Introduction
As one of the major malignancies in humans, lung
cancer is a leading cause of cancer mortality worldwide (1). It has been reported that the morbidity
and mortality of lung cancer has continued to increase in recent
years (2). According to pathological
characteristics, lung cancer can be divided into non-small cell
lung cancer (NSCLC) and small cell lung cancer (3), the former of which accounts for ~75–80%
of all lung cancer cases (4). At
present, the most effective therapy for patients with NSCLC is
surgery (5). Unfortunately, many
patients are diagnosed at an advanced stage, which results in
failed surgery (6). Although great
advances have been made in surgery, chemotherapy, radiotherapy and
targeting therapy, patients with NSCLC still experience limited
early prognosis and diagnosis methods, causing substantial
financial and psychological burdens (7). At the early onset of the disease, few
symptoms are noticed by patients with NSCLC (8). Therefore, it is important to quickly
and effectively identify possible biomarkers of NSCLC to develop
early preventative therapeutic methods.
MicroRNAs (miRNAs or miRs) are small non-coding RNAs
that exert extensive post transcriptional regulation by binding to
the 3′untranslated region (3′UTR) of target mRNAs (9,10). The
abnormal expression of microRNAs (miRNAs or miRs) have been
detected in various types of tumor, including NSCLC (11,12). For
example, miR-200c has been demonstrated to suppress NSCLC invasion
and metastasis by targeting ubiquitin-specific peptidase 25
(11). In addition, miR-377
suppresses NSCLC by decreasing the expression of astrocyte elevated
gene-1 (12). Circulating miRNAs,
including those in serum and plasma, have been demonstrated to
serve as potential noninvasive biomarkers for the early
identification of various types of cancer due to their long-term
stability in the circulatory system (13,14). In
NSCLC, the upregulation of plasma miR-21 has been demonstrated to
be effective for its early detection and chemosensitivity (15). In addition, circulating miR-29a and
miR-150 have been reported to be associated with the delivery dose
of thoracic radiation therapy in NSCLC (16).
The expression pattern of miR-484 has been revealed
to be significantly different in various types of tumor, including
breast cancer, cervical cancer and NSCLC (17,18). It
has been reported that enhanced miR-484 promotes the progression of
NSCLC by suppressing apoptotic protease activating factor-1
(19). However, to the best of our
knowledge, whether miR-484 could be used as a potential biomarker
for the early detection of NSCLC is yet to be elucidated. The aim
of the current study was to assess the expression and clinical
significance of serum miR-484 in patients with NSCLC.
Patients and methods
Patients and specimens
A total of 150 serum samples were collected from 150
patients with NSCLC (male, n=85; female, n=65; age, 56.8±13.9
years) at Laiyang Central Hospital (Laiyang, China) from Feb 2016
to Jan 2017. The diagnosis of all samples was histopathologically
confirmed by two pathologists at Laiyang Central Hospital. None of
the patients had received neoadjuvant chemotherapy or radiotherapy
prior to surgery. The clinicopathological results were determined
according to the classification of malignant tumors by the World
Health Organization (20). TNM was
staged (Stage I, n=45; Stage II, n=55; Stage III, n=27; Stage IV,
n=28) and the details are provided in Table I. Overall survival was defined as the
time interval between the date of surgery and the date of
mortality. An additional 50 healthy volunteers (24 males, 26
females; average age, 58.6±14.5 years) from the Physical
Examination Center of Laiyang Central Hospital between February
2016 and February 2017 were enrolled as the normal control group.
Informed consent was obtained from all participants prior to
enrollment and the study was approved by the Ethics Committee of
Laiyang Central Hospital (Laiyang, China). Peripheral blood was
collected in VP-AS109K Vacutainer tubes (Terumo Medical
Corporation, Tokyo, Japan) and incubated at room temperature for 30
min. To isolate serum, peripheral blood was centrifuged at 1,500 ×
g for 10 min at 4°C to. Serum was then further centrifuged at
20,000 × g for 10 min at 4°C to remove cell debris. Finally, serum
was divided into 200 µl aliquots and stored at −80°C until use.
Hemolyzed serum samples were excluded.
 | Table I.Association of microRNA-484 expression
and the clinicopathological variables of patients with non-small
cell lung cancer. |
Table I.
Association of microRNA-484 expression
and the clinicopathological variables of patients with non-small
cell lung cancer.
| Variables | Number | Low (n=68) | High (n=82) | P-value |
|---|
| Age |
|
|
| 0.825 |
|
<60 | 70 | 34 | 36 |
|
| ≥60 | 80 | 39 | 41 |
|
| Sex |
|
|
| 0.532 |
| Male | 85 | 43 | 42 |
|
|
Female | 65 | 32 | 33 |
|
| Tumor size |
|
|
| 0.278 |
| <5
cm | 100 | 53 | 47 |
|
| ≥5
cm | 50 | 24 | 26 |
|
| Histological
grade |
|
|
| 0.013 |
| I | 98 | 47 | 51 |
|
|
II–III | 52 | 25 | 27 |
|
| Lymph node
metastasis |
|
|
| 0.004 |
|
Yes | 50 | 24 | 26 |
|
| No | 100 | 53 | 47 |
|
| Distant
metastasis |
|
|
| 0.032 |
|
Yes | 52 | 23 | 32 |
|
| No | 98 | 45 | 53 |
|
| Clinical stage |
|
|
| 0.004 |
|
I–II | 95 | 55 | 40 |
|
|
III–IV | 55 | 32 | 23 |
|
Cell culture
Four NSCLC cell lines, including A549, NCI-H460, 95D
and H358, and a normal human bronchial epithelial cell line (16HBE)
were purchased from the Institute of Biochemistry and Cell Biology
of the Chinese Academy of Sciences (Shanghai, China). All cells
were cultured in RPMI 1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS) (Invitrogen; Thermo fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin in a humidified
incubator at 37°C with 5% CO2.
Transient transfection
An miR-484 inhibitor (5′-ATGGGAGGGGACTGAGCCTGA-3′)
or a negative control (NC; 5′-ACUAGUCGAUCUAUGUGUGAUATT-3′; both
Shanghai GenePharma Co., Ltd., Shanghai, China) was transfected
into 95D and H358 cells using 6 µl Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) per well in a 6-well
plate according to the manufacturers' protocol. 95D and H358 cells
(1×106 cells/well) were seeded in a six-well plate with
2 ml RPMI-1640 medium. After cells reached a 60% confluence, the
miR-484 inhibitor or NC was mixed with Lipofectamine®
2000 at room temperature for 20 min. Then, the mixture was added
into each well at a final concentration of 20 nM for 48 h and the
cells were collected for further analysis.
RNA extraction
To isolate the RNA (10 µg) from the serum samples (5
ml) or NSCLC cancer tissues or 95D and H358 cells transfected with
miR-484 inhibitor for 1, 2, 3, 4 and 5 days, RNAVzol LS (Vigorous
Biotechnology Co., Ltd., Beijing, China) was used in the present
study according to the manufacturer's protocol. To determine the
concentration and purity of the RNA samples, the
OD260/OD280 ratio was evaluated using a
microplate reader (Model 3550; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For RT, a total of 1 µg of RNA isolated from the
serum samples (5 ml) or NSCLC cancer tissues or 95D and H358 cells
was added to a TaqMan MicroRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). U6, a small nuclear
RNA, was used as an internal control (21). The primer sequences for reverse
transcription were as follows: miR-484,
5′-CGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACATCGG-3′; and U6,
5′-TCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAATATG-3′. The
primer sequences for qPCR were as follows: miR-484 forward,
5′-TCAGGCTCAGTCCCCTC-3′; U6, forward 5′-GCGTCGTGAAGCGTTC-3′; and
universal reverse primer, 5′-GTGCAGGGTCCGAGGT-3′.
PCR amplifications were performed using a 10 µl
reaction system, which contained 5 µl SYBR Green Supermix (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), 0.4 µl forward primer, 0.4
µl reverse primer, 2.2 µl double-distilled water and 2 µl template
cDNA using a Bio-Rad iCycler iQ Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.). The thermocycling conditions for qPCR
was listed as follows: Denaturation at 95°C for 10 min, followed by
40 cycles of annealing and elongation at 95°C for 15 sec and a
final extension at 60°C for 1 min. Relative levels of miR-484 were
determined using the 2−ΔΔCq method (22). In the present study, the cut off
value was defined as the mean value of serum miR-484 level in
patients with NSCLC, that was divided into high serum miR-484
(>16.06) and low miR-484 (≤16.06) levels.
Cell proliferation assay
To determine cell proliferation, a Cell Counting
kit-8 (CCK-8; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) was used. 95D and H358 cells were seeded into
96-well plates at a density of 2,000 cells/well. Subsequently, 95D
and H358 cells were transfected with a miR-484 inhibitor or an NC
for 1, 2, 3, 4 and 5 days as aforementioned. For the cell
proliferation assay, 10 µl of CCK-8 solution was added into each
well following 1, 2, 3, 4 and 5 days of incubation at 37°C for 15
min. WST-8 was metabolized in viable cells and produced a chromogen
that was detected at 450 nm using a Spectra Max M2
spectrophotometer (SpectraMax M2, Molecular Devices, LLC,
Sunnyvale, CA, USA).
Cell cycle analysis
For cell cycle analysis, 95D and H358 cells were
transfected with a miR-484 inhibitor or an NC for 48 h and then
harvested. Subsequently, cells were fixed with 70% ethanol at −20°C
for 24 h. Then, 95D and H358 (~1×106) cells were
trypsinized, washed twice with PBS and fixed in 70% ice-cold
ethanol for 1 h. To remove ethanol and displace sediment, samples
were centrifuged at 300 × g for 5 min at 4°C and were then exposed
to 100 mg/ml RNaseA (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
for 30 min at 37°C. Propidium iodide (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China) was used to stain cellular DNA at room
temperature for 10 min. Cell-cycle distributions were assessed
using a BD FACSCalibur flow cytometry system (BD Biosciences,
Franklin Lakes, NJ, USA) with ModFit software version 4.1 (Verity
Software House, Inc., Topsham, ME, USA).
Cell migration and invasion
assays
Cell migration assays were performed with Boyden
chambers (8-µm pore filter; Corning Inc., Corning, NY, USA). For
the cell invasion assay, filter surfaces were precoated with 1%
Matrigel (BD Biosciences). 95D and H358 cells were seeded in
six-well plates at a density of 105 cells/well in the
upper chamber with RPMI-1640 medium. 95D and H358 cells were also
transfected with a miR-484 inhibitor or an NC for 48 h in the
corresponding upper chamber. RPMI-1640 medium (600 µl) with 20% FBS
was plated in the lower chamber. Following incubation at 37°C for
48 h, cotton swabs were used to remove non-migratory and
non-invading cells. Migratory and invasive cells were then fixed in
methanol for 30 min at 37°C and stained with 0.5% crystal violet
for 1 h at 37°C. Stained cells were counted in 5 random fields
under a light microscope (magnification, ×40; XDS-500D; Shanghai
Caikon Optical Instrument Co., Ltd., Shanghai, China).
Statistical analysis
Data were analyzed using SPSS software (version
13.0; SPSS, Inc., Chicago, IL, USA). The relevant data were
expressed as the mean ± standard deviation. To examine differences
between two groups, a two-tailed unpaired Student's t-test was
used. For comparisons of more than two groups, one-way analysis of
variance followed by a Tukey's post hoc test were performed. The
χ2-test was used to assess the association between serum
miR-484 expression and clinicopathologic features of NSCLC. A
receiver operating characteristic curve (ROC) curve was created and
the area under the curve was determined to assess the specificity
and sensitivity of circulating miR-484 as a diagnostic biomarker. A
Cox proportional hazards model was used for multivariate analysis.
Multivariable Cox regression models were built for the analysis of
risk factors for survival outcomes between patients with NSCLC and
healthy controls. The comparison of overall survival rate between
patients with NSCLC and healthy controls was made using the
Kaplan-Meier method with a log-rank test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Serum miR-484 is increased in the
patients with NSCLC and NSCLC cell lines
The current study assessed levels of miR-484 in the
serum of patients with NSCLC and healthy controls. Compared with
the serum miR-484 levels of the healthy controls (1±1.23), miR-484
was significantly higher in patients with NSCLC (32.12±5.67;
Fig. 1A). The level of miR-484 was
then assessed according to TNM staging. Compared with stage I
(1±0.24), the level of miR-484 was increased in stage II
(2.43±0.43), stage III (4.21±0.78) and stage IV (6.35±0.82;
Fig. 1B). In addition, relative
miR-484 levels were assessed in various NSCLC cell lines. Compared
with the level of miR-484 in the 16HBE cells (1±0.12), the level of
miR-484 was significantly higher in A549 (2.56±0.34), H460
(3.45±0.27), 95D (5.67±0.68) and H358 (3.45±0.78) cells (Fig. 1C).
Serum miR-484 distinguishes patients
with NSCLC from healthy controls
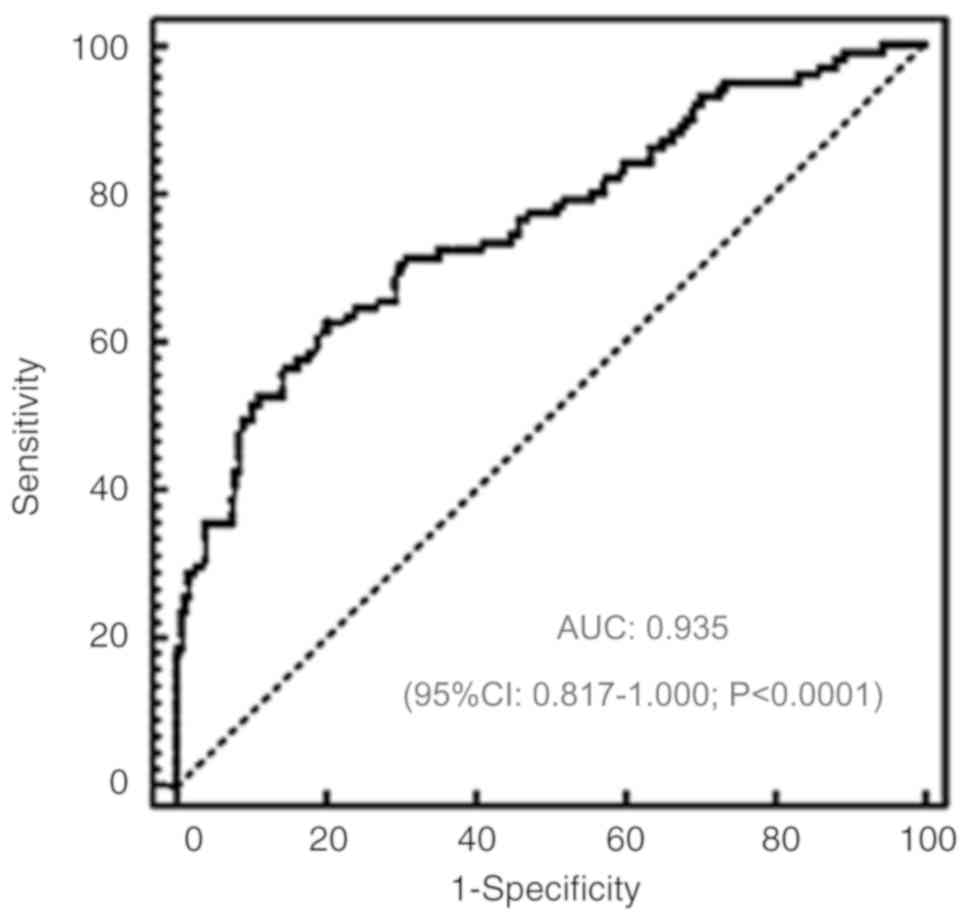
The diagnostic value of serum miR-484 in patients
with NSCLC was determined. ROC analysis revealed that serum miR-484
could distinguish patients with NSCLC from healthy controls, with
an ROC curve area of 0.935 (95% confidence interval: 0.817–1.000;
P<0.0001; Fig. 2).
Association of Serum miR-484 with the
clinicopathological characteristics of patients with NSCLC
The current study analyzed the clinical relevance of
serum miR-484 expression in patients with NSCLC. Patients with
NSCLC (n=150) were further divided into two groups based on their
average expression of serum miR-484: A high expression group (n=82)
and a low expression group (n=68). As presented in Table I, serum miR-484 was positively
associated with histological grade, lymph node metastasis, distant
metastasis and clinical stage. However, no association was
identified between serum miR-484 and patient sex, age, or tumor
size.
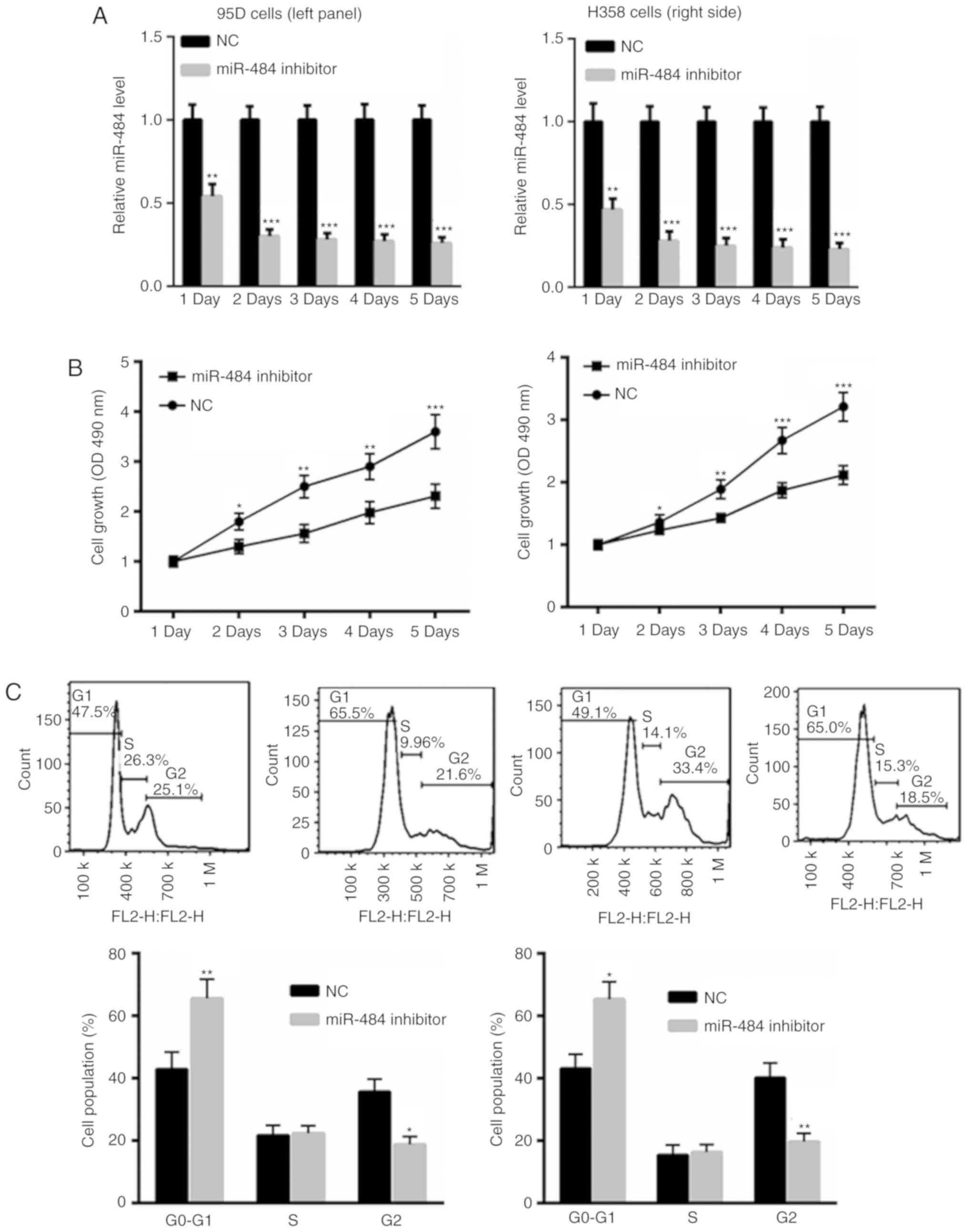
miR-484 inhibition suppresses NSCLC
cell proliferation and induces cell cycle arrest
To inhibit miR-484 levels, 95D and H358 cells were
transfected with a miR-484 inhibitor or an NC for 1, 2, 3, 4 and 5
days. As presented in Fig. 3A,
transfection with the miR-484 inhibitor significantly suppressed
miR-484 levels in 95D and H358 cells at each day of incubation when
compared with NC treated cells. The results also revealed the
reduced growth of 95D and H358 cells transfected with the miR-484
inhibitor compared with those transfected with the NC (Fig. 3B). Flow cytometric analysis
determined that the inhibition of miR-484 induced a more pronounced
cell cycle arrest in 95D and H358 cells compared with the NC group
(Fig. 3C). These data indicate the
tumor suppressive role of the miR-484 inhibitor in NSCLC cells.
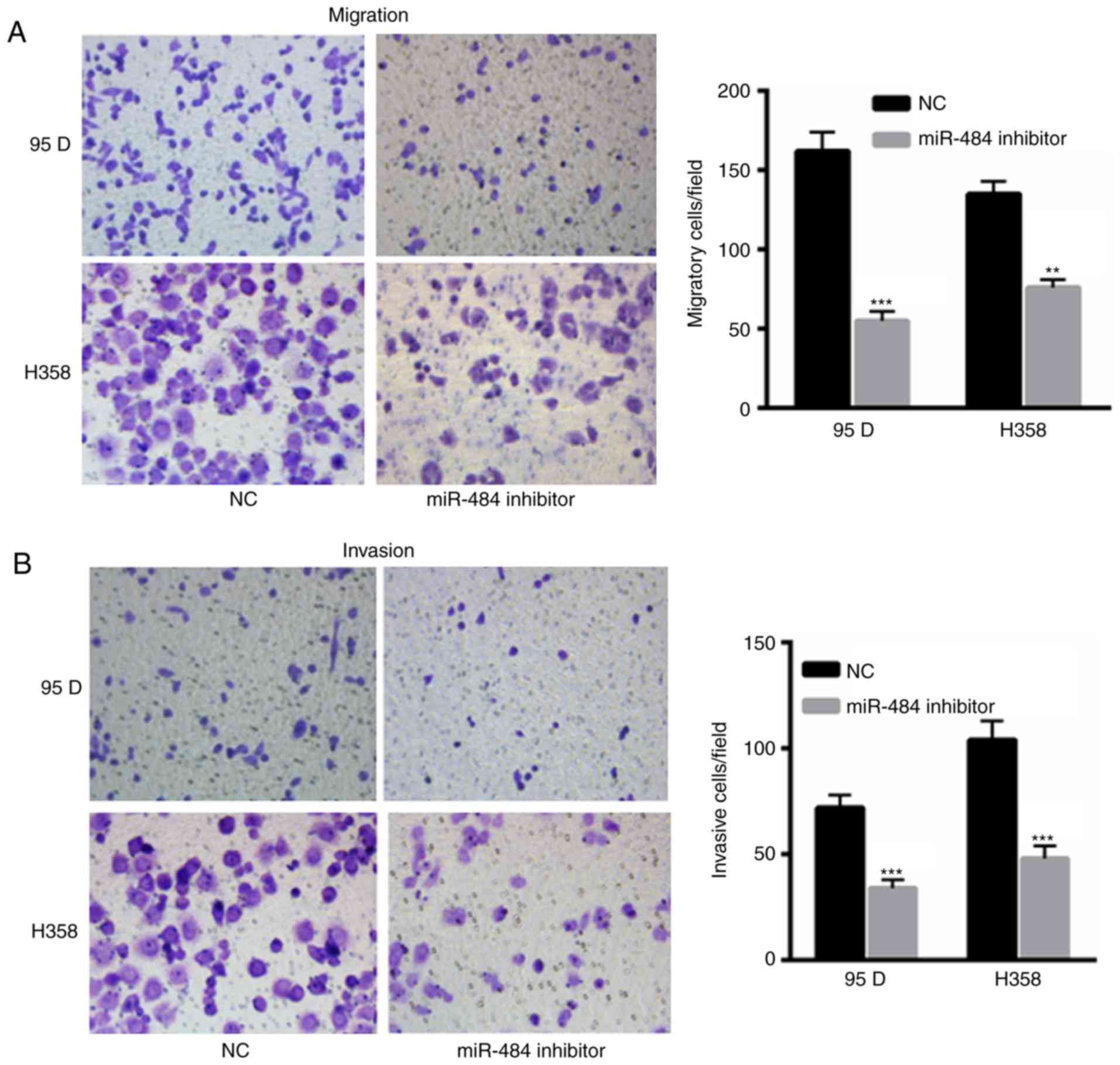
miR-484 inhibition reduces NSCLC cell
invasion and migration
The migration and invasion of 95D and H358 cells
transfected with miR-484 were decreased compared with those
transfected with the NC (Fig. 4A and
B). The results indicate the oncogenic role of miR-484 in the
development of NSCLC.
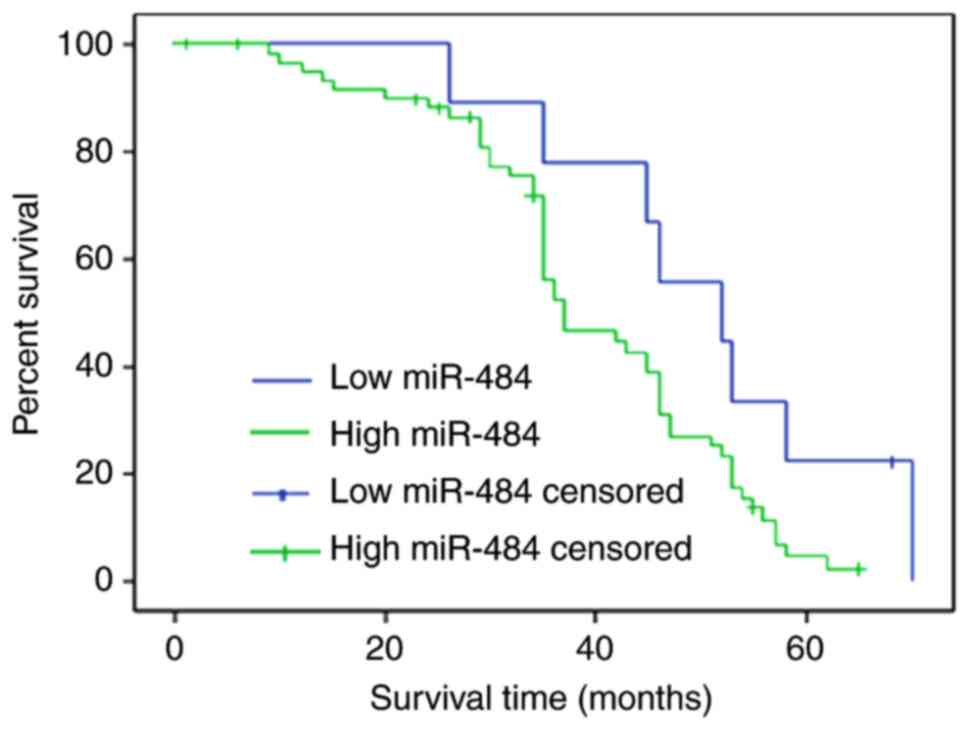
miR-484 upregulation predicts the
deterioration of NSCLC
The present study compared the overall survival rate
of patients with NSCLC and high or and low serum miR-484 levels.
The cut off value was defined as the mean value of serum miR-484 in
patients with NSCLC (high serum miR-484, >16.06; low miR-484,
≤16.06). Compared with low serum miR-484 patients, high serum
miR-484 NSCLC patients exhibited a shorter overall survival time
(Fig. 5). Univariate and
multivariate analysis was performed to assess whether serum miR-484
was an independent prognostic parameter. According to univariate
analysis, the following were significant risk factors for poor
survival: Lymph node metastasis, distant metastasis, clinical stage
and serum miR-484 (Table II).
According to multivariate analysis with Cox regression, lymph node
metastasis, distant metastasis, clinical stage and serum miR-484
were independent prognostic factors for NSCLC (Table II).
 | Table II.Univariate and multivariate analysis
of overall survival in patients with non-small cell lung cancer |
Table II.
Univariate and multivariate analysis
of overall survival in patients with non-small cell lung cancer
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | P-value | HR | P-value |
|---|
| Age | 1.534 | 0.421 | – | – |
| Sex | 1.321 | 0.578 | – | – |
| Tumor size | 1.678 | 0.113 | – | – |
| Histology type | 1.345 | 0.203 | – | – |
| Lymph node
metastasis | 3.932 | 0.012 | 3.245 | 0.024 |
| Distant
metastasis | 3.012 | 0.015 | 2.543 | 0.036 |
| Clinical stage | 3.934 | 0.006 | 3.465 | 0.009 |
| Serum miR-484 | 3.865 | 0.003 | 3.452 | 0.001 |
Discussion
NSCLC is one of the most common malignancies that
threatens human health and life (23). The major therapy methods for NSCLC
include radiotherapy and chemotherapy (24), but due to resistance, the mortality
rate of NSCLC remains high (25).
Furthermore, the underlying mechanism by which NSCLC is modulated
is not fully understood (25).
miRNAs are key regulators of NSCLC progression and therefore may
become an important therapeutic target (26). The abnormal expression of circulating
miRNA has also been widely reported in patients with NSCLC
(6,26).
Increasing evidence has indicate the important role
of miR-484 in various types of tumor (18,27,28). For
instance, increased miR-484 levels have been revealed to promote
glioma progression by regulating extracellular signal-regulated
kinase 1/2 signaling (27). In
metastatic renal cell carcinoma, miR-484 has been reported to be
increased and associated with tumor progression (28). However, in cervical cancer, miR-484
serves as a tumor suppressor by suppressing zinc finger
E-box-binding homeobox 1 and SMAD2 (18). Circulating miRNA exhibits a good
stability in serum, which is associated with the occurrence and
development of tumors (29). In
addition, serum samples are easy to obtain and as such may be used
for the early screening of tumors in healthy individuals (29). Chen et al (30) reported 10 differentially expressed
miRNAs in the serum of patients with NSCLC patients, including
miR-20a, miR-24, miR-145, miR-152, miR-199a-5p, miR-221, miR-222,
miR-223 and miR-320. The authors concluded that these may serve as
potential biomarkers for patients with NSCLC. However, to the best
of our knowledge, whether serum miR-484 is differentially expressed
in patients with NSCLC is yet to be elucidated, despite the fact
that a high miR-484 expression has been reported in NSCLC tissue
samples and cell lines (19).
To the best of our knowledge, the current study
demonstrated for the first time that serum miR-484 is increased in
patients with NSCLC compared with healthy controls. The clinical
relevance of serum miR-484 expression was also assessed in patients
with NSCLC. The results revealed that serum miR-484 was positively
associated with histological grade, lymph node metastasis, distant
metastasis and clinical stage. These data indicated that higher
miR-484 levels are associated with the severity of malignancy in
patients with NSCLC. Furthermore, based on the results of ROC
analysis, the data revealed that serum miR-484 could differentiate
patients with NSCLC from healthy controls, indicating that serum
miR-484 may serve as a potential non-invasive biomarker for
NSCLC.
The present study also analyzed the prognostic
significance of serum miR-484 in patients with NSCLC. A
Kaplan-Meier analysis with a log-rank test demonstrated that
patients with NSCLC and a high serum expression of miR-484
demonstrated significantly poorer overall survival rates than those
with low serum miR-484 levels. Additionally, according to the
multivariate Cox proportional hazards analysis, high serum miR-484
expression was independently associated with poor survival. The
current study therefore hypothesizes that serum miR-484 may be used
as an effective biomarker for the diagnosis and prognosis of
patients with NSCLC.
The present study has various limitations. Only a
limited number of samples were included in the current study and an
increased sample size should be used in the future to validate the
accuracy of miR-484 as a biomarker. Furthermore, more experiments
should be performed to confirm that miR-484 may be utilized to
screen patients with lung cancer from healthy individuals. For
instance, ROC analysis should be performed to analyze the
association between serum miR-484 and the TNM stage of patients,
which would validate whether miR-484 could be used as an early
biomarker for NSCLC.
In summary, the present study demonstrated that
miRNA-484 was upregulated in the serum of patients with NSCLC.
Increased miRNA-484 expression was also positively associated with
aggressive progression and poor prognosis. These results
demonstrate that serum miRNA-484 may be used as a potential
biomarker for patients with NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding obtained
from Laiyang Central Hospital (Laiyang, China; grant no.
LYCH-20180634).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ performed the experiments and analyzed the data.
CS performed RT-qPCR experiments. HG designed the experiments,
analyzed the data and gave approval for the publication of the
final version of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Laiyang Central Hospital (Laiyang, China) and
all patients provided written informed consent prior to
enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tian W, Wang G, Liu Y, Huang Z, Zhang C,
Ning K, Yu C, Shen Y, Wang M, Li Y, et al: The miR-599 promotes
non-small cell lung cancer cell invasion via SATB2. Biochem Biophys
Res Commun. 485:35–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang D, Ma J, Ji X, Xu F and Wei Y:
miR-141 regulation of EIF4E expression affects docetaxel
chemoresistance of non-small cell lung cancer. Oncol Rep.
37:608–616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang H, Shen Q, Zhang X, Yang C, Cui S,
Sun Y, Wang L, Fan X and Xu S: The long non-coding RNA XIST
controls non-small cell lung cancer proliferation and invasion by
modulating miR-186-5p. Cell Physiol Biochem. 41:2221–2229. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Wang Y, Sun D, Bu J, Ren F, Liu B,
Zhang S, Xu Z, Pang S and Xu S: miR-455-5p promotes cell growth and
invasion by targeting SOCO3 in non-small cell lung cancer.
Oncotarget. 8:114956–114965. 2017.PubMed/NCBI
|
|
5
|
Wang K, Dong L, Fang Q, Xia H and Hou X:
Low serum miR-98 as an unfavorable prognostic biomarker in patients
with non-small cell lung cancer. Cancer Biomark. 20:283–288. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Liu W, Zhang YP and Huang XR: The
miR-224 promotes non-small cell lung cancer cell proliferation by
directly targeting RASSF8. Eur Rev Med Pharmacol Sci. 21:3223–3231.
2017.PubMed/NCBI
|
|
7
|
Wang M, Meng B and Liu Y, Yu J, Chen Q and
Liu Y: MiR-124 inhibits growth and enhances radiation-induced
apoptosis in non-small cell lung cancer by inhibiting STAT3. Cell
Physiol Biochem. 44:2017–2028. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang P, Chen D, Ma H and Li Y: LncRNA
SNHG12 contributes to multidrug resistance through activating the
MAPK/Slug pathway by sponging miR-181a in non-small cell lung
cancer. Oncotarget. 8:84086–84101. 2017.PubMed/NCBI
|
|
9
|
Wang P, Deng Y and Fu X: MiR-509-5p
suppresses the proliferation, migration, and invasion of non-small
cell lung cancer by targeting YWHAG. Biochem Biophys Res Commun.
482:935–941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang SY, Li Y, Jiang YS and Li RZ:
Investigation of serum miR-411 as a diagnosis and prognosis
biomarker for non-small cell lung cancer. Eur Rev Med Pharmacol
Sci. 21:4092–4097. 2017.PubMed/NCBI
|
|
11
|
Li J, Tan Q, Yan M, Liu L, Lin H, Zhao F,
Bao G, Kong H, Ge C, Zhang F, et al: miRNA-200c inhibits invasion
and metastasis of human non-small cell lung cancer by directly
targeting ubiquitin specific peptidase 25. Mol Cancer. 13:1662014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng F, Zhang L, Shao Y, Ma Q and Lv H:
MicroRNA-377 inhibits non-small-cell lung cancer through targeting
AEG-1. Int J Clin Exp Pathol. 8:13853–13863. 2015.PubMed/NCBI
|
|
13
|
Weber DG, Brik A, Casjens S, Burek K,
Lehnert M, Pesch B, Taeger D, Brüning T and Johnen G; MoMar study
Group, : Are circulating microRNAs suitable for the early detection
of malignant mesothelioma? Results from a nested case-control
study. BMC Res Notes. 12:772019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
do Amaral AE, Cisilotto J, Creczynski-Pasa
TB and de Lucca Schiavon L: Circulating miRNAs in nontumoral liver
diseases. Pharmacol Res. 128:274–287. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei J, Gao W, Zhu CJ, Liu YQ, Mei Z, Cheng
T and Shu YQ: Identification of plasma microRNA-21 as a biomarker
for early detection and chemosensitivity of non-small cell lung
cancer. Chin J Cancer. 30:407–414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dinh TK, Fendler W, Chalubinska-Fendler J,
Acharya SS, O'Leary C, Deraska PV, D'Andrea AD, Chowdhury D and
Kozono D: Circulating miR-29a and miR-150 correlate with delivered
dose during thoracic radiation therapy for non-small cell lung
cancer. Radiat Oncol. 11:612016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye FG, Song CG, Cao ZG, Xia C, Chen DN,
Chen L, Li S, Qiao F, Ling H, Yao L, et al: Cytidine deaminase axis
modulated by miR-484 differentially regulates cell proliferation
and chemoresistance in breast cancer. Cancer Res. 75:1504–1515.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y, Xie H, Liu Y, Liu W, Liu M and Tang
H: miR-484 suppresses proliferation and epithelial-mesenchymal
transition by targeting ZEB1 and SMAD2 in cervical cancer cells.
Cancer Cell Int. 17:362017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li T, Ding ZL, Zheng YL and Wang W:
MiR-484 promotes non-small-cell lung cancer (NSCLC) progression
through inhibiting Apaf-1 associated with the suppression of
apoptosis. Biomed Pharmacother. 96:153–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osmani L, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ofori JK, Salunkhe VA, Bagge A, Vishnu N,
Nagao M, Mulder H, Wollheim CB, Eliasson L and Esguerra JL:
Elevated miR-130a/miR130b/miR-152 expression reduces intracellular
ATP levels in the pancreatic beta cell. Sci Rep. 7:449862017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang T, Liu X, Tian Q, Liang T and Chang
P: Increasing expression of miR-5100 in non-small-cell lung cancer
and correlation with prognosis. Eur Rev Med Pharmacol Sci.
21:3592–3597. 2017.PubMed/NCBI
|
|
24
|
Wang Y, Cong W, Wu G, Ju X, Li Z, Duan X,
Wang X and Gao H: MiR-376a suppresses the proliferation and
invasion of non-small-cell lung cancer by targeting c-Myc. Cell
Biol Int. 42:25–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Liu Z, Fang X and Yang H:
MiR-142-5p suppresses tumorigenesis by targeting PIK3CA in
non-small cell lung cancer. Cell Physiol Biochem. 43:2505–2515.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei K, Pan C, Yao G, Liu B, Ma T, Xia Y,
Jiang W, Chen L and Chen Y: MiR-106b-5p promotes proliferation and
inhibits apoptosis by regulating BTG3 in non-small cell lung
cancer. Cell Physiol Biochem. 44:1545–1558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yi R, Feng J, Yang S, Huang X, Liao Y, Hu
Z and Luo M: miR-484/MAP2/c-Myc-positive regulatory loop in glioma
promotes tumor-initiating properties through ERK1/2 signaling. J
Mol Histol. 49:209–218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Merhautova J, Hezova R, Poprach A,
Kovarikova A, Radova L, Svoboda M, Vyzula R, Demlova R and Slaby O:
miR-155 and miR-484 Are associated with time to progression in
metastatic renal cell carcinoma treated with sunitinib. Biomed Res
Int. 2015:9419802015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Halvorsen AR, Sandhu V, Sprauten M, Flote
VG, Kure EH, Brustugun OT and Helland Å: Circulating microRNAs
associated with prolonged overall survival in lung cancer patients
treated with nivolumab. Acta Oncol. 57:1225–1231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen X, Hu Z, Wang W, Ba Y, Ma L, Zhang C,
Wang C, Ren Z, Zhao Y, Wu S, et al: Identification of ten serum
microRNAs from a genome-wide serum microRNA expression profile as
novel noninvasive biomarkers for nonsmall cell lung cancer
diagnosis. Int J Cancer. 130:1620–1628. 2012. View Article : Google Scholar : PubMed/NCBI
|