Introduction
Transcatheter aortic valve implantation (TAVI) is
the recommended treatment option in patients with severe aortic
stenosis considered inoperable or at high surgical risk (1). The balloon-expandable Edward Sapien
(Edwards Lifesciences) and the self-expandable Medtronic CoreValve
(Medtronic) are the most commonly used aortic valves from the early
generation (2). Studies have shown
similar outcomes with both valves, in terms of mortality, rate of
stroke and post-operative functional status (3,4).
However, one of the major shortcomings of these early generation
valves is the presence of paravalvular leakage, which results in
moderate-severe aortic regurgitation and has been associated with
an increased late mortality (5,6). With
ongoing research and development with respect to TAVI, two
competing newer generation valves, the Sapien 3 (Edwards
Lifesciences) and CoreValve Evolut R (Medtronic) have been
introduced and are widely used in the Unites States (7). The Sapien 3 is a balloon-expandable
valve, comprising a nickel cobalt chromium frame, tri-leaflet
bovine pericardial tissue valve and a polyethylene terephthalate
sealing skirt. The Evolut R, is a self-expanding valve comprising a
tri-leaflet porcine pericardial tissue valve mounted within a
compressible Nitinol frame (8). Both
have been designed to further reduce the rate of paravalvular
leakage, conduction abnormalities and vascular complications
associated with TAVI (9,10).
Schulz et al (11), in a single center cohort study,
demonstrated a reduced rate of more-than-mild paravalvular
regurgitation and a greater device success with Evolut R, as
compared to the earlier generation CoreValve. Similarly, the
results of the study by Nijhoff et al (12) indicated the superiority of Sapien 3
over its predecessor, in terms of improved valve patency and
increased transfemoral access safety. With both the new-generation
valves becoming increasingly popular, it is important to determine
whether any one of the valve results in superior outcomes over the
other. While studies have compared the efficacy and safety of
Evolut R vs. Sapien 3, there is a lack of level 1 evidence in the
form of a systematic review and meta-analysis. Hence, the aim of
this study was to systematically search the literature and conduct
a meta-analysis comparing the clinical efficacy and safety of
Evolut R and Sapien 3 valves for TAVI.
Data and methods
This systematic review and meta-analysis were
conducted in accordance with the Meta-analyses Of Observational
Studies in Epidemiology (MOOSE) checklist (13). The research question to be answered
was the following: Whether there is any difference in the clinical
efficacy and safety profiles of Evolut R and Sapien 3 valves for
TAVI.
Search strategy
An electronic search of the PubMed, Biomed Central,
Scopus, Cochrane library and Google scholar databases was performed
for articles published up to June, 2019. Free text keywords and
MeSH terms were used in various combinations. Search keywords
included: ‘Transcatheter aortic valve SAPIEN 3’, ‘transcatheter
aortic valve Evolut R’, ‘TAVI’, ‘transcatheter aortic valve
implantation’, ‘self-expandable aortic valve’ OR ‘balloon
expandable aortic valve’. The references of included studies and
review articles on the subject were hand searched for the
identification of any additional studies.
Eligibility criteria
We searched for pertinent studies comparing the
clinical efficacy and safety of Evolut R and Sapien 3 valves for
TAVI. No restriction was placed on the type of study. Studies were
included if they met the following specifications: i) Compared
outcomes following TAVI with Evolut R and Sapien 3 valves; and ii)
predominant mode of TAVI was trans-femoral route (>80%). The
outcome assessment had to include any one of the following
variables: Mortality, stroke, paravalvular leakage, major vascular
complications and bleedings, and pacemaker implantation. Studies
excluded were the following: i) Studies not reporting separate
outcome data for Evolut R and Sapien 3 valves; ii) duplicate
reports; iii) case series and case reports with <10 patients;
and iv) non-English language studies and animal studies.
Data extraction and quality
assessment
Two independent reviewers performed the literature
search. Following the removal of duplicates, studies were
scrutinized by their title and abstracts to determine whether they
met the inclusion criteria. The full-texts of the selected articles
were then scanned for inclusion in the review. Any discrepancies
were resolved by discussion. Data were extracted from the included
trials by 2 independent reviewers using an abstraction form. The
following details were sourced: Authors, publication year, sample
size, demographic and baseline data of participants, TAVI
procedural details, and outcomes assessed. Two reviewers
independently assessed the risk of bias using the Newcastle-Ottawa
Scale for non-randomized studies (14).
Statistical analysis
Outcome data are presented as either the means ±
standard deviation (SD) for continuous variables or as the number
of events per group for categorical variables. Data extracted were
entered into Review Manager [RevMan, version 5.3; Nordic Cochrane
Centre (Cochrane Collaboration), Copenhagen, Denmark, 2014] for
meta-analysis. Considering the heterogeneity amongst studies, a
random-effects model was used to calculate the pooled effect size.
Categorical data were summarised using the Mantel-Haenszel odds
ratios (OR) and 95% confidence intervals (CI). The mean difference
(MD) with 95% CI was used to pool continuous variables.
Heterogeneity was calculated using the I2 statistic.
I2 values of 25–50% represented low, values of 50–75%
medium and >75% represented substantial heterogeneity. The
influence of each study on the pooled effect size was analyzed
using a sensitivity analysis. Using the one-study-out method, we
assessed whether deleting each individually would significantly
change the pooled results of the meta-analysis.
Results
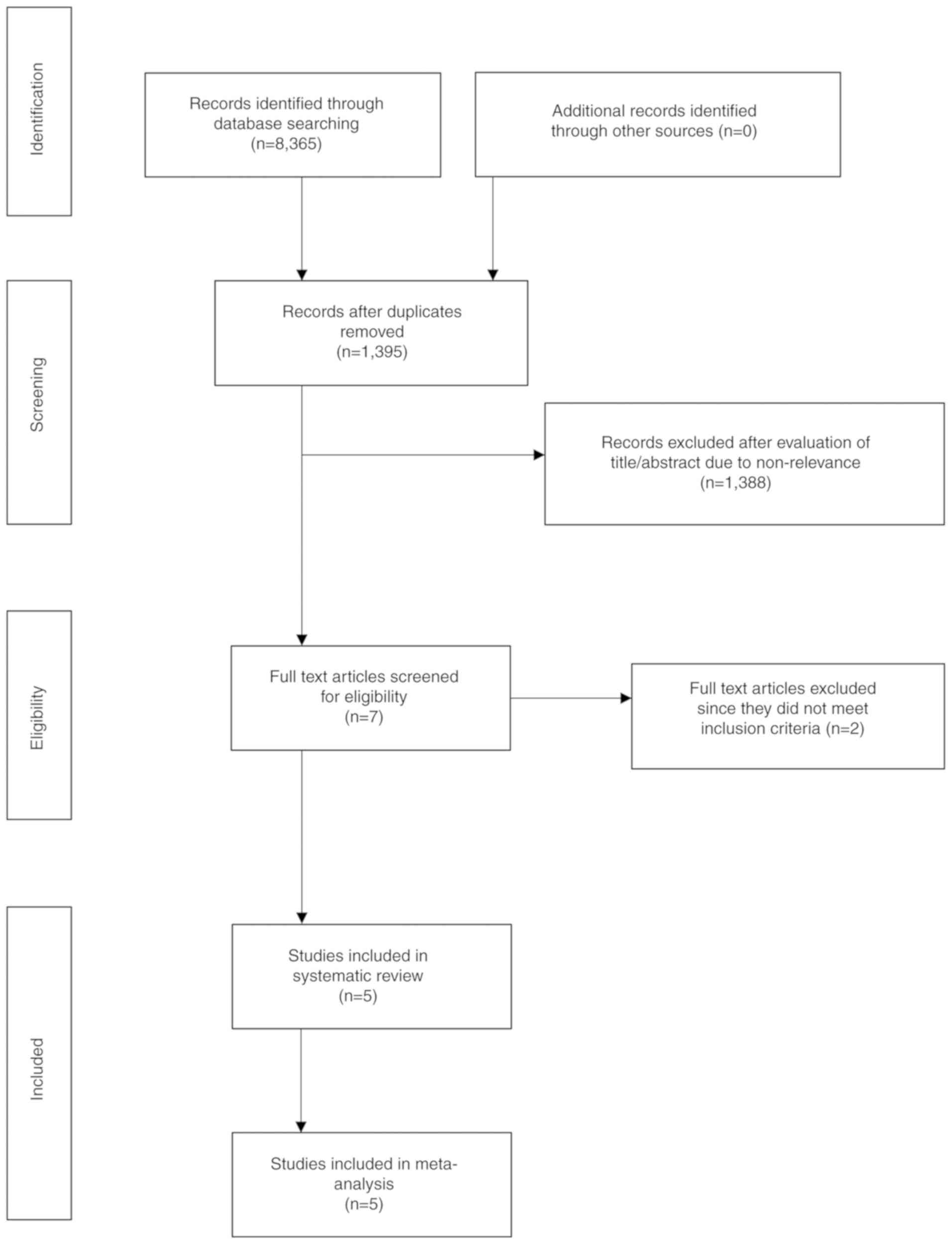
The search results are presented in Fig. 1. A total of seven articles were
analyzed by their full text. Two studies were excluded. In one
study (15), the outcome data for
Evolut R and Sapien 3 valves were not presented separately. Another
study (16) examined gender-based
differences in outcomes, in a cohort of Evolut R and Sapien 3 TAVI
patients without any comparison between the 2 valves. A total of 5
studies (8,17–20) met
the inclusion criteria and were included in the systematic review
and meta-analysis. No randomized controlled trials were available
for inclusion. Four (8,18–20) were
retrospective cohort studies, while one was a prospective study
(17). The prospective study
(17) compared outcomes between
Evolut R and Sapien 3 valves in patients with large annuli (≥26
mm). None of the remaining studies used any exclusion criteria
based on any patient characteristic.
Baseline and procedural
characteristics
A total of 795 patients underwent TAVI with Evolut
R, while 665 patients received the Sapien 3 valve in the included
studies. The baseline characteristics of the study participants are
presented in Table I. With the
exception of one study (20), there
were minimal differences between the baseline characteristics of
the 2 groups. In the study by Finkelstein et al (20), the groups were not matched for age,
sex, Society of Thoracic Surgeons (STS) score, hyperlipidemia,
ischemic heart disease, New York Heart Association (NYHA)
classification and left ventricular ejection fraction (LVEF). The
procedural details of the included studies are presented in
Table II. Two studies (17,18)
utilized transfemoral access in all patients, while in the
remaining studies, the transfemoral route was used in >89% of
the study participants. One study (17) compared the 34 mm Evolut R valve with
29 mm Sapien 3 valve. Multiple different-sized valves were used in
the remaining studies. The authors' assessment of the quality of
the included studies is presented in Data S1.
 | Table I.Baseline characteristics of patients
in the included studies. |
Table I.
Baseline characteristics of patients
in the included studies.
|
| Ben-Shoshan et
al (18) | Rogers et al
(8) | Eitan et al
(17) | Finkelstein et
al (20) | Enríquez-Rodríguez
et al (19) |
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Evolut R | SAPIEN 3 | Evolut R | SAPIEN 3 | Evolut R | SAPIEN 3 | Evolut R | SAPIEN 3 | Evolut R | SAPIEN 3 |
|---|
| No. of
patients | 108 | 124 | 74 | 183 | 37 | 55 | 512 | 223 | 64 | 80 |
| Age, years | 82.7 (5.8) | 82 (6.3) | 82 (8) | 81 (9) | 82.4 (5.8) | 80.9 (6.3) | 83 (79–87) | 81
(77–85)e | 84 (5) | 82 (6) |
| Male sex, % | 33 | 57e | 39.2 | 53.6e | 91.1 | 94.5 | 35 | 77e | 42 | 52 |
| Body surface area,
m2 | 1.77 (0.2) | 1.84 (0.19) | 1.8 (0.3) | 1.9
(0.3)e | NS | NS | NS | NS | 1.62 (0.2) | 1.72 (0.2) |
| BMI,
kg/m2 | 27.6 (5) | 26.9 (3.6) | NS | NS | 27 (4.5) | 26.4 (4.1) | 64%a | 71% | 26.8 (4.1) | 27 (3.8) |
| STS score, % | 4.27 (2.7) | 4.05(4.9) | 8.1 (4.6) | 6.5
(6.3)e | 4.6 (2.4) | 3.9 (2.5) | 3.5 (2.6–5.2) | 3.1
(2.5–4.6)e | 5.8 (5) | 6.2 (5) |
| Euro Score2, % | 5.4 (4.1) | 5.2 (5.7) | NS | NS | 5.7 (3.8) | 4.4 (3.6) | NS | NS | NS | NS |
| Hypertension,
% | 89 | 86 | 88.1 | 87.1 | 94.6 | 81.8 | 87 | 82 | 81 | 79 |
| Hyperlipidemia,
% | 77 | 76 | 83.3 | 87 | 78.4 | 56.4e | 74 | 65e | 47 | 45 |
| Diabetes Mellitus,
% | 40 | 40 | 30.3 | 33.5 | 32.4 | 25.5 | 40 | 40 | 34 | 39 |
| Ischemic heart
disease, % | 54 | 58 | 58.3 | 61.1 | NS | NS | 46 | 61e | NS | NS |
| Serum creatinine,
mg/dl | 1.08(0.76) | 1.18 (0.67) | NS | NS | 1.6 (1.2) | 1.3 (0.8) | NS | NS | NS | NS |
| Dialysis, % | NS | NS | 28.8 | 32.1 | 5.4 | 5.5 | 2 | 3 | 23c | 15 |
| COPD, % | NS | NS | 39.4 | 34.2 | 24.3 | 20 | 8 | 12 | 16 | 14 |
| Prior pacemaker,
% | 19 | 13 | 33.3 | 25.3 | 24.3 | 9.1e | NS | NS | NS | NS |
| Prior vascular
disease, % | NS | NS | 21.3 | 19 | 32.4 | 22.2 | 10 | 11 | 5 | 9 |
| NYHA Class III or
IV, % | 94 | 92 | 75.4 | 64.3 | 95 | 83 | 2.8
(0.7)b | 3
(0.7)e | 64 | 53 |
| Aortic valve area,
cm2 | 0.72
(0.18) | 0.72
(0.17) | 0.68 (0.13) | 0.7
(0.17) | NS | NS | 0.7 (0.6–0.8) | 0.7
(0.6–0.9)e | 0.68 (0.2) | 0.69 (0.2) |
| LVEF | 56.2 (9.7) | 55.6 (8.5) | 55 (12) | 53
(13) | 50 (13) | 52 (12) | 57 (11) | 54
(12)e | 5
(8)d | 13 (17) |
| Mean aortic valve
gradient, mmHg | 44.7
(15.5) | 46
(15) | 48.1 (12.2) | 45.8 (12) | 61.9 (16.2) | 65
(15.2) | 44 (35–54) | 44 (35–53) | 47 (14) | 45 (15) |
 | Table II.Procedural characteristics of the
included studies. |
Table II.
Procedural characteristics of the
included studies.
|
| Ben-Shoshan et
al (18) | Rogers et al
(8) | Eitan et al
(17) | Finkelstein et
al (20) | Enríquez-Rodríguez
et al (19) |
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Evolut R | Sapien 3 | Evolut R | Sapien 3 | Evolut R | Sapien 3 | Evolut R | Sapien 3 | Evolut R | Sapien 3 |
|---|
| No. of
patients | 108 | 124 | 74 | 183 | 37 | 55 | 512 | 223 | 64 | 80 |
| General anesthesia,
% | NS | NS | 14.9 | 9.3 | 94.5 | 78.2 | 7 | 9 | NS | NS |
| Transfemoral
access, % | 100 | 100 | 89.2 | 91.3 | 100 | 100 | 95 | 92 | 95 | 94 |
| Pre-dilation,
% | 25.9 | 72.5a | 36.7 | 65.4a | 81 | 14.5a | 21 | 63a | 39 | 36 |
| Post-dilation,
% | 23.1 | 7.2a | 42.6 | 18.4a | 32 | 5.5a | 35 | 10a | 22 | 2 |
| Valve sizes used,
mm | 23,26, 29 | 23,26,29 | 23,26,29,31 | 20,23,26,29 | 34 | 29 | 23,26,29 | 23,26,29 | 23,26,29 | 23,26,29 |
Outcomes
Device success and post-procedural
echocardiogram outcomes
Data on the outcomes of the included studies are
presented in Table III. Valve
Academic Research Consortium-2 consensus document (VARC-2)
(21)-defined device success was
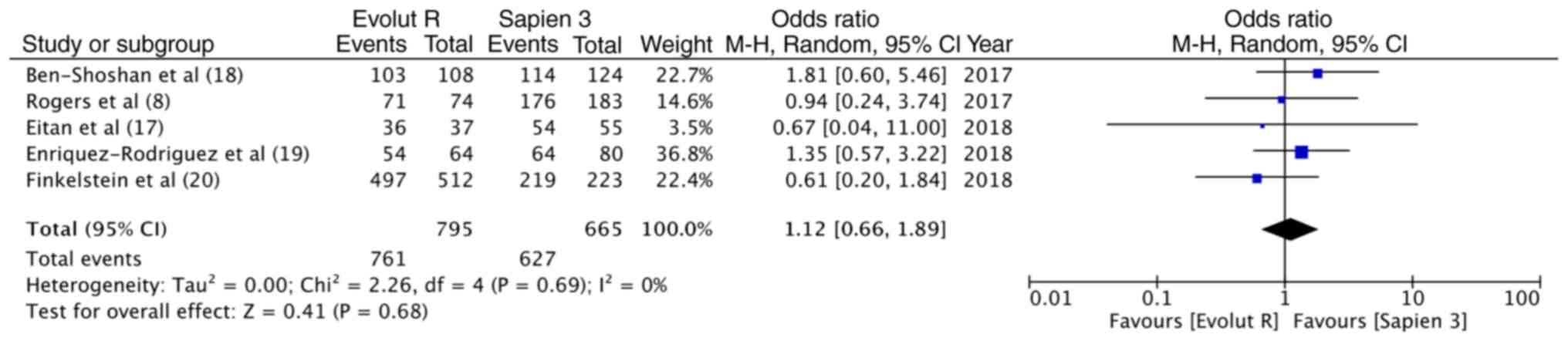
reported in all 5 studies. The overall device success with Evolut R
was 95.7% (761/795) and that with Sapien 3 was 94.2% (627/665).
Pooled data indicated no significant differences between the 2
valves (OR, 1.12, 95% CI, 0.66–1.89; P=0.68; I2=0%)
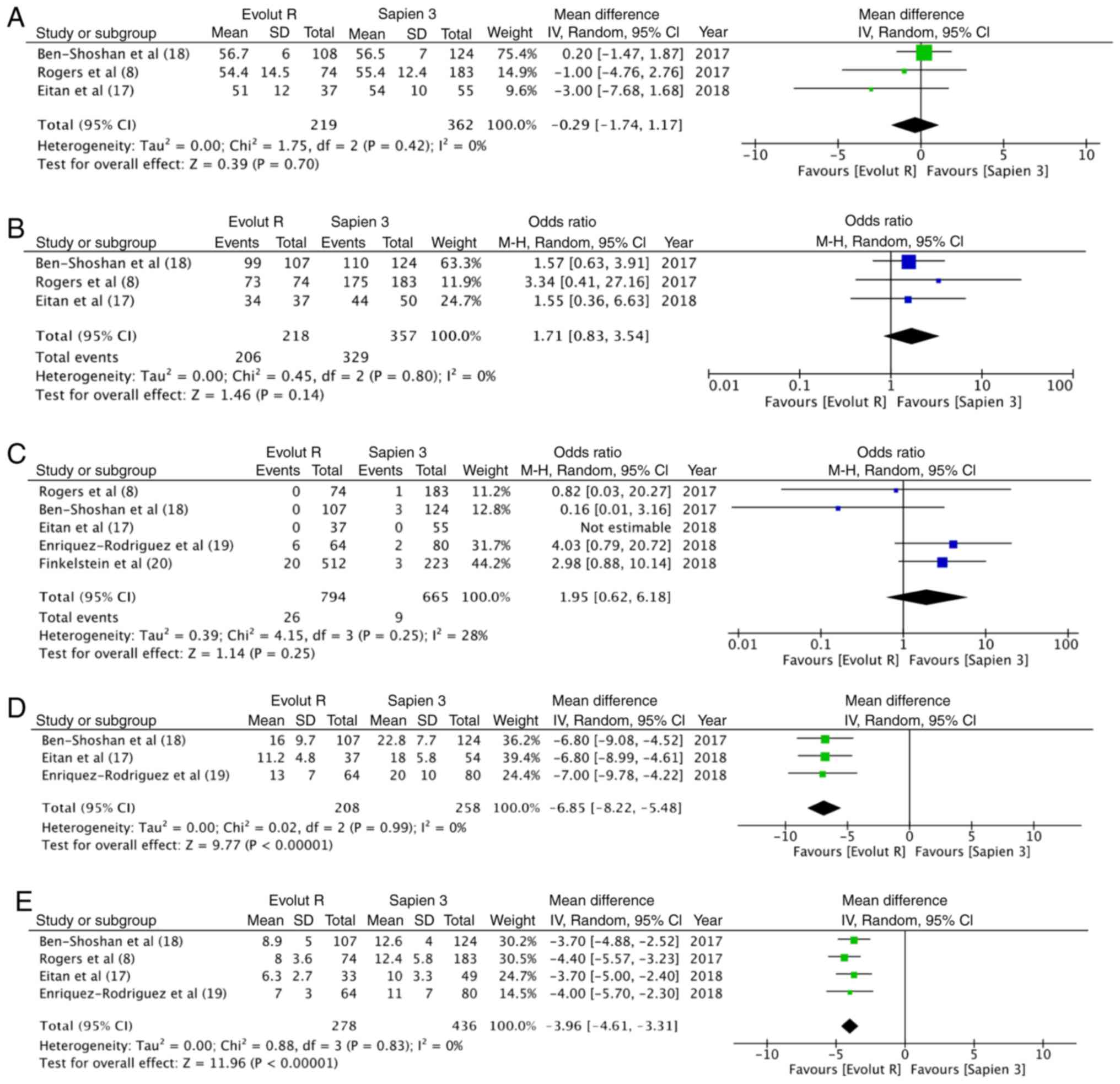
(Fig. 2). Data on post-procedural
LVEF were available from 3 studies. Meta-analysis indicated no
differences between the 2 groups (random; MD = −0.29; 95% CI, −1.74
to 1.17; P=0.70; I2=0%) (Fig.
3A). The incidence of none to mild paravalvular leakage did not
differ between the 2 groups (OR, 1.71; 95% CI, 0.83–3.54; P=0.14;
I2=0%) (Fig. 3B). In
total, 3.3% (26/794) patients had more than moderate paravalvular
regurgitation with Evolut R, while the same was reported in 1.4%
(9/665) patients with Sapien 3. Pooled analysis did not demonstrate
any statistically significant differences (OR, 1.95; 95% CI,
0.62–6.18; P=0.25; I2=28%) (Fig. 3C). Both the mean (random; MD = −3.96;
95% CI, −4.61 to −3.31; P<0.00001, I2=0%) (Fig. 3D) and peak (random; MD = −6.85; 95%
CI, −8.22 to −5.48; P<0.00001, I2=0%) (Fig. 3E) aortic valve gradients were
significantly lower with Evolut R.
 | Table III.Outcomes and complications of the
included studies. |
Table III.
Outcomes and complications of the
included studies.
|
| Ben-Shoshan et
al (18) | Rogers et al
(8) | Eitan et al
(17) | Finkelstein et
al (20) | Enríquez-Rodríguez
et al (19) |
|---|
|
|
|
|
|
|
|
|---|
| Outcome | Evolut R | Sapien 3 | Evolut R | Sapien 3 | Evolut R | Sapien 3 | Evolut R | Sapien 3 | Evolut R | Sapien 3 |
|---|
| Device success | 103 | 114 | 71 | 176 | 36 | 54 | 497 | 219 | 54 | 64 |
| LVEF | 56.7 (6) | 56.5 (7) | 54.4 (14.5) | 55.4 (12.4) | 51 (12) | 54 (10) | NS | NS | NS | NS |
| PVL (not mild) | 99 | 110 | 73 | 175 | 34 for n=37 | 44 for n=50 | NS | NS | NS | NS |
| PVL (more than
moderate) | 0 | 3 | 0 | 1 | 0 | 0 | 20 | 3 | 6 | 2 |
| Mean AV gradient
mmHg | 12.6 (4) | 8.9 (5) | 8 (3.6) | 12.4 (5.8) | 6.3 (2.7) for
n=33 | 10 (3.3) for
n=49 | NS | NS | 7 (3) | 11 (7) |
| Peak AV
gradient |
| mmHg | 22.8 (7.7) | 16 (9.7) | NS | NS | 11.2 (4.8) | 18 (5.8) for
n=54 | NS | NS | 13 (7) | 20 (10) |
|
| Immediate
post-procedural complications |
|
| Major vascular
complication | 2 | 4 | 3 | 4 | 0 | 2 | NS | NS | NS | NS |
| Life threatening
bleed | 3 | 1 | 2 | 6 | 0 | 1 | NS | NS | NS | NS |
| Pacemaker
implantation | 22 for n=88 | 26 for n=108 | 9 | 8 | NS | NS | 90 | 32 | 12 | 6 |
| Acute kidney
injury | 10 | 11 | 2 | 8 | 1 | 0 | NS | NS | NS | NS |
| Mortality | 1 | 0 | NS | NS | 0 | 1 | 5 | 0 | NS | NS |
| Stroke | 2 | 1 | 1 | 4 | 2 | 0 | NS | NS | NS | NS |
|
| 30-day
outcomes |
|
| Mortality | 2 | 0 | 1 | 3 | NS | NS | 8 | 3 | NS | NS |
| Stroke | 2 | 1 | NS | NS | NS | NS | 11 | 7 | 0 | 1 |
| Life-threatening
bleed | 3 | 1 | NS | NS | NS | NS | 8 | 6 | 1 | 2 |
| Major vascular
complication | 2 | 4 | NS | NS | NS | NS | 17 | 10 | 7 | 5 |
Immediate post-operative
complications
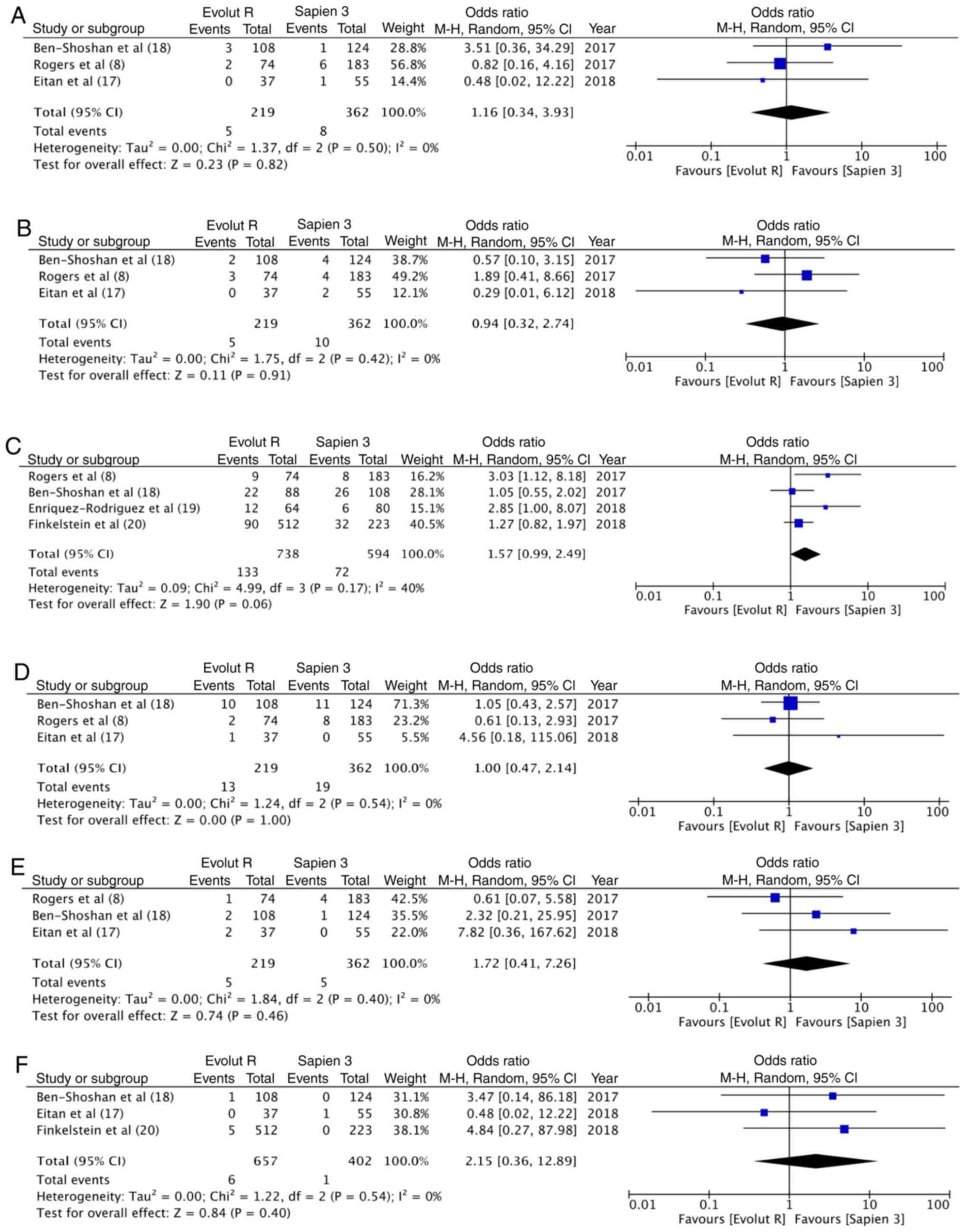
In total, 2.28% (5/219) of the patients had a
life-threatening bleed with Evolut R, while 2.2% (8/362) had the
same experience with Sapien 3; no statistically significant
differences were observed (OR, 1.16; 95% CI, 0.34–3.93; P=0.82;
I2=0%) (Fig. 4A). Data on
major vascular complication was reported by 3 studies. With 5/219
events (2.3%) with Evolut R and 10/362 events (2.8%) with Sapien 3,
there were no statistically significant differences between the 2
groups (OR, 0.94; 95% CI, 0.32–2.74; P=0.91; I2=0%)
(Fig. 4B). A greater percentage [18%
(133/738)] of patients underwent pacemaker implantation following
TAVI with Evolut R, as compared to 12% (72/594) with Sapien 3. This
difference, however, did not reach statistical significance (OR,
1.57, 95% CI, 0.99–2.49, P=0.06; I2=40%) (Fig. 4C). Pooled data from 3 studies on
acute kidney injury with 13/219 (5.9%) events with Evolut R and
19/362 (5.2%) events with Sapien 3, did not reveal any significant
differences between the 2 groups (OR, 1.00; 95% CI, 0.47–2.14;
P=1.00; I2=0%) (Fig. 4D).
Data on stroke and immediate mortality were reported by 3 studies.
With 5 cases of stroke in 219 patients (2.2%) in the Evolut R group
and 5 cases of stroke in 362 patients (1.4%) in the Sapien 3 group,
no statistically significant differences were noted (OR, 1.72; 95%
CI, 0.41–7.26; P=0.46; I2=0%) (Fig. 4E). Similarly, with 6 deaths out of
the 657 patients (0.9%) with Evolut R and 1 death out of the 402
patients (0.2%) with Sapien 3, the pooled analysis did not reveal
any significant difference (OR, 2.15; 95% CI, 0.36–12.89, P=0.40;
I2=0%) (Fig. 4F).
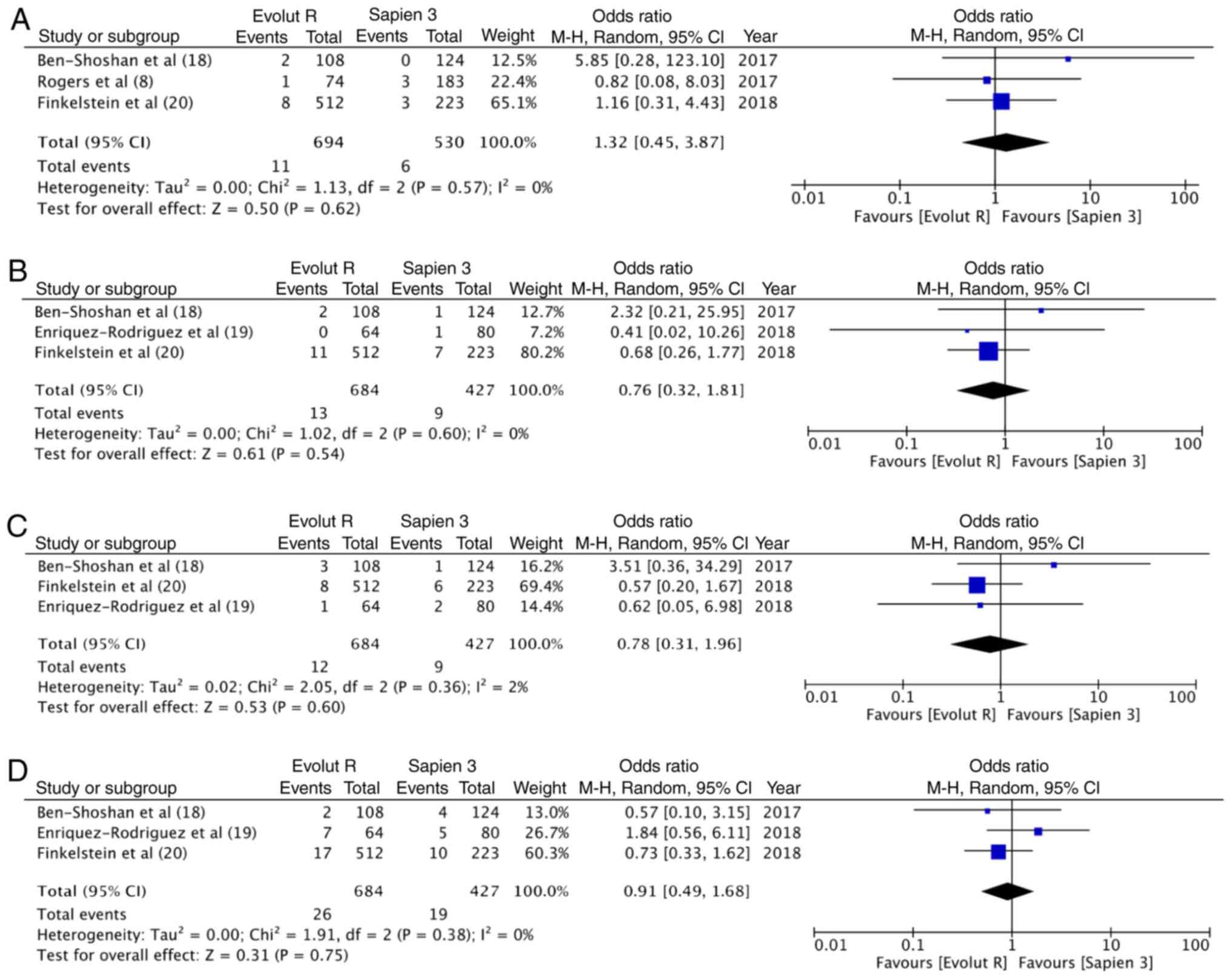
30-day outcomes
At 30 days, there were 11 deaths out of the 694
patients (1.6%) with Evolut R and 6 deaths out of the 530 patients
(1.1%) with Sapien 3. The difference was not statistically
significant (OR, 1.32; 95% CI, 0.45–3.87; P=0.62; I2=0%)
(Fig. 5A). Similarly, no differences
was observed in the 30-day stroke outcomes between the 2 groups
(OR, 0.76; 95% CI, 0.32–1.81; P=0.54; I2=0%) (Fig. 5B). In total, 12 patients had a
life-threatening bleed out of 684 patients in the Evolut R group
(1.8%), while 9 of the 427 patients experienced the same with
Sapien 3 (2.1%). Meta-analysis indicated no statistically
significant differences (OR, 0.78; 95% CI, 0.31–1.96; P=0.60;
I2=2%) (Fig. 5C). In
total, 3.8% patients (26/684) had major vascular complication with
Evolut R, while 4.4% (19/427) had the same experience with Sapien
3. Pooled analysis failed to demonstrate any significant difference
(OR, 0.91; 95% CI, 0.49–1.68; P=0.75; I2=0%) (Fig. 5D).
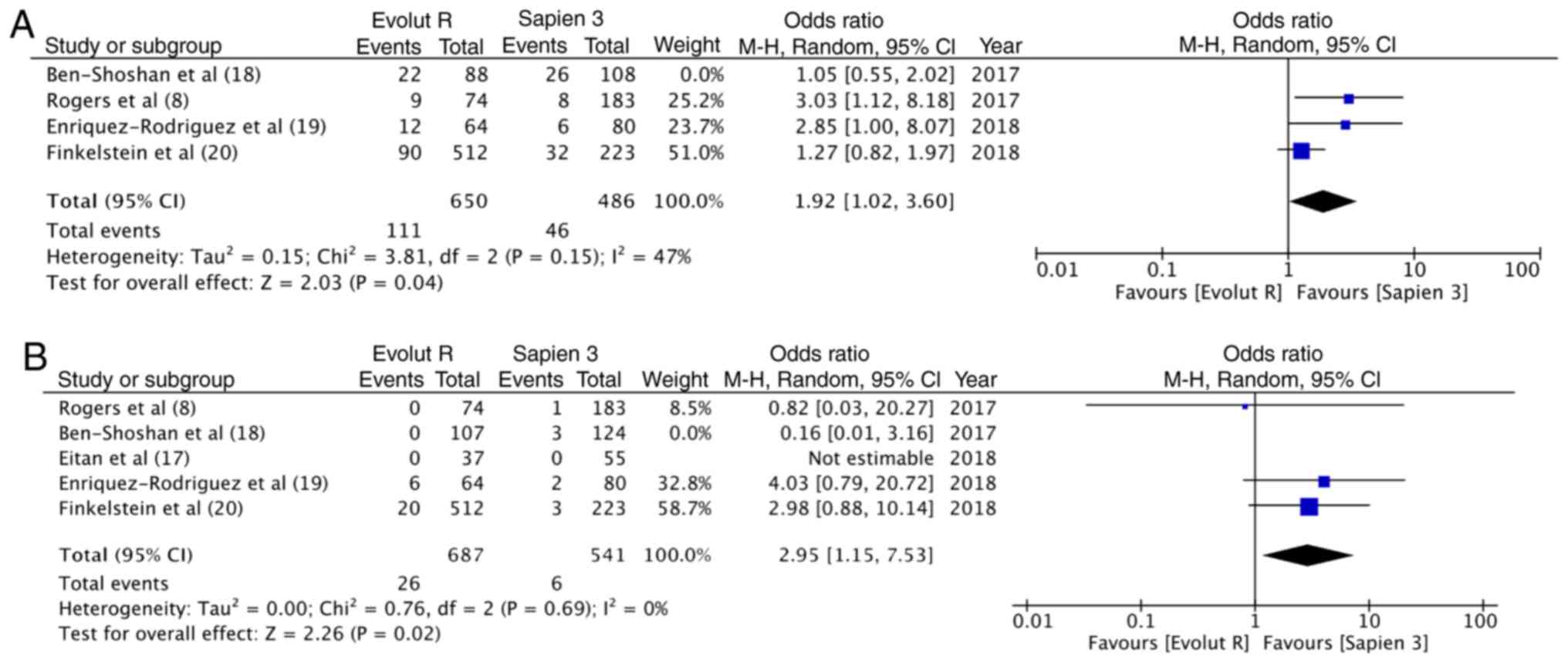
Sensitivity analysis
A sensitivity analysis was carried out wherein each
study was sequentially removed from the meta-analysis. It was found
that when the results of the study by Ben-Shoshan et al
(18) were removed, overall pooled
estimate for pace-maker implantation became statistically
significant in favor of Sapien 3 (OR, 1.92; 95% CI, 1.02–3.60;
P=0.04; I2=47%) (Fig.
6A). In addition, the results for more than moderate
paravalvular regurgitation became significant in favor of Sapien 3
(OR, 2.95; 95% CI, 1.15–7.53; P=0.02; I2=0%) (Fig. 6B). No other outcomes were changed
following the removal of any of the 5 included studies. Sensitivity
analysis with fixed model also did not influence any of the
outcomes.
Discussion
The primary objective of this study was to compare
the clinical outcomes and safety profile of the newer generation
Evolut R and Sapien 3 valves. An important criteria for such a
comparison is to have baseline similarity between the 2 groups. Our
literature search revealed only 5 studies reporting head-to-head
comparison of the 2 devices. With the exception of one study
(19), there were some baseline
differences between the 2 groups, which could have affected the
results of this meta-analysis. Mindful of this disparity, the
primary findings of this study can be summarized as follows: i)
VARC-2 success rates with both devices seem to be similar; ii)
there seem to be no difference in the incidence of mild
paravalvular leak with the 2 valves; however, evidence regarding
moderate to severe paravalvular leak is not clear; iii) aortic
valve gradients are significantly lower with Evolut R; iv) there
seems to be no difference in the short-term safety profile of the 2
devices.
All included studies reported outcomes based on the
VARC-2 guidelines (21), which
enabled the pooling of multiple variables. Device success rates,
defined as the absence of procedural mortality, correct positioning
of the valve, no prosthesis- patient mismatch, mean aortic valve
gradient <20 mmHg or peak velocity <3 m/sec, and no moderate
or severe prosthetic valve regurgitation (21), were reported by all 5 studies. Our
analysis revealed high rates of success with both devices, with no
statistically significant difference between the 2 groups. These
results are similar to studies comparing the former generation
Sapien/Sapien XT and CoreValve devices, which too were comparable
in terms of device success (4,22).
Pooled device success rates from our analysis are also proportional
with single arm trials. Kalra et al (23) reported a 91.3% success rate in 264
consecutive Evolut R implantations, while Husser et al
(24) reported a success rate of
97.6% with Sapien 3 valve in 244 patients.
A significant complication of TAVI is para-valvular
leak that can lead to increased mortality for both the
balloon-expandable and the self-expanding transcatheter heart
valves (25). A meta-analysis of
12,926 patients by Athappan et al reported a 11.7% incidence
of moderate to severe aortic regurgitation in patients treated with
the earlier generation Sapien or CoreValve devices (6). To overcome this drawback, newer
generation Evolut R and Sapien 3 valves were developed. These
devices are equipped with features for repositionability and
retrievability that allow for controlled deployment. While the
Evolut R is equipped with a longer porcine pericardial sealing
skirt, the Sapien 3 consists of an outer polyethylene terephthalate
cuff to reduce paravalvular regurgitation (9,26).
Researchers have reported up to a 50% reduction in moderate to
severe paravalvular leak with Evolut R, as compared to its
predecessor, the CoreValve (27).
Moderate to severe para-valvular leak has reduced from 6.9% with
SAPIEN XT to 1.6% with Sapien 3 (28). In this study, the incidence of
moderate to severe paravalvular leak was found to be extremely low
with a pooled frequency of 3.3% in the Evolut R group and 1.4% in
the Sapien 3 group. The statistically significant differences
between the 2 devices for this variable, are however, not clear.
Finkelstein et al (20) found
a higher rate of moderate to severe paravalvular leak in their
cohort of Evolut R patients. The difference was significant for the
angiographic outcome and only numerically higher for the
echocardiography outcome. The only case-matched study (19) in this review also reported a
significantly increased incidence of moderate paravalvular leak
with Evolut R. The lower rate of regurgitation with Sapien 3 was
attributed to the valves' greater radial force and its adaptability
to the aortic annulus (19). Robust
evidence on the actual difference between the 2 valves can only be
ascertained with prospective studies where the 2 groups are matched
for annulus diameter, valve size and valve calcification and
outcomes assessed at a central independent echocardiographic core
laboratory.
The difference in the hemodynamic performance of the
earlier generation Sapien and CoreValve is well documented. In a
case-matched study, the self-expanding CoreValve was associated
with a significantly lower residual gradient as compared to the
Sapien Valve (29). Similar to their
predecessors, our results indicate superior antegrade hemodynamic
performance of Evolut R as compared to the Sapien 3 device. The
difference is probably attributed to the supra-annular position of
the Evolut R leaflets, allowing lower resistance to the left
ventricle outflow and gradients (19).
Pacemaker implantation following TAVI varies across
studies and can be affected by institutional electrophysiology
protocols (30,31). Single arm studies report the
incidence of permanent pacemaker implantation with Evolut R to be
16.4–19.7% (23,26). Similarly, 14–17% of patients require
permanent pacemaker implantation with Sapien 3 device (9,24). In
this study, the pooled incidence of pacemaker implantation with
Evolut R was slightly higher at 18% as compared to 12% with the
Sapien 3 device. While the difference was not statistically
significant, evidence in not strong since sensitivity analysis
presented conflicting results. The additional skirt added to Sapien
3 device, to reduce paravalvular leakage, has been associated with
increased need of post-procedural pacemaker implantation. It is
postulated that higher local trauma delivered to the conduction
system with this addition results in greater requirement for
pacemaker implantation (20,32). On the contrary, the re-sheathable
delivery system of the Evolut-R may allow more accurate valve
positioning, potentially reducing trauma to the conduction system.
More studies are required to validate the comparison.
Vascular complications following TAVI is another
major problem, with initial rates of complications ranging from 1.9
to 17.3% (33). Important predictors
are small vessel diameters, presence of severe calcifications and
sheath-to-femoral artery ratio (33). Important changes in the design of the
newer generation valves have been made. The 14F expandable sheath
with Sapien 3 and the lower profile 14F equivalent sheath with the
Evolut R device have significantly reduced the incidence of major
vascular complications as compared to the earlier generation valves
(23,24). Our review found that the incidence of
major vascular complications and life-threatening bleeds with both
devices to be extremely low and comparable.
The short-term mortality rates with earlier
generation self-expanding and balloon-expanding valves have been
reported to be 6.8 and 7.1%, respectively (34). These rates have significantly
improved with both new-generation devices. The overall pooled
30-day mortality rates was found to be very low with no difference
between Evolut R and Sapien 3 (1.6 vs. 1.1%). 30-day stroke
outcomes also did not differ between the two devices.
The limitations of this study need to be enumerated.
Firstly, this is a meta-analysis of retrospective observational
studies. An inherent drawback of observational studies is a greater
probability of bias as compared to randomized controlled trials.
Secondly, this is a study-level meta-analysis, a patient level
meta-analysis would have provided stronger evidence. Thirdly, the
participants of the 2 groups were not matched for all baseline
characteristics. Only one-study included case-matched participants.
The results of this analysis, therefore, must be interpreted with
caution. Fourthly, only short-term outcomes were compared as
long-term data was not available. Lastly, not all studies utilized
transfemoral route in all patients. The use of other routes would
have introduced bias in the results.
Despite the limitations, the consistency of the
direction and magnitude of the overall effect, and stability of the
results after sensitivity analysis, provide some support to the
study's overall conclusions. To the best of our knowledge, this is
the first systematic review and meta-analysis comparing the Evolut
R and Sapien 3 device. Our results indicate, Evolut R and Sapien 3
valves may be comparable in terms of device success and short-term
complications. The difference between the 2 devices for
post-operative moderate to severe paravalvular leak and permanent
pacemaker implantation is not clear. There is a need for a large
multi-center randomized controlled trial to provide stronger
evidence on this subject.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH and JL designed the paper. CH and LX were
involved in literature search and data interpreted. JL was
responsible for the data analysis. CH and LX prepared the
manuscript. JL edited the manuscript. All authors have read and
approved the final manuscript. All authors have read and approved
the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith CR, Leon MB, Mack MJ, Miller DC,
Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et
al: Transcatheter versus surgical aortic-valve replacement in
high-risk patients. N Engl J Med. 364:2187–2198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adams DH, Popma JJ, Reardon MJ, Yakubov
SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr,
Kleiman NS, et al: Transcatheter aortic-valve replacement with a
self-expanding prosthesis. N Engl J Med. 370:1790–1798. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Collas VM, Dubois C, Legrand V, Kefer J,
De Bruyne B, Dens J, Rodrigus IE, Herijgers P and Bosmans JM;
Belgian TAVI Registry Participants, : Midterm clinical outcome
following Edwards SAPIEN or Medtronic Corevalve transcatheter
aortic valve implantation (TAVI): Results of the Belgian TAVI
registry. Catheter Cardiovasc Interv. 86:528–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chieffo A, Buchanan GL, Van Mieghem NM,
Tchetche D, Dumonteil N, Latib A, van der Boon RM, Vahdat O,
Marcheix B, Farah B, et al: Transcatheter aortic valve implantation
with the Edwards SAPIEN versus the medtronic CoreValve revalving
system devices. J Am Coll Cardiol. 61:830–836. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Belle E, Juthier F, Susen S,
Vincentelli A, Iung B, Dallongeville J, Eltchaninoff H, Laskar M,
Leprince P, Lievre M, et al: Postprocedural aortic regurgitation in
balloon-expandable and self-expandable transcatheter aortic valve
replacement procedures: Analysis of predictors and impact on
long-term mortality: Insights from the FRANCE2 registry.
Circulation. 129:1415–1427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Athappan G, Patvardhan E, Tuzcu EM,
Svensson LG, Lemos PA, Fraccaro C, Tarantini G, Sinning JM,
Nickenig G, Capodanno D, et al: Incidence, predictors, and outcomes
of aortic regurgitation after transcatheter aortic valve
replacement: Meta-analysis and systematic review of literature. J
Am Coll Cardiol. 61:1585–1595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grover FL, Vemulapalli S, Carroll JD,
Edwards FH, Mack MJ, Thourani VH, Brindis RG, Shahian DM, Ruiz CE,
Jacobs JP, et al: 2016 annual report of the society of thoracic
surgeons/American college of cardiology transcatheter valve therapy
registry. Ann Thorac Surg. 103:1021–1035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rogers T, Steinvil A, Buchanan K, Alraies
MC, Koifman E, Gai J, Torguson R, Okubagzi P, Ben-Dor I, Pichard A,
et al: Contemporary transcatheter aortic valve replacement with
third-generation balloon-expandable vs. self-expanding devices. J
Interv Cardiol. 30:356–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Binder RK, Rodés-Cabau J, Wood DA, Mok M,
Leipsic J, De Larochellière R, Toggweiler S, Dumont E, Freeman M,
Willson AB and Webb JG: Transcatheter aortic valve replacement with
the SAPIEN 3: A new balloon-expandable transcatheter heart valve.
JACC Cardiovasc Interv. 6:293–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manoharan G, Walton AS, Brecker SJ,
Pasupati S, Blackman DJ, Qiao H and Meredith IT: Treatment of
symptomatic severe aortic stenosis with a novel resheathable
supra-annular self-expanding transcatheter aortic valve system.
JACC Cardiovasc Interv. 8:1359–1367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schulz E, Jabs A, Gori T, von Bardeleben
S, Hink U, Kasper-König W, Vahl CF and Münzel T: Transcatheter
aortic valve implantation with the new-generation Evolut R™:
Comparison with CoreValve® in a single center cohort.
Int J Cardiol Heart Vasc. 12:52–56. 2016.PubMed/NCBI
|
|
12
|
Nijhoff F, Abawi M, Agostoni P, Ramjankhan
FZ, Doevendans PA and Stella PR: Transcatheter aortic valve
implantation with the new balloon-expandable Sapien 3 versus Sapien
XT valve system: A propensity score-matched single-center
comparison. Circ Cardiovasc Interv. 8:e0024082015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of Observational Studies in epidemiology: A
proposal for reporting. Meta-analysis Of observational studies in
epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analysesOttawa Hospital Research Institute; Ottawa, ON, 2013:
http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
|
|
15
|
Tham JLM, Adams H, Paleri S, Wright C,
Dimitriou J, Newcomb A, MacIsaac AI, Whitbourn RJ and Palmer SC:
Clinical outcomes of self-expandable vs. balloon-expandable TAVI
for severe aortic stenosis. Acta Cardiol. Apr 1–2019.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang TY, Gracia E, Callahan S, Bilfinger
T, Tannous H, Pyo R, Kort S, Skopicki H, Weinstein J, Patel N, et
al: Gender disparities in management and outcomes following
transcatheter aortic valve implantation with newer generation
transcatheter valves. Am J Cardiol. 123:1489–1493. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eitan A, Witt J, Stripling J, Haselbach T,
Rieß FC and Schofer J: Performance of the Evolut-R 34 mm versus
Sapien-3 29 mm in Transcatheter aortic valve replacement patients
with larger annuli: Early outcome results of Evolut-R 34 mm as
compared with Sapien-3 29 mm in patients with Annuli ≥26 mm.
Catheter Cardiovasc Interv. 92:1374–1379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ben-Shoshan J, Konigstein M, Zahler D,
Margolis G, Chorin E, Steinvil A, Arbel Y, Aviram G, Granot Y,
Barkagan M, et al: Comparison of the Edwards SAPIEN S3 versus
medtronic Evolut-R devices for transcatheter aortic valve
implantation. Am J Cardiol. 119:302–307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Enríquez-Rodríguez E, Amat-Santos IJ,
Jiménez-Quevedo P, Martín-Morquecho I, Tirado-Conte G,
Pérez-Vizcayno MJ, Gómez de Diego JJ, Arnold R, Aldazábal A, Rojas
P, et al: Comparison of the hemodynamic performance of the
balloon-expandable SAPIEN 3 versus self-expandable Evolut R
transcatheter valve: A case-matched study. Rev Esp Cardiol (Engl
Ed). 71:735–742. 2018.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Finkelstein A, Steinvil A, Rozenbaum Z,
Halkin A, Banai S, Barbash I, Guetta V, Segev A, Danenberg H, Orvin
K, et al: Efficacy and safety of new-generation transcatheter
aortic valves: insights from the Israeli transcatheter aortic valve
replacement registry. Clin Res Cardiol. 108:430–437. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kappetein AP, Head SJ, Généreux P, Piazza
N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE,
van Es GA, et al: Updated standardized endpoint definitions for
transcatheter aortic valve implantation: The valve academic
research Consortium-2 consensus document (VARC-2). Eur J
Cardiothorac Surg. 42:S45–S60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Pyxaras SA, Wolf A, Schmitz T and
Naber CK: Propensity-matched comparison between direct flow
medical, medtronic corevalve, and Edwards Sapien XT prostheses:
Device success, thirty-day safety, and mortality. Catheter
Cardiovasc Interv. 85:1217–1225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalra SS, Firoozi S, Yeh J, Blackman DJ,
Rashid S, Davies S, Moat N, Dalby M, Kabir T, Khogali SS, et al:
Initial experience of a second-generation self-expanding
transcatheter aortic valve: The UK & Ireland Evolut R
implanters' registry. JACC Cardiovasc Interv. 10:276–282. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Husser O, Pellegrini C, Kessler T,
Burgdorf C, Thaller H, Mayr NP, Ott I, Kasel AM, Schunkert H,
Kastrati A and Hengstenberg C: Outcomes after transcatheter aortic
valve replacement using a novel balloon-expandable transcatheter
heart valve: A single-center experience. JACC Cardiovasc Interv.
8:1809–1816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moretti C, D'Ascenzo F, Mennuni M, Taha S,
Brambilla N, Nijhoff F, Fraccaro C, Barbanti M, Tamburino C,
Tarantini G, et al: Meta-analysis of comparison between
self-expandable and balloon-expandable valves for patients having
transcatheter aortic valve implantation. Am J Cardiol.
115:1720–1725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Popma JJ, Reardon MJ, Khabbaz K, Harrison
JK, Hughes GC, Kodali S, George I, Deeb GM, Chetcuti S, Kipperman
R, et al: Early clinical outcomes after transcatheter aortic valve
replacement using a novel self-expanding bioprosthesis in patients
with severe aortic stenosis who are suboptimal for surgery: Results
of the Evolut R U.S. study. JACC Cardiovasc Interv. 10:268–275.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kowalewski M, Gozdek M, Raffa GM, Słomka
A, Zieliński K, Kubica J, Anisimowicz L, Kowalewski J, Landes U,
Kornowski R, et al: Transcathether aortic valve implantation with
the new repositionable self-expandable Medtronic Evolut R vs.
CoreValve system: Evidence on the benefit of a meta-analytical
approach. J Cardiovasc Med (Hagerstown). 20:226–236. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ando T, Briasoulis A, Holmes AA, Taub CC,
Takagi H and Afonso L: Sapien 3 versus Sapien XT prosthetic valves
in transcatheter aortic valve implantation: A meta-analysis. Int J
Cardiol. 220:472–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nombela-Franco L, Ruel M, Radhakrishnan S,
Webb JG, Hansen M, Labinaz M, Thompson C, Fremes S, Dumont E,
DeLarochellière R, et al: Comparison of hemodynamic performance of
self-expandable CoreValve versus balloon-expandable Edwards SAPIEN
aortic valves inserted by catheter for aortic stenosis. Am J
Cardiol. 111:1026–1033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khawaja MZ, Rajani R, Cook A, Khavandi A,
Moynagh A, Chowdhary S, Spence MS, Brown S, Khan SQ, Walker N, et
al: Permanent pacemaker insertion after CoreValve transcatheter
aortic valve implantation: Incidence and contributing factors (the
UK CoreValve collaborative). Circulation. 123:951–960. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Biner S, Michowitz Y, Leshem-Rubinow E,
Topilsky Y, Ben-Assa E, Shimiaie J, Banai S, Keren G, Steinvil A
and Finkelstein A: Hemodynamic impact and outcome of permanent
pacemaker implantation following transcatheter aortic valve
implantation. Am J Cardiol. 113:132–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Webb J, Gerosa G, Lefèvre T, Leipsic J,
Spence M, Thomas M, Thielmann M, Treede H, Wendler O and Walther T:
Multicenter evaluation of a next-generation balloon-expandable
transcatheter aortic valve. J Am Coll Cardiol. 64:2235–2243. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toggweiler S, Leipsic J, Binder RK,
Freeman M, Barbanti M, Heijmen RH, Wood DA and Webb JG: Management
of vascular access in transcatheter aortic valve replacement: Part
2: Vascular complications. JACC Cardiovasc Interv. 6:767–776. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Agarwal S, Parashar A, Kumbhani DJ,
Svensson LG, Krishnaswamy A, Tuzcu EM and Kapadia SR: Comparative
meta-analysis of balloon-expandable and self-expandable valves for
transcatheter aortic valve replacement. Int J Cardiol. 197:87–97.
2015. View Article : Google Scholar : PubMed/NCBI
|