Introduction
Bladder cancer (BCa) is the most common malignancy
of the urinary tract (1). In 2017,
there were ~79,030 new cases (4.6% of all cancer cases) of BCa in
the USA, with a mortality rate of 16,870 (2.8% of all cancer cases)
(2). Approximately 20–30% of all
newly diagnosed cases of BCa are muscle invasive bladder cancer
(MIBCa). Despite extensive cystectomy and adjuvant therapies,
including chemotherapy and radiotherapy, the 5-year overall
survival (OS) rate for patients with MIBCa is only 47% (3). Cisplatin-containing combination
chemotherapy with gemcitabine and cisplatin or methotrexate,
vinblastine, adriamycin and cisplatin is the standard treatment
used for patients with advanced or metastatic BCa. However, the OS
rate of patients with advanced MIBCa remains poor (4). Therefore, the identification of novel
biomarkers associated with diagnosis and disease progression is
required in order to select high-risk patients who may benefit from
combination-treatment strategies.
Thymosin β10 (TMSB10) is an important member of the
β-thymosin family, which was first isolated in 1966 by Goldstein
et al (5), in addition to
other lymphocytopoietic factors. TMSB10 functions as an
actin-sequestering protein involved in cell motility (6). In recent years, TMSB10 has been shown
to be associated with the occurrence and development of malignant
tumors. An accumulation of evidence suggests that the expression of
TMSB10 is significantly upregulated in six types of cancer,
including human melanoma, gastric, breast and lung cancer,
hepatocellular carcinoma and thyroid cancer (7–13), and
is involved in a number of cellular processes, including cell
proliferation, anti-apoptosis, angiogenesis and metastasis
(14,15). However, the expression and role of
TMSB10 in BCa have not been examined previously.
The present study reported for the first time, to
the best of our knowledge, that TMSB10 is frequently overexpressed
in BCa and that it exhibits clinical significance in BCa, as a high
expression of TMSB10 was associated with significantly poorer OS.
Knockdown of the expression of TMSB10 significantly suppressed BCa
cell migration and invasion. Therefore, assessing the tumor
expression of TMSB10 may improve disease prognosis and assist in
the identification of patients who may benefit from combination
treatment.
Materials and methods
Aim, design and setting of the
study
The present study aimed to assess the expression of
TMSB10 in BCa cell lines and human tissues and to examine the
association between TMSB10 and the clinicopathological features and
outcomes of patients with BCa. The present study performed in
vitro analysis and retrospective analysis of clinical specimens
obtained from 101 patients treated at a single institution in
China.
Tissue specimens and patient
information
Paraffin-embedded BCa tissue samples from a total of
101 cases were clinically and histologically diagnosed at Guangdong
Second Provincial General Hospital (Guangzhou, China) between 2002
and 2014. The institutional Ethics Committee approved the study and
written informed consent was obtained from each patient.
The age distribution of the patients was between 37
and 82 years, and the mean age was 60.4 years. According to the
2009 World Health Organization (WHO) 7th edition of the TNM
classification system (16), 48
patients had primary non-MIBCa and 53 had MIBCa. The pathological
grades were classified according to the WHO classification criteria
for urothelial cancer. The histological diagnosis was evaluated
according to the WHO 2004 guidelines. Grades I, II and III indicate
well, moderately, and poorly differentiated tumors, respectively.
Grades I and II were classified as low-grade urothelial carcinomas,
whereas grade III was classified as high-grade tumors. In the
present study, the 101 cases of BCa were divided into 33 cases of
high-grade urothelial carcinoma and 68 cases of low-grade
urothelial carcinoma. All clinical and pathological features of the
patients are shown in Table I.
 | Table I.Correlation between TMSB10 and
clinicopathological characteristics of patients with bladder
cancer. |
Table I.
Correlation between TMSB10 and
clinicopathological characteristics of patients with bladder
cancer.
|
|
| Expression of
TMSB10 |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | n | Low, n (%) | High, n (%) | P-value
(χ2 test)a |
|---|
| Age (years) |
|
|
|
|
|
<65 | 52 | 21 (40.4) | 31 (59.6) | 0.191 |
| ≥65 | 49 | 25 (51.0) | 24 (49.0) |
|
| Gender |
|
|
|
|
| Male | 58 | 28 (48.3) | 30 (51.7) | 0.331 |
|
Female | 43 | 18 (41.9) | 25 (58.1) |
|
| Histological
grade |
|
|
|
|
| GI | 68 | 31 (45.6) | 37 (54.4) | 0.580 |
|
GII–III | 33 | 15 (45.5) | 18 (54.5) |
|
| T stage |
|
|
|
|
|
Ta-T1 | 48 | 27 (56.3) | 21 (43.8) | 0.032 |
|
T2-T4 | 53 | 19 (35.8) | 34 (64.2) |
|
| Distant
metastasis |
|
|
|
|
|
Absent | 90 | 41 (45.6) | 49 (54.4) | 0.625 |
|
Present | 11 | 5 (45.5) | 6 (54.5) |
|
| Multiplicity |
|
|
|
|
|
Single | 71 | 33 (46.5) | 38 (53.5) | 0.473 |
|
Multiple | 30 | 13 (43.3) | 17 (56.7) |
|
Microarray data process
The microarray data process and evaluation of
microarray datasets [Gene expression Omnibus (GEO) accession nos.
GSE3167 and GSE13507] from the BCa and control samples were
retrieved from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). Integrative
analyses were subsequently performed using the Cancer Genome Atlas
(TCGA, http://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga)
data for bladder urothelial carcinoma (TCGA, Provisional).
Cell lines and transfection
T24, 5637, UM-UC-3, HCV-29 and EJ cells
(authenticated via short tandem repeat analysis) were purchased
from the Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences and were grown in RPMI 1640 (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Corning, Inc.) in 5% CO2 at 37°C. The normal cells
comprised primary cells, which were extracted from patients devoid
of BCa.
The sequences of oligonucleotides 1 and 2 targeting
TMSB10 mRNA, were as follows: RNA i#1: sense
5′-GAAAUCGCCAGCUUCGAUATT-3′ and antisense
5′-UAUCGAAGCUGGCGAUUUCTT-3′. RNAi#2: sense
5′-CGACCAAAGAGACCAUUGATT-3′ and antisense
5′-UCAAUGGUCUCUUUGGUCGTT-3′; These siRNAs were synthesized by
Shanghai GenePharma Co., Ltd. Approximately 2×105 cells
per well were seeded in a 6-well tissue culture dish on the day
before transfection. Transfection with 50 nmol of the small
interfering (si)RNAs was performed according to the manufacturer's
instructions using Lipofectamineä transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). The transfection was performed 48
h prior to subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA samples from the cell lines and freshly
frozen tissues were isolated using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) following the manufacturer's
recommendations. The extracted RNA was pretreated with RNase-free
DNase and 2.0 µg of total RNA was reverse transcribed into
complementary DNA (cDNA) using iScript™ cDNA synthesis Kit (Life
Science Research; Bio-Rad, Laboratories, Inc.) according to
manufacturer's protocols. To amplify TMSB10 cDNA, the
following real-time RT-qPCR cycling conditions were used: Initial
denaturation step at 95°C for 10 min, followed by 28 cycles of
denaturation at 95°C for 60 sec, primer annealing at 58°C for 30
sec and extension at 72°C for 30 sec. The cycle program was
terminated with a final extension step at 72°C for 5 min, and the
products were stored at 4°C. The primers were designed using Primer
Express software v. 2.0 (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with the following sequences: TMSB10,
forward 5′-TGGCAGACAAACCAGACATGG-3′ and reverse
5′-CGAAGAGGACGGGGGTAGG-3′; and ACTB, forward
5′-CGTGGACATCCGCAAAGA-3′ and reverse 5′-GAAGGTGG-ACAGCGAGGC-3′. The
relative mRNA expression levels were calculated by the
2−ΔΔCq method and normalized to ACTB (17), where Ct represents the quantification
cycle for each transcript. Each experiment was performed in
triplicate.
Western blotting
The samples were prepared for immunoblotting as
previously described. The cells cultured at 70–80% confluence were
washed twice with ice-cold phosphate-buffered saline (PBS) and
lysed on ice in radio-immunoprecipitation assay buffer (Cell
Signaling Technology, Inc.) with complete protease inhibitor
cocktail (Roche Applied Science). The concentration of total
protein was determined using Bicinchoninic Acid Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc). The samples were
subsequently heated for 10 min at 98°C. The freshly-frozen tissue
samples were homogenized in liquid nitrogen and lysed using
SDS-PAGE sample buffer. The protein concentrations were determined
with the Bradford assay reagent (Bio-Rad Laboratories, Inc.). Equal
quantities of protein (30 µg) were separated on a 10.5% SDS
polyacrylamide gel and subjected to electrophoresis. The gels were
transferred onto PVDF membranes (Immobilon P; Millipore Corp.;
Merck KGaA) and the membranes were blocked using 5% fat-free milk
in Tris-buffered saline containing 0.1% Tween-20 for 1 h at room
temperature. The membranes then were probed with anti-TMSB10 (cat.
no. ab14338; 1:1,000 dilution; Abcam) or anti-GAPDH (cat. no.
ab181602; 1:10,000 dilution; Abcam) at 4°C overnight. Membranes
were washed for three times with Tris-buffered saline and
Polysorbate 20 and incubated with horseradish peroxidase-conjugated
goat anti-rabbit IgG (cat. no. 2030230; 1:3,000 dilution;
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. The bands were detected using enhanced
chemiluminescence reagent (Amersham; GE Healthcare Life Sciences)
following the manufacturer's protocols and quantified via
densitometry using Image J software (V1.8.0; National Institutes of
Health).
Transwell invasion assay
The T24 and EJ cells were initially transfected with
TMSB10 siRNA or control siRNA. A total of 5×104
transfected cells were seeded onto a thick layer of Matrigel in
Transwell inserts (BD Biosciences) and cultured for an additional
24 h. The invasive cells that adhered to the lower surface of the
filter were washed with PBS, fixed with 4% paraformaldehyde and
stained with 0.05% crystal violet. The invasive cells were counted
under a light microscope (magnification, ×100; Carl Zeiss,
Inc.).
Wound healing assay
A total of 1×106 transfected cells were
seeded and allowed grow at 70–80% confluence. The cells were
subsequently starved for 24 h. The cell monolayers were wounded
with a sterile plastic tip and cultured in serum-free medium. Cell
migration was monitored every 12 h using light microscopy
(magnification, ×100; Nikon Corporation). The Image J software
(V1.8.0; National Institutes of Health) was used to quantify the
percentage of cell wound closure, in a blinded manner.
Immunohistochemical (IHC)
analysis
The protein expression of TMSB10 was examined in the
101 human BCa tissue specimens via immunohistochemistry. Samples
were fixed in 4% paraformaldehyde buffer at 25°C for 24 h. After
embedding in paraffin sections (4 µm thick) were incubated at 60°C
for 1 h. The sections were deparaffinized with xylene twice for 15
min, rehydrated in a descending alcohol series (100, 95 and 70%
ethanol; each rinse, 5 min), placed in EDTA antigen retrieval
buffer and microwaved. Endogenous peroxidase activity was inhibited
using a solution of 3% hydrogen peroxide in methanol and
non-specific binding was blocked using 1% bovine serum albumin.
Samples were subsequently incubated with anti-TMSB10 rabbit
polyclonal antibodies (cat. no. ab14338; 1:50; Abcam) at 4°C
overnight. Normal goat serum was used as a negative control in
place of primary antibodies. After washing with (PBS) 4 times (each
wash, 4 min), sections were incubated at room temperature for 30
min with biotinylated anti-rabbit secondary antibodies (1:50; cat.
no. ab6720; Abcam) and stained for 0.5–1 min with The EnVision™
Detection System (cat. no. K5007, Dako; Agilent Technologies,
Inc.). Samples were then treated with hematoxylin for 30 sec at
25°C. Following dehydration and soaking in xylene, the stained
slices were sealed and observed under a light microscope
(magnification, ×200 and ×400).
The degree of immunostaining was scored by two
independent observers. The proportion of TMSB10-expressing cells
was scored as 1 (<25% positive tumor cells), 2 (25–50%), 3
(50–75%) or 4 (>75%). Staining intensity was scored as 0 (no
staining), 1 (weak, light yellow), 2 (moderate, yellowish-brown),
and 3 (strong, brown). Staining intensity and the proportion of
positive cells in each section were multiplied (to obtain values of
0, 1, 2, 3, 4, 6, 8, 9 or 12). Tumor samples with scores >6 were
considered to exhibit a high expression and samples with scores ≤6
were determined to have a low TMSB10 expression. The AxioVision
Rel.4.6 computerized image analysis system, with assistance from
the automatic measurement program (Carl Zeiss), was used to analyze
inconsistencies in IHC stain intensities in tumors and normal
tissues.
Statistical analysis
All statistical analyses were conducted using SPSS
(version 19.0) software (IBM Corp.). Data were expressed as the
mean ± standard deviation and compared using a paired t-test
between two groups. One-way ANOVA followed by an LSD post-hoc test
was used for comparisons among groups. The cutoff value for TMSB10
was selected using receiver operating characteristic (ROC) curve
analysis. The associations between the expression of TMSB10 and
clinicopathological features were examined using Pearson's
χ2 test or Fisher's exact test. OS was measured from the
initiation of treatment until patient mortality. The survival
curves were plotted using the Kaplan-Meier method and compared
using the log-rank test. Univariate and multivariate Cox regression
models were used to evaluate prognostic significance. P<0.05 was
considered to indicate a statistically significant difference.
Results
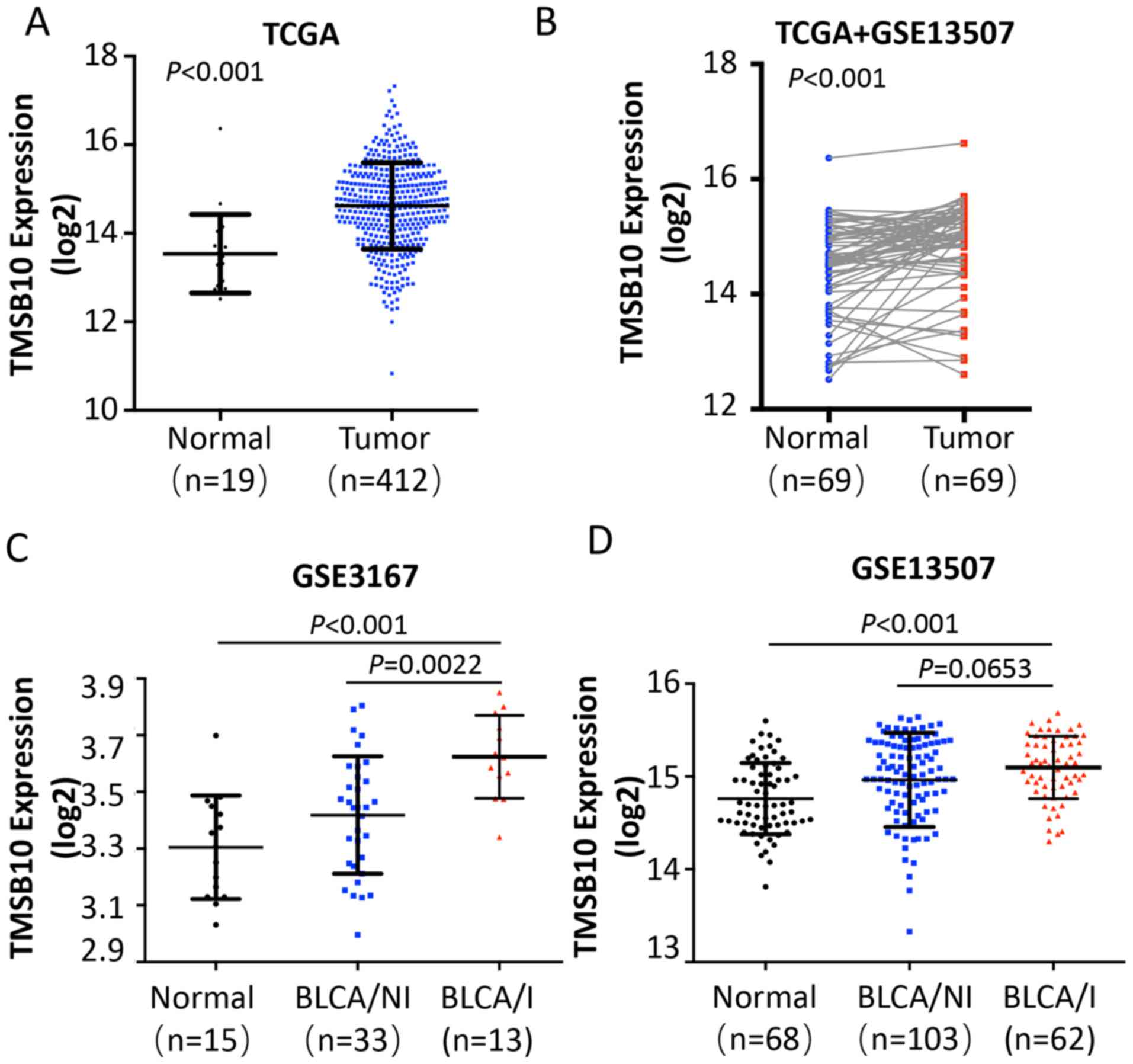
TMSB10 is overexpressed in human BCa
tissues, notably in MIBCa
To determine the clinical significance of TMSB10 in
BCa, publicly available microarray data (TCGA data, and GSE3167 and
GSE13507 data for BCa) were initially analyzed. The analysis
revealed that the expression of TMSB10 was upregulated in BCa
tissues compared with that noted in adjacent normal tissues (ANT)
(Fig. 1A and B). Of note, the
expression of TMSB10 was considerably higher with regard to MIBCa
(Fig. 1C and D).
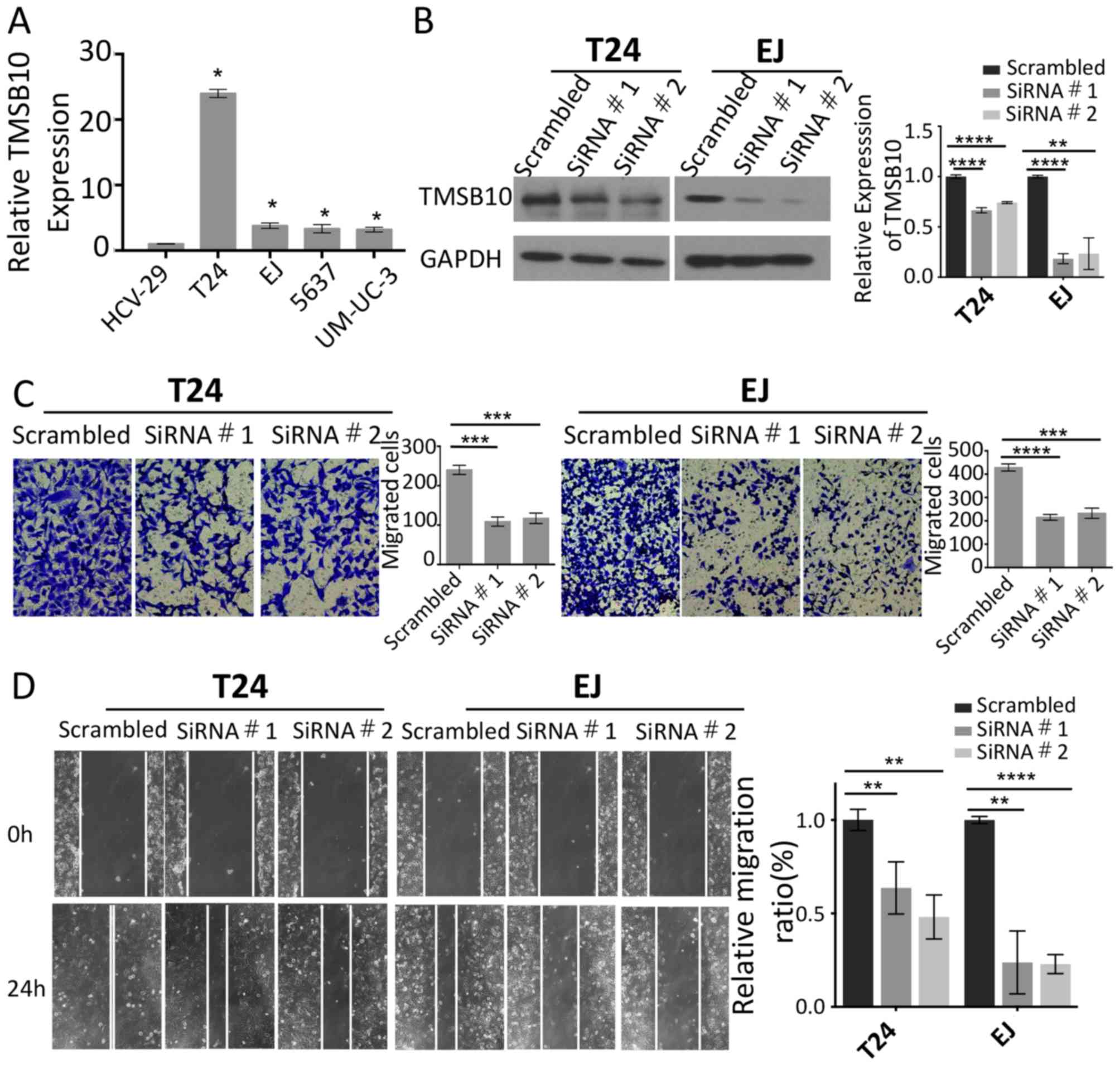
TMSB10 is overexpressed in BCa cell
lines and its knockdown suppresses BCa cell migration and
invasion
To investigate the expression levels of TMSB10 and
its effects on the metastatic ability of BCa cells, the mRNA
expression of TMSB10 was measured in four BCa cell lines
(EJ, T24, 5637 and UM-UC-3) and one normal bladder epithelial cell
line (HCV-29). The results indicated that TMSB10 was
overexpressed in all four BCa cell lines compared with the
corresponding expression of this gene in the HCV-29 cell line
(Fig. 2A). The expression of
TMSB10 was elevated in the T24 and EJ cells. Therefore,
TMSB10 was silenced in these cells in order to assess cell
motility. Efficient knockdown of the expression of TMSB10 was
confirmed by western blotting (Fig.
2B). Wound healing and migration assays were performed, and it
was shown that the knockdown of TMSB10 reduced the number of
invasive cells and significantly suppressed mobility compared with
the corresponding parameters noted in the vector control cells
(Fig. 2C and D).
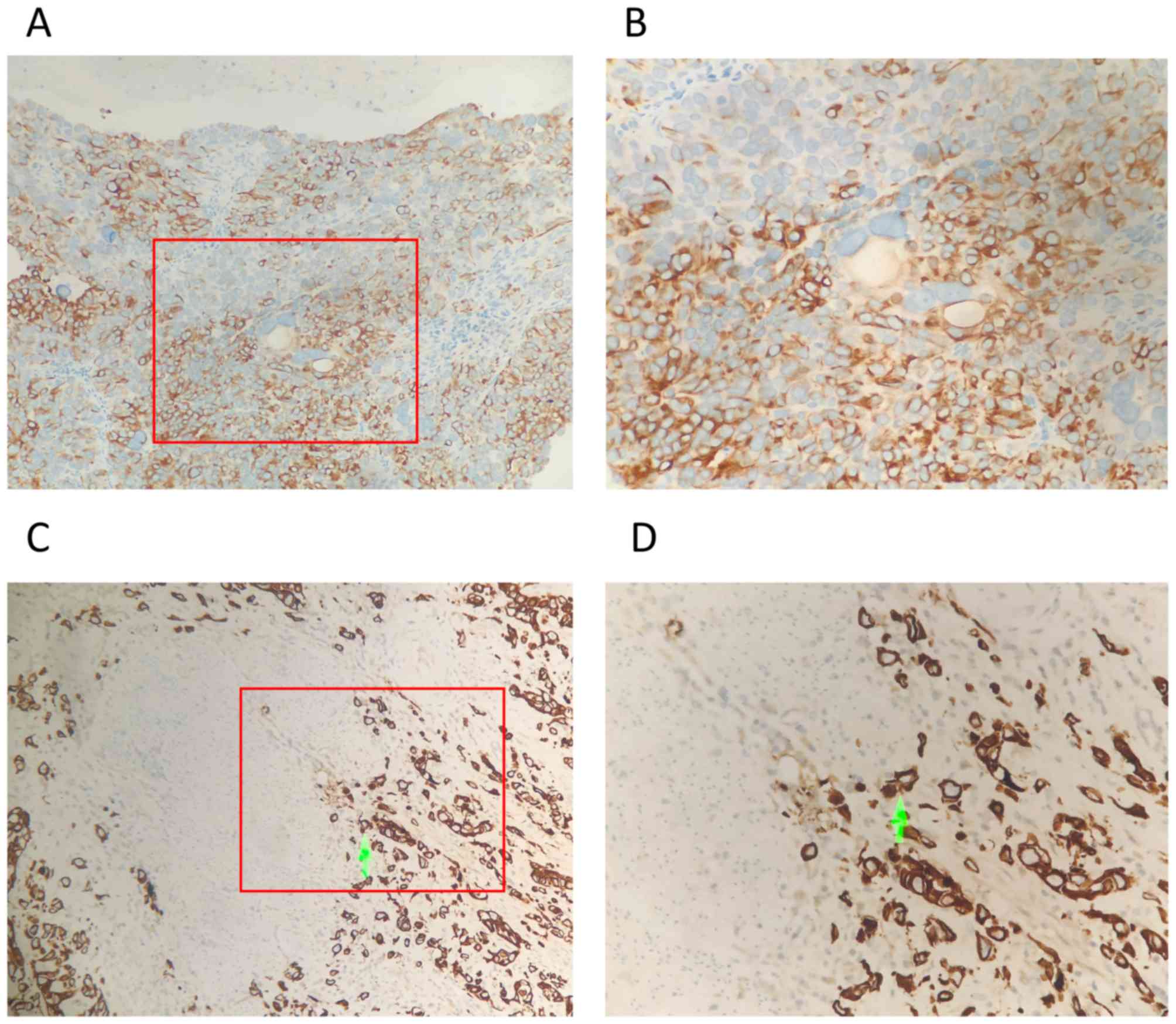
Expression of TMSB10 correlates with
the clinicopathological features of BCa
The association between the protein expression of
TMSB10 and the clinicopathological features of BCa were further
assessed using 101 archived paraffin-embedded human BCa specimens.
The percentage of TMSB10 protein overexpression in patients with
BCa was 54.5% (55/101; Fig. 3A-D).
By contrast, 30.0% (6/20) of the adjacent normal tissues exhibited
overexpression of TMSB10 protein, which was significantly lower
than that noted in the BCa tissues (P<0.05, Table II). A positive association between
clinicopathological features and the overexpression of TMSB10
protein was noted for muscular invasion (absent vs. present,
P<0.05). A previous study suggested that the incidence of UCB
markedly increases after the age of 65 years (18). Therefore, the age of 65 was used as a
cut-off threshold in the present study to monitor the correlation
between TMSB10 and age. However, based on the analysis, no
significant associations were found between the protein expression
of TMSB10 and age (≥65 vs. <65 years, P>0.05), gender (male
vs. female, P>0.05), histological grade (GI vs. GII–III,
P>0.05), distant metastases (absent vs. present, P>0.05) or
multiplicity (single vs. multiple, P>0.05) (Table I).
 | Table II.Protein expression of TMSB10 is
increased in bladder cancer. |
Table II.
Protein expression of TMSB10 is
increased in bladder cancer.
|
|
| Protein expression
of TMSB10 |
|
|---|
|
|
|
|
|
|---|
| Group | n | Low, n (%) | High, n (%) | P-value
(χ2 test)a |
|---|
| Tumor tissues | 101 | 46 (45.5) | 55 (54.5) | 0.039 |
| Normal tissues | 20 | 14 (70.0) | 6
(30.0) |
|
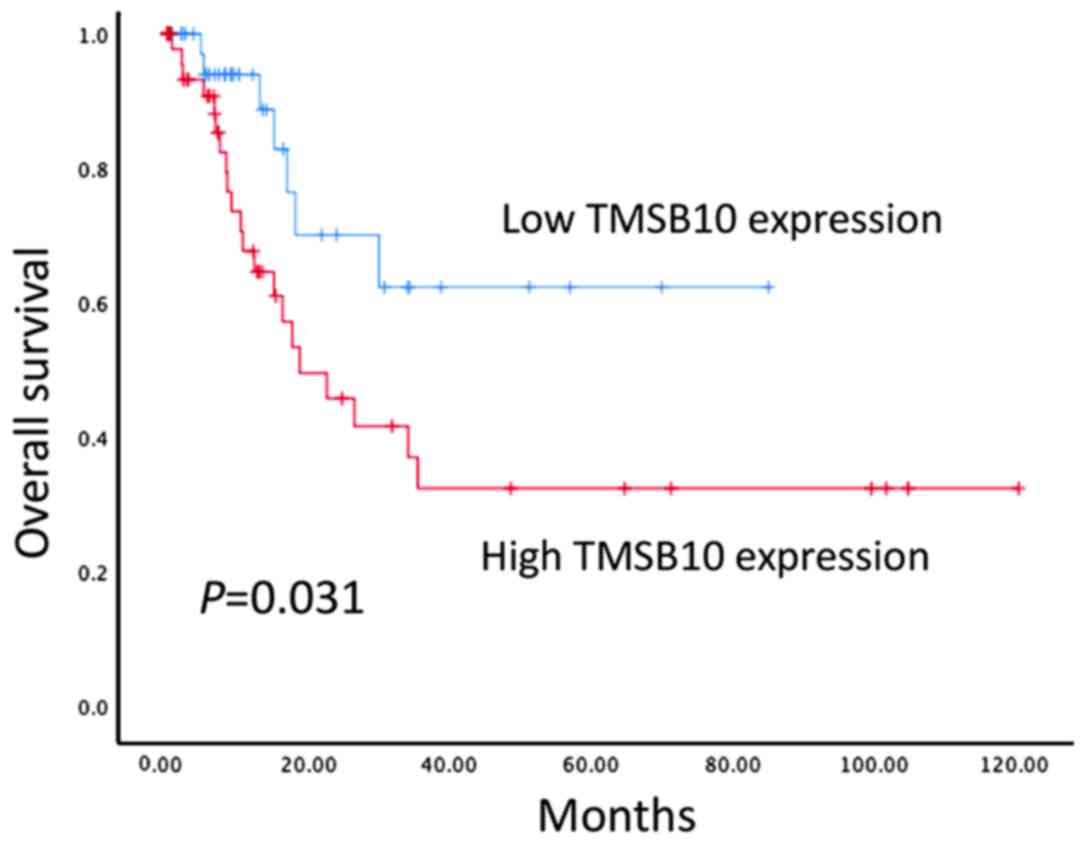
High expression of TMSB10 is
associated with poor survival and prognosis in BCa
The prognostic value of the protein expression of
TMSB10 in patients with BCa was further examined in 101 BCa tissue
samples using immunohistochemical techniques. Kaplan-Meier survival
analysis indicated that patients with high protein expression
levels of TMSB10 exhibited lower OS compared with patients with low
protein expression levels of TMSB10 (P<0.05, Fig. 4). In addition, univariate analysis
was performed and identified the following seven prognostic
parameters: Age, gender, histological grade, distant metastasis,
multiplicity, muscular invasion and protein expression of TMSB10.
It was found that the overexpression of TMSB10 protein was an
independent risk factor for poor disease prognosis (P<0.05;
Table III). However, no
significant associations were found between the protein expression
of TMSB10 and age, histological grade or distant metastases when
all data were separated according to gender (Tables SI–IV).
 | Table III.Summary of univariate and
multivariate Cox regression analyses of overall survival duration
in patients with bladder cancer. |
Table III.
Summary of univariate and
multivariate Cox regression analyses of overall survival duration
in patients with bladder cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) |
P-valuea | HR (95% CI) |
P-valuea |
|---|
| Age (≥65/<65
years) | 0.859
(0.400–1.842) | 0.696 |
|
|
| Gender
(female/male) | 0.766
(0.362–1.620) | 0.484 |
|
|
| Histological grade
(GI/GII–III) | 1.359
(0.596–3.103) | 0.466 |
|
|
| T stage
(T2-T4/Ta-T1) | 2.351
(1.081–5.113) | 0.031 | 2.338
(1.062–5.146) | 0.035 |
| Distant metastasis
(present/absent) | 3.015
(1.216–7.478) | 0.017 | 3.402
(1.341–8.632) | 0.010 |
| Multiplicity
(multiple/single) | 1.568
(0.722–3.405) | 0.225 |
|
|
| TMSB10
(high/low) | 0.402
(0.171–0.946) | 0.037 | 0.418
(0.177–0.988) | 0.047 |
Discussion
The role of TMSB10 in tumor metastasis is
controversial. It has been shown that TMSB10 is downregulated in
ovarian cancer compared with its expression in normal ovarian
tissues (19), whereas others have
shown that this protein acts as a tumor suppressor by disrupting
the actin structure and inhibiting the Ras signaling pathway
(20). However, the majority of
studies indicate that TMSB10 serves an oncogenic role. The
overexpression of TMSB10 is correlated with distant metastases and
lymph node metastases in lung cancer. In addition, the expression
of TMSB10 has been significantly associated with other factors,
including VEGF, VEGF-C, and microvascular and lymphatic vessel
density in lung cancer (21).
Califano et al (13) found
that the overexpression of TMSB10 correlated with the anaplastic
histotype of thyroid tumors, which is considered the most
aggressive thyroid histotype. The same study showed that the gene
expression of TMSB10 was associated with the oncogenic
transformation of highly malignant rat thyroid epithelial cells
caused by several viruses (13).
High expression levels of TMSB10 have also been associated with
lymph node metastases in papillary thyroid carcinoma (22). Recent studies by Zhang et al
reported that the expression of TMSB10 correlated with
clinicopathological features, poor prognosis and distant metastases
in patients with breast cancer. In addition, they found that TMSB10
promoted the proliferation, invasion and migration of breast cancer
cells in vitro and in vivo via the AKT/FOXO signaling
pathway (9). To the best of our
knowledge, the present study is the first to assess the expression
and association of TMSB10 with the clinicopathological features of
BCa.
In the present study, following the analysis of
publicly available microarray data, it was found that the mRNA
expression levels of TMSB10 in BCa were significantly higher
than those in adjacent noncancerous tissues. It is noteworthy that
the overexpression of TMSB10 was associated with MIBCa. Similarly,
the mRNA levels of TMSB10 were overexpressed in all four of
the BCa cell lines assessed compared with those noted in the HCV-29
normal bladder epithelial cell line. In addition, knocking down the
expression of TMSB10 caused a marked reduction in the migration and
invasion of BCa cell lines.
High expression levels of TMSB10 were observed in
54.5% (55/101) of BCa tissues in the present study by
immunohistochemical detection. In addition, the overexpression of
TMSB10 protein was positively associated with cancer cell invasion
to the muscularis mucosae. These results support the hypothesis
that TMSB10 may be an oncogene in BCa and further suggested
that it may serve an important role in the metastasis of BCa. The
results also revealed that the expression of TMSB10 correlated with
OS and that it was an independent prognostic factor for low OS
rates in patients with BCa. The present study suggested that TMSB10
may serve a crucial role in muscle invasion of bladder tissues and
that high expression levels may represent a novel indicator for
muscle invasion in BCa and poor prognosis of the disease.
The present study has limitations, such as the
retrospective study design and the lack of experimental evidence to
support a specific regulatory mechanism between TMSB10 and the
activation of BCa cell metastasis. Furthermore, the processes of
invasion and apoptosis were not thoroughly addressed. Therefore,
the examination of further subjects is required in order to
determine the exact role of TMSB10 in a carcinogenic environment.
In addition, the cohort size was not sufficiently large to support
the use of TMSB10 as a prognostic marker. However, similar
conclusions may be deduced following the investigation of TMSB10 in
larger sample sizes of BCa specimens, based on the survival curve
shown in the present study. In the future, follow up of the
patients is planned to examine differences in recurrence, kidney
function and infection in returning patients with disease
progression.
In conclusion, the present study is the first, to
the best of our knowledge, to report that TMSB10 is frequently
overexpressed and has considerable clinical applications in BCa. A
high expression of TMSB10 was associated with significantly lower
OS. In addition, the assessment of tumor expression of TMSB10 may
improve prognosis and assist in the identification of patients who
may benefit from combination treatment. The in vitro
analyses demonstrated that the migration and invasion of cancer
cells were significantly reduced by silencing TMSB10.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from
Guangdong Medical Research Foundation of China (grant no.
A2018326).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
BW and GY conceived and designed the study, and
analyzed and interpreted the results. BW, ZW and TZ performed
experiments and wrote this manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The institutional Ethics Committee of Guangdong
Second Provincial General Hospital (Guangdong, China) approved the
study and written informed consent was obtained from each
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bellmunt J and Petrylak DP: New
therapeutic challenges in advanced bladder cancer. Semin Oncol.
39:598–607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldstein AL, Slater FD and White A:
Preparation, assay, and partial purification of a thymic
lymphocytopoietic factor (thymosin). Proc Natl Acad Sci USA.
56:1010–1017. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Erickson-Viitanen S, Ruggieri S, Natalini
P and Horecker BL: Thymosin beta 10, a new analog of thymosin beta
4 in mammalian tissues. Arch Biochem Biophys. 225:407–413. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weterman MA, van Muijen GN, Ruiter DJ and
Bloemers HP: Thymosin beta-10 expression in melanoma cell lines and
melanocytic lesions: A new progression marker for human cutaneous
melanoma. Int J Cancer. 53:278–284. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oien KA, Vass JK, Downie I, Fullarton G
and Keith WN: Profiling, comparison and validation of gene
expression in gastric carcinoma and normal stomach. Oncogene.
22:4287–4300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Ren D, Guo L, Wang L, Wu S, Lin
C, Ye L, Zhu J, Li J, Song L, et al: Thymosin beta 10 is a key
regulator of tumorigenesis and metastasis and a novel serum marker
in breast cancer. Breast Cancer Res. 19:152017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu CR, Ma CS, Ning JY, You JF, Liao SL
and Zheng J: Differential thymosin beta 10 expression levels and
actin filament organization in tumor cell lines with different
metastatic potential. Chin Med J (Engl). 117:213–218.
2004.PubMed/NCBI
|
|
11
|
Theunissen W, Fanni D, Nemolato S, Di
Felice E, Cabras T, Gerosa C, Van Eyken P, Messana I, Castagnola M
and Faa G: Thymosin beta 4 and thymosin beta 10 expression in
hepatocellular carcinoma. Eur J Histochem. 58:22422014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takano T, Hasegawa Y, Miyauchi A,
Matsuzuka F, Yoshida H, Kuma K and Amino N: Quantitative analysis
of thymosin beta-10 messenger RNA in thyroid carcinomas. Jpn J Clin
Oncol. 32:229–232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Califano D, Monaco C, Santelli G, Giuliano
A, Veronese ML, Berlingieri MT, de Franciscis V, Berger N, Trapasso
F, Santoro M, et al: Thymosin beta-10 gene overexpression
correlated with the highly malignant neoplastic phenotype of
transformed thyroid cells in vivo and in vitro. Cancer Res.
58:823–828. 1998.PubMed/NCBI
|
|
14
|
Sribenja S, Li M, Wongkham S, Wongkham C,
Yao Q and Chen C: Advances in thymosin beta10 research:
Differential expression, molecular mechanisms, and clinical
implications in cancer and other conditions. Cancer Invest.
27:1016–1022. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sribenja S, Wongkham S, Wongkham C, Yao Q
and Chen C: Roles and mechanisms of β-thymosins in cell migration
and cancer metastasis: An update. Cancer Invest. 31:103–110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International against cancer (UICC). TNM classification of
malignant tumours. 7th edn. New York. Wiley2009.
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SH, Zhang W, Choi JJ, Cho YS, Oh SH,
Kim JW, Hu L, Xu J, Liu J and Lee JH: Overexpression of the
thymosin beta-10 gene in human ovarian cancer cells disrupts
F-actin stress fiber and leads to apoptosis. Oncogene.
20:6700–6706. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SH, Son MJ, Oh SH, Rho SB, Park K, Kim
YJ, Park MS and Lee JH: Thymosin {beta}(10) inhibits angiogenesis
and tumor growth by interfering with Ras function. Cancer Res.
65:137–148. 2005.PubMed/NCBI
|
|
21
|
Gu Y, Wang C, Wang Y, Qiu X and Wang E:
Expression of thymosin beta10 and its role in non-small cell lung
cancer. Hum Pathol. 40:117–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang XJ, Su YR, Liu D, Xu DB, Zeng MS and
Chen WK: Thymosin beta 10 correlates with lymph node metastases of
papillary thyroid carcinoma. J Surg Res. 192:487–493. 2014.
View Article : Google Scholar : PubMed/NCBI
|