Introduction
At present, liver transplantation is one of the most
effective treatments for pediatric end-stage liver diseases. With
developments in surgical procedures and immune suppressive
medication, the short-term and long-term survival rates of
transplant recipients and grafts have markedly increased (1). However, infectious complications
following pediatric liver transplantation have been noted as
important causes of patient mortality, as they are major factors
that affect survival (2). Therefore,
early diagnosis and the treatment of infectious complications may
markedly improve the survival of pediatric patients following liver
transplantation.
Few studies (3,4) have
monitored the immune status of pediatric patients after liver
transplantation. The majority of investigations into patients'
immune status after living-donor liver transplantation (LDLT) are
conducted in adult patients. Patients receiving a liver transplant
are administered immunosuppressants for life following the
procedure; thus, it is critical to evaluate the post-transplant
immune status of patients to aid clinical management. Monitoring
the blood concentration of immunosuppressants is a method used to
indirectly determine the immunological status of patients at
post-transplantation stage, but this does not reflect their actual
immune status. The Immuknow cell function assay may be used to
measure CD4+ T lymphocyte ATP levels and directly
monitor the immune status of patients. A number of studies revealed
that bacterial and viral infections may suppress the function of
CD4+ T lymphocytes in adult patients following liver
transplantation (5,6). However, the clinical association
between CD4+ T lymphocyte ATP levels and infection in
pediatric LDLT remains unknown. The present study aimed to evaluate
the clinical relevance between the value of CD4+ T
lymphocyte ATP levels and post-transplant infection for pediatric
patients who have undergone LDLT, based on the retrospective
analysis of data from pediatric post-transplant patients with or
without episodes of infection.
Materials and methods
Patients and samples
A retrospective analysis of 66 pediatric LDLT
patients from the Tianjin First Central Hospital, enrolled between
June and December 2017, was conducted. All patients who received
liver transplantation were aged 0–3 years, and were administered
post-transplant immunosuppressive agents, including tacrolimus,
mycophenolate mofetil and methylprenol. The exclusion criteria
included treatment with tacrolimus for <3 months or
administration of other immunosuppressive agents during the course
of treatment, secondary liver transplantation or combined organ
transplantation, and follow-up of <3 months (Table I).
 | Table I.Characteristics of pediatric
living-donor liver transplantation recipients (n=66). |
Table I.
Characteristics of pediatric
living-donor liver transplantation recipients (n=66).
| Characteristics | Data at
transplantation |
|---|
| Age [mean ± SD
(range)] | 9.3±11.7 (5–36) |
| 0–6
months, n (%) | 28 (42.4) |
| 7–12
months, n (%) | 24 (36.4) |
| 1–2
years, n (%) | 9 (13.6) |
| 2–3
years, n (%) | 5 (7.6) |
| Sex, n |
|
|
Male/female | 34/32 |
| Blood type
combination, n |
|
|
Identical/compatible/incompatible | 41/15/10 |
| Follow-up period,
years [mean ± SD (range)] | 1.6±0.9
(0.8–3.4) |
| Primary diagnosis of
recipient |
|
|
Congenital biliary atresia and
biliary cholestatic cirrhosis | 63 |
| Alagille
syndrome | 1 |
|
Budd-Chiari syndrome | 1 |
|
Methylmalonic acidemia and
liver cirrhosis | 1 |
Based on whether the patients were diagnosed with
infection post-liver transplantation, the patients were divided
into the infection group (28 cases; 19 male and 9 female) and the
non-infection group (38 cases; 13 male and 25 female). The original
diagnosis was for congenital biliary atresia. The criteria of
diagnosis for infection were systemic inflammatory response
syndrome-positive suspect lesions or infectious agent-positive
(pathogenic microorganisms, imaging examination or biopsy), as well
as effective anti-infection treatments (7).
Analysis of peripheral blood
CD4+ T lymphocyte ATP levels
Assays were conducted according to the
manufacturer's protocol (Cylex, Inc.). Briefly, 2–5 ml whole blood
was collected from fasting patients into heparin sodium
anticoagulant tubes, followed by addition of 25 µl
phytohemagglutinin solution (Cylex, Inc.) and incubation for 15–18
h at 37°C in a 5% CO2 incubator. After incubation, 50 µl
anti-CD4 monoclonal antibody coupling magnetic beads (Cylex, Inc.)
were added to isolate CD4+ T lymphocytes. After washing
(200 µl/time, three times; Cylex, Inc.), the cells were lysed
(Cylex, Inc.) and the released intracellular ATP was measured by
luminometry using the luciferin/luciferase mixture (Cylex, Inc.).
The concentration of ATP was calculated from a calibration curve
generated with calibrators (0, 1, 10, 100 and 1,000 ng/ml).
Blood tacrolimus monitoring
The whole blood samples of 200 µl were fully mixed
with 200 µl whole blood precipitators and centrifuged (9,500 × g; 4
min; room temperature). The concentration of tacrolimus in blood
was determined via a fluorescence polarization immunoassay (Abbott,
Inc), and the concentration/dose (C0/D) was calculated
based on the administered dose.
Clinical data collection
A total of 462 peripheral blood samples were
collected from patients with LDLT pre-transplant, as
aforementioned, and at 1–4 weeks and 2 and 3 months
post-transplant. The ATP values of CD4+ T cells and
tacrolimus concentration were determined for each specimen. The
serum C0/D ratio was calculated. The lymphocyte counts
were detected using a Sysmex hematology analyzer (XS-800i; Sysmex,
Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
(version 20.0, IBM Corp.) and GraphPad Prism (version 5.0; GraphPad
Software, Inc.) software. The normality test and homogeneity test
of variance were applied to the continuous variable data, and a
Student's t-test was used to analyze data with normal distribution.
The results were expressed as the mean ± standard deviation. For
data that did not have normal distribution, a Mann-Whitney test was
conducted and data were presented as median values (interquartile
range). Pearson's correlation analysis was used to determine the
association between tacrolimus blood concentration and ATP levels.
Receiver operating characteristic (ROC) curves were generated to
analyze decreases in ATP levels to predict the sensitivity and
specificity of infection. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of cellular immunity
responses between the infection and non-infection groups
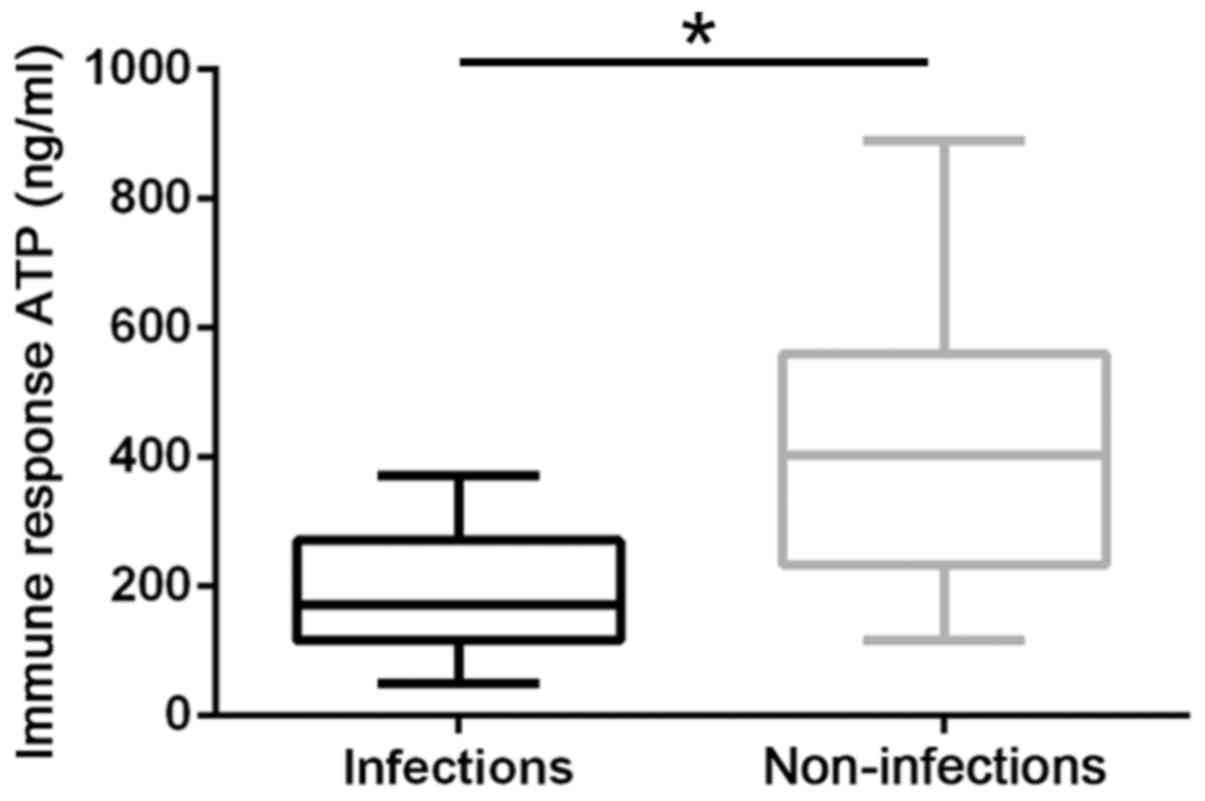
The pre-transplant CD4+ T lymphocyte ATP
levels of the 66 children who underwent liver transplantation were
302.5±195.7 ng/ml. The post-transplant CD4+ T lymphocyte
ATP levels were 188.6±93.5 and 424.4±198.1 ng/ml for the infection
and non-infection group, respectively. The ATP levels of the
infection group were significantly lower compared with those of the
non-infection group (P<0.05; Fig.
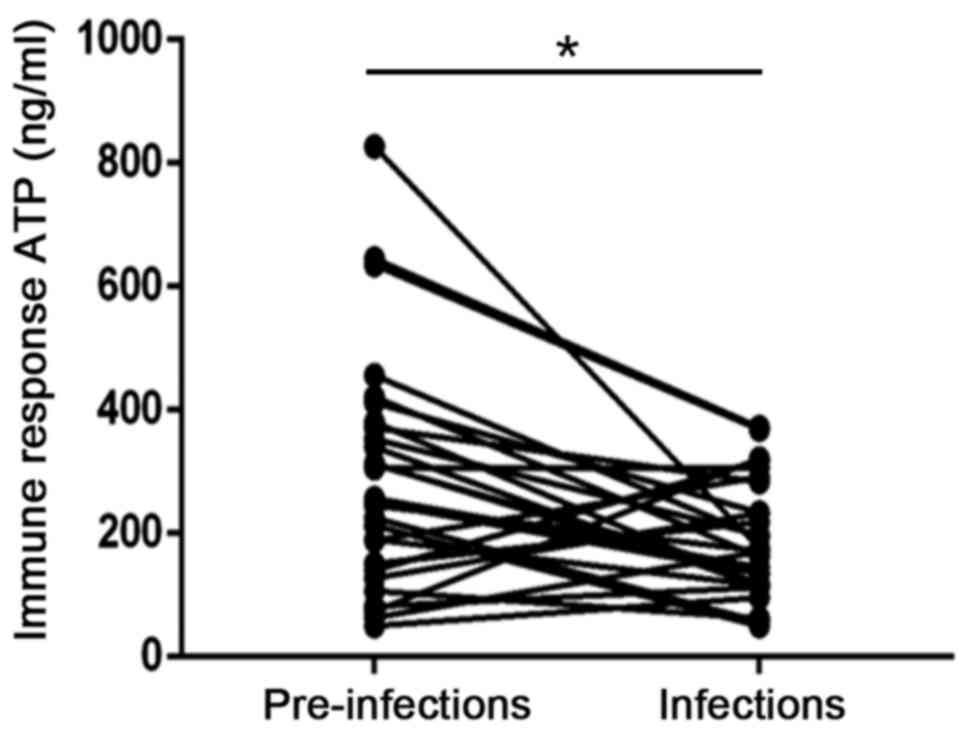
1). In addition, the ATP levels of the infection group
significantly decreased after infection. (P<0.05; Fig. 2).
Analysis of the types of
infection
A total of 28 LDLT patients displayed different
degrees and sites of infection that occurred between 2 weeks and 6
months post-transplantation. Among these patients, 14 cases of
pulmonary infection (50%), 4 cases of intra-abdominal infection
(14.3%), 6 cases of bloodstream infection (21.4%) and other
infections (14.3%) were reported. Bacteriological analysis revealed
6 cases of Klebsiella pneumoniae, 6 cases of Enterococcus
faecium, 4 cases of Acinetobacter baumannii and 3 cases
of Staphylococcus epidermidis. Fungal infections included 1
case of Stenotrophomonas maltophilia, 1 case of Candida
parapsilosis, 1 case of Candida tropicalis and 1 case of
Candida albicans. Viral infections included 4 cases of
cytomegalovirus and 1 case of Epstein-Barr virus (Table II).
 | Table II.Specific types of bacterial, fungal
and viral infections following pediatric liver transplantation. |
Table II.
Specific types of bacterial, fungal
and viral infections following pediatric liver transplantation.
| Type of
infection | Number of
examinations |
|---|
| Bacterial | 19 |
|
Klebsiella
pneumoniae | 6 |
|
Enterococcus
faecium | 6 |
|
Acinetobacter
baumannii | 4 |
|
Staphylococcus
epidermidis | 3 |
| Fungal | 4 |
|
Stenotrophomonas
maltophilia | 1 |
|
Candida
parapsilosis | 1 |
|
Candida tropicalis | 1 |
|
Candida albicans | 1 |
| Viral | 5 |
|
Cytomegalovirus | 4 |
|
Epstein-Barr virus | 1 |
Immuknow analysis of ATP levels and
immunosuppressant serum trough C0/D ratio correlation
analysis
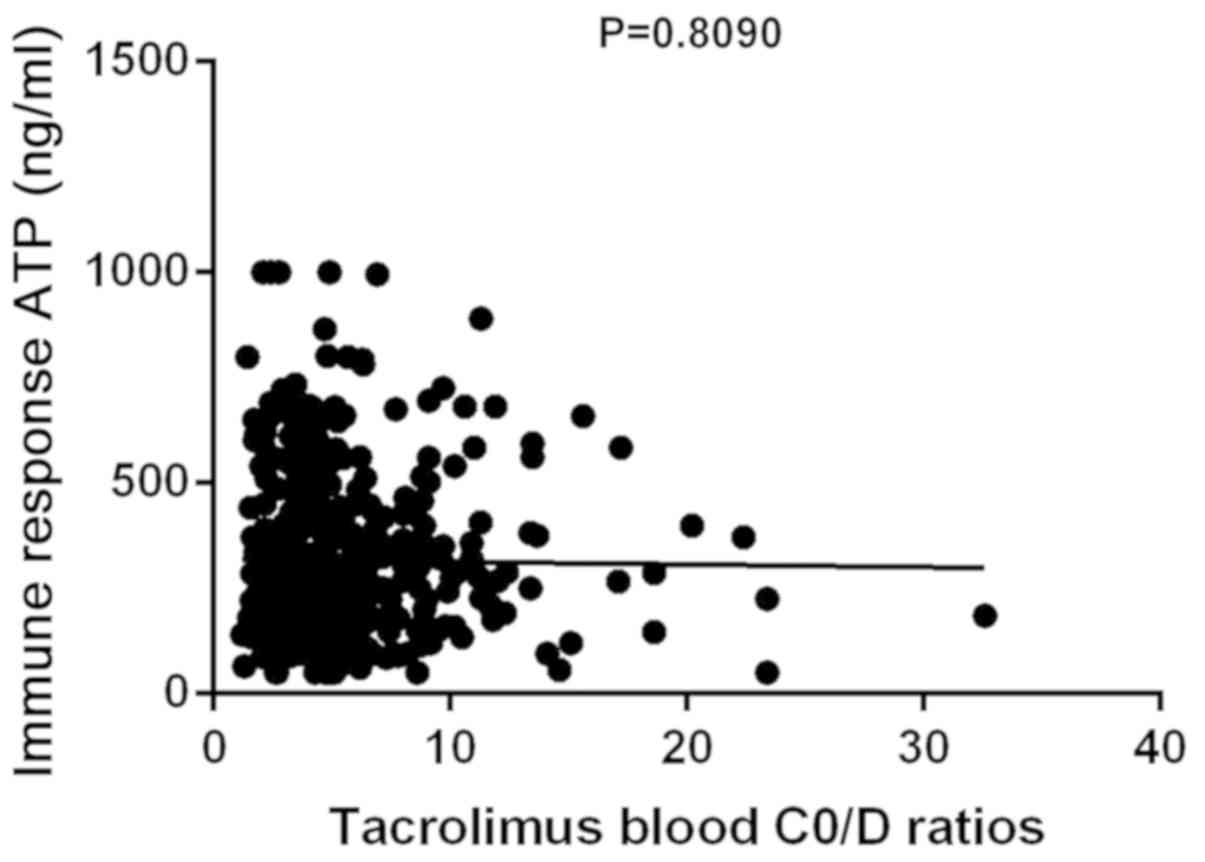
A total of 396 peripheral blood samples were
collected from 66 patients at weeks 1–4, and at 2 and 3 months
following liver transplantation. The trough tacrolimus (FK506)
C0/D ratio and ATP levels were determined at the same
time. The mean FK506 C0/D ratio was 5.6±3.8 ng/ml and
the mean ATP value was 313.9±195.1 ng/ml. No correlation was
observed between the trough tacrolimus C0/D ratio and
ATP levels (P>0.05, R2=0.0001484; Fig. 3).
Correlation between CD4+ T
cell ATP levels and lymphocyte count
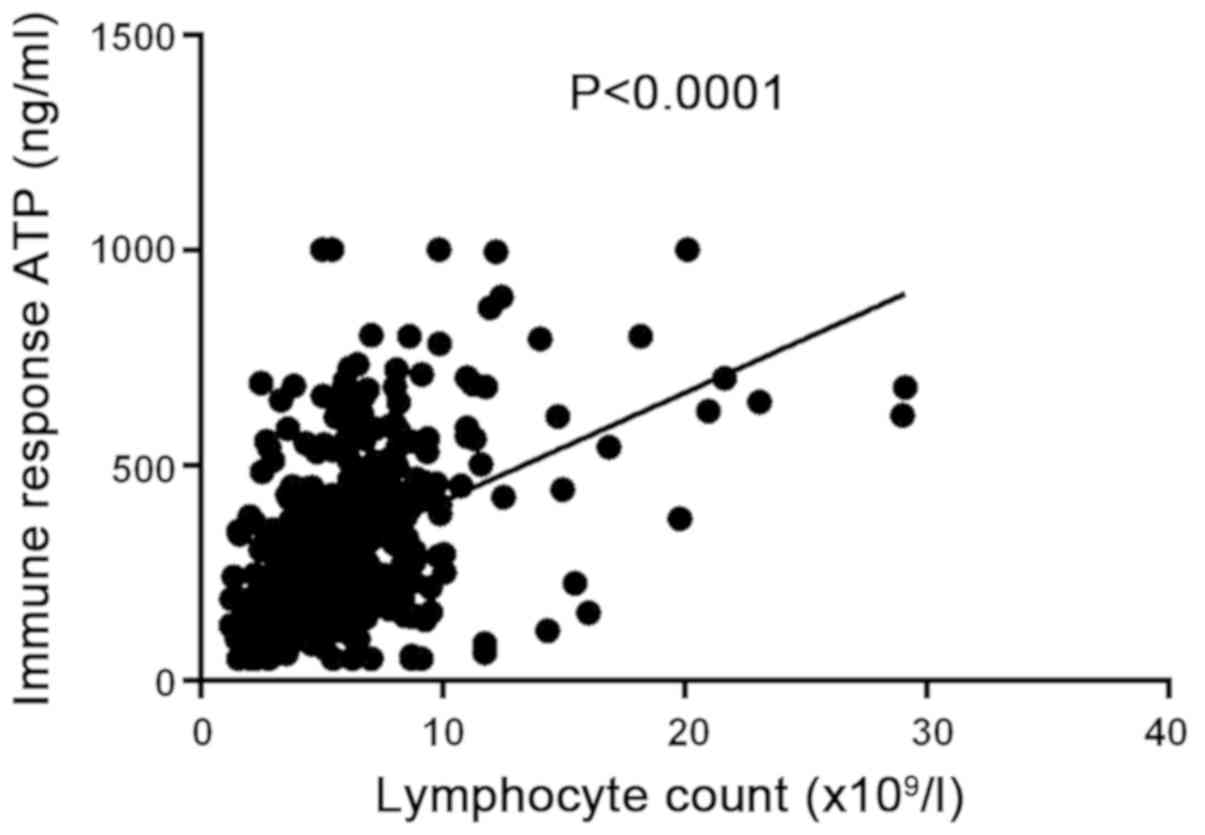
The ATP levels of the 66 children with LDLT were
measured using 396 samples obtained at weeks 1–4, and at 2 and 3
months post-transplantation. The mean ATP levels was 313.9±195.1
ng/ml and the mean lymphocyte count was 6.05±3.6×109/l.
Correlation analysis revealed a positive correlation between the
CD4+ T cell ATP levels and the total lymphocyte count
from each specimen (P<0.05, R2=0.2149; Fig. 4).
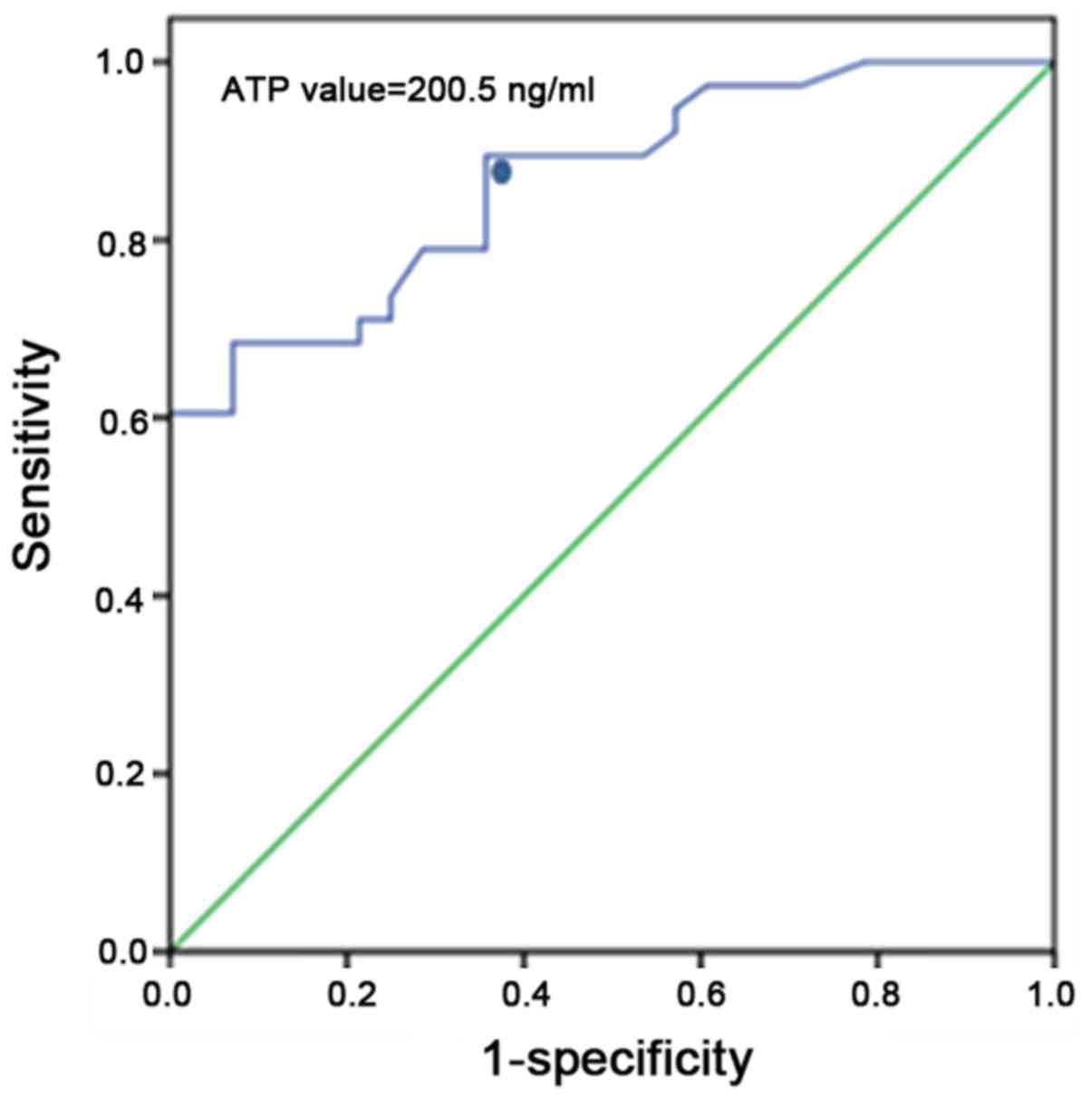
ROC curve analysis for the sensitivity
and specificity of infections
A ROC curve was generated to determine a reference
ATP level for the diagnosis of infection. The results revealed
that, when ATP levels were set at 200.5 ng/ml for the ROC curve
analysis of patients diagnosed with infection, the sensitivity and
specificity were 89.5 and 64.3%, respectively; the area under the
ROC curve was 0.867 (Fig. 5).
Discussion
In the present study, immune cell function following
transplantation was evaluated by determining the function of the
transplanted organ, using detection of the lymphocyte subsets, and
the blood concentration of immunosuppressants; however, the
specificity and sensitivity of these two methods for the
post-transplantation evaluation of cellular immune function are low
(8). As a technique to evaluate
cellular immune function, the clinical value of the Immuknow assay
lies with permitting assessment of the risk of infection or
rejection in transplant recipients.
The ATP levels of LDLT patients in the infection
group were found to be significantly lower compared with those in
the non-infection group; there was a statistically significant
difference in the ATP levels pre- and post-transplant in the
infection group. In the present study, low values on the T cell
immune function assay were associated with the susceptibility to
infection; however, controversial findings have been reported
(9,10). In the present study, the ATP levels
in the infection group were significantly lower compared with the
non-infection group. Kobashigawa et al (11) and Mandras et al (12) analyzed the post-transplant Immuknow
assay-derived data of heart transplant recipients, and revealed
that the mean ATP levels of patients at the time of infection were
significantly lower compared with those in patients without
infection. Naderi et al (13)
conducted an Immuknow analysis using samples from 113 kidney
transplant patients and reported that the intracellular ATP
concentrations were significantly lower in patients who suffered
from infection vs. the renal transplant recipients with stable
graft function. Of note, the iATP levels were increased in those
that had experienced an episode of allograft rejection.
Additionally, the Immuknow assay has been proposed to predict the
risk of post-transplant infection more effectively than predicting
rejection (14).
In the present study, a variety of bacterial, fungal
and viral infections were observed in the infection group (Table I). Regarding the diagnosis of
bacterial infections, positive etiological identification may aid
analysis; however, the rate of positive bacterial culture is low,
time consuming, and specimens are not easily obtained, whereas
other factors notably inhibit correct and timely diagnosis.
Chiereghin et al (15)
analyzed 98 symptomatic infections in 202 transplant patients,
retrospectively within 1 year post-operation. The results revealed
77 (57.1%) bacterial, 45 (33.3%) viral and 13 (9.6%) fungal
infections, with the bacterial infections mainly comprising
Escherichia coli (21 strains) and Klebsiella
pneumoniae (19 strains). In addition, bacteria were determined
to be the cause of most symptomatic infections and occur more
frequently in the first month after transplantation (15). Furthermore, the ATP levels of
CD4+ T lymphocytes in patients with bacterial and fungal
infections were significantly lower compared with those in
uninfected patients, whereas the intracellular ATP levels in
patients with viral infections did not differ significantly from
those of uninfected patients. Furthermore, several studies have
determined that alterations in the abundance of CD4+ T
lymphocytes in the peripheral blood are associated with the outcome
of severe lung infections following early renal transplantation.
The incidence of cytomegalovirus pneumonia in CD4+ T
lymphocyte-depleted patients was significantly increased compared
with those possessing stable CD4+ T lymphocyte counts
(16,17).
Considering the immature state of children's immune
system and the administration of immunosuppressants following liver
transplantation, pediatric patients who have undergone this
procedure are at high risk of contracting a variety of infections.
The incidence of pulmonary infections is particularly high among
such patients, and is an important factor affecting the rates of
patient and graft survival (18). It
was observed that patients with infection had reduced lymphocyte
counts; a positive correlation between CD4+ T lymphocyte
ATP levels and lymphocyte counts was also reported. Thus, the
Immuknow assay combined with the monitoring of lymphocyte count may
have the potential to determine the risk for contracting
opportunistic infections, and may reflect the patient immune
status, providing a good basis for developing individualized
treatment regimens.
It is widely known that administering appropriate
doses of immunosuppressive agents is crucial for improving
allograft outcome; however, the blood concentration of drugs is not
directly correlated with the dose of drug administered due to
individual pharmacokinetic differences and variations in the
methods used for their detection (19). On the contrary, under conditions of
hyper- and hypo-immune suppression, detrimental effects on the
graft may occur, and increase the risk of infection and graft
rejection (20). The present study,
along with other reports, support the hypothesis that monitoring
the ATP concentrations of CD4+ T cells may aid in
distinguishing between hyper- and hypo immunity for the
identification of LDLT patients at risk of infection or graft
rejection, and may be applied to increase the efficacy of
immunosuppressive therapies (21).
The present study also demonstrated that Immuknow
assay-derived data were not correlated with the serum
immunosuppressive drug C0/D ratio of the patients, but
were positively correlated with the lymphocyte count, indicating
that modifications in administering immunosuppressive agents should
be considered with respect to patient immune status. A prospective
study conducted by Ravaioli et al (22) adjusted for the clinical benefits of
immunosuppressive therapy in patients who had undergone liver
transplantation based on Immuknow assays. In that study, the dose
of tacrolimus was reduced by 25% when the ATP value was <130
ng/ml (weak immune cell response), but was increased by 25% when
the ATP value was >450 ng/ml (strong immune cell response).
CD4+ T lymphocytes play important roles
in initiating immune responses, and the activity of these cells may
reflect the status of the body's immune function (23,24). As
the majority of immune cell functions have been directly and
indirectly associated with intracellular ATP activity, measuring
the ATP levels of CD4+ T lymphocytes may be used to
assess body immune function.
In conclusion, the Immuknow assay may be employed to
evaluate the functional status of immune cells in pediatric
patients following LDLT to predict the risk of infection. The
application of this assay may permit individualized adjustments in
the administration of immunosuppressive agents for patients to
achieve an immune status that may reduce the risk of opportunistic
infections and promote graft survival.
Acknowledgements
Not applicable.
Funding
The present study was supported by Tianjin Natural
Science Foundation (grant no. 17JCYBJC27500).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
DHL designed the experiments. WL performed the
experiments, analyzed the data and wrote the first draft of the
manuscript. KW collected the data and revised the draft. YHZ and
GPS performed the measurement of CD4+ T lymphocyte ATP
levels and other tests. WG interpreted the patient data regarding
pediatric liver transplantation and diagnosed the clinical cases.
All authors discussed the results and reviewed the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants or their parent/guardian in the case of children under
18 years of age, or patients otherwise considered minors under
local legislation. The present study was approved by the Medical
Ethics Committee of Tianjin First Central Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rawal N and Yazigi N: Pediatric liver
transplantation. Pediatr Clin North Am. 64:677–684. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu H and Gao W: Risk factors of bacterial
nosocomial infection after pediatric liver transplantation.
Zhonghua Er Ke Za Zhi. 55:593–596. 2017.(In Chinese). PubMed/NCBI
|
|
3
|
Xue F, Zhang J, Han L, Li Q, Xu N, Zhou T,
Xi Z, Wu Y and Xia Q: Immune cell functional assay in monitoring of
adult liver transplantation recipients with infection.
Transplantation. 89:620–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Te HS, Dasgupta KA, Cao D, Satoskar R,
Mohanty SR, Reau N, Millis JM and Jensen DM: Use of immune function
test in monitoring immunosuppression in liver transplant
recipients. Clin Transplant. 26:826–832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shapiro R: End-stage renal disease in
2010: Innovative approaches to improve outcomes in transplantation.
Nat Rev Nephrol. 7:68–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brick C, Atouf O, Benseffaj N and
Essakalli M: Rejection of kidney graft: Mechanism and prevention.
Nephrol Ther. 7:18–26. 2011.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
People's Republic of China Ministry of
Health, . Hospital infection diagnostic criteria (trial). Natl Med
J China. 81:314–320. 2001.
|
|
8
|
Kidney Disease: Improving Global Outcomes
(KDIGO) Transplant Work Group: KDIGO clinical practice guideline
for the care of kidney transplant recipients. Am J Transplant. 9
(Suppl 3):S1–S155. 2009. View Article : Google Scholar
|
|
9
|
Ben-Youssef R, Baron PW, Sahney S,
Weissman J, Baqai W, Franco E, Kore A, Trimzi M and Ojogho O: The
impact of intercurrent EBV infection on ATP levels in
CD4+ T cells of pediatric kidney transplant recipients.
Pediatr Transplant. 13:851–855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bennett WM, Meyer L, Ridenour J and Batiuk
TD: Surveillance and modification of immunosuppression minimizes BK
virus nephropathy. Am J Nephrol. 32:10–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobashigawa JA, Kiyosaki KK, Patel JK,
Kittleson MM, Kubak BM, Davis SN, Kawano MA and Ardehali AA:
Benefit of immune monitoring in heart transplant patients using ATP
production in activated lymphocytes. J Heart Lung Transplant.
29:504–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mandras SA, Crespo J and Patel HM:
Innovative application of immunologic principles in heart
transplantation. Ochsner J. 10:231–235. 2010.PubMed/NCBI
|
|
13
|
Naderi H, Pourmand G, Dehghani S,
Nikoueinejad H, Jafari M and Tajik N: Monitoring cellular immune
function of renal transplant recipients based on adenosine
triphosphate (ATP) production by mitogen- induced CD4+ T
helper cells. Biomed Pharmacother. 107:1402–1409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Millán O, Sánchez-Fueyo A, Rimola A,
Guillen D, Hidalgo S, Benitez C, Campistol JM and Brunet M: Is the
intracellular ATP concentration of CD4+ T-Cells a
predictive biomarker of immune status in stable transplant
recipients? Transplantation. 88 (Suppl 3):S78–S84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiereghin A, Petrisli E, Ravaioli M,
Morelli MC, Turello G, Squarzoni D, Piccirilli G, Ambretti S,
Gabrielli L, Pinna AD, et al: Infectious agents after liver
transplant: Etiology, timeline and patients' cell-mediated immunity
responses. Med Microbiol Immunol. 206:63–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang B, Liu D, Wu JQ, Zhou JX, Li C and
Meng SD: Clinical significance of monitoring CD4+ T
lymphocytes in patients with cytomegalovirus pneumonia after renal
transplantation. J South Med Univ. 29:1176–1178. 2009.
|
|
17
|
Xiong HY, Zhang L, Wang LM, Kang YD, Zhou
MS, Zhou L, Zhang ZZ, Han S, Fu SX, Yuan Q, et al: Clinical
significance of peripheral blood CD4+T-lymphocyte count
in patients with severe pulmonary infection after renal
transplantation. Chin J Organ Transplant. 6:334–337. 2009.
|
|
18
|
Oh SH, Kim KM, Kim DY, Lee YJ, Rhee KW,
Jang JY, Chang SH, Lee SY, Kim JS, Choi BH, et al: Long-term
outcomes of pediatric living donor liver transplantation at a
single institution. Pediatr Transplant. 14:870–878. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Venkataramanan R, Shaw LM, Sarkozi L,
Mullins R, Pirsch J, MacFarlane G, Scheller D, Ersfele D, Frick M,
Fitzsimmons WE, et al: Clinical utility of monitoring tacrolimus
blood concentrations in liver transplant patients. J Clin
Pharmacol. 41:542–551. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naderi H, Naiafi A, Khoshroo M and Tajik
N: Development of an immune function assay by measuring
intracellular adenosine triphosphate (iATP) levels in
mitogen-stimulated CD4+ T lymphocytes. J Immunoassay
Immunochem. 37:407–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Serban G, Whittaker V, Fan J, Liu Z, Manga
K, Khan M, Kontogianni K, Padmanabhan A, Cohen D, Suciu-Foca N, et
al: Significance of immune cell function monitoring in renal
transplantation after Thymoglobulin induction therapy. Hum Immunol.
70:882–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ravaioli M, Neri F, Lazzarotto T, Bertuzzo
VR, Di Gioia P, Stacchini G, Morelli MC, Ercolani G, Cescon M,
Chiereghin A, et al: Immunosuppression modifications based on an
immune response assay: Results of a randomized controlled trial.
Transplantation. 99:1625–1632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kobayashi S, Soyama A, Takatsuki M, Hidaka
M, Adachi T, Kitasato A, Kinoshita A, Hara T, Kanetaka K, Fujita F,
et al: Relationship between immune function recovery and infectious
complications in patients following living donor liver
transplantation. Hepatol Res. 46:908–915. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo Y, Ji WB, Duan WD, Shi XJ and Zhao ZM:
Delayed introduction of immunosuppressive regimens in critically
ill patients after liver transplantation. Hepatobiliary Pancreat
Dis Int. 16:487–492. 2017. View Article : Google Scholar : PubMed/NCBI
|