Introduction
Psoriasis is an inflammatory condition with chronic
evolution, multifactorial etiology and complex pathogenesis,
involving genetic, immunological and environmental factors. Skin
lesions, erythematous-squamous plaques with inconstant
osteo-articular and nail involvement may be associated with
systemic damage, such as cardiovascular diseases, metabolic
syndrome or autoimmune disorders (1,2).
Psoriasis is a disease with a severe psychosocial impact,
comparable to diabetes and cancer (3,4).
The treatment is adapted to the extent of the
lesions, the duration of the disease evolution, the comorbidities
and the general biological status of the patient. In mild or
moderate clinical forms of psoriasis, with lesions not exceeding
10% of the skin surface, local therapy is recommended. In severe
forms of psoriasis, with extensive lesions or resistant to either
topical therapy or UVA/NB-UVB therapy, a systemic treatment with
antiproliferative medicines (methotrexate, retinoids) or
immunomodulators, such as cyclosporine or fumaric acid is
established (5,6). Lack of response constitutes an
eligibility criterion for biological therapy (7).
Biological agents are a class of revolutionary
pharmaceuticals useful in many immunological-mediated diseases.
Those intended for psoriasis include selective inhibiting
biological agents of anti-TNFα tumor necrosis factor, of
interleukin 12/23 or interleukin 17A (8,9). The
longest experience has been gained by anti-TNFα agents (infliximab,
etanercept, adalimumab).
Despite the modern therapeutic options being
destined to patients with psoriasis, obtaining satisfactory
clinical and biological results requires optimal patient compliance
with the treatment. The term ‘compliance’ has been criticized for
having an authoritative message and referring in particular to the
rigor with which a patient is following the prescribed treatment,
not to the doctor's additional recommendations on appropriate diet
or change of lifestyle (10). For
these reasons, the term adhesion or even therapeutic alliance is
currently preferred to suggest an approach that indicates a high
degree of involvement of both the patient and the physician in the
therapeutic act (11). As an
extended definition, adherence is expressed in patients who have
been treated for a certain period of time and who have followed
both the indications and the doctor's prescription following
treatment without interruption. According to the World Health
Organization (WHO), adherence is ‘the extent to which the behavior
of a person taking medication, following a diet, making lifestyle
changes corresponds to the recommendations accepted by the
physician’ (12).
Biological agents, targeted, rational and long-term
individualized therapy are able to increase adherence to treatment
for patients with psoriasis, due to the ease of drug
administration, the costs incurred by the national health insurance
system, the clinical effectiveness shortly after treatment
initiation, the long-term safety profile. Non-adherence to
biological therapy is found, however, correlated with a number of
factors that involve both the patient and the attending physician
(13,14).
This study aimed to identify the main factors
involved in the non-adherence of patients with psoriasis to
anti-TNFα biological therapy, as well as to analyze the optimal
methods of combating this phenomenon with negative echoes in terms
of disease evolution over time.
Patients and methods
The current retrospective, observational study
includes patients with moderate-severe psoriasis vulgaris treated
with biological anti-TNFα agents. The study group encompassed 84
patients who underwent therapy with anti-TNFα biological agents in
the Department of Dermatology at CF University Hospital Iasi (Iasi,
Romania) between January 2009 and October 2018. The Ethics
Committee of the Railways University Hospital Iasi (Iasi, Romania)
approved the current study. The patients were aged 9–82 years, 48
women and 35 men, 66 from urban areas and 18 from rural areas.
Depending on the biological agent administered, the
patients were divided into 3 groups: group 1 included 34 patients
who received Infliximab (5 mg/kg iv every 8 weeks), group 2
consisted of 26 patients treated with Adalimumab (40 mg
subcutaneously every 2 weeks) and group 3 had 24 patients treated
with Etanercept (50 mg subcutaneously every week). The patients'
eligibility was based on the inclusion and exclusion criteria
regarding the general protocol for biological therapy. The analysis
of the clinical and paraclinical parameters during the biological
therapy allowed the evaluation of the adherence to treatment, as
well as the investigation of the factors responsible for
non-adherence.
Statistical analysis
Descriptive and analytical methods were used in the
statistical analysis. Once the data were collected, in an
accessible form to ensure their informational character, the
processing was performed. The confidence intervals, at the
significance threshold of 95%, were used in the data presentation,
and the χ2 and Student's t-tests were used to assess the
differences. The data were centralized in EXCEL and SPSS 13.0
databases and processed with the statistical functions they are
suitable for. In calculating the significant difference between two
environments, the Student's t-test takes into account the
variability measurement and the weight of the observations. The
accepted significance threshold was 95%. χ2 test is a
nonparametric test comparing two or more frequencies distributed
from the same population; it applies when the expected events are
excluded.
Results
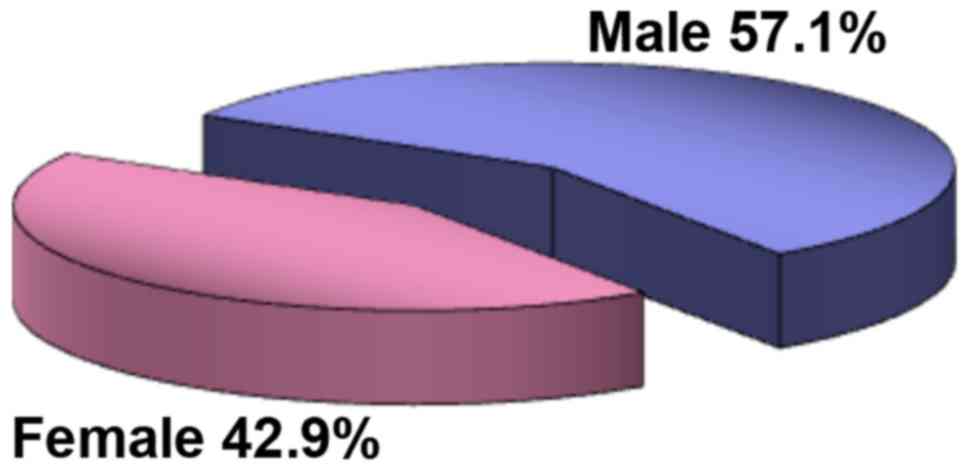
From an epidemiological point of view, the study
group consisted predominantly of male patients (57.1%) with a M/F
ratio of 1.4/1 (Fig. 1). Most
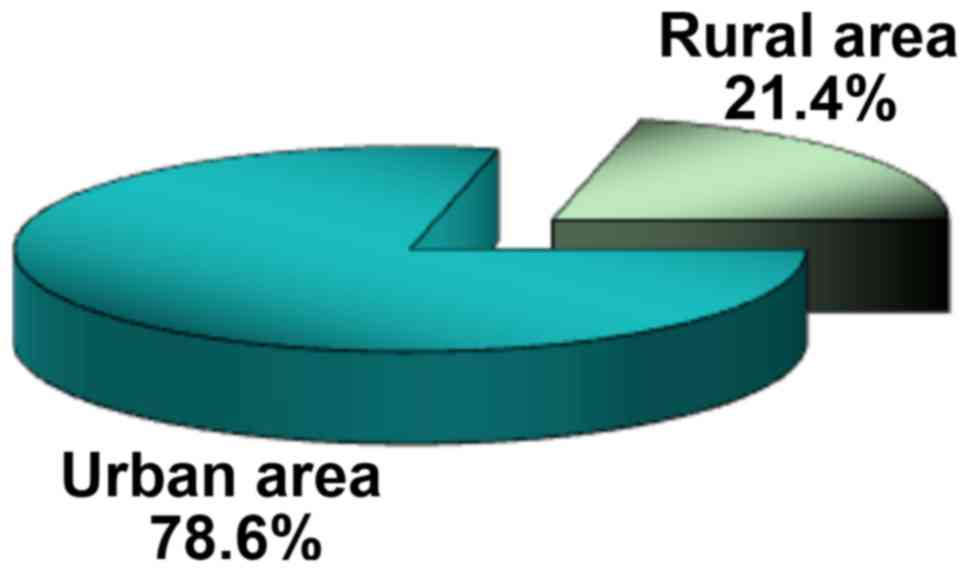
subjects were from the urban area, the U/R ratio being 3.7/1
(Fig. 2). The distribution by areas
of origin and sex did not show significant differences: 75% of
women and 83.3% of men came from the urban environment (P=0.514),
statistically revealing the homogeneity of the study group.
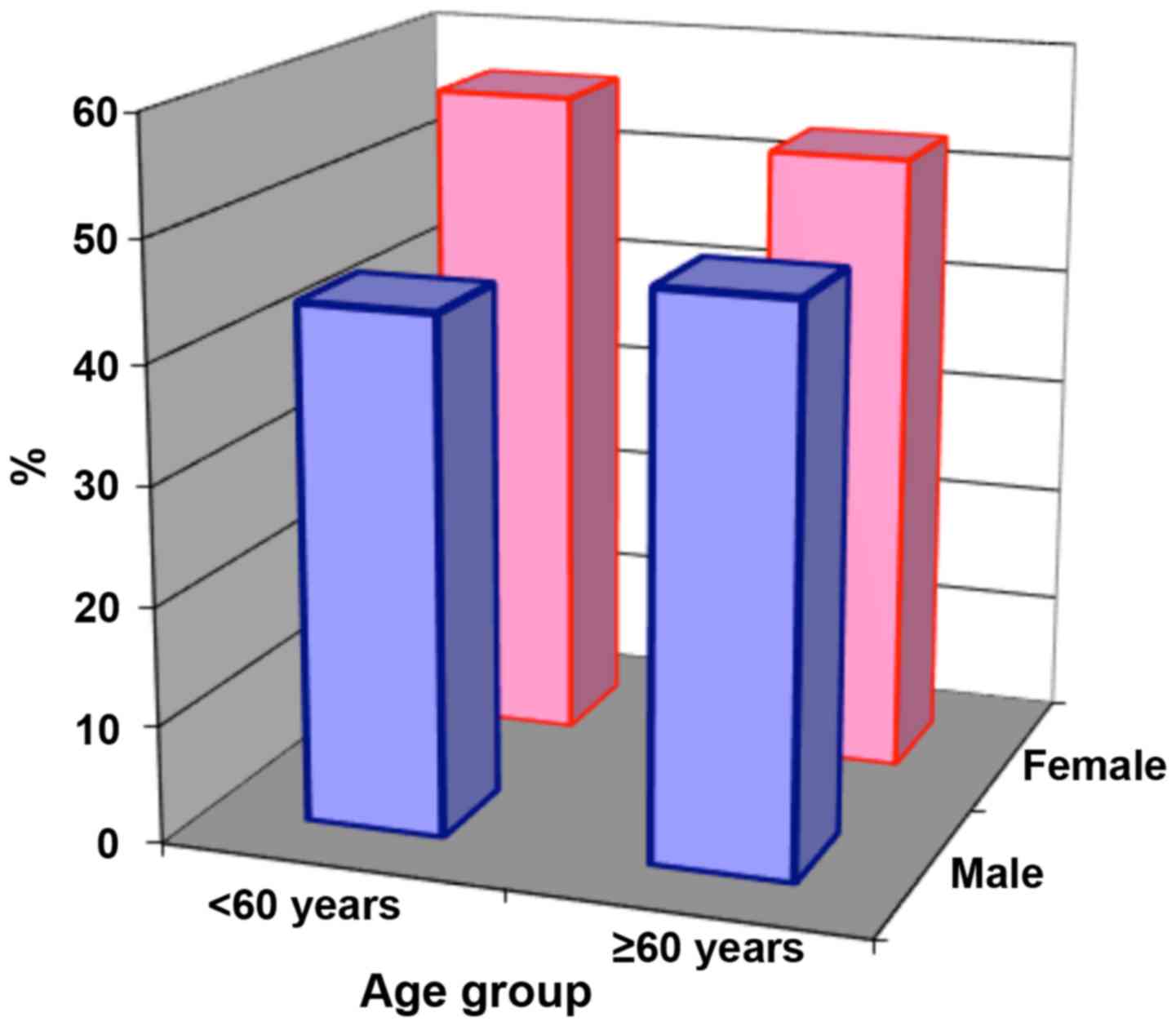
Distribution by age group reveals the high
proportion of cases in the 60–69 age group, both in women and men.
Since the mean age of the group was 58.88 years and the highest
weight of cases is found in patients >60 years (54.8%), we chose
this age as reference in the statistical processing (Fig. 3).
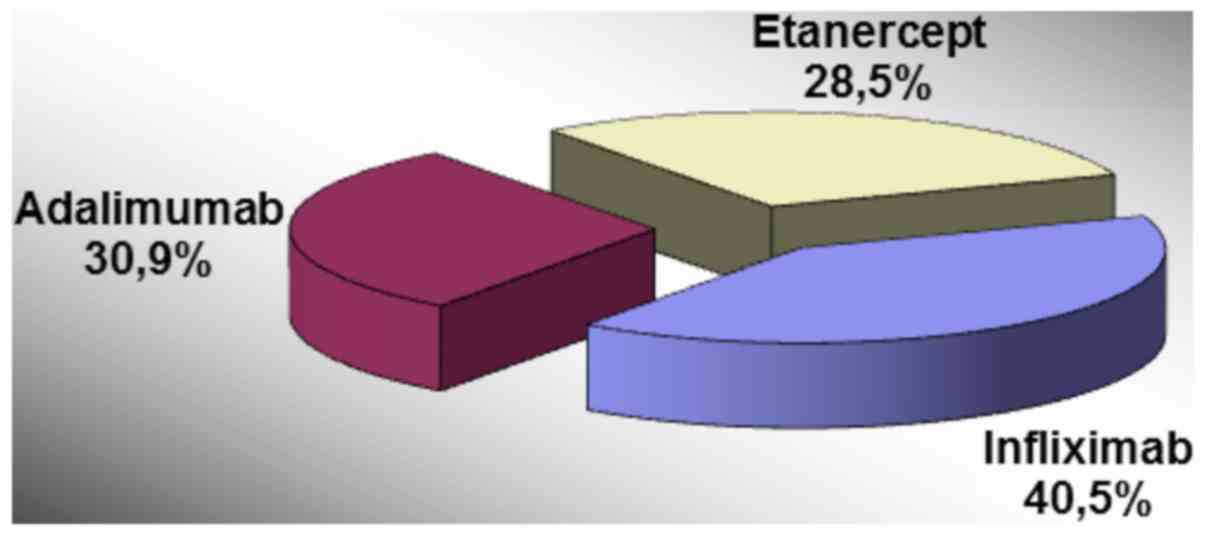
Infliximab was used in 40.5% of the cases, the
majority in men (72.7 vs. 27.3%, P=0.038) and Adalimumab was
recommended in 30.9% of the patients, frequently women (60 vs. 40%,
P=0.038) (Fig. 4).
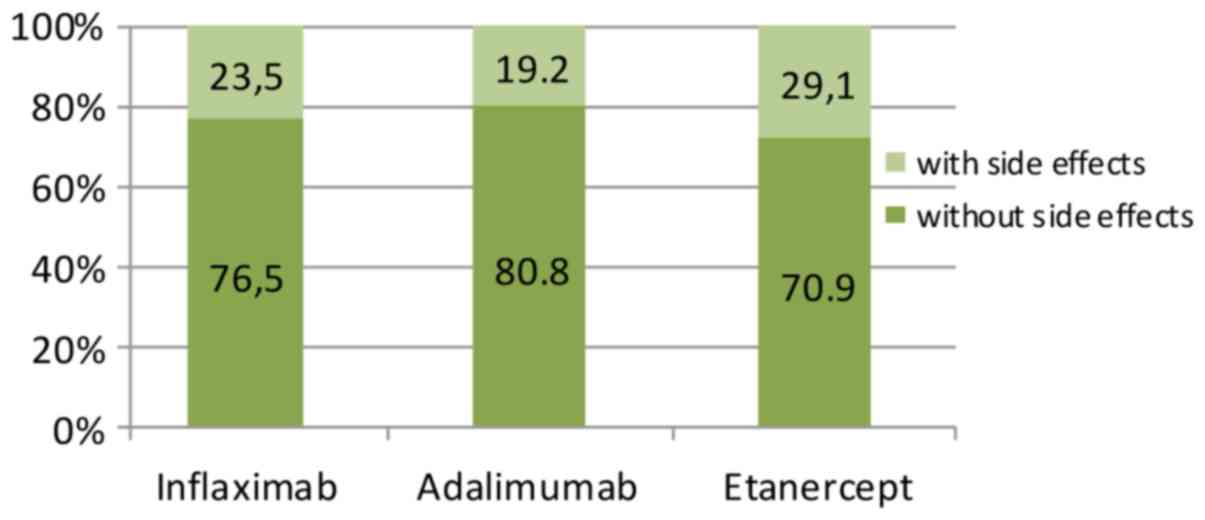
Discontinuation of therapy was observed in 20 of the
84 patients enrolled in the study (22.6%) for reasons related
either to therapy (adverse events) or to the patient. The highest
rate of treatment discontinuation was observed in the Etanercept
group (29.1), followed by the Infliximab group (23.5%) and
Adalimumab group (19.2%) (Fig.
5).
In most cases, the reasons for discontinuing
biological therapy were represented by adverse events. In the
Infliximab group these were: allergic reactions (angioedema and
deep urticaria) in three cases (8.82% of all patients treated with
Infliximab) representing 3.57% of the total number of patients
enrolled in the study, NYHA class III heart failure one patient
(2.94% of all patients in the Infliximab group), viral hepatitis
with C virus in one patient (2.94% of all patients treated with
Infliximab). One patient developed paradoxical psoriasis and
cataract (2.94% of all patients treated with Infliximab). Home
relocation was the reason for discontinuing the therapy in one
patient (2.94% of all patients in the Infliximab group). Patients
who discontinued Infliximab accounted for 8.3% of the total number
of patients.
In the Adalimumab group, the therapy was
discontinued in the context of the following adverse events:
polydiscitis in one case (3.84% of all patients in the Adalimumab
group), infectious endocarditis in one case (3.84%), laryngeal
tuberculosis, one case (3.84%), hypereosinophilic syndrome, one
case (3.84%) and breast cancer, one case (3.84%), each adverse
event representing 1.19% of the total. Patients who discontinued
Adalimumab accounted for 5.95% of the total number of patients.
In the Etanercept group, adverse events that led to
the interruption of therapy were: eosinophilic cellulitis in two
cases (8.33% of all patients in the Etanercept group), accounting
for 2.38% of the total number of patients, colon cancer occurred in
one patient (4.16% of all patients in the Etanercept group),
representing 1.19% of the total number of patients, alopecia areata
in one case (4.16%), pancreatitis in one case (4.16%) and
thrombocytopenia in one case (4.16%). Pregnancy occurred during
biologic therapy in one patient (4.16% of all patients in the
Etanercept group) and led to interruption of Etanercept. Patients
who interrupted Etanercept accounted for 8.3% of the total number
of patients (Table I).
 | Table I.Distribution of events leading to
discontinuation of therapy. |
Table I.
Distribution of events leading to
discontinuation of therapy.
|
| Infliximab
(n=8/34) | Adalimumab
(n=5/26) | Etanercept
(n=7/24) |
|---|
|
|
|
|
|
|---|
| Adverse effects | % of Infliximab
group | % of all
patients | % of Adalimumab
group | % of all
patients | % of Etanercept
group | % of all
patients |
|---|
| Angioedema
urticarial | 8.82 | 3.57 | – | – | – | – |
| Infectious
endocarditis |
|
| 3.84 | 1.19 | – | – |
| Hypereosinophilic
syndrome | – | – | 3.84 | 1.19 | – | – |
| Paradoxical
psoriasis | 2.94 | 1.19 |
|
|
|
|
|
|
|
| – | – | – | – |
| Cataract | 2.94 | 1.19 |
|
|
|
|
| Eosinophilic
cellulite | – | – | – | – | 8.33 | 2.38 |
| IC class III
NYHA | 2.94 | 1.19 | – | – | – | – |
| Hepatitis C
virus | 2.94 | 1.19 | – | – | – | – |
| Tuberculous
laryngitis | – | – | 3.84 | 1.19 | – | – |
| Thrombocytopenia | – | – | – | – | 4.16 | 1.19 |
| Pancreatitis | – | – | – | – | 4.16 | 1.19 |
| Breast cancer | – | – | 3.84 | 1.19 | – | – |
| Colon cancer | – | – | – | – | 4.16 | 1.19 |
| Polydiscitis | – | – | 3.84 | 1.19 | – | – |
| Move home | 2.94 | 1.19 | – | – | – | – |
| Pregnancy | – | – | – | – | 4.16 | 1.19 |
| Alopecia | – | – | – | – | 4.16 | 1.19 |
| Total adverse
effects | 23.5 | 9.52 | 19.2 | 5.95 | 29.1 | 8.33 |
Overall adverse events that led to treatment
discontinuation occurred in 52.6% of men and 47.4% of women
(P=0.179). There were no significant differences in the reason for
non-adherence to treatment based on the background (P=0.280). In
conclusion, the study group had a predominantly male distribution
(57.1%), from urban areas (68.6%) aged between 28 and 82 years.
Statistically, the homogeneity of the group by sex, age groups, and
background was demonstrated. Non-adherence to therapy was noted in
22.6% of the patients, most commonly in those treated with
Etanercept (29.1%). The adherence rate for the entire patient
population was of 76.2%, statistical analysis revealing a lack of
direct correlation between the incidence of adverse events and
biologic therapy (P=0.00213).
Discussion
Biological therapy in psoriasis is a modern
treatment alternative with long-term proven efficacy and safety
profile. Clinical experience with biological agents from the
anti-TNFα class allows them to be categorized as superior to
conventional systemic therapy for moderate-severe psoriasis. Rapid
remission of symptomatology, its maintenance over treatment, easy
administration, significant improvement in the quality of life are
the arguments that reinforce this idea (15,16).
The adhesion of patients with psoriasis to anti-TNFα
therapy is an essential condition for obtaining and maintaining the
expected therapeutic outcome, but also a long-term indicator of
patient satisfaction. The risk of reactivating the disease in the
context of cessation of treatment confers a key role in maintaining
the favorable clinical effects to the notion of adherence to
therapy (17,18).
The rate of adherence to biological therapy is
clearly superior compared to adherence to classical topical or
systemic treatments, but the literature data on this topic varies,
depending on the size of the group studied and the biological agent
used (19).
Thus, a 2017 study conducted in Germany for 31
months in 13 dermatology centers showed a rate of adhesion to
anti-TNFα therapy of ~85%. Other studies concluded that adherence
rates ranged between 65 and 96.7% one year after initiation of
anti-TNFα therapy with a 40–70% decrease in adherence after 4 years
of therapy (20). Another large
study on 747 patients concludes on a high adherence to Infliximab
of 70% after 4 years of treatment as compared to Etanercept and
Adalimumab with a 40% adherence rate (21).
The results of the current study, which included 84
patients treated with anti-TNFα (Infliximab, Adalimumab,
Etanercept) over the past 10 years, showed an overall 76.2%
adherence rate, lowest in patients treated with Etanercept (70.9%).
Relative to the anti-TNF agent used, the highest adherence rate was
recorded in Adalimumab (80.8%), followed by Infliximab (76.5%) and
Etanercept (70.9%). We noted differences between the rates of
adhesion to therapy with different anti-TNFα agents, but with no
statistical significance (P=0.00213).
The main adverse events of anti-TNFα therapy
reported in literature are cardiovascular diseases, increased risk
of latent tuberculosis reactivation, increased risk of infections,
malignancy, injection site reactions, haematological disorders
(22,23). These events may be a factor of
non-adherence to therapy. In our study, patients treated with
Infliximab experienced: allergic reactions (8.8%), cardiotoxicity
(NYHA class III congestive heart failure 2.9%), hepatitis C (2.9%),
results which are comparable to those published in other studies
(23,24).
The most common adverse events reported during
treatment with Adalimumab are minor injection site reactions,
occurring in 12–37% of the treated cases. In addition, Adalimumab
increases the risk of infection or reactivation of chronic latent
infections, such as viral hepatitis or TB (22). Only one case of tuberculous infection
(tuberculous laryngitis (3.8%) and a bacterial endocarditis (3.8%)
were recorded in our study during Adalimumab therapy.
Numerous research studies focused on the efficacy
and safety profile of Etanercept. The main side effects to
Etanercept reported in the literature are local reactions on
injection site (eosinophilic cellulitis) and the risk of infection.
In our study, 8.3% of the patients treated with Etanercept
developed eosinophilic cellulitis. Other events recorded during
Etanercept therapy were: malignancies (4.1% colon cancer), alopecia
areata (4.1%), and pancreatitis (4.1%). Various studies have
focused on the analysis of long-term safety of Etanercept
administration and concluded on a similar rate of side effects
after 96 and 144 weeks of monitoring. They have also concluded that
the rate of major adverse events, such as severe infections and
tumors, is higher than in the general population (24,25).
On the one hand, adherence to long-term treatment is
influenced by its nature; on the other hand, it is influenced by
the patient's compliance based on an optimal physician-patient
relationship. Two cases of non-adherence due to factors independent
of therapy (home relocation and pregnancy) were reported in our
study. There were no matters involving refusal to follow the
prescribed treatment, under or overdosing, intermittent dosing,
premature discontinuation, addition or waiver of medicine without
medical advice, non-compliance to the protocol regimen. Serious
side events were rare and difficult to interpret widely on a small
batch of patients.
Other non-adherence reasons reported in literature
are loss of insurance, long-term remission of the skin lesions or
lack of confidence in pharmaceutical drugs. Even though vitamins,
minerals and herb supplements (zinc, selenium, B12, fish oil,
curcumin, aloe vera extract) are sometimes prescribed in psoriasis
due to their mainly antioxidant action, we consider it a
complementary therapeutical instrument (26,27).
The case with PR and aggravated psoriasis under
Infliximab therapy benefited from switch to anti-IL17 therapy.
Potential causes for paradoxical psoriasis under TNFα are the
imbalance of the key cytokines in the pathophysiological chain of
the disease (TNFα, IFNα, IL12/23, IL17), the differences between
the immunological properties of monoclonal antibodies and soluble
TNFα receptor, as well as the change of the immunological profile
from Th1 to Th2, with the consequent increase in antibody
production (28,29).
Malignancy caused interruption of anti-TNF biologic
therapy in two patients in the study (breast cancer:
Adalimumab/colon cancer: Etanercept), but it is difficult to
interpret the contribution of biologic therapy in the
etiopathogenesis of these neoplasms (30)]. Prior to the biological treatment, no
tumor markers were used to stratify the risk of patients treated
with anti-TNF to develop a form of cancer. Studies do not indicate
a clear correlation between anti-TNFα treatment and an increased
risk of malignancy, which allows us to interpret the occurrence of
these events independently of the biological therapy for
psoriasis.
In conclusion, adherence is a complex process, which
represents an important parameter in assessing the long-term
effectiveness of biological therapy. Improved adhesion is based on
the patient's transformation into an active co-participant to
his/her own healing through an optimal communication with the
treating physician, as well as on an easy way to carry out the
treatment, on its safety and the application of pharmacovigilance
principles.
Acknowledgements
Professional editing, linguistic and technical
assistance performed by Individual Service Provider Irina Radu,
certified translator in Medicine and Pharmacy.
Funding
No funding was received.
Availability of data and materials
The data that support the findings of this study are
available from the archives of the Railways University Hospital
Iasi, (Iasi, Romania), but restrictions apply to the availability
of these data which are not publicly available. Data are, however,
available from the authors upon reasonable request and with
permission from the Railways University Hospital Iasi.
Author's contributions
MM, MPT, ER, TT contributed equally to acquisition,
analysis and systematization of data, manuscript writing and
critical revision of it for important intellectual content. All the
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Railways University
Hospital Iasi (Iasi, Romania) approved the current study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schadler ED, Ortel B and Mehlis SL:
Biologics for the primary care physician: Review and treatment of
psoriasis. Dis Mon. 65:51–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55 (Suppl):1191–1196.
2014.PubMed/NCBI
|
|
3
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Căruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in
vivo reflectance confocal microscopy. Exp Ther Med.
15:1241–1246. 2018.PubMed/NCBI
|
|
4
|
Negrei C, Ginghină O, Căruntu C, Burcea
Dragomiroiu GTA, Jinescu G and Boda D: Investigation relevance of
methotrexate polyglutamates in biological systems by high
performance liquid chromatography. Rev Chim. 66:766–768. 2015.
|
|
5
|
Negrei C, Arsene AL, Toderașcu CD, Boda D
and Ilie M: Acitretin treatment in psoriasis may influence the cell
membrane fluidity. Farmacia. 60:767–771. 2012.
|
|
6
|
Negrei C, Căruntu C, Ginghina O,
Dragomiroiu GTAB, Toderascu CD and Boda D: Qualitative and
quantitative determination of methotrexate polyglutamates in
erythrocytes by high performance liquid chromatography. Rev Chim.
66:607–610. 2015.
|
|
7
|
Sbidian E, Chaimani A, Garcia-Doval I, Do
G, Hua C, Mazaud C, Droitcourt C, Hughes C, Ingram JR, Naldi L, et
al: Systemic pharmacological treatments for chronic plaque
psoriasis: A network meta-analysis. Cochrane Database Syst Rev.
12:CD0115352017.PubMed/NCBI
|
|
8
|
Olteanu R, Zota A and Constantin M:
Biosimilars: An update on clinical trials (review of published and
ongoing studies. Acta Dermatovenerol Croat. 25:57–66.
2017.PubMed/NCBI
|
|
9
|
Olteanu R, Constantin MM, Zota A,
Dorobantu DM, Constantin T, Serban ED, Balanescu P, Mihele D and
Gheuca-Solovastru L: Original clinical experience and approach to
treatment study with interleukine 12/23 inhibitor in
moderate-to-severe psoriasis patients. Farmacia. 64:918–921.
2016.
|
|
10
|
Zambrano R, Duitama JF, Posda JI and
Flórez JF: Perception of adherence to treatment among patients with
cardiovascular risk factors. Rev Fac Nac Salud Publica. 30:163–174.
2012.
|
|
11
|
Tatu AL and Nwabudike LC:
Metoprolol-associated onset of psoriatic arthropathy. Am J Ther.
24:e370–e371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sabate E: Adherence to Long-Term
Therapies: Evidence for ActionWorld Health Organization; Geneva:
2003, https://www.who.int/chp/knowledge/publications/adherence_report/en/
|
|
13
|
Boda D, Negrei C, Nicolescu F and Balalau
C: Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
14
|
Radtke MA and Augustin M: Economic
considerations in psoriasis management. Clin Dermatol. 26:424–431.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ross C, Marshman G, Grillo M and Stanford
T: Biological therapies for psoriasis: Adherence and outcome
analysis from a clinical perspective. Australas J Dermatol.
57:137–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raval K, Lofland JH, Waters H and Piech
CT: Disease and treatment burden of psoriasis: Examining the impact
of biologics. J Drugs Dermatol. 10:189–196. 2011.PubMed/NCBI
|
|
17
|
Schaarschmidt ML, Kromer C, Herr R,
Schmieder A, Goerdt S and Peitsch WK: Treatment satisfaction of
patients with psoriasis. Acta Derm Venereol. 95:572–578. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zschocke I, Ortland C and Reich K:
Evaluation of adherence predictors for the treatment of moderate to
severe psoriasis with biologics: The importance of
physician-patient interaction and communication. J Eur Acad
Dermatol Venereol. 31:1014–1020. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doshi JA, Takeshita J, Pinto L, Li P, Yu
X, Rao P, Viswanathan HN and Gelfand JM: Biologic therapy
adherence, discontinuation, switching, and restarting among
patients with psoriasis in the US Medicare population. J Am Acad
Dermatol. 74:1057–1065.e4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Umezawa Y, Nobeyama Y, Hayashi M, Fukuchi
O, Ito T, Saeki H and Nakagawa H: Drug survival rates in patients
with psoriasis after treatment with biologics. J Dermatol.
40:1008–1013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gniadecki R, Kragballe K, Dam TN and Skov
L: Comparison of drug survival rates for adalimumab, etanercept and
infliximab in patients with psoriasis vulgaris. Br J Dermatol.
164:1091–1096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim WB, Marinas JE, Qiang J, Shahbaz A,
Greaves S and Yeung J: Adverse events resulting in withdrawal of
biologic therapy for psoriasis in real-world clinical practice: A
Canadian multicenter retrospective study. J Am Acad Dermatol.
73:237–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaushik SB and Lebwohl MG: Psoriasis:
Which therapy for which patient: Focus on special populations and
chronic infections. J Am Acad Dermatol. 80:43–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martín B, Sánchez-Carazo JL,
Pérez-Ferriols A, Laguna C, Oliver V and Alegre V: Clinical
experience with etanercept in the treatment of psoriasis. Actas
Dermosifiliogr. 99:540–545. 2008.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sehgal VN, Pandhi D and Khurana A:
Biologics in dermatology: An integrated review. Indian J Dermatol.
59:425–441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niculet E, Neculia GV, Tatu AL and Buzia
OD: Curcumin-extraction, physical and chemical analysis, formulas
and control. Basic methods for further research. Mater Plast.
55:672–675. 2018.
|
|
27
|
Nwabudike LC and Tatu AL: Using
complementary and alternative medicine for the treatment of
psoriasis: A step in the right direction. JAMA Dermatol.
155:6362019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Căruntu C, Boda D, Dumitrașcu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomarkers Med. 9:513–528. 2015. View Article : Google Scholar
|
|
29
|
Ilie MA, Căruntu C, Lixandru D, Tampa M,
Georgescu SR, Constantin MM, Constantin C, Neagu M, Zurac SA and
Boda D: In vivo confocal laser scanning microscopy imaging
of skin inflammation: Clinical applications and research
directions. Exp Ther Med. 17:1004–1011. 2019.PubMed/NCBI
|
|
30
|
Căruntu C, Boda D, Musat S, Căruntu A and
Mandache E: Stress-induced mast cell activation in glabrous and
hairy skin. Mediators Inflamm. 2014:1059502014. View Article : Google Scholar : PubMed/NCBI
|