Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies (1). HCC has the
fifth highest incidence and the third highest mortality of all
malignancies worldwide (1,2), leading to >600,000 deaths annually
(3). However, effective treatment
for HCC remains elusive (4).
Liver resection and transplantation are currently
the primary methods for treating HCC (5). However, due to unobservable symptoms in
the early stages of HCC pathogenesis, most patients are diagnosed
with HCC in the advanced stages (4).
Surgical intervention for advanced HCC is not effective and
chemotherapy is the only therapeutic option (6). However, traditional chemotherapeutic
agents, including cisplatin, doxorubicin and fluorouracil, produce
unsatisfactory outcomes, adverse side effects and drug resistance
(6). With the emergence of targeted
drugs such as small molecule kinase inhibitors, considerable
progress has been made in the treatment of HCC (7). For patients with HCC, where surgery is
not recommended, palliative treatment using sorafenib is currently
the only clinical solution (8).
However, since some patients with HCC also exhibit insensitivity or
resistance to sorafenib, other chemotherapeutic options are
required (8).
Gefitinib is a small molecule inhibitor of epidermal
growth factor receptor (EGFR) (9).
As a transmembrane protein, EGFR can induce cell proliferation by
phosphorylating mitogen-activated protein kinase (MAPK), Akt and
JNK (10–12). Previous studies have confirmed that
increased expression of EGFR is an important feature to the
occurrence and development of HCC. In particular, Schiffer et
al (13) has found that
gefitinib inhibited hepatoma cell proliferation, however, any
further mechanism remains unclear.
Genistein, also known as
5,7,4′-trihydroxyisoflavone, is an isoflavone originally found in
Glycine max (14), which has
demonstrated the potential to reduce the risk of developing liver,
lung and breast cancer (15). In
hepatoma cells, a previous study has found that genistein treatment
inhibited proliferation whilst promoting apoptosis (16) and inhibited EGFR activation (17). However, to the best of our knowledge,
no study on the combined effects of gefitinib and genistein on
liver cancer has been conducted.
Therefore, the purpose of the present study was to
investigate the effect of gefitinib and genistein on the physiology
of hepatocellular carcinoma cells and to investigate the mechanism
by testing the Akt/Erk/mTOR pathway.
Materials and methods
Cell culture
The HCC cell line Hep3B was purchased from American
Type Culture Collection (cat. no. ATCC® HB-8064). The
cells were cultured in RPMI 1640 medium supplemented with 10% fetal
bovine serum (FBS) and 100 U/ml of penicillin-streptomycin
maintained in a humidified atmosphere at 37°C under 5%
CO2. RPMI 1640, FBS and penicillin-streptomycin were
purchased from Gibco (Thermo Fisher Scientific, Inc.). Hep3B cells
(5×103 cells/well) were cultured with different
concentrations of genistein (10, 20, 40, 80 and 160 µM; cat. no.
G0272; Tokyo Chemical Industry Development Co., Ltd.) or gefitinib
(1, 2.5, 5, 10 and 20 µM; cat. no. S1025, Selleck Chemicals) for 48
h at 37°C, in order to calculate the IC50 values. Hep3B
cells were cultured in the presence of PBS (control group), 120 µM
genistein (genistein group), 12 µM gefitinib (gefitinib group) and
127.6 µM genistein + 9.8 µM gefitinib (combination group) at 37°C,
with the inhibition of cell growth analyzed at 0, 12, 24, 36, 48,
60 and 72 h for each group.
Cell cytotoxicity assay
Cell viability (5×103 cells/well) treated
with genistein (10, 20, 40, 80 and 160 µM) or gefitinib (1, 2.5, 5,
10 and 20 µM) for 48 h at 37°C was detected by using cell counting
kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology). Diluted
CCK-8 reagent was added and cultured at 37°C in a humidified
atmosphere under 5% CO2 for 4 h. Optical density in each
well at 450 nm was subsequently measured using a microplate reader
(ELx800™; Omega Bio-Tek, Inc.). The cellular proliferation
inhibition rates were calculated as: (1-OD/OD0 µM)
×100%.
Flow cytometry assay
After the treatment of 120 µM genistein (genistein
group), 12 µM gefitinib (gefitinib group) or 127.6 µM genistein +
9.8 µM gefitinib (combination group) and culturing for 72 h
(5×103 cells/well), cell apoptosis was measured using BD
Pharmingen™ PE Annexin V-FITC/PI Apoptosis Detection Kit I (BD
Biosciences) according to manufacturer's protocol. The samples were
incubated at room temperature in the dark for 10 min. BD
FACSCalibur™ Flow Cytometer and BD FACStation™ Software v6.1 × (BD
Biosciences) was used to analyze cell apoptosis.
Western blot analysis
Cells were lysed using NP40 lysis buffer (Beyotime
Institute of Biotechnology). The supernatant was collected by
centrifuging the cell lysate at 10,000 × g at 4°C for 15 min.
Bicinchoninic acid assay was used to determine the protein
concentration. The proteins (20 µg/lane) were separated using 10%
SDS-PAGE followed by transfer onto PVDF membranes. The membranes
were blocked with 5% fat-free milk, diluted in PBS, at room
temperature for 2 h prior to incubation with primary antibodies
against anti-pro-caspase-3, (1:800, cat. no. ab13847),
anti-cleaved-caspase-3 (1:600; cat. no. ab49822), anti-cleaved-poly
ADP ribose polymerase (cleaved-PARP; 1:700; cat. no. ab32064),
anti-EGFR (1:2,000; cat. no. ab32562), anti-platelet-derived growth
factor α (PDGF; 1:500; cat. no. ab38562), anti-pan-Akt (Akt; 1:800;
cat. no. ab8805), anti-phospho-pan-Akt (phospho T308; p-Akt; 1:800,
cat. no. ab38449), anti-Erk1/2 (1:800, cat. no. ab54230),
anti-p-Erk1/2 (1:600; cat. no. ab201015), anti-mTOR (1:800; cat.
no. ab2732) or anti-p-mTOR (1:600; cat. no. ab109268) at 4°C
overnight. All primary antibodies were purchased from Abcam. The
membranes were subsequently incubated with goat anti-mouse IgG
(1:8,000; cat. no. ab6785; Abcam), rabbit anti-mouse IgG, (1:9,000;
cat. no. ab99697; Abcam), mouse anti-rabbit IgG (1:7,000; cat. no.
BA1034; Invitrogen; Thermo Fisher Scientific, Inc.) and donkey
anti-rabbit IgG (1:5,000; cat. no. NL004; R&D Systems, Inc.)
secondary antibodies at room temperature for 1.5 h. Protein bands
were visualized using BeyoECLMoon ECL reagent (Beyotime Institute
of Biotechnology) and Quantity One v4.6.2 (Bio-Rad Laboratories,
Inc.) quantitative analysis software.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol and
quantified using a NanoDrop spectrometer (Thermo Fisher Scientific,
Inc.). RNA was reverse transcribed into cDNA at 42°C for 30 min and
85°C for 5 min, using the iScript™ cDNA Synthesis kit (Bio-Rad
Laboratories, Inc.) according to the manufacturer's protocol. qPCR
was then performed using FastStart Universal SYBR® Green
Master kit (Roche Diagnostics) in the ABI StepOne system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to
manufacturers' protocols. Each reaction system was prepared as
follows: cDNA template, 2.5 µl; forward primers (10 µM), 1 µl;
reverse primers (10 µM), 1 µl; 2X SYBR® Green master
mix, 10 µl and ddH2O, 5.5 µl. The following
thermocycling conditions were used for the qPCR: Initial
denaturation for 2 min at 95°C; 40 cycles of 15 sec at 95°C, 25 sec
at 60°C and 60 sec at 72°C. Expression levels were quantified using
the 2−ΔΔCq method with GAPDH as the internal reference
(18). The list of primers used for
this study is included in Table
I.
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
|
| Primer sequence
(5′-3′) |
|---|
|
|
|
|---|
| Primer name | Forward | Reverse |
|---|
| EGFR |
AACACCCTGGTCTGGAAGTACG |
TCGTTGGACAGCCTTCAAGACC |
| PDGF |
GAGGAAGCCGAGATGCCCC |
TGCTGTGGATCTGACTTCGAG |
| GAPDH |
AGTATGACTCCACTCACGGC |
CACCAGTAGACTCCACGACA |
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using SPSS 20 software (IBM
Corp.). One-way ANOVA followed by Tukey's multiple comparisons post
hoc test was performed to analyze differences between experimental
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
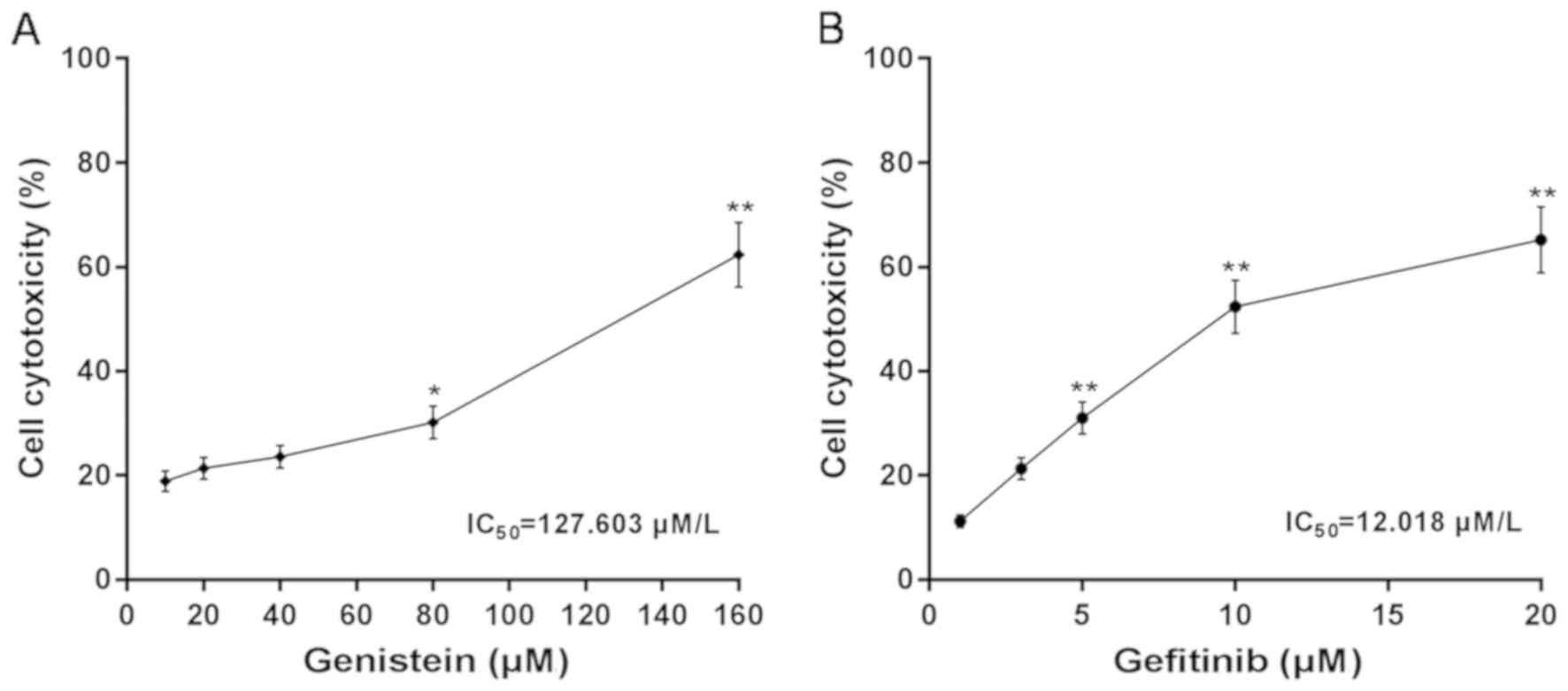
IC50 determination of
genistein and gefitinib
By measuring Hep3B cell cytotoxicity under different
concentrations of genistein and gefitinib after 48 h, the
IC50 values of genistein and gefitinib were calculated
to be 127.603 and 9.818 µM, respectively (Fig. 1A and B). These concentrations were
therefore used for subsequent experiments.
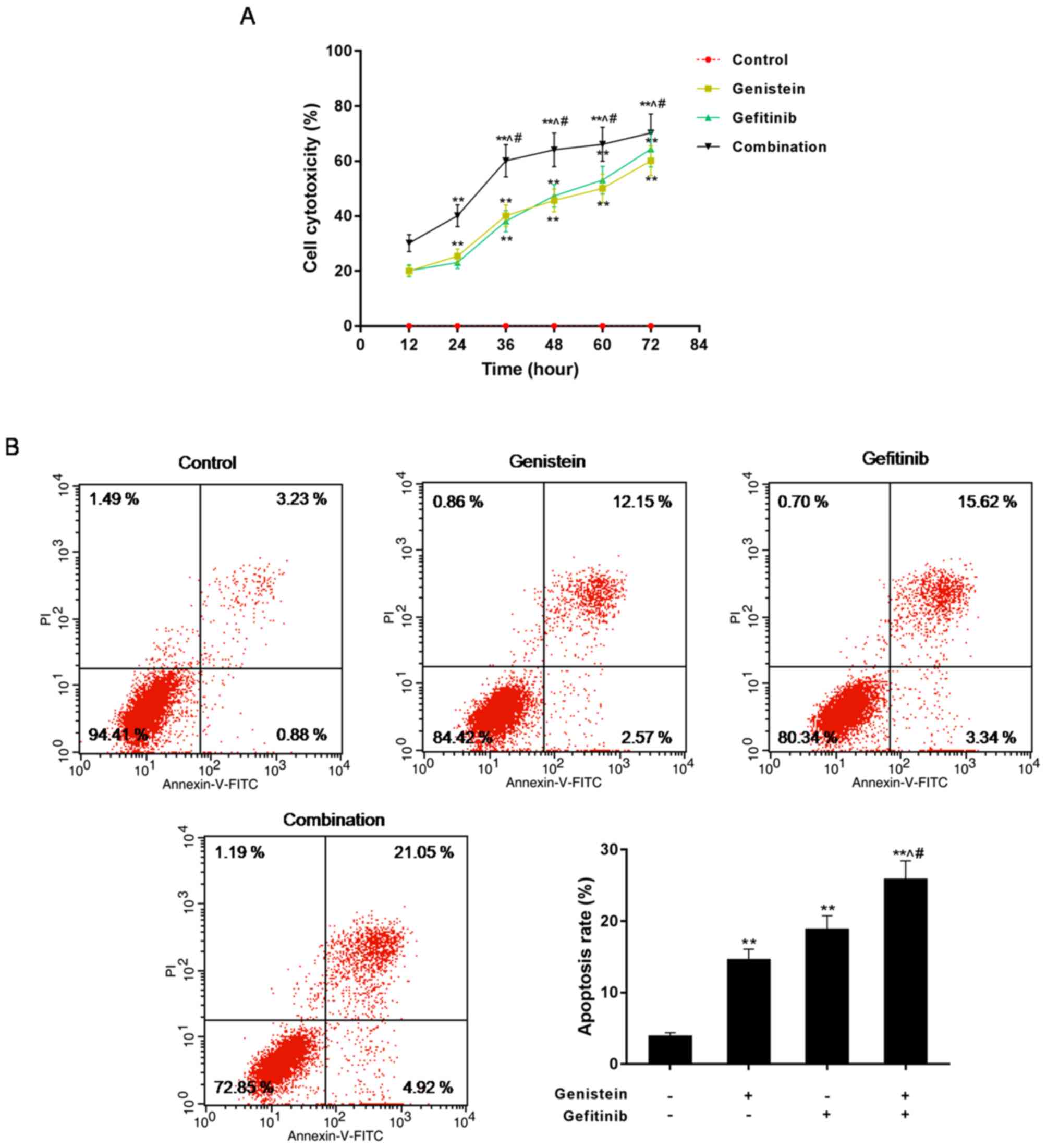
Apoptosis of Hep3B cells induced by
genistein and gefitinib
Comparing the cytotoxicity (killing the cells) of
the control, genistein, gefitinib and combination groups over a
72-h time course, it was found that the cytotoxicity in the
combination group was the highest (Fig.
2A). Flow cytometry was applied to detect the apoptosis of the
control, genistein, gefitinib, and combination groups, after cells
were cultured for 72 h. The apoptosis in the genistein alone and
gefitinib alone groups were significantly higher compared with that
in the control group, whereas that of the combination group was the
highest compared with the other three groups (Fig. 2B). This suggests that genistein in
combination with gefitinib enhanced hep3b cell apoptosis.
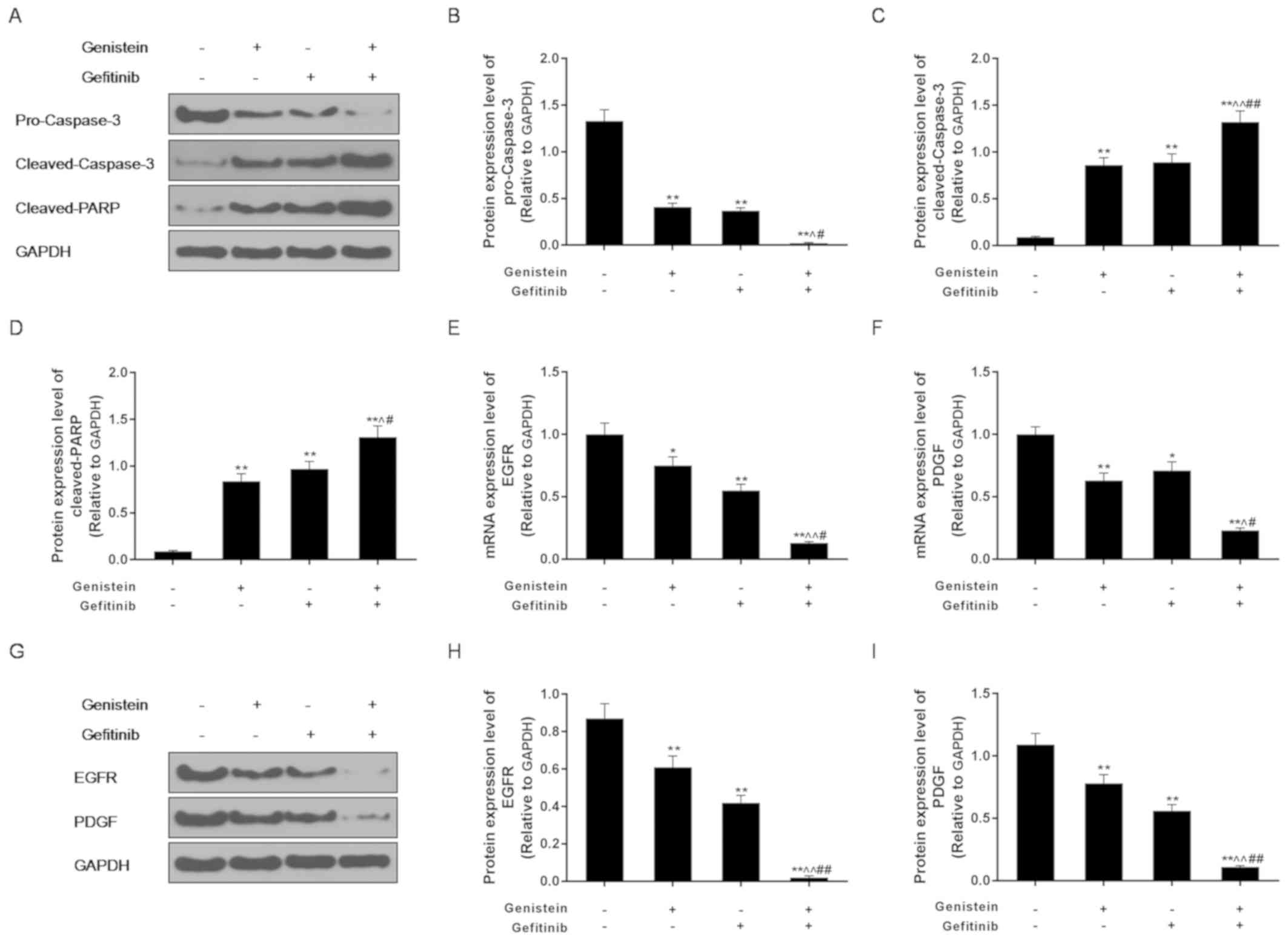
Effects of genistein and gefitinib on
the activity of EGFR, PDGF, and the expression of proteins
associated with apoptosis
Expression levels of pro-caspase-3,
cleaved-caspase-3, cleaved-PARP, EGFR and PDGF were detected using
western blot analysis. The expression levels of pro-caspase-3
protein was significantly reduced in the genistein alone and
gefitinib alone groups, whilst the protein expression levels of
cleaved-caspase-3 and cleaved-PARP were significantly increased
compared with the control group (Fig.
3A-D). When genistein and gefitinib were combined together,
these observed effects on the expression of proteins associated
with apoptosis were significantly enhanced compared with when
either drug was used alone (Fig.
3A-D).
RT-qPCR and western blotting results revealed that
either genistein or gefitinib treatment alone significantly
downregulated the expression of EGFR and PDGF mRNA and protein,
which was significantly enhanced by the combined addition of
genistein and gefitinib (Fig. 3E-I).
This suggest that that the enhanced effects of genistein in
combination with gefitinib were achieved by inhibiting EGFR and
PDGF expression and promoting the expression of pro-apoptotic
proteins.
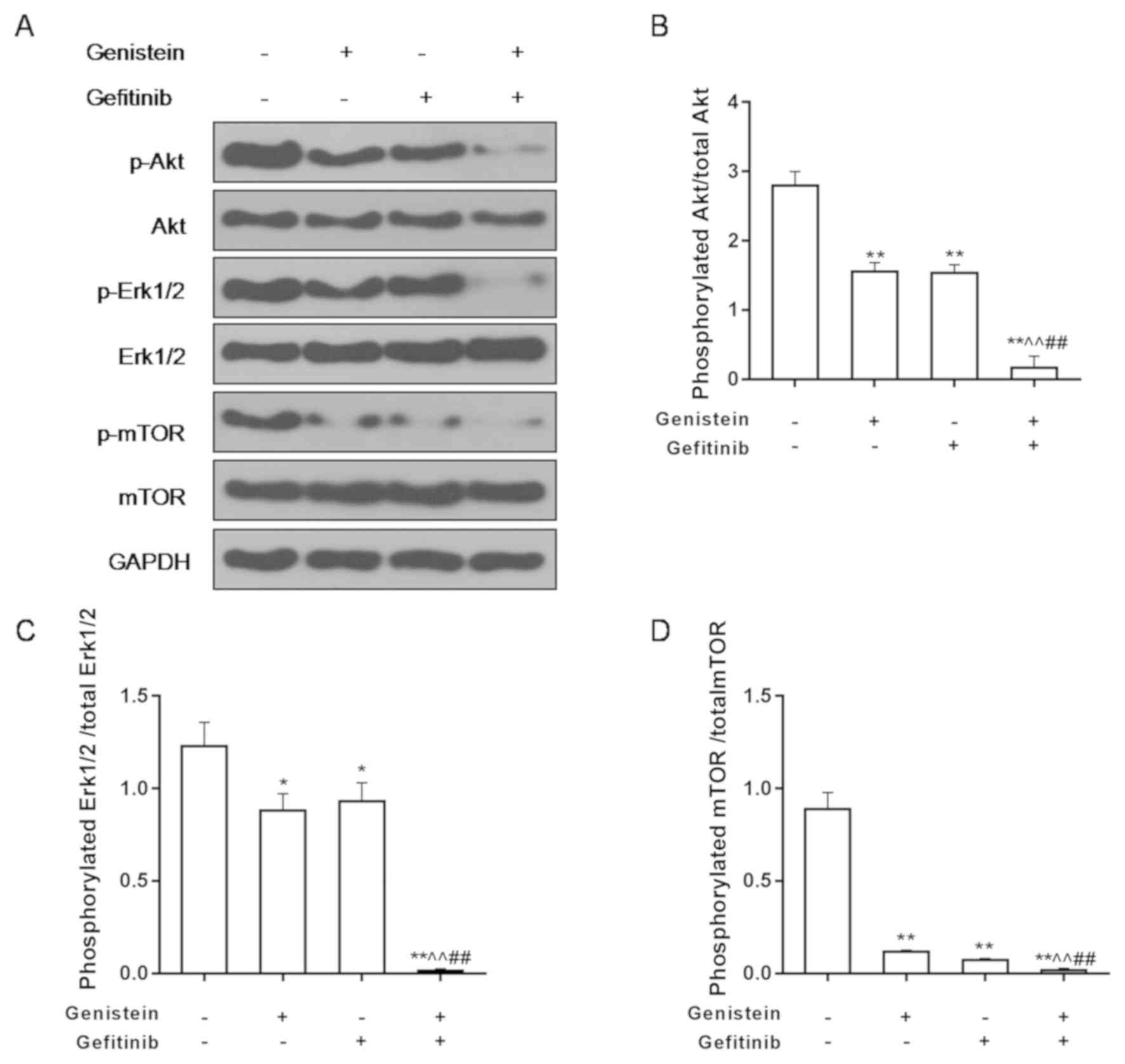
Effects of genistein and gefitinib on
the Akt/Erk/mTOR pathway
Treatment with genistein alone, gefitinib alone or
both in combination did not have statistically significant effects
on the expression of total Akt, Erk and mTOR proteins (Fig. 4A-D). However, Akt, Erk and mTOR
phosphorylation levels were significantly lower in genistein or
gefitimib alone group, compared with control group. In addition,
Akt, Erk and mTOR phosphorylation levels were significantly lower
in the combination group compared with control, genistein or
gefitinib alone, while Akt, Erk and Mtor phosphorylation levels
were significantly lower in genistein or gefitinib groups compared
with control group (Fig. 4A-D).
These results suggest that genistein in combination with gefitinib
can significantly reduce the Akt/Erk/mTOR pathway activity.
Discussion
Gefitinib is a small-molecule antitumor drug that
can selectively inhibit EGFR and inhibit tumor cell proliferation
to promote apoptosis (19). Indeed,
gefitinib has demonstrated a high efficacy in treating lung and
gastric cancer (20,21). A recent study has shown that
gefitinib exhibited inhibitory effects on HCC (22). By contrast, genistein is an
isoflavone compound and an inhibitor of tyrosine protein kinase
(TPK) (14). Studies have shown that
inhibiting TPK activity using genistein prevented EGFR-mediated
receptor autophosphorylation and mitotic signal transduction
(23,24). Genistein has been studied in various
malignancies, including breast, lung and prostate cancer (25–27).
However, only a limited number of studies have been conducted on
the treatment of genistein on HCC.
The occurrence and development of HCC is closely
associated with the abnormal expression of a number of proteins. In
particular, one study has confirmed that the overexpression of EGFR
was associated with the occurrence and development of liver cancer
(28), where higher levels of EGFR
expression were associated with poorer prognosis and higher
recurrence rates in poorly differentiated HCC (29). Combination therapy is the most
important method for treating advanced HCC (30), and to the best of our knowledge,
there is currently no study investigating the effects of genistein
in combination with gefitinib on HCC. Therefore, in the present
study the effect of genistein combined with gefitinib on the
proliferation and apoptosis of the HCC cell line Hep3B was
investigated. Both drugs significantly inhibited Hep3B cell
viability and promoted apoptosis, and these effects were enhanced
when these drugs were used in combination. These results suggest
that combined genistein and gefitinib treatment exerted increased
anti-proliferative and pro-apoptotic effects, compared with
genistein or gefitinib alone. In addition, a previous study has
also shown that combined genistein and gefitinib treatment enhanced
the effect of growth inhibition and apoptosis on non-small cell
lung cancer, where the strongest synergistic effect was observed at
low concentrations (31).
To explore the role of genistein in combination with
gefitinib in cell proliferation and apoptosis further the
expression levels of proteins associated with apoptosis, EGFR and
PDGF, were determined. The caspase protein family serves important
roles in apoptosis. Caspase-3 is the activator of apoptosis where
it can enzymatically cleave PARP (32). Downstream, PARP is a multifunctional
post-translational modification enzyme that recognizes structurally
damaged DNA fragments. PARP cleavage by caspase-3 increases cell
instability and promotes apoptosis (32,33). The
results of the present study revealed that genistein in combination
with gefitinib displayed the strongest effect on the activation of
caspase-3 and PARP compared with the other three groups tested,
which caused PARP to lose its enzymatic activity and promote
apoptosis. EGFR and PDGF have been demonstrated to promote the
division of epithelial cells and proliferation of liver cancer
cells (34–36). The present study demonstrated that
the expression levels of EGFR and PDGF in the combination group
were significantly lower compared with genistein and gefitinib
groups alone. Previous studies have found that gefitinib inhibited
proliferation by suppressing EGFR (37) whereas genistein has also been
demonstrated to inhibit EGFR and PDGF in tumor cells (38,39).
These results suggest that genistein promoted the effects of
gefitinib by inhibiting the expression of EGFR and PDGF.
The downstream mechanism by which genistein in
combination with gefitinib inhibited HCC Hep3B cell growth was
subsequently explored by assessing the Akt/Erk/mTOR pathway.
Gefitinib and genistein have been previously observed to act on
EGFR, where the PI3K/Akt/mTOR and MAPK signaling pathways were the
most important signal transduction pathways downstream of EGFR
(40,41). Therefore, the effects of genistein
and gefitinib on the Akt/Erk/mTOR signaling were investigated in
the current study. Total Akt, Erk and mTOR protein levels remained
relatively stable in all treatment groups; however, their
phosphorylation levels decreased significantly. The levels of
p-Akt, p-Erk, and p-mTOR in the combination group were
significantly lower compared with genistein or gefitinib alone.
Akt, alternatively known as protein kinase B, is an important
downstream molecule of PI3K and serves an important role in the
regulation of cell growth, proliferation, survival and glucose
metabolism. Akt can modulate mTOR either directly or through Erk
(42,43). mTOR is a type of serine/threonine
kinase and activated mTOR promotes the phosphorylation of
substrates S6 kinase (S6K) and 4E binding protein 1 (42). As both substrates are key regulators
of protein translation, their phosphorylation leads to the
initiation and increase in ribosomal protein synthesis. When mTOR
activation is inhibited, cells undergo cell cycle arrest at the G1
phase and apoptosis (44). Previous
studies have shown that genistein and gefitinib inhibited cell
proliferation by reducing mTOR phosphorylation in cervical cancer
cells and breast cancer cells (45,46). The
present study suggested that the combination of genistein and
gefitinib synergistically exerted anti-proliferative and
pro-apoptotic effects by inhibiting the activation of the
Akt/Erk/mTOR pathway. Indeed, the Akt-mTOR-p70 S6K pathway has been
reported to be overactivated and may serve as a potential target in
HCC therapy (47). Therefore, p70S6K
activity may also have been inhibited in this study. In addition, a
previous study has shown that the Akt/Erk/mTOR pathway was
associated with cell autophagy in cancer cells (12). Therefore, it would be beneficial to
investigate the effect of genistein and gefitinib on HCC cell
autophagy in the future. Genistein has been reported to exhibit
multi-targeted biological and molecular effects on cancer cells
(15), and the specific inhibition
of tyrosine-specific protein kinases is one of the best documented
(14). Therefore, it would be of
interest to validate the mechanism in which genistein acts to
enhance the antitumor effects of gefitinib.
In conclusion, genistein in combination with
gefitinib could synergistically inhibit HCC cell proliferation and
promote apoptosis, in a more powerful manner compared with either
applied alone. Such a phenomenon may be associated with the
inhibition of the Akt/Erk/mTOR pathway. The present study provides
support for the clinical application of genistein-gefitinib
combination treatment on HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhejiang Science
and Technology Department Public Welfare Project (grant no.
2016C37149).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT and MW performed western blot analysis and
RT-qPCR. HH and JZ performed flow cytometry assay. YH detected cell
cytotoxicity. YC and HP analyzed data and wrote the manuscript. All
authors read and approved the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hartke J, Johnson M and Ghabril M: The
diagnosis and treatment of hepatocellular carcinoma. Semin Diagn
Pathol. 34:153–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Costentin C: Hepatocellular carcinoma
surveillance. Presse Med. 46:381–385. 2017.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang JF, Lao YC, Yuan BH, Yin J, Liu X,
Chen L and Zhong JH: Treatment of hepatocellular carcinoma with
portal vein tumor thrombus: Advances and challenges. Oncotarget.
8:33911–33921. 2017.PubMed/NCBI
|
|
4
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsueh KC, Lee TY, Kor CT, Chen TM, Chang
TM, Yang SF and Hsieh CB: The role of liver transplantation or
resection for patients with early hepatocellular carcinoma. Tumour
Biol. 37:4193–4201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hollebecque A, Malka D, Ferte C, Ducreux M
and Boige V: Systemic treatment of advanced hepatocellular
carcinoma: From disillusions to new horizons. Eur J Cancer.
51:327–339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qi XS, Guo XZ, Han GH, Li HY and Chen J:
MET inhibitors for treatment of advanced hepatocellular carcinoma:
A review. World J Gastroenterol. 21:5445–5453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdel-Rahman O and Fouad M:
Sorafenib-based combination as a first line treatment for advanced
hepatocellular carcinoma: A systematic review of the literature.
Crit Rev Oncol Hematol. 91:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu BN, Yan HQ, Wu X, Pan ZH, Zhu Y, Meng
ZW, Zhou QH and Xu K: Apoptosis induced by benzyl isothiocyanate in
gefitinib-resistant lung cancer cells is associated with Akt/MAPK
pathways and generation of reactive oxygen species. Cell Biochem
Biophys. 66:81–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao ZQ, Yu ZY, Li J and Ouyang XN:
Gefitinib induces lung cancer cell autophagy and apoptosis via
blockade of the PI3K/AKT/mTOR pathway. Oncol Lett. 12:63–68. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu PH, Kuo TC, Chang KC, Chang CH and Chu
CY: Gefitinib-induced epidermal growth factor receptor-independent
keratinocyte apoptosis is mediated by the JNK activation pathway.
Br J Dermatol. 164:38–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schiffer E, Housset C, Cacheux W, Wendum
D, Desbois-Mouthon C, Rey C, Clergue F, Poupon R, Barbu V and
Rosmorduc O: Gefitinib, an EGFR inhibitor, prevents hepatocellular
carcinoma development in the rat liver with cirrhosis. Hepatology.
41:307–314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akiyama T, Ishida J, Nakagawa S, Ogawara
H, Watanabe S, Itoh N, Shibuya M and Fukami Y: Genistein, a
specific inhibitor of tyrosine-specific protein kinases. J Biol
Chem. 262:5592–5595. 1987.PubMed/NCBI
|
|
15
|
Banerjee S, Li Y, Wang Z and Sarkar FH:
Multi-targeted therapy of cancer by genistein. Cancer Lett.
269:226–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Li J, Dai W, Zhang Q, Feng J, Wu L,
Liu T, Yu Q, Xu S, Wang W, et al: Genistein suppresses aerobic
glycolysis and induces hepatocellular carcinoma cell death. Br J
Cancer. 117:1518–1528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gruca A, Krawczyk Z, Szeja W, Grynkiewicz
G and Rusin A: Synthetic genistein glycosides inhibiting EGFR
phosphorylation enhance the effect of radiation in HCT 116 colon
cancer cells. Molecules. 19:18558–18573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu JF, Ji J, Dong SY, Li BB, Yu ML, Wu DD,
Tao L and Tong XH: Gefitinib enhances oxaliplatin-induced apoptosis
mediated by Src and PKC-modulated gap junction function. Oncol Rep.
36:3251–3258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kishida O, Miyazaki Y, Murayama Y, Ogasa
M, Miyazaki T, Yamamoto T, Watabe K, Tsutsui S, Kiyohara T,
Shimomura I and Shinomura Y: Gefitinib (‘Iressa’, ZD1839) inhibits
SN38-triggered EGF signals and IL-8 production in gastric cancer
cells. Cancer Chemother Pharmacol. 55:584–594. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang T, Gu J, Liu T, Ma H, Ma X, Tao J,
Jin Y and Liang X: Expression of acetaldehyde dehydrogenase in
gefitinib-resistant human lung adenocarcinoma HCC-827/GR cells.
Zhongguo Fei Ai Za Zhi. 21:431–436. 2018.(In Chinese). PubMed/NCBI
|
|
23
|
Nakashima S, Koike T and Nozawa Y:
Genistein, a protein tyrosine kinase inhibitor, inhibits
thromboxane A2-mediated human platelet responses. Mol Pharmacol.
39:475–480. 1991.PubMed/NCBI
|
|
24
|
Papazisis KT, Zambouli D, Kimoundri OT,
Papadakis ES, Vala V, Geromichalos GD, Voyatzi S, Markala D,
Destouni E, Boutis L and Kortsaris AH: Protein tyrosine kinase
inhibitor, genistein, enhances apoptosis and cell cycle arrest in
K562 cells treated with gamma-irradiation. Cancer Lett.
160:107–113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Duursen MB, Nijmeijer SM, de Morree
ES, de Jong PC and van den Berg M: Genistein induces breast
cancer-associated aromatase and stimulates estrogen-dependent tumor
cell growth in in vitro breast cancer model. Toxicology. 289:67–73.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geller J, Sionit L, Partido C, Li L, Tan
X, Youngkin T, Nachtsheim D and Hoffman RM: Genistein inhibits the
growth of human-patient BPH and prostate cancer in histoculture.
Prostate. 34:75–79. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tian T, Li J, Li B, Wang Y, Li M, Ma D and
Wang X: Genistein exhibits anti-cancer effects via down-regulating
FoxM1 in H446 small-cell lung cancer cells. Tumour Biol.
35:4137–4145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han C, Michalopoulos GK and Wu T:
Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor
tyrosine kinases and enhances invasiveness in human hepatocellular
carcinoma cells. J Cell Physiol. 207:261–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Ma XP, Shi Z, Zhang P, Ding DL,
Huang HX, Saiyin HG, Chen TY, Lu PX, Wang NJ, et al: Epidermal
growth factor receptor pathway polymorphisms and the prognosis of
hepatocellular carcinoma. Am J Cancer Res. 5:396–410.
2014.PubMed/NCBI
|
|
30
|
Lin J, Wu L, Bai X, Xie Y, Wang A, Zhang
H, Yang X, Wan X, Lu X, Sang X and Zhao H: Combination treatment
including targeted therapy for advanced hepatocellular carcinoma.
Oncotarget. 7:71036–71051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu H, Cheng H, Ren Y, Liu ZG, Zhang YF
and De Luo B: Synergistic inhibitory effects by the combination of
gefitinib and genistein on NSCLC with acquired drug-resistance in
vitro and in vivo. Mol Biol Rep. 39:4971–4979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo H, Liang H, Chen J, Xu Y, Chen Y, Xu
L, Yun L, Liu J, Yang H, Liu L, et al: Hydroquinone induces TK6
cell growth arrest and apoptosis through PARP-1/p53 regulatory
pathway. Environ Toxicol. 32:2163–2171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu J, Zhang X, Wang H, Ge S, Gao T, Song
L, Wang X, Li H, Qin Y and Zhang Z: HCRP1 downregulation promotes
hepatocellular carcinoma cell migration and invasion through the
induction of EGFR activation and epithelial-mesenchymal transition.
Biomed Pharmacother. 88:421–429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lv X, Fang C, Yin R, Qiao B, Shang R, Wang
J, Song W, He Y and Chen Y: Agrin para-secreted by PDGF-activated
human hepatic stellate cells promotes hepatocarcinogenesis in vitro
and in vivo. Oncotarget. 8:105340–105355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang R, Li Y, Hou Y, Yang Q, Chen S, Wang
X, Wang Z, Yang Y, Chen C, Wang Z and Wu Q: The
PDGF-D/miR-106a/Twist1 pathway orchestrates epithelial-mesenchymal
transition in gemcitabine resistance hepatoma cells. Oncotarget.
6:7000–7010. 2015.PubMed/NCBI
|
|
37
|
Ni J, Zhou LL, Ding L, Zhao X, Cao H, Fan
F, Li H, Lou R, Du Y, Dong S, et al: PPARγ agonist efatutazone and
gefitinib synergistically inhibit the proliferation of
EGFR-TKI-resistant lung adenocarcinoma cells via the
PPARgamma/PTEN/Akt pathway. Exp Cell Res. 361:246–256. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakamura H and Wang Y, Kurita T, Adomat H,
Cunha GR and Wang Y: Genistein increases epidermal growth factor
receptor signaling and promotes tumor progression in advanced human
prostate cancer. PLoS One. 6:e200342011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Little PJ, Getachew R, Rezaei HB,
Sanchez-Guerrero E, Khachigian LM, Wang H, Liao S, Zheng W,
Ballinger ML and Osman N: Genistein inhibits PDGF-stimulated
proteoglycan synthesis in vascular smooth muscle without blocking
PDGFβ receptor phosphorylation. Arch Biochem Biophys. 525:25–31.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ono M and Kuwano M: Molecular mechanisms
of epidermal growth factor receptor (EGFR) activation and response
to gefitinib and other EGFR-targeting drugs. Clin Cancer Res.
12:7242–7251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tanjak P, Thiantanawat A, Watcharasit P
and Satayavivad J: Genistein reduces the activation of AKT and
EGFR, and the production of IL6 in cholangiocarcinoma cells
involving estrogen and estrogen receptors. Int J Oncol. 53:177–188.
2018.PubMed/NCBI
|
|
42
|
Bodine SC, Stitt TN, Gonzalez M, Kline WO,
Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC,
Glass DJ and Yancopoulos GD: Akt/mTOR pathway is a crucial
regulator of skeletal muscle hypertrophy and can prevent muscle
atrophy in vivo. Nat Cell Biol. 3:1014–1019. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Missiaglia E, Dalai I, Barbi S, Beghelli
S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A,
delle Fave G, et al: Pancreatic endocrine tumors: Expression
profiling evidences a role for AKT-mTOR pathway. J Clin Oncol.
28:245–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sahin K, Tuzcu M, Basak N, Caglayan B,
Kilic U, Sahin F and Kucuk O: Sensitization of cervical cancer
cells to cisplatin by genistein: The role of NFκB and Akt/mTOR
signaling pathways. J Oncol. 2012:4615622012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Block M, Gründker C, Fister S, Kubin J,
Wilkens L, Mueller MD, Hemmerlein B, Emons G and Günthert AR:
Inhibition of the AKT/mTOR and erbB pathways by gefitinib,
perifosine and analogs of gonadotropin-releasing hormone I and II
to overcome tamoxifen resistance in breast cancer cells. Int J
Oncol. 41:1845–1854. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li W, Tan D, Zhang Z, Liang JJ and Brown
RE: Activation of Akt-mTOR-p70S6K pathway in angiogenesis in
hepatocellular carcinoma. Oncol Rep. 20:713–719. 2008.PubMed/NCBI
|