Introduction
As a common neuropsychiatric disorder, depression is
one of the major causes of disability worldwide (1). Depression is characterized by a variety
of symptoms, including persistent low mood, cognitive impairment,
intellectual ability retardation and somatic symptoms (2). With the rapid social and economic
development, stress and adversity are becoming more severe, which
may lead to depression. According to the prediction of WHO, the
incidence of depression increases 113% annually and is expected to
become the second largest disease burden worldwide by the end of
2020 (3). Although the cause of
depression is poorly understood, stress is considered to be the
main contributing factor leading to biochemical alterations in the
brain that present as the clinical symptoms of depression (4,5). To
date, >20 types of animal models of depression have been
established, and the chronic unpredictable mild stress (CUMS)
model, originally developed by Willner et al (6), is the most widely used model. At
present, tricyclic antidepressants, monoamine oxidase inhibitors
and selective 5-hydroxytryptamine reuptake inhibitors are the most
commonly used drugs for the treatment of depression; however, their
effect is not satisfactory. Therefore, identifying drugs with high
efficiency and low toxicity for the treatment of depression is
currently urgent.
β-asarone (also known as
cis-2,4,5-trimethoxy-1-allyl phenyl) is the main active ingredient
of the traditional Chinese medicinal herb Acorus tatarinowii
Schott. β-asarone has various pharmacological properties, and can
easily pass through the blood-brain barrier and be distributed in
the brain (7). Recent data have
demonstrated that β-asarone can significantly affect the central
nervous system and serves an important role in relieving neuronal
apoptosis (8–10). Studies have also evaluated the
anti-tumor effect of β-asarone (11,12).
Furthermore, it has been reported that β-asarone has antidepressive
effects (13–17); however, the precise role and
underlying mechanisms of these effects remain largely unclear.
Therefore, the current study aimed to investigate
the effects of β-asarone administration in a rat model of
depression induced by CUMS and to further explore the underlying
molecular mechanisms.
Materials and methods
Depression model establishment
In total, 120 healthy 6-week-old male Sprague-Dawley
rats (180–210 g) were obtained from the Vital River Laboratory
Animal Technology Co., Ltd. (Beijing, China). The rats were fed
under standard conduction (12-h light/dark cycle; 55±5% humidity;
22±2°C), and were provided with food and water ad libitum. All
animal experiments were conducted according to the Guidelines for
the Care and Use of Laboratory Animals by the National Institutes
of Health. The present study was approved by the Ethics Committee
of Qiqihar Medical University (Qiqihar, China).
The rat model of depression was established by CUMS
treatment for 6 weeks as described in a previous study (18). Briefly, rats were group-housed and
allowed to adapt to the environment for 1 week. Next, rats were
single-housed and subjected to a variety of mild stressors for 6
weeks, with the exception of the control group rats, which were
undisturbed in their cages in a separated room throughout the
following 6 weeks. The mild stressors were as follows: Food
deprivation for 24 h; water deprivation for 24 h; overnight
illumination; cage tilt (45°) for 7 h; soiled cage (200 ml water in
100 g sawdust bedding); foreign object exposure; light/dark
perversion; hanging the rat on a balance bar with rope for 10 min;
physical restraint for 3 h; 1-min tail pinch (1 cm from the
beginning of the tail); 5-min oscillation; and exposure to white
noise for 1 h. To ensure the unpredictability of the experiment,
all stressors were performed randomly. Two or three types of
stimuli were randomly scheduled daily for a total of 28 days. The
same stimulus was not repeated for 3 consecutive days.
Rats were randomly divided into six groups (n=20 per
group), as follows: i) Control group, unstressed + saline vehicle
(0.01 ml/g body weight); ii) model group, CUMS + saline vehicle
(0.01 ml/g body weight); iii) CUMS + 12.5 mg/kg/day β-asarone
treatment group; iv) CUMS + 25 mg/kg/day β-asarone treatment group;
v) CUMS + 50 mg/kg/day β-asarone treatment group; and vi) CUMS + 20
mg/kg/day fluoxetine treatment group, serving as the positive
control. Beginning on week 4, the rats were orally administered
with β-asarone or fluoxetine every day for 3 weeks. β-asarone and
fluoxetine hydrochloride were obtained from Hubei Bangsun Chemical
Co., Ltd. (Wuhan, China). At the end of the treatment, behavioral
tests were conducted, and the hippocampal tissues of the rats were
collected (week 6 after CUMS) according to the previous study
(18,19). Rats were decapitated after
anesthetization with 5% chloral hydrate (350 mg/kg;
intraperitoneally). No rats exhibited signs of peritonitis
following the administration of anesthetic.
Open field test
The open field test was performed as previously
described (20). The open field
device used was a four-sided black metallic enclosure (100×100×40
cm) with a white open floor and was divided into 25 equal sectors
by red lines. Each rat was placed alone in the center of the arena
and was allowed to explore freely for 5 min. The frequency of
rearing (rat erected on its hind legs) and number of crossings (rat
entered into a new sector with four paws) were recorded.
Forced swimming test
The forced swimming test was performed according to
a previously described method, with minor modifications (21). Briefly, each rat was forced to swim
in a cylindrical glass container (height, 50 cm; diameter, 20 cm)
with 30 cm of water (22±1°C) for 6 min. In the last 4 min, the
immobile time (rat floating in the water without swimming) was
recorded.
Sucrose preference test
The sucrose preference test was performed every week
[from the beginning of the experiment (week 1) to week 6 (after the
last treatment)], as described previously (22). The sucrose preference ratio was
calculated as follows: Sucrose preference value (%)=Sucrose intake
(g) × 100%/[sucrose intake (g) + water intake (g)].
Detection of the apoptosis in the
hippocampus
Extraction of hippocampal tissue from rats of
different groups and single cell suspension preparation were
performed according to a previous study (23). Briefly, hippocampal tissue was
extracted on ice, washed with cold saline, and then a 200 mesh
sieve was used to mechanically dissociate the tissues into a single
cell suspension. Subsequently, the cell suspension was centrifuged
at 4°C for 5 min at 670.8 × g, and the supernatant was discarded.
Finally, to detect the apoptosis in the hippocampus of rats, an
Annexin V-FITC Apoptosis Detection kit (Cell Signaling Technology,
Inc., Danvers, MA, USA) was used as per the manufacturer's
protocol. Flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA)
was then used to analyze cell apoptosis. Tests were repeated three
times.
Reverse transcription-quantitative
polymerase chain reaction (qPCR)
To extract the total RNA from hippocampal tissues,
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used in line with the manufacturer's
protocol. Total RNA concentration was detected using Nanodrop2000
(Thermo Fisher Scientific, Inc.). cDNA was then generated using the
TaqMan microRNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.), following the manufacturer's protocol.
Subsequently, the TaqMan® Universal PCR Master Mix kit
(Thermo Fisher Scientific, Inc.) was utilized to examine the gene
expression. The qPCR amplification conditions were as follows: 95°C
for 10 min, followed by 37 cycles of 95°C for 10 sec and 60°C for
60 sec. The primer sequences were obtained as required, and were as
follows: cAMP response element binding protein (CREB) forward,
5′-ACAGATTGCCACATTAGC-3′, and reverse, 5′-CTCCTCCCTGGGTAATGG-3′;
brain-derived neurotrophic factor (BDNF) forward,
5′-CCCTTCTACACTTTACCTCTTG-3′, and reverse,
5′-GTTTCACCCTTTCCACTCCTA-3′; tropomyosin receptor kinase B (Trk-B)
forward, 5′-GGGGCTTATGCTTGCTGGTC-3′, and reverse,
5′-CTCTGGGTCAATGCTGTTAGGTT-3′; B-cell lymphoma-2 (Bcl-2) forward,
5′-GGGACGCGAAGTGCTATTGGTA-3′, and reverse,
5′-CAGGCTGGAAGGAGAAGATGC-3′; Bcl-2-associated death promoter (Bad)
forward, 5′-ACACGCCCTAGGCTTGAGGA-3′, and reverse,
5′-GGCTCAAACTCTGGGATCTGGA-3′; and GAPDH forward,
5′-GACAACTTTGGCATCGTGGA-3′, and reverse,
5′-ATGCAGGGATGATGTTCTGG-3′. The 2−ΔΔCq method was
applied for relative gene expression quantification (24).
Western blot analysis
To measure the protein levels of CREB, BDNF, Trk-B,
Bcl-2, Bad, extracellular signal-regulated kinase (ERK) and
phosphorylated (p)-ERK in the hippocampus of rats from different
groups, western blot analysis was conducted. Total proteins from
the hippocampus of rats were extracted using a
radioimmunoprecipitation assay buffer (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China). The protein
quantification was calculated using the BCA assay (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. An
equal amount of protein samples (25 µg/lane) was resolved by 10%
SDS-PAGE and then blotted onto polyvinylidene difluoride membranes.
The membranes were first blocked with 5% skim milk at room
temperature for 2 h, and then incubated with a primary antibody
against CREB (cat. no. 9197; 1:1,000; Cell Signaling Technology,
Inc.), BDNF (cat no. Ab108319; 1:1,000; Abcam), Trk-B (cat. no.
4607; 1:1,000; Cell Signaling Technology, Inc.), Bcl-2 (cat. no.
4223; 1:1,000; Cell Signaling Technology, Inc.), Bad (cat. no.
9239; 1:1,000; Cell Signaling Technology, Inc.), ERK (cat. no.
4695; 1:1,000; Cell Signaling Technology, Inc.), p-ERK (cat. no.
4370; 1:1,000; Cell Signaling Technology, Inc.) or β-actin (cat.
no. 4970; 1:1,000; Cell Signaling Technology, Inc.) at 4°C
overnight. Subsequently, the membranes were incubated with
horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibodies (cat. no. 7074; 1:2,000; Cell Signaling Technology,
Inc.) at room temperature for 4 h. To visualize the protein blots,
an enhanced chemiluminescence detection kit (Cell Signaling
Technology, Inc.) was used in accordance with the manufacturer's
protocol. ImageJ 1.38X (National Institutes of Health, Bethesda,
MD, USA) was used to perform densitometry.
Statistical analysis
All experiments were repeated three times. Data are
displayed as the mean ± standard deviation. SPSS version 17.0
software (SPSS, Inc., Chicago, IL, USA) was used for data analysis.
Student's t-test or one-way analysis of variance followed by
Tukey's test was used to assess comparisons between groups.
P<0.05 was considered to denote a statistically significant
difference.
Results
β-asarone ameliorates body weight
reduction induced by CUMS
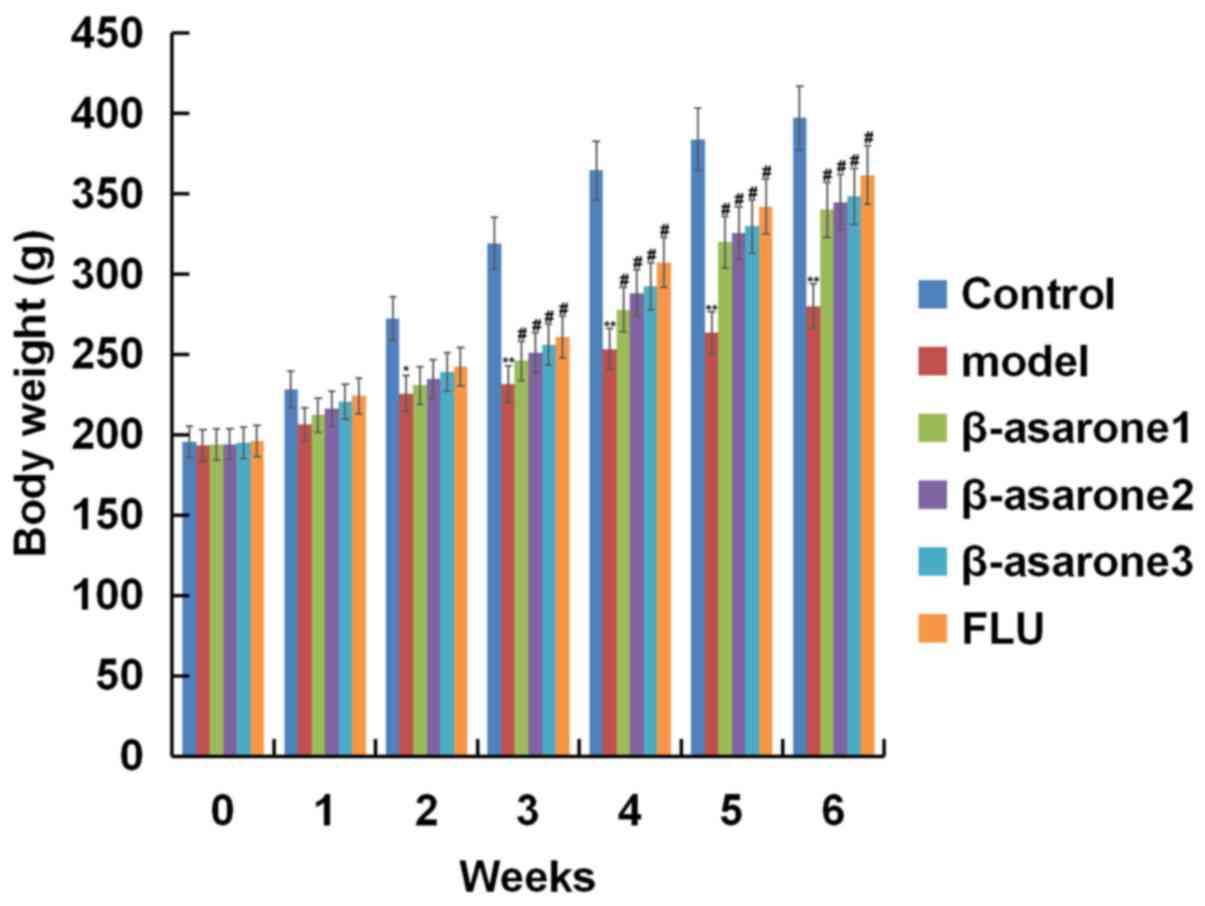
As shown in Fig. 1,
at the beginning of the test, the body weight of rats in different
groups was similar. After rats were subjected to CUMS for 2 weeks,
their body weight was significantly decreased in comparison with
that in the control group (P<0.05). Treatment with different
concentrations of β-asarone (12.5, 25 and 50 mg/kg/day) and with 20
mg/kg/day fluoxetine significantly ameliorated the reduction in the
body weight that was induced by CUMS. β-asarone administered at 50
mg/kg/day exhibited the most marked effect on the body weight of
CUMS induced rats.
β-asarone ameliorates
depression-associated symptoms induced by CUMS
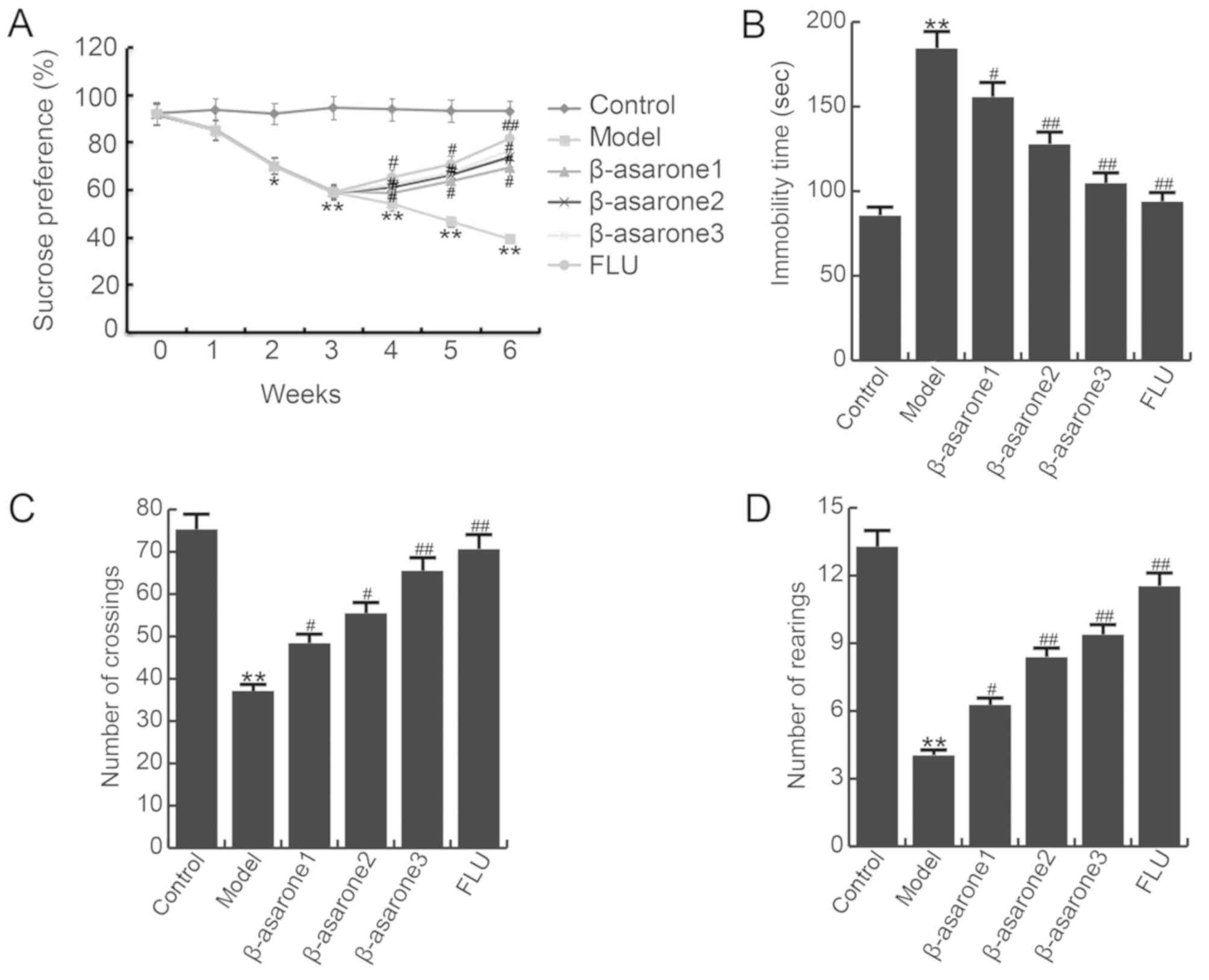
To investigate the effects of β-asarone on
CUMS-induced depression-like behavior, the sucrose preference,
forced swimming and open field tests were conducted in the present
study. As shown in Fig. 2A, a
significant decrease in sucrose intake was observed in the model
group when compared with the control group, and (12.5, 25 and 50
mg/kg/day) β-asarone or 20 mg/kg/day fluoxetine treatment
significantly increased the sucrose intake. In addition, the
immobility time in the forced swimming test was significantly
enhanced in rats of the model group, while (12.5, 25, 50 mg/kg/day)
β-asarone or 20 mg/kg/day fluoxetine treatment notably reduced this
enhancement (Fig. 2B). Furthermore,
the locomotor activity was detected in the open field test, and the
results are shown in Fig. 2C and D.
Compared with the control group, the crossing and rearing number in
the model rats was significantly decreased, while treatment with
(12.5, 25 and 50 mg/kg/day) β-asarone or 20 mg/kg/day fluoxetine
significantly eliminated these changes. Furthermore, 50 mg/kg/day
β-asarone exhibited the most marked effect on CUMS induced
depression-associated symptoms in rats. The findings indicated that
the β-asarone and fluoxetine treatments significantly relieved
CUMS-induced depression.
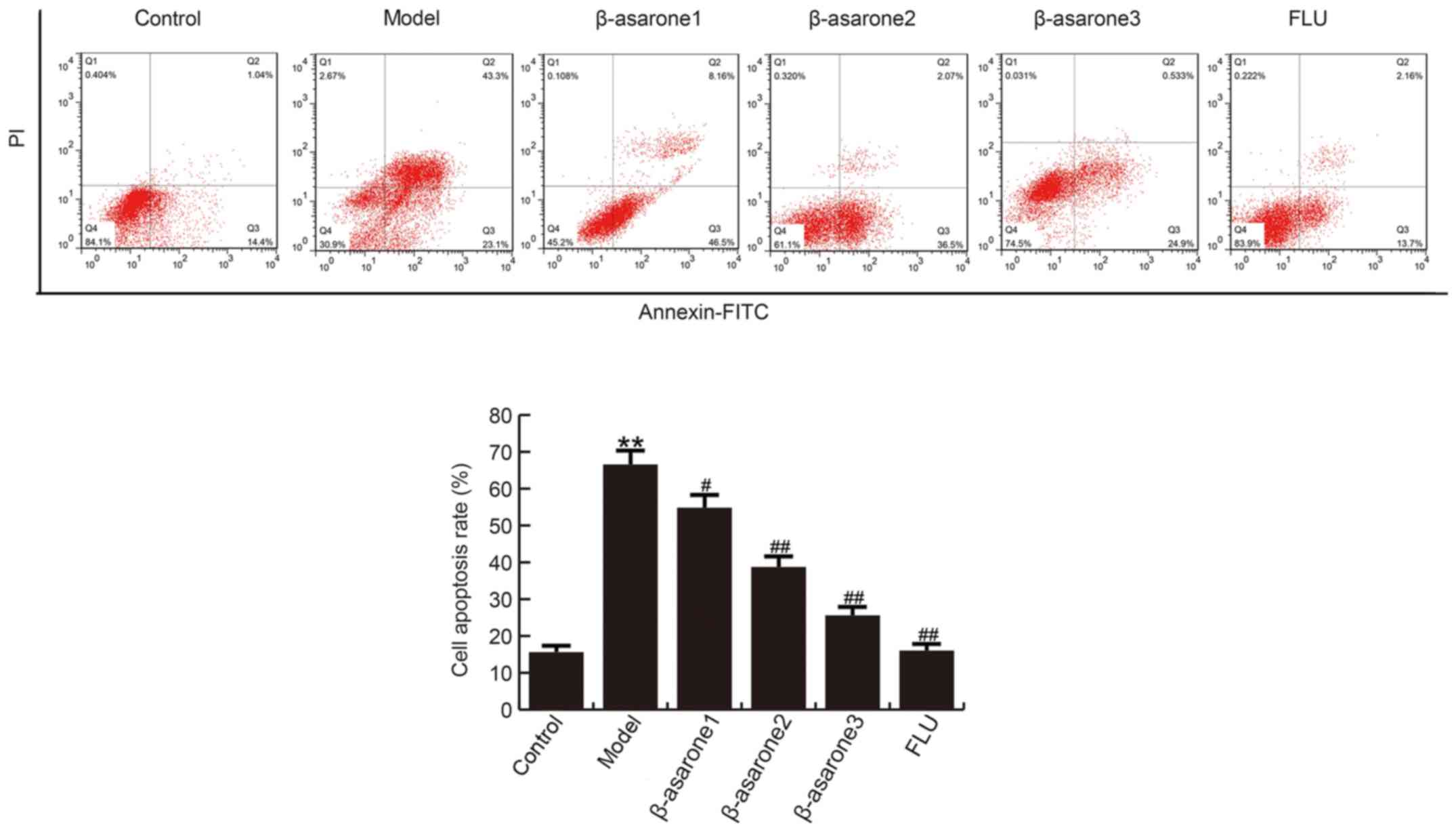
β-asarone prevents CUMS-induced
apoptosis in the hippocampus
The findings demonstrated that, compared with the
control group, the apoptosis rate of hippocampal neuronal cells
significantly increased in rats of the model group. By contrast,
(12.5, 25 and 50 mg/kg/day) β-asarone and 20 mg/kg/day fluoxetine
treatment significantly decreased the apoptosis rate in hippocampal
cells as compared with that observed in the model group (Fig. 3). β-asarone administered at 50
mg/kg/day exhibited the most marked effect on CUMS-induced
apoptosis in the hippocampus of rats.
β-asarone enhances CREB, BDNF and
Trk-B levels in the hippocampus of CUMS-treated rats
The effect of β-asarone on the levels of CREB, BDNF
and Trk-B in the hippocampus of rats subjected to CUMS was
determined. As shown in Fig. 4,
compared with the control group, the mRNA and protein levels of
CREB, BDNF and Trk-B were significantly decreased in the
hippocampus of rats in the model group; however, (12.5, 25 and 50
mg/kg/day) β-asarone and 20 mg/kg/day fluoxetine treatment
significantly inhibited this reduction. β-asarone administered at
50 mg/kg/day exhibited the most marked effect on CREB, BDNF and
Trk-B levels in the hippocampus of CUMS-treated rats.
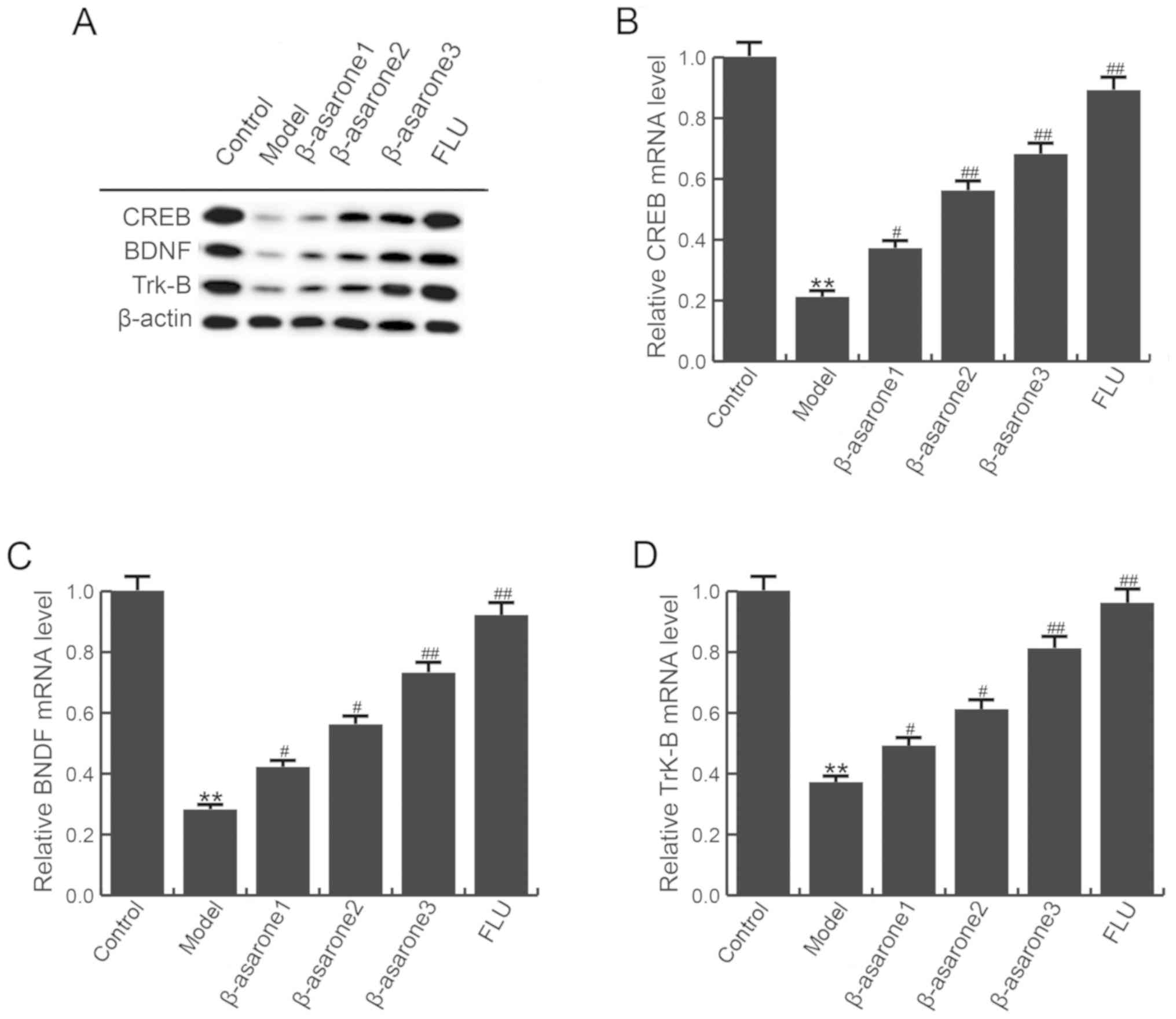 | Figure 4.β-asarone increases CREB, BDNF and
Trk-B levels in the hippocampus of chronic unpredictable mild
stress-treated rats. (A) Western blot analysis to examine CREB,
BDNF and Trk-B protein levels. (B) CREB, (C) BDNF and (D) Trk-B
mRNA levels in different groups were detected by reverse
transcription-quantitative polymerase chain reaction. Data are
expressed as the mean ± standard deviation. **P<0.01 vs. control
group; #P<0.05 and ##P<0.01, vs. model group. β-asarone1/2/3,
depression model rats treated with 12.5, 25 or 50 mg/kg β-asarone,
respectively; FLU, fluoxetine-treated depression rats; CREB, cAMP
response element binding protein; BDNF, brain-derived neurotrophic
factor; Trk-B, tropomyosin receptor kinase B. |
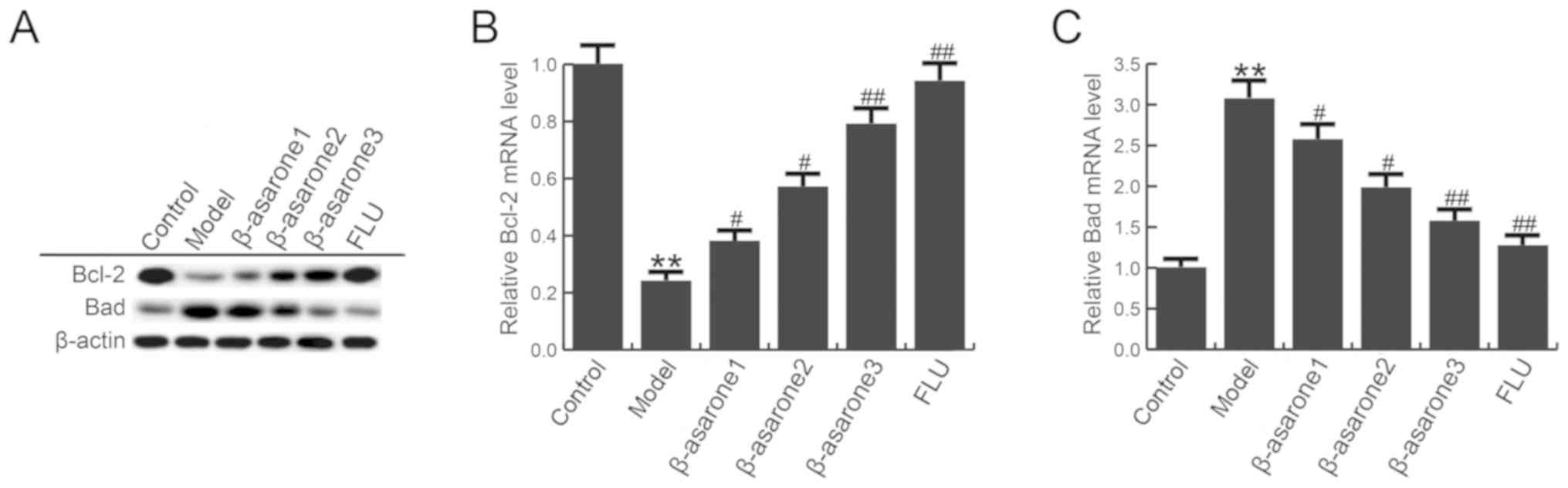
β-asarone enhances Bcl-2 level and
reduces Bad in the hippocampus of CUMS-treated rats
The effect of β-asarone on the levels of Bcl-2 and
Bad in the hippocampus of rats in the model group was also
analyzed. As shown in Fig. 5,
compared with the control group, the mRNA and protein levels of
Bcl-2 were significantly decreased, while Bad levels were increased
in the hippocampus of rats in the model group. However, (12.5, 25
and 50 mg/kg/day) β-asarone and 20 mg/kg/day fluoxetine treatment
significantly eliminated these changes in Bcl-2 and Bad levels.
β-asarone administered at 50 mg/kg/day exhibited the most marked
effect on Bcl-2 and Bad levels in the hippocampus of CUMS-treated
rats.
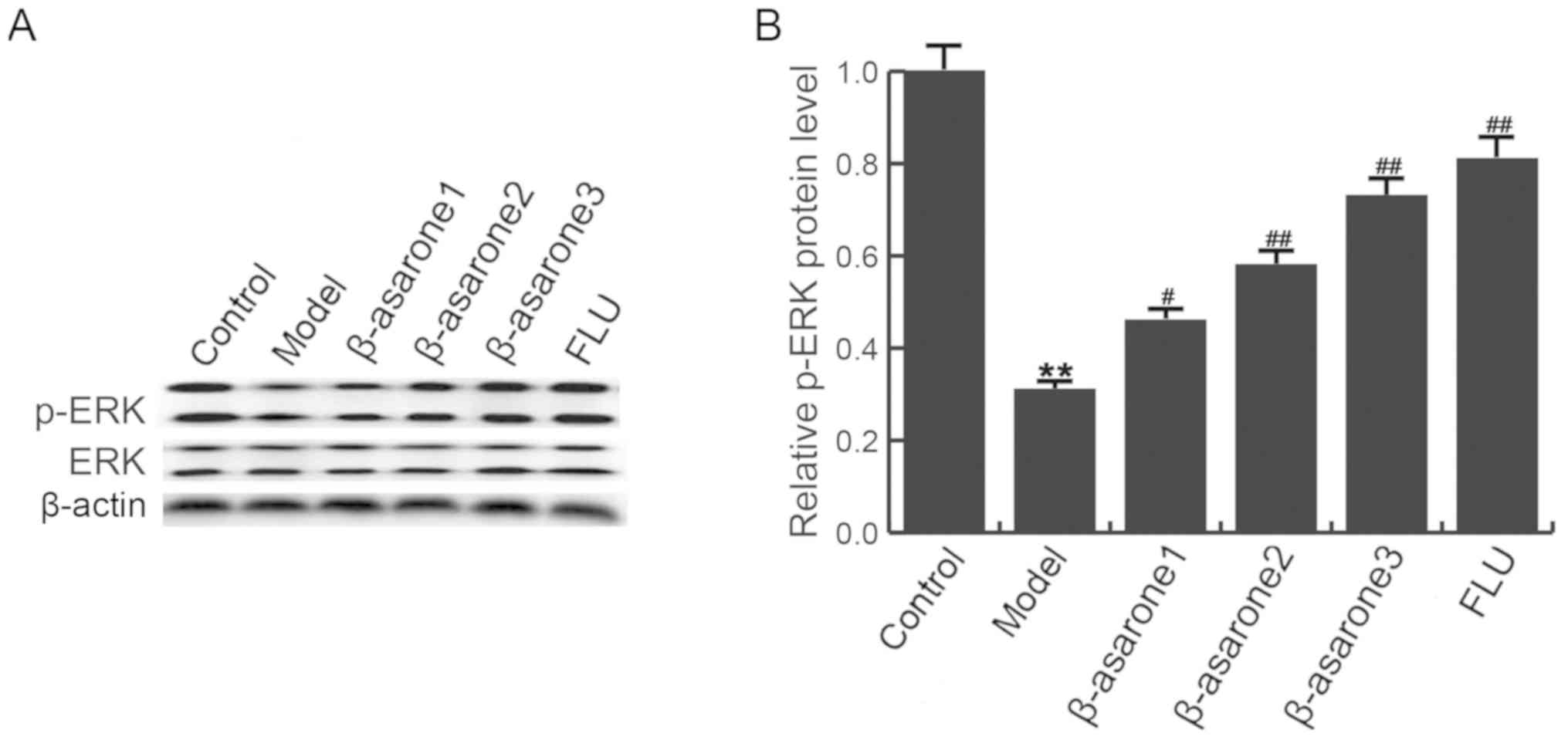
β-asarone increases ERK
phosphorylation in the hippocampus of CUMS-treated rats
The ERK signaling pathway serves an important role
by mediating the protective responses to stress (25). As shown in Fig. 6, CUMS decreased the phosphorylation
of ERK in the hippocampus of rats, while (12.5, 25 and 50
mg/kg/day) β-asarone and 20 mg/kg/day fluoxetine treatment
significantly increased the ERK phosphorylation as compared with
that in the CUMS model group. β-asarone administered at 50
mg/kg/day had the most marked effect on p-ERK level in the
hippocampus of CUMS-treated rats.
Discussion
Depression is a complex disorder that has become an
important social problem due to the severe effect it has on the
health and quality of life of patients (1). Thus far, drug treatment has been proven
to be an effective method for depression treatment (26). Therefore, seeking novel and effective
drugs for the treatment of depression is urgent.
β-asarone is the major active ingredient extracted
from Acorus tatarinowii Schott. Acorus tatarinowii contains
volatile oils, consisting mainly of 8.8–13.7% α-asarone and
63.2–81.2% β-asarone, and has been used in traditional Chinese
medicine formulas to relieve learning and memory deficits (27,28).
β-asarone can easily pass through the blood-brain barrier (7), and various studies have indicated that
it has various pharmacological properties, including an
anti-cancer, anti-inflammatory and neuroprotective effect (12,29,30). In
addition, β-asarone was able to relieve cognitive impairments in
Alzheimer's disease (30),
Parkinson's disease (31), and
neuroinflammatory disease (32).
Previous studies have also suggested the antidepressive effects of
β-asarone (13–17). A study by Dong et al (33) suggested that β-asarone can reverse
CUMS-induced depression-like behavior and induce hippocampal
neurogenesis in rats. Nevertheless, to the best of our knowledge,
the molecular mechanisms underlying the effect of β-asarone in
depression are not fully elucidated. Therefore, clarification of
these mechanisms was attempted in the present study, and a rat
model of depression induced by CUMS was established. Our
investigation differed from the study of Dong et al
(33) in various aspects: Firstly,
in the current study, rats were subjected to different
concentrations of β-asarone (12.5, 25 and 50 mg/kg), whereas the
study by Dong et al only examined the dose of 25 mg/kg
β-asarone; secondly, fluoxetine (20 mg/kg) was used as the positive
control drug in the present study, while this was not utilized in
the previous study; and finally, the effect on the body weight and
apoptosis in the hippocampus of depression rats was also explored
in the present study. Consistent with the findings of the previous
study (33), the current study also
found that β-asarone significantly mitigated CUMS-induced
depression-like behavior.
Hippocampal neuronal damage may be one of factors
that trigger depression (1). Maggio
and Segal (34) demonstrated that
hippocampal neurons in a stress-depression rat model had a
decreased proliferation ability, dendritic atrophy, increased
apoptosis of pyramidal neurons and decreased hippocampal volume in
rats with recurrent depression. The majority of current
antidepressant drugs or methods exert their effect by promoting
neuronal regeneration in the adult brain (35). Consistent with a previous study
(23), the results of the present
study indicated that CUMS treatment significantly induced
hippocampal neuronal cell apoptosis, while β-asarone significantly
decreased hippocampal neuronal cell apoptosis in CUMS rats.
BDNF, a recognized molecular marker of
neuroplasticity, is one of the important members of the family of
neurotrophic factors and is widely distributed in the central
nervous system of mammals, particularly in the hippocampus, cortex
and amygdala (36). Injection of
BDNF in the brain to activate endogenous adult neural precursor
cells can produce antidepressive effects (37). As one of the third messengers in the
nucleus, CREB serves an important role in neurogenesis and
neuroplasticity. CREB-mediated transcriptional alterations and
central nervous system diseases are closely associated, such as
cognitive decline, depression and cerebral ischemia (38). Activation of CREB in the hippocampal
dentate gyrus of the adult rat hippocampus promotes neurogenesis,
including cell proliferation and increased cell survival (39). At the same time, CREB also
participates in the regulation of antidepressant therapy, and its
activation mediates the change of neuronal plasticity induced by
the antidepressant treatment. CREB achieves its function primarily
through modulation of the target genes that are critical for the
maintenance of synaptic function and cell survival. BDNF is one of
the most important target genes of CREB and serves an important
role in the pathological process of depression and antidepressant
treatment. Furthermore, ERK1/2 is one of the mitogen-activated
protein kinases involved in numerous cellular processes, including
long-term neuronal plasticity and survival (40). Besides, the ERK signaling pathway is
particularly important in mediating the protective responses to
stress (25). ERK1/2 is upstream to
CREB in the control of neurogenesis (23,41). In
the present study, the effect of β-asarone on the ERK1/2-CREB-BDNF
pathway was further investigated. Consistent with a previous study
(33), β-asarone was found to
enhance p-ERK1/2, CREB, BDNF and Trk-B levels in the hippocampus of
CUMS-treated rats. In addition, the anti-apoptotic gene Bcl-2 and
pro-apoptotic gene Bad, which serve critical roles in apoptosis
regulation, were analyzed in the current study. It was observed
that β-asarone enhanced Bcl-2 level and inhibited Bad level in the
hippocampus of CUMS-treated rats. The data indicated that β-asarone
activated the ERK1/2-CREB-BDNF pathway, enhanced Bcl-2 expression
and reduced Bad expression in the hippocampus of depression rats;
however, the causal association remains unclear in vivo.
This also requires in-depth investigation, thus, further research
on this topic will be conducted in future studies.
In conclusion, the results of the present study
indicated that β-asarone exerted an antidepressive effect on a rat
model of depression induced by CUMS, and that the underlying
molecular mechanism may be associated with the activation of the
ERK signaling pathway. Therefore, β-asarone may be a novel and
effective agent for the treatment of depression.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Heilongjiang
Provincial Education Department (grant no. 2016-KYYWF-0881).
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD and WC designed the current study and drafted the
manuscript. HD, WC, XG, and YW collected the data and performed
statistical analyses. ST, QL and CL performed statistical analysis
and interpreted the data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Qiqihar Medical University (Qiqihar, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alonso M, Vianna MR, Depino AM, Mello e
Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I and
Medina JH: BDNF-triggered events in the rat hippocampus are
required for both short- and long-term memory formation.
Hippocampus. 12:551–560. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu JL, Yu SY, Wu SH and Bao AM: A
sensitive and practical RP-HPLC-FLD for determination of the low
neuroactive amino acid levels in body fluids and its application in
depression. Neurosci Lett. 616:32–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kendler KS and Gardner CO: Dependent
stressful life events and prior depressive episodes in the
prediction of major depression: The problem of causal inference in
psychiatric epidemiology. Arch Gen Psychiatry. 67:1120–1127. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albert PR, Benkelfat C and Descarries L:
The neurobiology of depression-revisiting the serotonin hypothesis.
I. Cellular and molecular mechanisms. Philos Trans R Soc Lond B
Biol Sci. 367:2378–2381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manji HK, Drevets WC and Charney DS: The
cellular neurobiology of depression. Nat Med. 7:541–547. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Willner P, Towell A, Sampson D,
Sophokleous S and Muscat R: Reduction of sucrose preference by
chronic unpredictable mild stress, and its restoration by a
tricyclic antidepressant. Psychopharmacology (Berl). 93:358–364.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang YQ, Shi C, Liu L and Fang RM:
Analysis of transformation and excretion of β-asarone in rabbits
with GC-MS. Eur J Drug Metab Pharmacokinet. 37:187–190. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang S, Xue ZF, Huang LP, Fang RM, He YP,
Li L and Fang YQ: Dynamic expressions of Beclin 1 and tyrosine
hydroxylase in different areas of 6-hydroxydopamine-induced
Parkinsonian rats. Cell Mol Neurobiol. 33:973–981. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Fang YQ, Xue ZF, He YP, Fang RM and
Li L: Beta-asarone attenuates ischemia-reperfusion-induced
autophagy in rat brains via modulating JNK, p-JNK, Bcl-2 and Beclin
1. Eur J Pharmacol. 680:34–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Geng Y, Li C, Liu J, Xing G, Zhou L, Dong
M, Li X and Niu Y: Beta-asarone improves cognitive function by
suppressing neuronal apoptosis in the beta-amyloid hippocampus
injection rats. Biol Pharm Bull. 33:836–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang N, Zhang Q, Ning B, Luo L and Fang Y:
β-Asarone promotes Temozolomide's entry into glioma cells and
decreases the expression of P-glycoprotein and MDR1. Biomed
Pharmacother. 90:368–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu J, Zhang XX, Sun QM, Chen M, Liu SL,
Zhang X, Zhou JY and Zou X: β-Asarone inhibits gastric cancer cell
proliferation. Oncol Rep. 34:3043–3050. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu DS, Xing GH, Lu CF, et al: Effect of
β-asarone on the changes of specific enolase in hippocampus and
serum neurons of CUMS rats. Chin J Gerontol. 36:1040–1041. 2016.(In
Chinese).
|
|
14
|
Cai ZZ, Jin M, Yang PP, Li CC, Dong HY,
Zhao AM and Zhang XJ; Qiqihaer Medical College, : Effects of
β-asarone on expression of endoplasmic reticulum stress regulators
GRP78, ATF6 and XBP1 in hippocampus of depressive rats. Chin J New
Drugs. 26:1053–1058. 2017.
|
|
15
|
Zhao CM, Zhang XJ, Dong HY, et al: Effects
of β-asarone on behavior and MKP-1, MSK-1, CREB and Bcl-2 in
hippocampus of depressive rats. Chin J Exp Tradit Med Formulae.
16:272–277. 2013.(In Chinese).
|
|
16
|
Wang JP, Dong HY, Zhao CM, et al: Effects
of β-asarone on behavior and biological clock related gene
expression in depressive rats. Chin J Exp Tradit Med Formulae.
2:170–173. 2015.(In Chinese).
|
|
17
|
Dong HY, Zhang C, Wang JP, et al: Effects
of β-asarone on the expression of circadian rhythm cycle gene Per1
in depressive rats. Chin J New Drugs. 7:823–826. 2015.(In
Chinese).
|
|
18
|
Deng XY, Li HY, Chen JJ, Li RP, Qu R, Fu Q
and Ma SP: Thymol produces an antidepressant-like effect in a
chronic unpredictable mild stress model of depression in mice.
Behav Brain Res. 291:12–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng XY, Xue JS, Li HY, Ma ZQ, Fu Q, Qu R
and Ma SP: Geraniol produces antidepressant-like effects in a
chronic unpredictable mild stress mice model. Physiol Behav.
152:264–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang D, An SC and Zhang X: Prevention of
chronic stress-induced depression-like behavior by inducible nitric
oxide inhibitor. Neurosci Lett. 433:59–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurhe Y, Radhakrishnan M and Gupta D:
Ondansetron attenuates depression co-morbid with obesity in obese
mice subjected to chronic unpredictable mild stress; an approach
using behavioral battery tests. Metab Brain Dis. 29:701–710. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li YC, Shen JD, Li J, Wang R, Jiao S and
Yi LT: Chronic treatment with baicalin prevents the chronic mild
stress-induced depressive-like behavior: Involving the inhibition
of cyclooxygenase-2 in rat brain. Prog Neuropsychopharmacol Biol
Psychiatry. 40:138–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li W, Zhu Y, Saud SM, Guo Q, Xi S, Jia B,
Jiao S, Yang X, Lu J, Song S and Tu Y: Electroacupuncture relieves
depression-like symptoms in rats exposed to chronic unpredictable
mild stress by activating ERK signaling pathway. Neurosci Lett.
642:43–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leem YH, Yoon SS, Kim YH and Jo SA:
Disrupted MEK/ERK signaling in the medial orbital cortex and dorsal
endopiriform nuclei of the prefrontal cortex in a chronic restraint
stress mouse model of depression. Neurosci Lett. 580:163–168. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho H, Son SJ, Kim S and Park J: A
randomized comparison of medication and cognitive behavioral
therapy for treating depression in low-income young minority women.
Med Sci Monit. 22:4947–4953. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rajput SB, Tonge MB and Karuppayil SM: An
overview on traditional uses and pharmacological profile of Acorus
calamus Linn. (Sweet flag) and other Acorus species. Phytomedicine.
21:268–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JH, Hahm DH, Lee HJ, Pyun KH and Shim
I: Acori graminei rhizoma ameliorated ibotenic acid-induced amnesia
in rats. Evid Based Complement Alternat Med. 6:457–464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang W and Teng J: β-asarone prevents
Aβ25-35-induced inflammatory responses and autophagy in SH-SY5Y
cells: Down expression Beclin-1, LC3B and up expression Bcl-2. Int
J Clin Exp Med. 8:20658–20663. 2015.PubMed/NCBI
|
|
30
|
Liu SJ, Yang C, Zhang Y, Su RY, Chen JL,
Jiao MM, Chen HF, Zheng N, Luo S, Chen YB, et al: Neuroprotective
effect of β-asarone against Alzheimer's disease: Regulation of
synaptic plasticity by increased expression of SYP and GluR1. Drug
Des Devel Ther. 10:1461–1469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang QS, Wang ZH, Zhang JL, Duan YL, Li
GF and Zheng DL: Beta-asarone protects against MPTP-induced
Parkinson's disease via regulating long non-coding RNA MALAT1 and
inhibiting α-synuclein protein expression. Biomed Pharmacother.
83:153–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim HW, Kumar H, Kim BW, More SV, Kim IW,
Park JI, Park SY, Kim SK and Choi DK: β-Asarone
(cis-2,4,5-trimethoxy-1-allyl phenyl), attenuates pro-inflammatory
mediators by inhibiting NF-κB signaling and the JNK pathway in LPS
activated BV-2 microglia cells. Food Chem Toxicol. 72:265–272.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong H, Gao Z, Rong H, Jin M and Zhang X:
β-asarone reverses chronic unpredictable mild stress-induced
depression-like behavior and promotes hippocampal neurogenesis in
rat. Molecules. 19:5634–5649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maggio N and Segal M: Differential
modulation of long-term depression by acute stress in the rat
dorsal and ventral hippocampus. J Neurosci. 29:8633–8638. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Piubelli C, Vighini M, Mathé AA, Domenici
E and Carboni L: Escitalopram modulates neuron-remodelling proteins
in a rat gene-environment interaction model of depression as
revealed by proteomics. Part I: Genetic background. Int J
Neuropsychopharmacol. 14:796–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
You J, Yuan Y, Zhang Z, Zhang X, Li H and
Qian Y: A preliminary association study between brain-derived
neurotrophic factor (BDNF) haplotype and late-onset depression in
mainland Chinese. J Affect Disord. 120:165–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Castrén E and Rantamäki T: The role of
BDNF and its receptors in depression and antidepressant drug
action: Reactivation of developmental plasticity. Dev Neurobiol.
70:289–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren X, Dwivedi Y, Mondal AC and Pandey GN:
Cyclic-AMP response element binding protein (CREB) in the
neutrophils of depressed patients. Psychiatry Res. 185:108–112.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oury F, Yadav VK, Wang Y, Zhou B, Liu XS,
Guo XE, Tecott LH, Schutz G, Means AR and Karsenty G: CREB mediates
brain serotonin regulation of bone mass through its expression in
ventromedial hypothalamic neurons. Genes Dev. 24:2330–2342. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou X, Moon C, Zheng F, Luo Y, Soellner
D, Nuñez JL and Wang H: N-methyl-D-aspartate-stimulated ERK1/2
signaling and the transcriptional up-regulation of
plasticity-related genes are developmentally regulated following in
vitro neuronal maturation. J Neurosci Res. 87:2632–2644. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ha S and Redmond L: ERK mediates activity
dependent neuronal complexity via sustained activity and
CREB-mediated signaling. Dev Neurobiol. 68:1565–1579. 2008.
View Article : Google Scholar : PubMed/NCBI
|