Introduction
Complications of diabetes include neuropathy,
retinopathy, nephropathy and, most notably, macroangiopathy
(1,2), which is considered to be the major
cause of death among patients with diabetes (3). Type 2 diabetes worsens as it progresses
from the postprandial hyperglycemic stage to the fasting
hyperglycemic stage. Postprandial hyperglycemia promotes the onset
and progression of diabetes (4) and,
by inducing oxidative stress, likely causes macroangiopathy
(5,6). Hence, suppression of postprandial
hyperglycemia may help prevent diabetes, its progression and the
occurrence of its complications.
Numerous diverse factors affect postprandial blood
glucose levels, including insulin, the incretins [gastric
inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1)],
the gastric emptying rate, the glycemic index and digestive
enzymes. Inadequate secretion of insulin and the incretins elevates
postprandial blood glucose levels (7), and insulin secretion is particularly
low in Japanese patients with diabetes (8). Recent studies suggest that the order in
which food is consumed limits postprandial glucose excursions.
Protein and olive oil promote the secretion of incretins (9–12), while
dietary fiber delays gastric emptying (13). Changing the order in which protein or
olive oil and fiber are ingested or ingesting them prior to meals
may curb postprandial glucose excursions.
Previous studies by our group reported that the
consumption of a green salad, which has a low glycemic index, prior
to meals limits postprandial glucose excursions (14,15). A
further study by our group then focused on vegetable juice, which
is easier to prepare and ingest than a green salad, demonstrating
that the consumption of 200 ml vegetable juice 30 min prior to the
consumption rice significantly attenuated increases in blood
glucose levels (16). However, the
mechanism has remained elusive.
The present study focused on the sugar content of
vegetable juice and investigated the effect of the sugars on blood
glucose and insulin levels when ingested prior to a standard
meal.
Materials and methods
Test foods
For the present study, as in the previous study by
our group (16), a commercially
available brand of vegetable juice (Yasai Ichinichi Kore Ippon;
Kagome Co., Ltd.) was used. Additional test substances were glucose
(Yoshida Pharmaceutical Company, Ltd.), D(−)-fructose and sucrose
(Fujifilm Wako Pure Chemical Corp.), and pre-packaged cooked rice
(Sato No Gohan; Sato Foods Co., Ltd.). The content of sugar
(glucose, fructose, sucrose) of the vegetable juice is presented in
Table I. The amount of carbohydrates
per 200 ml was 14.6 g, whereas the total amount of sugar determined
was 11.4 g, as described previously (16). A sugar solution with the same
glucose, fructose and sucrose sugar compositions as the vegetable
juice was prepared.
 | Table I.Nutritional content of the vegetable
juice used in the present study (per 200 ml). |
Table I.
Nutritional content of the vegetable
juice used in the present study (per 200 ml).
| Parameters | Value |
|---|
| Energy (kcal) | 71 |
| Protein (g) | 2.1 |
| Fat (g) | 0 |
| Carbohydrates
(g) | 14.6 |
| Sugars,
total (g) | 11.4 |
| Sucrose
(g) | 5.6 |
| Glucose
(g) | 2.8 |
| Fructose
(g) | 3.0 |
| Dietary fiber
(g) | 1.9 |
| Calcium (mg) | 53 |
| Potassium (mg) | 830 |
| Citric acid (mg) | 1,190 |
| Malic acid (mg) | 514 |
| Total polyphenols (mg
GAE) | 130 |
Subjects
Subjects were recruited from Josai University in
November 2014 and the study was conducted at the Laboratory of Drug
Safety Management of Josai University. The study cohort comprised
10 healthy individuals (7 males and 3 females) aged 20–29 years.
The sample size was determined based on a previous study by our
group (16), and as in that study,
none of the subjects exhibited any impaired glucose tolerance in
tests performed during the previous year (Table II). Subjects participated in all
three trials with a one-week washout period between each trial.
 | Table II.Descriptive characteristics of the
subjects. |
Table II.
Descriptive characteristics of the
subjects.
| Item | Total (n=10) | Males (n=7) | Females (n=3) |
|---|
| Age (years) | 22.9±1.6 | 23.0±2.1 | 22.7±0.9 |
| Body height
(cm) | 169.5±7.0 | 173.1±4.6 | 161.0±3.6 |
| Body weight
(kg) | 62.1±10.1 | 67.5±6.6 | 49.3±2.5 |
| BMI
(kg/m2) | 21.5±2.1 | 22.5±1.7 | 19.0±0.2 |
| HbA1c (%) | 5.2±0.3 | 5.2±0.3 | 5.2±0.1 |
The present study was approved by the Life Sciences
Research Ethics Committee of Josai University (Sakadoshi, Japan;
date of approval, October 20, 2014; approval no. H26-5) and
informed consent was obtained from all subjects.
Experimental protocol
The subjects consumed a 106.2 g cooked rice (sugar
content, 35.4 g) 30 min after drinking 200 ml of either water
(control group), the sugar solution (SS group) or vegetable juice
(VJ group). The study was performed using the randomized crossover
method. A random participant table was created using a computer
program and the order of the tests was randomly determined. The
nutritional content of the test meal and the beverages is provided
in Table III.
 | Table III.Nutritional content in the three
groups. |
Table III.
Nutritional content in the three
groups.
| Group | Protein (g) | Fat (g) | Available CHO
(g) | Fiber (g) |
|---|
| Control | 2.17 | 0.3 | Total, 35.4 | 0.3 |
|
|
|
| Rice, 35.4 |
|
| SS | 2.17 | 0.3 | Total, 46.8 | 0.3 |
|
|
|
| Rice, 35.4 |
|
|
|
|
| Sugar solution,
11.4 (glucose, 2.8; |
|
|
|
|
| fructose, 3.0;
sucrose, 5.6) |
|
| VJ | 4.27 | 0.3 | Total, 50 | 2.2 |
|
|
|
| Rice, 35.4 |
|
|
|
|
| Vegetable juice,
14.6 |
|
The subjects were prohibited from eating and
drinking anything but water from 9 pm on the night prior to the
experiment until the start of the experiment. All tests were
conducted at 9 am. It took <3 min for all subjects to consume
the test beverage and <5 min to eat the cooked rice.
Blood was sampled nine times: Immediately prior to
drinking the test beverage (−30 min), prior to eating the rice (0
min), and 15, 30, 45, 60, 90, 120 and 180 min after eating the
rice. Blood was collected from a fingertip using a lancet (Medisafe
FineTouch; Terumo Corp.). Blood glucose levels were measured
enzymatically using a self-testing blood glucose monitor (Glutest
Neo Alpha; Sanwa Kagaku Kenkyusyo, Co., Ltd.). For the measurement
of insulin levels, 100 µl of blood was transferred from a capillary
tube (Hematlon-L®; Minato Medical Co., Ltd.) to a
microtube; the blood was centrifuged (2,610 × g, 4°C, 5 min), and
25 µl of the supernatant (plasma) was immediately frozen at −80°C.
Insulin levels were later measured using an insulin kit (YK060
Insulin ELISA kit®; Yanaihara Institute Inc.) according
to the manufacturer's protocol.
Data analysis
Changes in blood glucose and insulin levels were
calculated by subtracting the blood glucose and plasma insulin
values measured prior to intake of the test beverages from the
values measured at the various time-points after the subjects
consumed the cooked rice. The maximum change in concentration,
which may be at any time-point up to 180 min after consumption of
the test beverages, was defined as ΔCmax. Incremental
areas under the curves (IAUCs) were calculated using the trapezoid
formula to assess the kinetics of the changes in blood glucose and
insulin levels.
Statistical analysis
Statistical analysis was performed using
Statsel3® software (OMS Publishing Inc.). Differences in
ΔCmax, IAUCs, and changes in postprandial blood glucose
and insulin levels were examined using one-way repeated analysis of
variance. F-tests with significant results were followed by a
Tukey-Kramer test. P<0.05 was considered to indicate a
statistically significant difference.
Results
General characteristics
There were no dropouts in any test group and no side
effects were reported. There were no differences in blood glucose
and plasma insulin levels between males and females.
Blood glucose levels
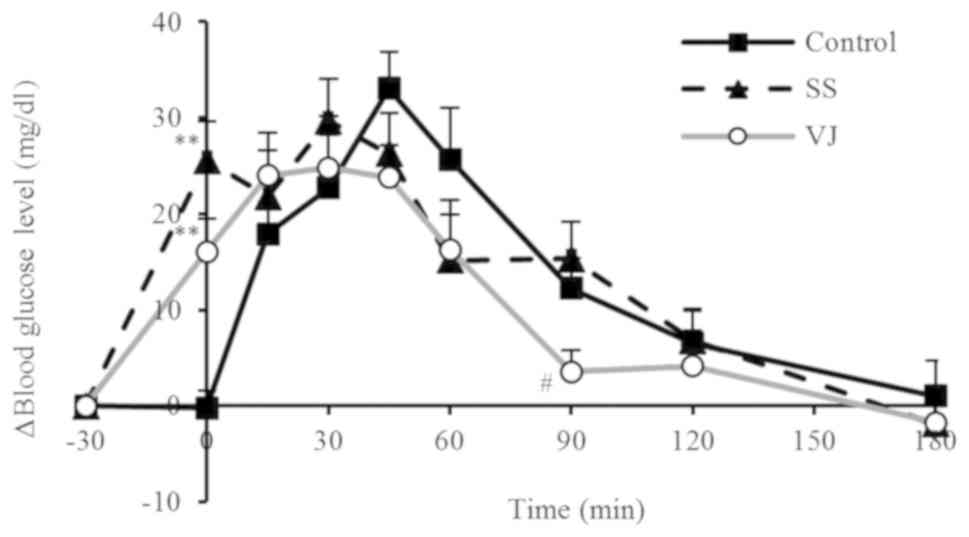
The changes in blood glucose levels in the different
groups are displayed in Fig. 1 and
the ΔCmax values and IAUCs are provided in Table IV. In the control group, the blood
glucose levels rose rapidly after the subjects consumed the rice,
peaking at 45 min. In the SS and VJ groups, the blood glucose
levels rose after the subjects consumed their respective beverages
(sugar solution or vegetable juice). These groups subsequently had
significantly higher values at 0 min (prior to consuming rice)
compared with those in the control group (P<0.01) and the blood
glucose levels reached a peak at 30 min. Furthermore, the increase
tended to be more gradual in the VJ group, and the VJ group had a
significantly lower value at 90 min compared with that in the SS
group (P<0.05). The ΔCmax of the VJ group was lower
than that of the control and SS groups, but the difference was not
significant. The IAUC in the SS group was obviously but
insignificantly higher than that in the control group, while the
IAUC was identical in the VJ and control groups.
 | Table IV.Kinetic parameters of blood
glucose. |
Table IV.
Kinetic parameters of blood
glucose.
| Group | ΔCmax
(mg/dl) | IAUC (mg ×
min/dl) |
|---|
| Control | 36.2±3.2 | 2287.1±348.4 |
| SS | 36.2±3.9 | 3018.2±466.5 |
| VJ | 33.0±3.6 | 2331.3±416.0 |
Plasma insulin levels
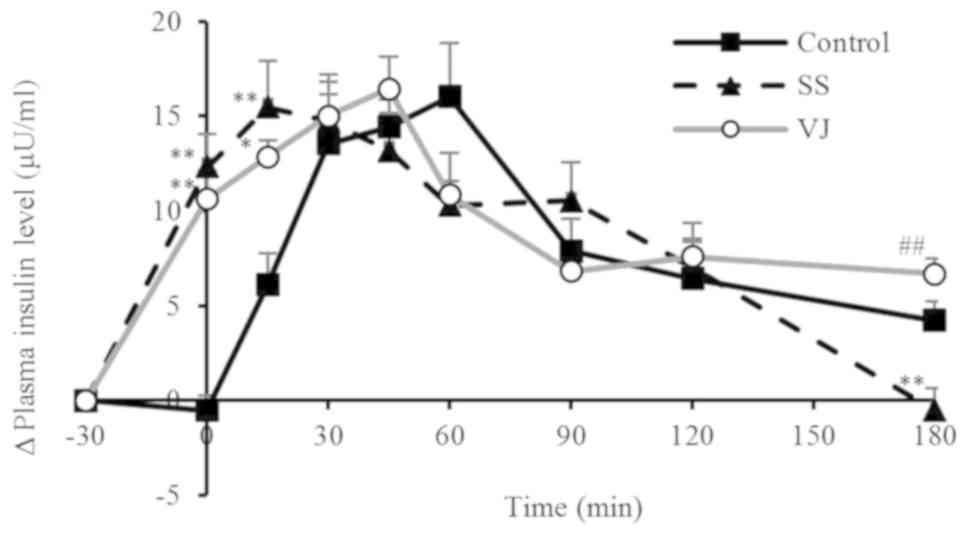
The changes in plasma insulin levels in the
different groups are presented in Fig.
2 and the ΔCmax values and IAUCs are provided in
Table V. In the control group, the
plasma insulin levels rose rapidly after the subjects consumed the
rice, peaking at 60 min. In the SS group, the plasma insulin levels
rose after the subjects consumed the sugar solution, peaking at 15
min. The levels at 0 and 15 min were significantly higher in the SS
group compared with those in the control group (P<0.01), whereas
the levels at 180 min were significantly lower in the VJ group
compared with those in the SS group (P<0.01). In the VJ group,
the plasma insulin levels rose after the subjects consumed the
vegetable juice, peaking at 45 min. The plasma insulin levels then
declined until 90 min and subsequently leveled off. Levels at 0 and
15 min were significantly higher in the VJ group than those in the
control group (P<0.01 and P<0.05, respectively), whereas the
levels at 180 min were significantly higher in the VJ group
compared with those in the SS group (P<0.01). All three groups
had essentially the same ΔCmax. The SS and VJ group had
higher IAUCs than the control group, but the differences were not
significant.
 | Table V.Kinetic parameters of plasma
insulin. |
Table V.
Kinetic parameters of plasma
insulin.
| Group | ΔCmax
(mg/dl) | IAUC (mg ×
min/dl) |
|---|
| Control | 21.2±3.0 | 1534.9±174.4 |
| SS | 20.3±1.8 | 1820.7±130.8 |
| VJ | 19.0±1.9 | 1895.4±181.6 |
Discussion
In the present study, the effects of pre-prandial
vegetable juice ingestion vs. sugars alone on postprandial blood
glucose and insulin levels were assessed and the effect of the
sugar contained in vegetable juice was clarified.
A previous study by our group suggests that
vegetable juice intake and vegetable salad intake suppress
postprandial hyperglycemia via different mechanisms (16). The major potential mechanisms of
suppression are as follows: i) Enhanced secretion of insulin, GIP
and GLP-1 by sugars; ii) reduced digestion and absorption of the
sugars by dietary fibers; and iii) reduced digestion and absorption
of the sugars by organic acids and polyphenols. Among these
mechanisms, the latter two are peculiar to vegetable juice. In the
manufacturing process of the vegetable juice used in the present
study, insoluble dietary fiber is removed as pomace; hence, most of
the dietary fiber in the juice (1.9 g) is presumably
water-soluble.
The indigestible dextrin component of water-soluble
dietary fiber has been reported to suppress postprandial blood
hyperglycemia in several ways (17–20). For
instance, it inhibits glucose absorption after sucrose or maltose
intake via the disaccharidase-associated transport system, and it
mechanically stimulates the digestive tract to induce the secretion
of GLP-1. Secreted GLP-1 lowers blood glucose levels by promoting
insulin secretion from pancreatic β-cells and simultaneously
inhibiting glucagon secretion (21).
The microchorionic membrane disaccharide-degrading enzyme is a
component of the disaccharidase-associated transport system.
Organic acids and polyphenols suppress the activity of digestive
enzymes, including α-amylase and α-glucosidase (22,23).
Polyphenols also inhibit the activity of sodium-glucose transport
protein 1 (24–26).
In the present study, blood glucose levels rose
within 30 min after the consumption of the sugar solution or
vegetable juice (0 min). However, the increase was less pronounced
in the VJ group than in the SS group, presumably owing to the
dietary fiber, organic acids and polyphenols in the vegetable
juice. The rapid increase in blood glucose levels in the SS and VJ
groups may be responsible for the significantly higher plasma
insulin levels in these groups compared with those in the control
group at 15 min; at this time-point, the plasma insulin levels
tended to be higher in the SS group compared with those in the VJ
group.
At 90 min, the blood glucose level was significantly
lower in the VJ group compared with that in the SS group. Moreover,
at 180 min, the plasma insulin level was significantly higher in
the VJ group compared with that in the SS group. These results are
different from those of a previous study by our group (16), where the group equivalent to the VJ
group had blood glucose levels that declined from 60 to 120 min,
and the 180-min insulin level was the same as the level after
fasting. This result may also reflect differences in the amount or
types of intestinal microflora in the study populations. Dietary
fiber promoting insulin secretion via a mechanism involving the
intestinal microflora has become a popular concept in recent years.
Previous studies have indicated that the intake of a small amount
of dietary fiber increases the microflora-mediated production of
short-chain fatty acids (27), which
in turn promote insulin secretion via secretion of GLP-1 (28,29).
Although the present study and the previous study by our group
(16) assessed healthy adults with
no glucose intolerance, neither of them examined the intestinal
microflora. Hence, further studies assessing the involvement of the
microflora are required in the future.
The vegetable juice and sugar solution used in the
present study contained fructose and sucrose. In the study by Yau
et al (30), healthy subjects
consumed sugar solutions containing glucose (39.6 g), fructose
(36.0 g), sucrose (36.0 g), or glucose (19.8 g) plus fructose (18.0
g), and the levels of blood glucose, insulin, GIP and GLP-1 were
measured after ingestion. The levels of insulin, GIP and GLP-1
peaked at 20–30 min after the intake of each sugar solution. In the
present study, the high plasma insulin level at 0 min in the VJ
group may have suppressed the rise in the blood glucose level
following rice consumption. Furthermore, Moore et al
(31) reported that low-dose
fructose improves the glycemic response to an oral glucose load in
normal adults without significantly enhancing the insulin or
triglyceride response. Regarding the potential mechanism, fructose
may activate glucokinase and promote glucose uptake in the liver
(32,33). The vegetable juice and sugar solution
used in the present study each contained 3 g of fructose, and it is
possible that the fructose may have inhibited, at least in part,
the postprandial increase in blood glucose levels in the SS and VJ
groups. It may therefore be suggested that fructose increased
glucose uptake by the liver, consequently stimulating glucose
metabolism.
Although the VJ and SS groups consumed sugar (14.6
and 11.4 g, respectively), whereas the control group did not,
ΔCmax was low in all groups with no significant
inter-group difference, and the IAUCs for blood glucose were also
similar. These results are reminiscent of the blood sugar
transition indicated in the previous study by our group (16) and highly support the contention that
vegetable juice suppresses postprandial hyperglycemia. Given the
similar effects in the VJ and SS groups, it is likely that the
sugars contained in the vegetable juice were mainly responsible for
its suppressive effects on blood glucose levels following rice
consumption.
The present study was performed to determine the
mechanism whereby vegetable juice attenuates the increase in
postprandial glucose levels. Blood glucose and plasma insulin
levels were compared after intake of vegetable juice or a sugar
solution with the same sugar composition as the vegetable juice.
Blood glucose kinetic parameters had similar values despite the
fact that the VJ group consumed more sugar than the SS group. In
the VJ group of the present study, the same postprandial blood
sugar rise inhibitory effect as that in the previous study by our
group (16) was observed. The VJ
group exhibited similar changes in blood glucose levels to those in
the SS group, and there were no significant differences in blood
glucose kinetic parameters, although the VJ group had a somewhat
low value. From this, it may be deduced that the effect of the
sugar contained in vegetable juice is largely responsible for the
suppression of the postprandial increase in blood glucose in the VJ
group. Furthermore, since the insulin level in the VJ group and the
SS group also exhibited the same transitional trend, it may be
indicated that the change in the insulin secretion pattern due to
the intake of glucose contributes to the suppression of
postprandial blood glucose elevation. However, in the present
study, the blood glucose level in the VJ group was significantly
lower when compared with the SS group at 90 min. This result
differed to those obtained in a previous study, where no
significant difference was observed (16). This may be due to the influence of
the subjects' intestinal environment and insulin secretion ability.
In addition, the present study was performed on healthy
individuals, and thus, the effect on diabetic patients remains
elusive. Based on the results of the study, vegetable juice may
contribute to the prevention of diabetes.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YI, IM and IK conceived the current study and
provided technical support. NK, NI and IK were responsible for the
acquisition, analysis and interpretation of the data and
contributed to the drafting of the manuscript and its critical
revision for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Life Sciences
Research Ethics Committee of Josai University (Sakadoshi, Japan;
date of approval: October 20, 2014; approval no. H26-5). All
procedures were performed in accordance with the ethical standards
of the responsible committee on human experimentation
(institutional and national) and/or with the Helsinki Declaration
of 1964 and its later versions. Informed consent was obtained from
all subjects prior to their inclusion in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GIP
|
gastric inhibitory polypeptide
|
|
GLP-1
|
glucagon-like peptide-1
|
|
IAUC
|
incremental area under the curve
|
|
ΔCmax
|
maximum change in concentration
|
|
SS group
|
sugar solution group
|
|
VJ group
|
vegetable juice group
|
References
|
1
|
Diabetes Control and Complications Trial
Research Group, ; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford
O, Davis M, Rand L and Siebert C: The effect of intensive treatment
of diabetes on the development and progression of long-term
complications in insulin-dependent diabetes mellitus. N Engl J Med.
329:977–986. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohkubo Y, Kishikawa H, Araki E, Miyata T,
Isami S, Motoyoshi S, Kojima Y, Furuyoshi N and Shichiri M:
Intensive insulin therapy prevents the progression of diabetic
microvascular complications in Japanese patients with
non-insulin-dependent diabetes mellitus: A randomized prospective
6-year study. Diabetes Res Clin Pract. 28:103–117. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DECODE Study Group and the European
Diabetes Epidemiology Group, : Glucose tolerance and cardiovascular
mortality: Comparison of fasting and 2-hour diagnostic criteria.
Arch Intern Med. 161:397–405. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monnier L, Colette C, Dunseath GJ and
Owens DR: The loss of postprandial glycemic control precedes
stepwise deterioration of fasting with worsening diabetes. Diabetes
Care. 30:263–269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ceriello A, Falleti E, Motz E, Taboga C,
Tonutti L, Ezsol Z, Gonano F and Bartoli E: Hyperglycemia-induced
circulating ICAM-1 increase in diabetes mellitus: The possible role
of oxidative stress. Horm Metab Res. 30:146–149. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nappo F, Esposito K, Cioffi M, Giugliano
G, Molinari AM, Paolisso G, Marfella R and Giugliano D:
Postprandial endothelial activation in healthy subjects and in type
2 diabetic patients: Role of fat and carbohydrate meals. J Am Coll
Cardiol. 39:1145–1150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holst JJ and Gromada J: Role of incretin
hormones in the regulation of insulin secretion in diabetic and
nondiabetic humans. Am J Physiol Endocrinol Metab. 287:E199–E206.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshinaga H and Kosaka K: Heterogeneous
relationship of early insulin response and fasting insulin level
with development of non-insulin-dependent diabetes mellitus in
non-diabetic Japanese subjects with or without obesity. Diabetes
Res Clin Pract. 44:129–136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Almario RU, Buchan WM, Rocke DM and
Karakas SE: Glucose-lowering effect of whey protein depends upon
clinical characteristics of patients with type 2 diabetes. BMJ Open
Diabetes Res Care. 5:e0004202017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amankwaah AF, Sayer RD, Wright AJ, Chen N,
McCrory MA and Campbell WW: Effects of higher dietary protein and
fiber intakes at breakfast on postprandial glucose, insulin, and
24-h interstitial glucose in overweight adults. Nutrients.
9:E3522017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jakubowicz D, Froy O, Ahrén B, Boaz M,
Landau Z, Bar-Dayan Y, Ganz T, Barnea M and Wainstein J: Incretin,
insulinotropic and glucose-lowering effects of whey protein
pre-load in type 2 diabetes: A randomised clinical trial.
Diabetologia. 57:1807–1811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu T, Little TJ, Bound MJ, Borg M, Zhang
X, Deacon CF, Horowitz M, Jones KL and Rayner CK: A protein preload
enhances the glucose-lowering efficacy of vildagliptin in type 2
diabetes. Diabetes Care. 39:511–517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu K, Ke MY, Li WH, Zhang SQ and Fang XC:
The impact of soluble dietary fibre on gastric emptying,
postprandial blood glucose and insulin in patients with type 2
diabetes. Asia Pac J Clin Nutr. 23:210–218. 2014.PubMed/NCBI
|
|
14
|
Kanamoto I, Inoue Y, Moriuchi T, Yamada Y,
Imura H and Sato S: Effect of differences in low glycemic index
food intake sequence on plasma glucose profile. J Jpn Diabetes Soc.
53:96–101. 2010.
|
|
15
|
Kasuya N, Ohta S, Takanami Y, Kawai Y,
Inoue Y, Murata I and Kanamoto I: Effect of low glycemic index food
and postprandial exercise on blood glucose level, oxidative stress
and antioxidant capacity. Exp Ther Med. 9:1201–1204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kasuya N, Okuyama M, Yoshida K, Sunabori
S, Suganuma H, Murata I, Inoue Y and Kanamoto I: Prior or
concomitant drinking of vegetable juice with a meal attenuates
postprandial blood glucose elevation in healthy young adults. Food
Nutr Sci. 7:797–806. 2016.
|
|
17
|
Nakajima H: Effect of indigestible dextrin
on changes in postprandial blood glucose. J Osaka Aoyama
University. 1:1–8. 2008.(In Japanese).
|
|
18
|
Kondo A, Kurihara S, Sato H and Ishitani
K: Effects on a soft drink containing indigestible dextrin on
postprandial blood glucose levels in healthy volunteer. J
Integrated Study Diet Habits. 14:221–225. 2004. View Article : Google Scholar
|
|
19
|
Beppu H and Watanabe H: Influence of sugar
loading on blood glucose suppression by indigestible dextrin.
Seikatsu Eisei. 55:3–14. 2011.(In Japanese).
|
|
20
|
Wakabayashi S, Kishimoto S, Nanbu S and
Matsuoka A: Effects of Indigestible dextrin on postprandial rise in
blood glucose levels in man. J Japanese Assoc Diet Fiber Res.
3:13–19. 1999.(In Japanese).
|
|
21
|
Pereira DF, Cazarolli LH, Lavado C,
Mengatto V, Figueiredo MS, Guedes A, Pizzolatti MG and Silva FR:
Effects of flavonoids on α-glucosidase activity: Potential targets
for glucose homeostasis. Nutrition. 27:1161–1167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tadera K, Minami Y, Takamatsu K and
Matsuoka T: Inhibition of alpha-glucosidase and alpha-amylase by
flavonoids. J Nutr Sci Vitaminol (Tokyo). 52:149–153. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Williamson G: Possible effects of dietary
polyphenols on sugar absorption and digestion. Mol Nutr Food Res.
57:48–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Welsch CA, Lachance PA and Wasserman BP:
Dietary phenolic compounds: Inhibition of Na+-dependent D-glucose
uptake in rat intestinal brush border membrane vesicles. J Nutr.
119:1698–1704. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li JM, Che CT, Lau CBS, Leung PS and Cheng
CHK: Inhibition of intestinal and renal Na+-glucose cotransporter
by naringenin. Int J Biochem Cell Biol. 38:985–995. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bahadoran Z, Mirmiran P and Azizi F:
Dietary polyphenols as potential nutraceuticals in management of
diabetes: A review. J Diabetes Metab Disord. 12:432013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sasaki D, Sasaki K, Ikuta N, Yasuda T,
Fukuda I, Kondo A and Osawa R: Low amounts of dietary fibre
increase in vitro production of short-chain fatty acids without
changing human colonic microbiota structure. Sci Rep. 8:4352018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tolhurst G, Heffron H, Lam YS, Parker HE,
Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F and
Gribble FM: Short-chain fatty acids stimulate glucagon-like
peptide-1 secretion via the G-protein-coupled receptor FFAR2.
Diabetes. 61:364–371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimura I, Ozawa K, Inoue D, Imamura T,
Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, et
al: The gut microbiota suppresses insulin-mediated fat accumulation
via the short-chain fatty acid receptor GPR43. Nat Commun.
4:18292013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yau AM, McLaughlin J, Gilmore W, Maughan
RJ and Evans GH: The acute effects of simple sugar ingestion on
appetite, gut-derived hormone response, and metabolic markers in
men. Nutrients. 9:E1352017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moore MC, Cherrington AD, Mann SL and
Davis SN: Acute fructose administration decreases the glycemic
response to an oral glucose tolerance test in normal adults. J Clin
Endocrinol Metab. 85:4515–4519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shiota M, Galassetti P, Monohan M, Neal DW
and Cherrington AD: Small amounts of fructose markedly augment net
hepatic glucose uptake in the conscious dog. Diabetes. 47:867–873.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shiota M, Moore MC, Galassetti P, Monohan
M, Neal DW, Shulman GI and Cherrington AD: Inclusion of low amounts
of fructose with an intraduodenal glucose load markedly reduces
postprandial hyperglycemia and hyperinsulinemia in the conscious
dog. Diabetes. 51:469–478. 2002. View Article : Google Scholar : PubMed/NCBI
|