|
1
|
Smith CR, Leon MB, Mack MJ, Miller DC,
Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et
al: Transcatheter versus surgical aortic-valve replacement in
high-risk patients. N Engl J Med. 364:2187–2198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adams DH, Popma JJ, Reardon MJ, Yakubov
SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr,
Kleiman NS, et al: Transcatheter aortic-valve replacement with a
self-expanding prosthesis. N Engl J Med. 370:1790–1798. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Collas VM, Dubois C, Legrand V, Kefer J,
De Bruyne B, Dens J, Rodrigus IE, Herijgers P and Bosmans JM;
Belgian TAVI Registry Participants, : Midterm clinical outcome
following Edwards SAPIEN or Medtronic Corevalve transcatheter
aortic valve implantation (TAVI): Results of the Belgian TAVI
registry. Catheter Cardiovasc Interv. 86:528–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chieffo A, Buchanan GL, Van Mieghem NM,
Tchetche D, Dumonteil N, Latib A, van der Boon RM, Vahdat O,
Marcheix B, Farah B, et al: Transcatheter aortic valve implantation
with the Edwards SAPIEN versus the medtronic CoreValve revalving
system devices. J Am Coll Cardiol. 61:830–836. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Belle E, Juthier F, Susen S,
Vincentelli A, Iung B, Dallongeville J, Eltchaninoff H, Laskar M,
Leprince P, Lievre M, et al: Postprocedural aortic regurgitation in
balloon-expandable and self-expandable transcatheter aortic valve
replacement procedures: Analysis of predictors and impact on
long-term mortality: Insights from the FRANCE2 registry.
Circulation. 129:1415–1427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Athappan G, Patvardhan E, Tuzcu EM,
Svensson LG, Lemos PA, Fraccaro C, Tarantini G, Sinning JM,
Nickenig G, Capodanno D, et al: Incidence, predictors, and outcomes
of aortic regurgitation after transcatheter aortic valve
replacement: Meta-analysis and systematic review of literature. J
Am Coll Cardiol. 61:1585–1595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grover FL, Vemulapalli S, Carroll JD,
Edwards FH, Mack MJ, Thourani VH, Brindis RG, Shahian DM, Ruiz CE,
Jacobs JP, et al: 2016 annual report of the society of thoracic
surgeons/American college of cardiology transcatheter valve therapy
registry. Ann Thorac Surg. 103:1021–1035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
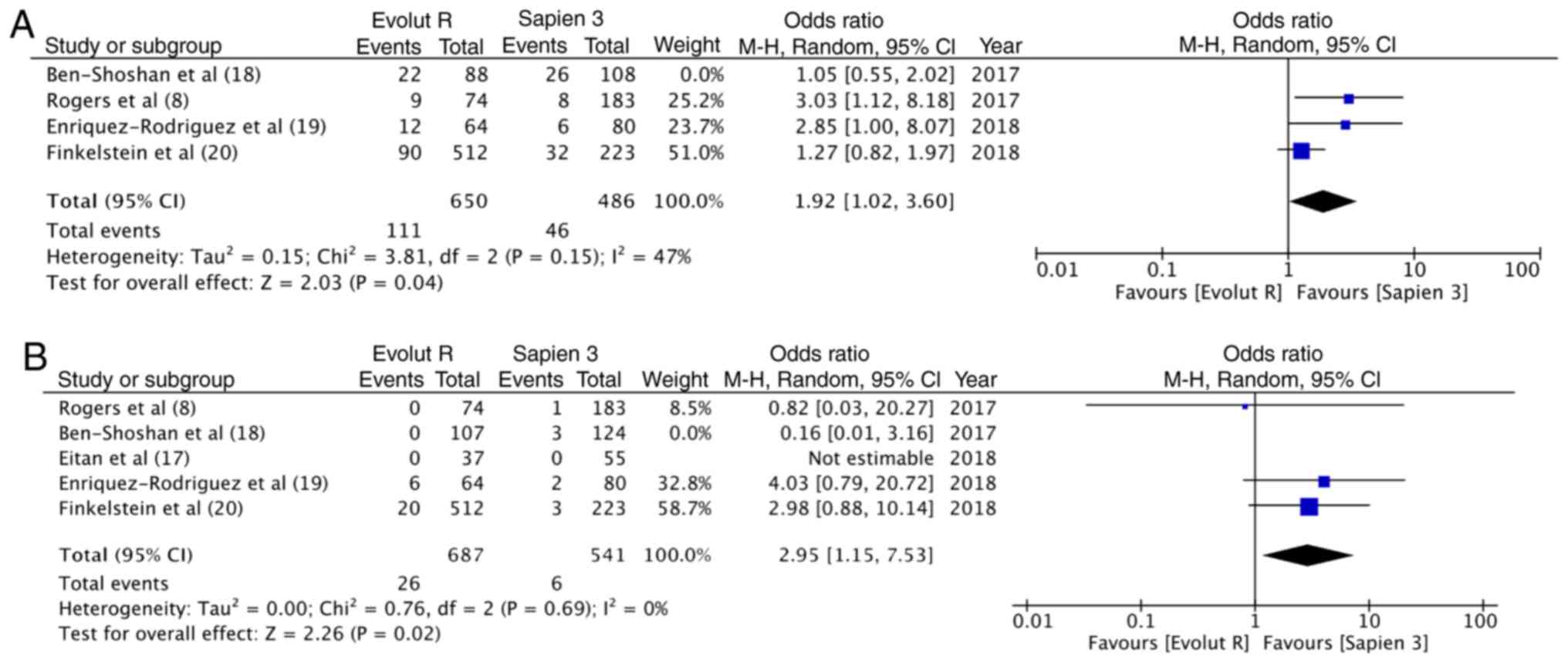
Rogers T, Steinvil A, Buchanan K, Alraies
MC, Koifman E, Gai J, Torguson R, Okubagzi P, Ben-Dor I, Pichard A,
et al: Contemporary transcatheter aortic valve replacement with
third-generation balloon-expandable vs. self-expanding devices. J
Interv Cardiol. 30:356–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Binder RK, Rodés-Cabau J, Wood DA, Mok M,
Leipsic J, De Larochellière R, Toggweiler S, Dumont E, Freeman M,
Willson AB and Webb JG: Transcatheter aortic valve replacement with
the SAPIEN 3: A new balloon-expandable transcatheter heart valve.
JACC Cardiovasc Interv. 6:293–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manoharan G, Walton AS, Brecker SJ,
Pasupati S, Blackman DJ, Qiao H and Meredith IT: Treatment of
symptomatic severe aortic stenosis with a novel resheathable
supra-annular self-expanding transcatheter aortic valve system.
JACC Cardiovasc Interv. 8:1359–1367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schulz E, Jabs A, Gori T, von Bardeleben
S, Hink U, Kasper-König W, Vahl CF and Münzel T: Transcatheter
aortic valve implantation with the new-generation Evolut R™:
Comparison with CoreValve® in a single center cohort.
Int J Cardiol Heart Vasc. 12:52–56. 2016.PubMed/NCBI
|
|
12
|
Nijhoff F, Abawi M, Agostoni P, Ramjankhan
FZ, Doevendans PA and Stella PR: Transcatheter aortic valve
implantation with the new balloon-expandable Sapien 3 versus Sapien
XT valve system: A propensity score-matched single-center
comparison. Circ Cardiovasc Interv. 8:e0024082015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of Observational Studies in epidemiology: A
proposal for reporting. Meta-analysis Of observational studies in
epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analysesOttawa Hospital Research Institute; Ottawa, ON, 2013:
http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
|
|
15
|
Tham JLM, Adams H, Paleri S, Wright C,
Dimitriou J, Newcomb A, MacIsaac AI, Whitbourn RJ and Palmer SC:
Clinical outcomes of self-expandable vs. balloon-expandable TAVI
for severe aortic stenosis. Acta Cardiol. Apr 1–2019.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang TY, Gracia E, Callahan S, Bilfinger
T, Tannous H, Pyo R, Kort S, Skopicki H, Weinstein J, Patel N, et
al: Gender disparities in management and outcomes following
transcatheter aortic valve implantation with newer generation
transcatheter valves. Am J Cardiol. 123:1489–1493. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
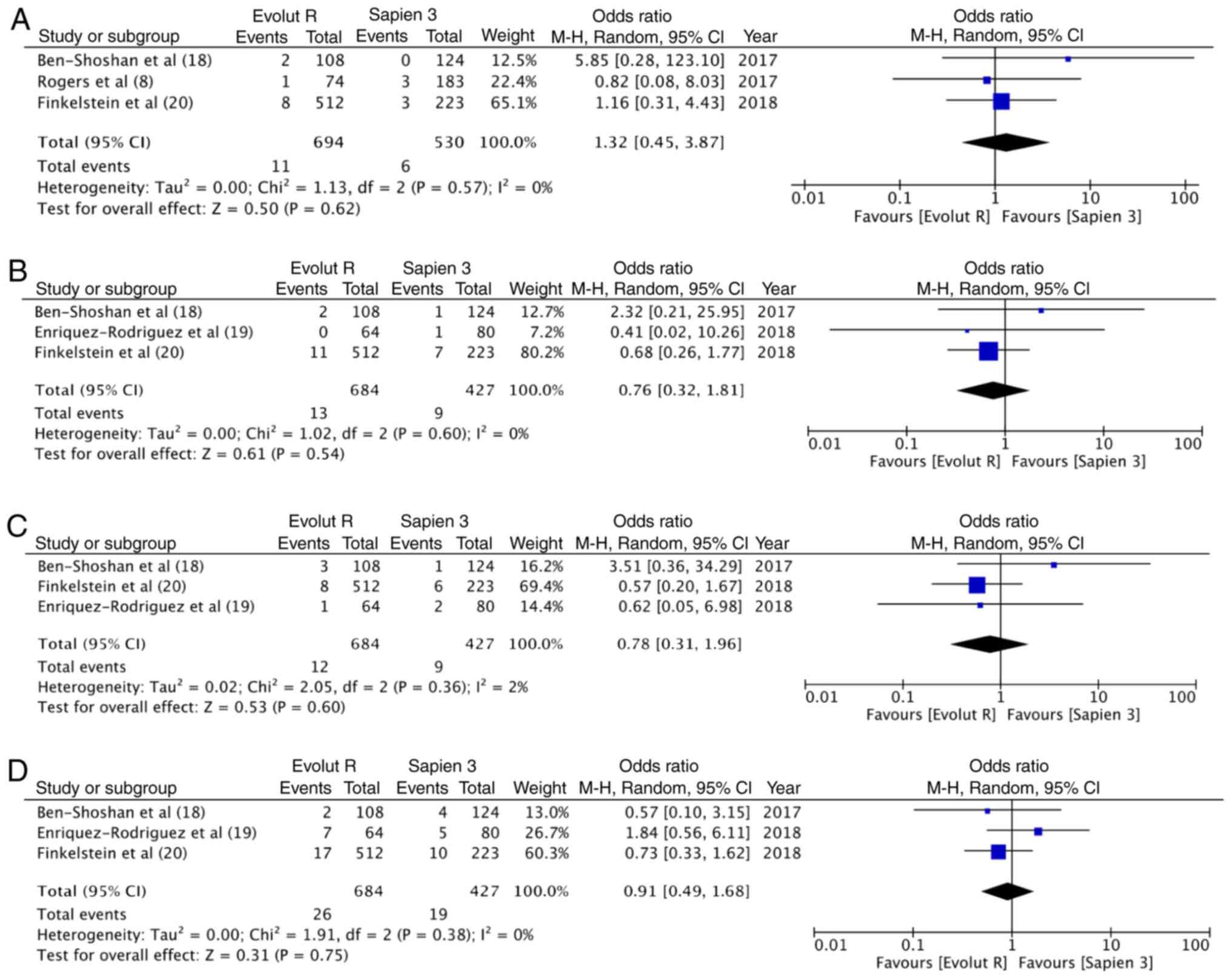
Eitan A, Witt J, Stripling J, Haselbach T,
Rieß FC and Schofer J: Performance of the Evolut-R 34 mm versus
Sapien-3 29 mm in Transcatheter aortic valve replacement patients
with larger annuli: Early outcome results of Evolut-R 34 mm as
compared with Sapien-3 29 mm in patients with Annuli ≥26 mm.
Catheter Cardiovasc Interv. 92:1374–1379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ben-Shoshan J, Konigstein M, Zahler D,
Margolis G, Chorin E, Steinvil A, Arbel Y, Aviram G, Granot Y,
Barkagan M, et al: Comparison of the Edwards SAPIEN S3 versus
medtronic Evolut-R devices for transcatheter aortic valve
implantation. Am J Cardiol. 119:302–307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Enríquez-Rodríguez E, Amat-Santos IJ,
Jiménez-Quevedo P, Martín-Morquecho I, Tirado-Conte G,
Pérez-Vizcayno MJ, Gómez de Diego JJ, Arnold R, Aldazábal A, Rojas
P, et al: Comparison of the hemodynamic performance of the
balloon-expandable SAPIEN 3 versus self-expandable Evolut R
transcatheter valve: A case-matched study. Rev Esp Cardiol (Engl
Ed). 71:735–742. 2018.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Finkelstein A, Steinvil A, Rozenbaum Z,
Halkin A, Banai S, Barbash I, Guetta V, Segev A, Danenberg H, Orvin
K, et al: Efficacy and safety of new-generation transcatheter
aortic valves: insights from the Israeli transcatheter aortic valve
replacement registry. Clin Res Cardiol. 108:430–437. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kappetein AP, Head SJ, Généreux P, Piazza
N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE,
van Es GA, et al: Updated standardized endpoint definitions for
transcatheter aortic valve implantation: The valve academic
research Consortium-2 consensus document (VARC-2). Eur J
Cardiothorac Surg. 42:S45–S60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Pyxaras SA, Wolf A, Schmitz T and
Naber CK: Propensity-matched comparison between direct flow
medical, medtronic corevalve, and Edwards Sapien XT prostheses:
Device success, thirty-day safety, and mortality. Catheter
Cardiovasc Interv. 85:1217–1225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalra SS, Firoozi S, Yeh J, Blackman DJ,
Rashid S, Davies S, Moat N, Dalby M, Kabir T, Khogali SS, et al:
Initial experience of a second-generation self-expanding
transcatheter aortic valve: The UK & Ireland Evolut R
implanters' registry. JACC Cardiovasc Interv. 10:276–282. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Husser O, Pellegrini C, Kessler T,
Burgdorf C, Thaller H, Mayr NP, Ott I, Kasel AM, Schunkert H,
Kastrati A and Hengstenberg C: Outcomes after transcatheter aortic
valve replacement using a novel balloon-expandable transcatheter
heart valve: A single-center experience. JACC Cardiovasc Interv.
8:1809–1816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moretti C, D'Ascenzo F, Mennuni M, Taha S,
Brambilla N, Nijhoff F, Fraccaro C, Barbanti M, Tamburino C,
Tarantini G, et al: Meta-analysis of comparison between
self-expandable and balloon-expandable valves for patients having
transcatheter aortic valve implantation. Am J Cardiol.
115:1720–1725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Popma JJ, Reardon MJ, Khabbaz K, Harrison
JK, Hughes GC, Kodali S, George I, Deeb GM, Chetcuti S, Kipperman
R, et al: Early clinical outcomes after transcatheter aortic valve
replacement using a novel self-expanding bioprosthesis in patients
with severe aortic stenosis who are suboptimal for surgery: Results
of the Evolut R U.S. study. JACC Cardiovasc Interv. 10:268–275.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kowalewski M, Gozdek M, Raffa GM, Słomka
A, Zieliński K, Kubica J, Anisimowicz L, Kowalewski J, Landes U,
Kornowski R, et al: Transcathether aortic valve implantation with
the new repositionable self-expandable Medtronic Evolut R vs.
CoreValve system: Evidence on the benefit of a meta-analytical
approach. J Cardiovasc Med (Hagerstown). 20:226–236. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ando T, Briasoulis A, Holmes AA, Taub CC,
Takagi H and Afonso L: Sapien 3 versus Sapien XT prosthetic valves
in transcatheter aortic valve implantation: A meta-analysis. Int J
Cardiol. 220:472–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nombela-Franco L, Ruel M, Radhakrishnan S,
Webb JG, Hansen M, Labinaz M, Thompson C, Fremes S, Dumont E,
DeLarochellière R, et al: Comparison of hemodynamic performance of
self-expandable CoreValve versus balloon-expandable Edwards SAPIEN
aortic valves inserted by catheter for aortic stenosis. Am J
Cardiol. 111:1026–1033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khawaja MZ, Rajani R, Cook A, Khavandi A,
Moynagh A, Chowdhary S, Spence MS, Brown S, Khan SQ, Walker N, et
al: Permanent pacemaker insertion after CoreValve transcatheter
aortic valve implantation: Incidence and contributing factors (the
UK CoreValve collaborative). Circulation. 123:951–960. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Biner S, Michowitz Y, Leshem-Rubinow E,
Topilsky Y, Ben-Assa E, Shimiaie J, Banai S, Keren G, Steinvil A
and Finkelstein A: Hemodynamic impact and outcome of permanent
pacemaker implantation following transcatheter aortic valve
implantation. Am J Cardiol. 113:132–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Webb J, Gerosa G, Lefèvre T, Leipsic J,
Spence M, Thomas M, Thielmann M, Treede H, Wendler O and Walther T:
Multicenter evaluation of a next-generation balloon-expandable
transcatheter aortic valve. J Am Coll Cardiol. 64:2235–2243. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toggweiler S, Leipsic J, Binder RK,
Freeman M, Barbanti M, Heijmen RH, Wood DA and Webb JG: Management
of vascular access in transcatheter aortic valve replacement: Part
2: Vascular complications. JACC Cardiovasc Interv. 6:767–776. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Agarwal S, Parashar A, Kumbhani DJ,
Svensson LG, Krishnaswamy A, Tuzcu EM and Kapadia SR: Comparative
meta-analysis of balloon-expandable and self-expandable valves for
transcatheter aortic valve replacement. Int J Cardiol. 197:87–97.
2015. View Article : Google Scholar : PubMed/NCBI
|