an independent
predictor of adverse clinical outcomes in patients with clear-cell
renal cell carcinoma. Tumour Biol. 37:15193–15201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu D, Pavlidis P, Thamadilok S, Redwood E,
Fox S, Blekhman R, Ruhl S and Gokcumen O: Recent evolution of the
salivary mucin MUC7. Sci Rep. 6:317912016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mahomed F: Recent advances in mucin
immunohistochemistry in salivary gland tumors and head and neck
squamous cell carcinoma. Oral Oncol. 47:797–803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagashio R, Ueda J, Ryuge S, Nakashima H,
Jiang SX, Kobayashi M, Yanagita K, Katono K, Satoh Y, Masuda N, et
al: Diagnostic and prognostic significances of MUC5B and TTF-1
expressions in resected non-small cell lung cancer. Sci Rep.
5:86492015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar MH, Sanjai K, Kumarswamy J,
Keshavaiah R, Papaiah L and Divya S: Expression of MUC1 mucin in
potentially malignant disorders, oral squamous cell carcinoma and
normal oral mucosa: An immunohistochemical study. J Oral Maxillofac
Pathol. 20:214–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thakur A, Tupkari JV, Joy T, Kende PP,
Siwach P and Ahire MS: Expression of mucin-1 in oral squamous cell
carcinoma and normal oral mucosa: An immunohistochemical study. J
Oral Maxillofac Pathol. 22:210–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Micel LN, Tentler JJ, Smith PG and
Eckhardt GS: Role of ubiquitin ligases and the proteasome in
oncogenesis: Novel targets for anticancer therapies. J Clin Oncol.
31:1231–1238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beaty TH, Taub MA, Scott AF, Murray JC,
Marazita ML, Schwender H, Parker MM, Hetmanski JB, Balakrishnan P,
Mansilla MA, et al: Confirming genes influencing risk to cleft lip
with/without cleft palate in a case-parent trio study. Hum Genet.
132:771–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Chen Y, Han J, Meng Q, Xi Q, Wu G
and Zhang B: DCAF4L2 promotes colorectal cancer invasion and
metastasis via mediating degradation of NFκB negative regulator
PPM1B. Am J Transl Res. 8:405–418. 2016.PubMed/NCBI
|
|
33
|
Miller RE, Uwamahoro N and Park JH: PPM1B
depletion in U2OS cells supresses cell growth through RB1-E2F1
pathway and stimulates bleomycin-induced cell death. Biochem
Biophys Res Commun. 500:391–397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saitoh E, Taniguchi M, Ochiai A, Kato T,
Imai A and Isemura S: Bioactive peptides hidden in human salivary
proteins. J Oral Biosciences. 59:71–79. 2017. View Article : Google Scholar
|
|
35
|
Saitoh E, Sega T, Imai A, Isemura S, Kato
T, Ochiai A and Taniguchi M: The PBII gene of the human salivary
proline-rich protein P-B produces another protein, Q504X8, with an
opiorphin homolog, QRGPR. Arch Oral Biol. 88:10–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lang Z, Wu Y, Pan X, Qu G and Zhang T:
Study of differential gene expression between invasive
multifocal/multicentric and unifocal breast cancer. J BUON.
23:134–142. 2018.PubMed/NCBI
|
|
37
|
Kuemmel A, Simon P, Breitkreuz A, Röhlig
J, Luxemburger U, Elsässer A, Schmidt LH, Sebastian M, Sahin U,
Türeci Ö and Buhl R: Humoral immune responses of lung cancer
patients against the Transmembrane phosphatase with TEnsin homology
(TPTE). Lung Cancer. 90:334–341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yao J, Caballero OL, Yung WK, Weinstein
JN, Riggins GJ, Strausberg RL and Zhao Q: Tumor subtype-specific
cancer-testis antigens as potential biomarkers and
immunotherapeutic targets for cancers. Cancer Immunol Res.
2:371–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cuffel C, Rivals JP, Zaugg Y, Salvi S,
Seelentag W, Speiser DE, Liénard D, Monnier P, Romero P, Bron L and
Rimoldi D: Pattern and clinical significance of cancer-testis gene
expression in head and neck squamous cell carcinoma. Int J Cancer.
128:2625–2634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Piotti KC, Scognamiglio T, Chiu R and Chen
YT: Expression of cancer/testis (CT) antigens in squamous cell
carcinoma of the head and neck: Evaluation as markers of squamous
dysplasia. Pathol Res Pract. 209:721–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li B, Zhu J and Meng L: High expression of
ACTL8 is poor prognosis and accelerates cell progression in head
and neck squamous cell carcinoma. Mol Med Rep. 19:877–884.
2019.PubMed/NCBI
|
|
42
|
Freitas M, Malheiros S, Stávale JN, Biassi
TP, Zamunér FT, de Souza Begnami M, Soares FA and Vettore AL:
Expression of cancer/testis antigens is correlated with improved
survival in glioblastoma. Oncotarget. 4:636–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mahmoud AM: Cancer testis antigens as
immunogenic and oncogenic targets in breast cancer. Immunotherapy.
10:769–778. 2018. View Article : Google Scholar : PubMed/NCBI
|