Introduction
Percutaneous nephrolithotomy (PCNL) is a common
procedure for large-volume renal calculus disease (1). Due to the refinement of technology,
improved surgical instrumentation and increasing experience, the
safety and efficacy of PCNL has increased in the last two decades.
However, the risk of significant complications, including renal
hemorrhage remains a problem. The majority of the bleeding may be
managed conservatively with bed rest and transfusion. However,
massive or continuous renal hemorrhage often requires expeditious
intervention (2,3). Surgical exploration as a therapeutic
option to stop bleeding may lead to nephrectomy. Renal artery
embolization has been used for controlling renal hemorrhage since
the 1970s (4,5). This technique has advanced with the
introduction of smaller delivery catheters and more precise embolic
agents (6). It is now possible to
perform superselective renal arterial embolization (SRAE), which
minimizes the loss of renal function (7,8).
However, when initial embolization fails, patients may require a
second superselective embolization or surgical exploration,
presenting a challenge for urologists and healthcare providers. A
detailed examination of the literature revealed a relative paucity
of studies focusing on the causes initial SRAE failure after PCNL.
In the present study, clinical data was retrospectively analyzed to
examine the incidence and management of the complication, and to
identify any risk factors that may predict the possibility of this
event.
Materials and methods
Patients
A total of 3,300 PCNL procedures were performed in
The First Affiliated Hospital of Zhejiang University (Zhejiang,
China) between August 2005 to June 2016. The records of patients
who had undergone renal arteriography for renal hemorrhage
following PCNL were respectively reviewed. The inclusion criterion
was severe post-PCNL renal hemorrhage requiring SRAE. Exclusion
criteria were no arterial lesion on renal arteriogram, and the use
of anticoagulant drugs or abnormal blood coagulation. The study was
approved by Research Ethics Committee of The First Affiliated
Hospital of Zhejiang University (approval number, 2018-1056).
A total of 98 patients underwent SRAE due to
uncontrolled renal hemorrhage following PCNL, including 15 patients
transferred from other hospitals. There were 77 males and 21
females. The mean patient age was 51.9 years (26–77 years). The
following variables were identified for each patient: Clinical
presentation; comorbidities; hemoglobin concentration; requirement
of pre-embolization blood transfusion; timing of embolization;
embolization agents; injury mechanism and location;
post-embolization transfusion requirement; and long-term
outcome.
Techniques
The PCNL was performed by 3 specialists in The First
Affiliated Hospital of Zhejiang University. The technique involved
percutaneous puncture using an 18G gauge coaxial needle under
ultrasound guidance. The nephrostomy track was dilated up to 26F
using metal telescopic dilation, Amplatz serial dilation or a
single-step balloon dilator. A smaller 16-18F tract was employed in
patients with a small renal stone. A single tract or multiple
tracts were used depending on the complex of stones. Pneumatic,
holmium laser or ultrasonic lithotripsy was used for calculus
disintegration at the discretion of the surgeon. A nephrostomy tube
was placed at the end of the procedure. A KUB film was obtained 48
h after the procedure to confirm the stone clearance status. The
tube was removed after 2 weeks if there were no complications or
residual stones.
Mild bleeding is self-limiting and resolves with
conservative measures, including bed rest, tamponade by clamping
the nephrostomy tube and adequate hydration. If conservative
treatment fails, patients with progressive decline of hemoglobin
undergo a computed tomographic angiography (CTA) to define the
optimal therapeutic strategy on the basis of the extent and origin
of bleeding (arterial or venous). If renal artery injuries are
confirmed, the patients proceed to renal arteriography with or
without embolization.
The technical details of SRAE were described
previously (8). Briefly, a 5F
angiographic catheter was introduced into the renal artery via the
transfemoral route. A global angiogram was performed to identify
any lesions, which were additionally confirmed by selective renal
arteriography. Embolic materials, metallic coils or polyvinyl
alcohol (PVA) particles, were then deployed. Renal arteriogram was
repeated to demonstrate a small avascular segment and patency of
the rest of the vessels. All procedures were performed by 2 senior
interventional radiologists.
Statistical analysis
To assess the risk factors for failure of initial
SRAE, data from the patients requiring repeated SRAE were compared
with those of the patients with successful initial SRAE using
two-tailed t-tests for age, and χ2 analysis for other
variables. Multivariate logistic regression analysis were applied
in a stepwise backward manner to verify the independent risk
factors, with a significance level of P<0.05. Various clinical
factors including patients age and sex, comorbidities (hypertension
and diabetes), kidney side, percutaneous tract size, stone size,
tract dilation methods, and the details of the angiographic data
[number of bleeding sites, arteriovenous fistula (AVF) and vascular
aberration/tortuosity] were assessed. All analyses were conducted
using SPSS software v16 (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
All 98 patients had basically normal INR levels, and
the platelet level was slightly decreased in 2 patients. A total of
43 patients exhibited bleeding from the left kidney, and 55 from
the right. Blood transfusion was required for 42 patients prior to
first embolization. The mean time between the surgery and first
embolization procedures was 8.1 days (1–22 days). Of the 98
patients, 65 developed a pseudoaneurysm, 6 had an arteriovenous
fistula, and 11 patients exhibited both. Free extravasation was
observed in 11 patients; 8 of these patients exhibited coexisting
pseudoaneurysm. Besides, renal vascular aberration or tortuosity
were encountered in 10 patients. For embolization, metallic coils
were used in all procedures, with the exception of 2 patients using
PVA particles. Of the 15 patients from other hospitals, 5 patients
had left kidney bleeding, and 10 had right. A total of 9 patients
had a pseudoaneurysm, 2 had an arteriovenous fistula, 3 had both a
pseudoaneurysm and an arteriovenous fistula, and 1 had free
extravasation.
SRAE
Complete resolution of bleeding was observed in 81
patients, and initial SRAE failure occurred in 17 (17.3%) patients
with recurrent hemorrhage. A second embolization was performed in
16 patients, and 1 patient had open exploration for deep suture of
bleeding site due to renal rupture on CT image, but the bleeding
did not cease following surgery until the second embolization was
performed 7 days later. Of the 17 cases of SRAE failure, the
angiography revealed a new bleeding site in 7 patients, while the
arteries were incompletely occluded in 10 patients with metallic
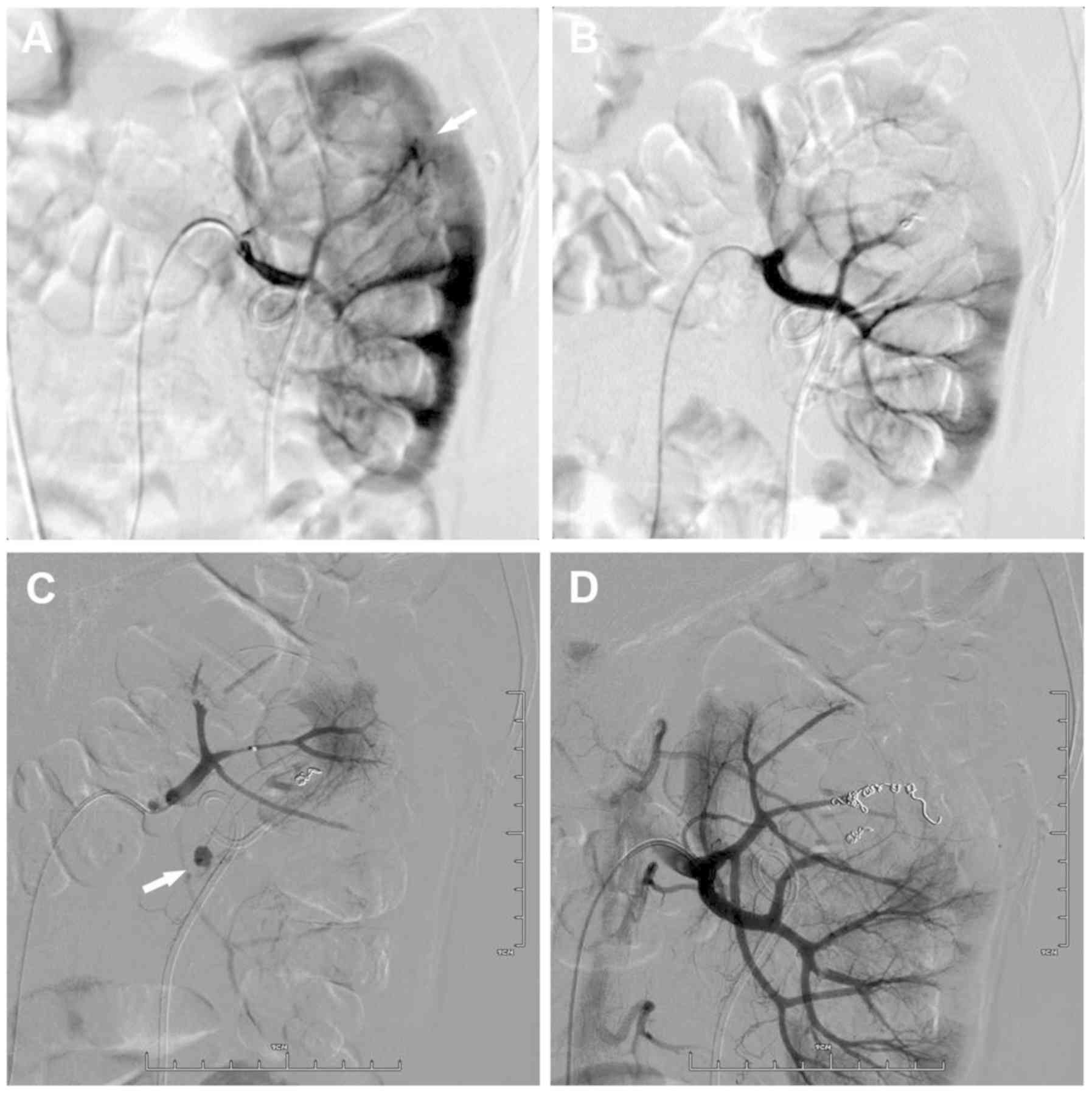
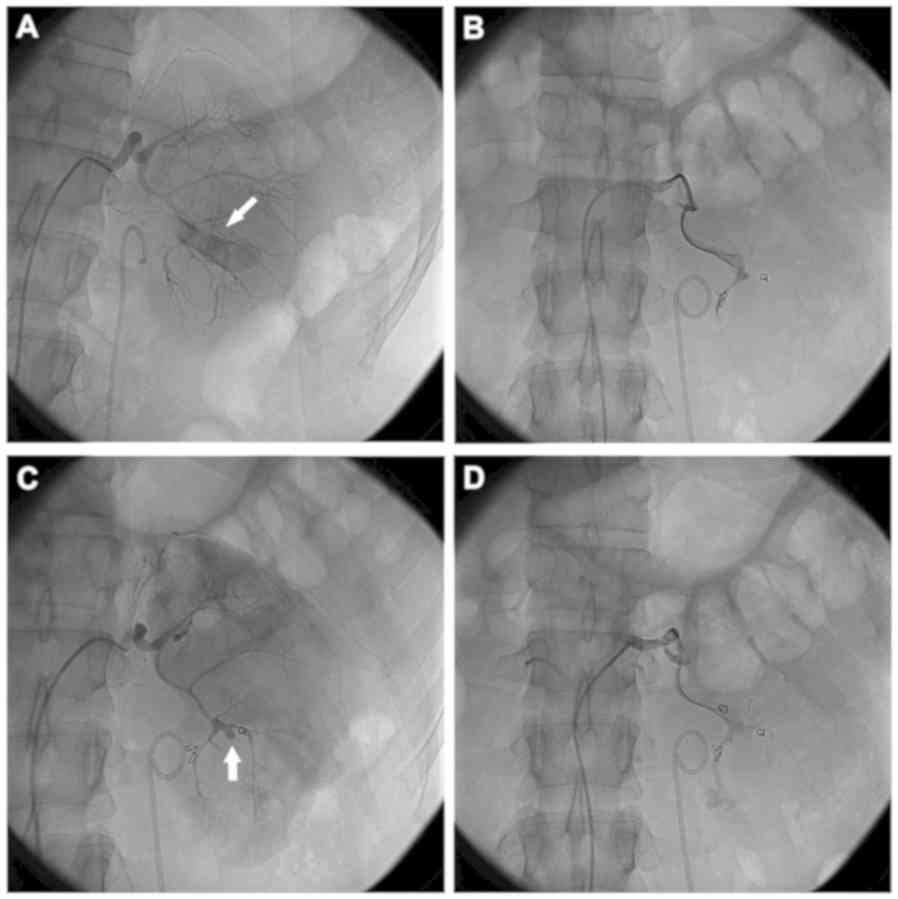
coils in place. Figs. 1 and 2 demonstrate the bleeding sites on initial
and repeat renal arteriograms from 2 patients. Complete resolution
of bleeding was observed in 16 patients following re-embolization.
The remaining 1 patient, who had renal vascular tortuosity, with
repeated embolization with metallic coils 5 times (the first 3
times were performed in other institutions and 2 times in The First
Affiliated Hospital of Zhejiang University) was finally managed by
conservative therapy with repeated transfusion. All the patients
had blood transfusion following initial treatment failure.
Follow-up
The follow-up of 17 patients with repeated SRAE was
presented in Table I. A total of 3
patients had low-grade fever, and 2 had flank pain. These symptoms
disappeared 2–5 days after symptomatic treatment. The mean
follow-up was 20 months (3–54 months). All had follow-up imaging of
a renal ultrasonography or contrast CT, in addition to serum
creatinine test. There was no global kidney atrophy or renal
abscesses observed. There was no renovascular hypertension in any
patient (5 patients had hypertension prior to embolization).
 | Table I.Follow-up of patients with repeated
superselective renal arterial embolization. |
Table I.
Follow-up of patients with repeated
superselective renal arterial embolization.
| Patients (n=17) | Count |
|---|
| Follow up, months
[mean (range)] | 20 (3–54) |
| Fever | 3 |
| Flank pain | 2 |
| Hypertension | 0 |
| Global renal
atrophy | 0 |
| Renal abscess | 0 |
Risk factors for initial failure of
SRAE
The univariate analyses results are presented in
Table II, to compare between
patients experiencing initial treatment failure and those who did
not. Statistical significance was suggested in the univariate
analyses for tract size, number of bleeding sites and renal
vascular aberration/tortuosity. Patient age, sex, kidney side,
hypertension, diabetes, stone size, tract dilation methods and AVF
at initial SRAE were not potential risk factors for initial
treatment failure. Multivariate analyses confirmed that the 3
factors identified in the univariate analysis were independent risk
factors (Table III).
 | Table II.Univariate analysis of risk factors
for initial failure of SRAE after PCNL. |
Table II.
Univariate analysis of risk factors
for initial failure of SRAE after PCNL.
| Variable | Success | Failures | χ2 | OR (95% CI) | P-value |
|---|
| Sex |
| Male | 62 | 15 |
| – |
|
|
Female | 19 | 2 | 1.141 | 0.44 (0.09–2.08) | 0.285 |
| Kidney side |
| Left | 33 | 10 |
| – |
|
|
Right | 48 | 7 | 1.866 | 0.48 (0.17–1.39) | 0.172 |
| Hypertension |
| No | 56 | 11 |
| – |
|
| Yes | 25 | 6 | 0.128 | 1.22 (0.41–3.67) | 0.721 |
| Diabetes |
| No | 75 | 16 |
| – |
|
| Yes | 6 | 1 | 0.049 | 0.78 (0.09–6.94) | 0.824 |
| Stone size, cm |
|
<3 | 50 | 11 |
| – |
|
| ≥3 | 31 | 6 | 0.053 | 0.88 (0.30–2.62) | 0.818 |
| Tract size |
|
Mini-PCNL | 33 | 1 |
| – |
|
|
Standard | 48 | 16 | 7.536 | 11.00
(1.39–87.03) | 0.006 |
| Dilation method |
|
Telescopic/serial
dilation | 52 | 8 |
| – |
|
| Balloon
dilation | 29 | 9 | 1.739 | 2.107
(0.70–5.79) | 0.187 |
| No. of bleeding
sites at initial SRAE |
| 1 | 76 | 12 |
| – |
|
| ≥2 | 5 | 5 | 8.282 | 6.33
(1.59–25.20) | 0.004 |
| AVF at initial
SRAE |
| No | 67 | 14 |
| – |
|
|
Yes | 14 | 3 | 0.001 | 1.03
(0.26–4.05) | 0.971 |
| Renal vascular
aberration/tortuosity |
| No | 76 | 12 |
| – |
|
|
Yes | 5 | 5 | 8.282 | 6.33
(1.59–25.20) | 0.004 |
 | Table III.Multivariate analysis of risk factors
for initial failure of SRAE after PCNL. |
Table III.
Multivariate analysis of risk factors
for initial failure of SRAE after PCNL.
| Variable | OR (95% CI) | P-value |
|---|
| Tract size | 12.23
(1.18–126.42) | 0.036 |
| No. of bleeding
sites at initial SRAE | 10.86
(1.90–62.03) | 0.007 |
| Renal vascular
aberration/tortuosity | 6.73
(1.37–33.15) | 0.019 |
Discussion
The introduction of smaller delivery catheters and
more precise embolic agents has markedly improved the morbidity
associated with SRAE (9), and it has
continued to gain popularity as a minimally invasive approach for
various urological conditions, including arteriovenous
malformations, medical renal disease, angiomyolipomas and
preoperative infarction of renal cell carcinoma (10). Although extracorporeal shock wave
lithotripsy (ESWL) and flexible ureteroscopic stone removal are
widely used treatment modalities for renal stones, PCNL is required
for large and complex renal calculi (1). However, PCNL does carry a risk of
post-operative renal bleeding (11–13). The
iatrogenic renal artery injuries following PCNL now represent a
significant proportion of the indications for SRAE (8,10,14). The
present study describes the use of SRAE in 98 patients from The
First Affiliated Hospital of Zhejiang University, with particular
emphasis on the risk factors of initial treatment failure.
The reported incidence of post-PCNL bleeding
requiring angiographic embolization is 0.8–2.4% (11,15–17). In
the present study, out of the 3,300 PCNL procedures performed
during the study period, 83 patients required SRAE (2.5%), and the
incidence was slightly increased compared with previous studies
(11,15–17). The
discrepancy of incidence between studies may be attributed to the
different indications for SRAE. For example, patients with severe
hematuria with a fall in hematocrit fall in blood pressure,
recurrent clot retention, and/or a requirement for inotropes to
maintain hemodynamic stability, will undergo angiography and
subsequent embolization. In the present study, if renal arterial
injuries (pseudoaneurysm, arteriovenous fistula or free
extravasation) were confirmed on CTA following severe hemorrhage,
the patient was treated with SRAE immediately. However, spontaneous
cessation of hemorrhage may occur for small renal arterial
injuries, resulting in the relatively high rate of embolization in
the present study cohort. Various risk factors have been proposed
for predicting severe bleeding due to PCNL, including access needle
size, number of punctures, staghorn stones, solitary kidney or
history of urinary tract infection (16–19).
Identification of risk factors affecting the incidence of
hemorrhage recurrence following SRAE is also of the utmost
importance, as failure of initial SRAE may be life threatening,
resulting in significant pressure on the patient and surgical team.
However, one study by Zeng et al (20) identified 3 risk factors for initial
treatment failure, including multiple percutaneous access sites,
>2 bleeding sites identified on renal angiogram and gelatin
sponge alone used as the embolic material. By contrast, the data
from the present study revealed that large tract size, multiple
bleeding sites and vascular aberration/tortuosity were significant
predictors of initial treatment failure.
The degree of dilation of the tract is one of the
factors responsible for bleeding. Previous evidence suggested that
decreasing the tract size for PCNL may decrease blood loss and
morbidity (21). Desai et al
(22,23) even developed a 3.5-F ultra-thin
telescope method, termed ‘ultra-mini percutaneous nephrolithotomy’.
In the present study, a significantly increased risk of repeated
SRAE for patients who had undergone standard PCNL (26F) as compared
with mini-PCNL (16F-18F) was observed. However, the most important
disadvantages of mini-PCNL are the long surgery times and the more
advanced technical skill required. It is difficult to determine
exactly which tract size is most appropriate for PCNL. On the basis
of the results of the present study, and the information from
previous studies, we propose avoidance of large tract size for
relatively small stone burden, in an attempt to decrease
retreatment rate of SRAE, particularly in cases with
non-hydronephrotic systems and those with a narrow
infundibulum.
An additional risk factor for initial treatment
failure observed in the present study was multiple bleeding sites.
The renal arterial lesions may develop in a number of different
sites, particularly in multi-tract PCNL, though all the patients
with repeated SRAE underwent one-tract PCNL. In the patients in the
present study, a global arteriogram was usually performed at the
end of the procedure. However, it was possible that certain
bleeding sites may have been omitted when the number of lesions was
too high. In addition, temporary spasm of the involved artery,
which is the first response of the blood vessel to injury, would be
difficult to identify on an arteriogram. Therefore, a second
session of SRAE was often required following this event.
In the present study, the vascular aberration or
tortuosity was also a significant risk factor for the failure of
initial SRAE. Vascular aberration or tortuosity would add time,
complexity and risk to the procedure. Navigating a guiding wire
across highly tortuous segments of the renal artery is challenging
and requires multiple attempts, and tends to induce severe
vasospasm and arterial dissections, particularly when the wire
catches the wall of the vessel at an abrupt 180° or 360° turn. In
certain cases, a distal position cannot be achieved and the
operator is forced to attempt embolization from a more proximal
parent artery, which increases the risk of treatment failure and
complication.
Akman et al (24) demonstrated that the risk of major
complications, in particular hemorrhage, was significantly
increased during PCNL in patients with diabetes mellitus,
hypertension, and the metabolic syndrome. Kukreja et al
(18) evaluated factors affecting
blood loss during PCNL in a prospective study. They identified that
diabetes was one of the risk factors associated with significantly
increased blood loss during PCNL. Associated arteriosclerosis in
patients with diabetes and hypertension may make these patients
more prone to iatrogenic arterial injuries secondary to
endovascular treatment, and impair the self-healing properties of
the arterial wall, due to the loss of its normal muscle and elastic
layers (25), resulting in recurrent
hemorrhage following initial SRAE. However, patients with
comorbidities including diabetes mellitus and hypertension appeared
to have no propensity to treatment failure in our series. Balloon
dilation is generally considered to have an improved hematologic
morbidity profile compared with the other dilation methods, as it
is radial without the risk of perforation of any structures ahead
or surrounding the tract, which is a risk of multi-incremental
methods. In the present study, there was no significant difference
of re-treatment rate between the balloon dilation and
telescopic/serial dilation groups. There have been studies
suggesting that the experience of the interventional radiologists
is associated with endovascular procedural outcomes, and the risk
of complications with coil embolization appears to decrease
markedly in correspondence with the level of physician experience.
However, all the procedures in the present study were performed by
2 senior interventional radiologists, who were highly experienced
in other endovascular techniques at study onset; therefore, the
physician experience was not considered to be as a risk factor in
the analysis.
The present study has several limitations, including
the nonrandomized and retrospective nature of the present study,
the unequal patient number in the different groups, and procedures
performed with different surgeon experience from different
institutes. Due to limited samples within the subgroups, the power
of statistical analyses for certain variables may have been low,
and potentially relevant factors may have been overlooked. In
addition, follow-up of differential renal function using
radioisotope renal scan was not available. Therefore, other studies
are required to address these limitations.
In conclusion, SRAE is recommended for patients who
have uncontrolled hemorrhage following PCNL, and patients
experiencing initial treatment failure may be successfully managed
with repeated SRAE. The results of the present study indicated that
tract size, number of bleeding sites and renal vascular
aberration/tortuosity significantly predicted the occurrence of
initial treatment failure of SRAE. No significant association was
identified between the other variables. The results of the present
study may assist interventional radiologists in the planning and
execution of SRAE in the treatment of PCNL.
Acknowledgements
Not applicable.
Funding
The present study is supported by grants from
Zhejiang Provincial Medical Science Foundation of China (grant no.
2013KYB086) and the Natural Science Foundation of Zhejiang Province
(grant no. LY18H160010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QM was involved in acquisition, analysis and
interpretation of the data. QM, CW and BS contributed to the design
and the conception of the study and interpretation of the data. GC
and FT conceived the study and participated in its design and
coordination. QM and BS drafted the manuscript.
Ethics approval and consent to
participate
The study was approved by Research Ethics Committee
of The First Affiliated Hospital of Zhejiang University (approval
number, 2018-1056).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AVF
|
arteriovenous fistula
|
|
ESWL
|
extracorporeal shock wave
lithotripsy
|
|
CTA
|
computed tomographic angiography
|
|
PCNL
|
percutaneous nephrolithotomy
|
|
SRAE
|
superselective renal arterial
embolization
|
References
|
1
|
Preminger GM, Assimos DG, Lingeman JE,
Nakada SY, Pearle MS and Wolf JS Jr; AUA Nephrolithiasis Guideline
Panel, : Chapter 1: AUA guideline on management of staghorn
calculi: Diagnosis and treatment recommendations. J Urol.
173:1991–2000. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin X, Murat FJ, Feitosa LC, Rouvière
O, Lyonnet D, Gelet A and Dubernard J: Severe bleeding after
nephrolithotomy: Results of hyperselective embolization. Eur Urol.
37:136–139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gallucci M, Fortunato P, Schettini M and
Vincenzoni A: Management of hemorrhage after percutaneous renal
surgery. J Endourol. 12:509–512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bookstein JJ and Ernst CB: Vasodilatory
and vasoconstrictive pharmacoangiographic manipulation of renal
collateral flow. Radiology. 108:55–59. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chuang VP, Reuter SR, Walter J, Foley WD
and Bookstein JJ: Control of renal hemorrhage by selective arterial
embolization. Am J Roentgenol Radium Ther Nucl Med. 125:300–306.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beaujeux R, Saussine C, al-Fakir A,
Boudjema K, Roy C, Jacqmin D and Bourjat P: Superselective
endo-vascular treatment of renal vascular lesions. J Urol.
153:14–17. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El Tayeb MM, Knoedler JJ, Krambeck AE,
Paonessa JE, Mellon MJ and Lingeman JE: Vascular complications
after percutaneous nephrolithotomy: 10 years of experience.
Urology. 85:777–781. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang C, Mao Q, Tan F and Shen B:
Superselective renal artery embolization in the treatment of renal
hemorrhage. Ir J Med Sci. 183:59–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keller FS: Interventional radiology: New
paradigms for the new millennium. J Vasc Interv Radiol. 11:677–681.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwartz MJ, Smith EB, Trost DW and
Vaughan ED Jr: Renal artery embolization: Clinical indications and
experience from over 100 cases. BJU Int. 99:881–886. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keoghane SR, Cetti RJ, Rogers AE and
Walmsley BH: Blood transfusion, embolisation and nephrectomy after
percutaneous nephrolithotomy (PCNL). BJU Int. 111:628–632. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ganpule AP, Shah DH and Desai MR:
Postpercutaneous nephrolithotomy bleeding: Aetiology and
management. Curr Opin Urol. 24:189–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rastinehad AR, Andonian S, Smith AD and
Siegel DN: Management of hemorrhagic complications associated with
percutaneous nephrolithotomy. J Endourol. 23:1763–1767. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jain V, Ganpule A, Vyas J, Muthu V, Sabnis
RB, Rajapurkar MM and Desai MR: Management of non-neoplastic renal
hemorrhage by transarterial embolization. Urology. 74:522–526.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kessaris DN, Bellman GC, Pardalidis NP and
Smith AG: Management of hemorrhage after percutaneous renal
surgery. J Urol. 153:604–608. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JK, Kim BS and Park YK: Predictive
factors for bleeding during percutaneous nephrolithotomy. Korean J
Urol. 54:448–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El-Nahas AR, Shokeir AA, El-Assmy AM,
Mohsen T, Shoma AM, Eraky I, El-Kenawy MR and El-Kappany HA:
Post-percutaneous nephrolithotomy extensive hemorrhage: A study of
risk factors. J Urol. 177:576–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kukreja R and Desai M, Patel S, Bapat S
and Desai M: Factors affecting blood loss during percutaneous
nephrolithotomy: Prospective study. J Endourol. 18:715–722. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Srivastava A, Singh KJ, Suri A, Dubey D,
Kumar A, Kapoor R, Mandhani A and Jain S: Vascular complications
after percutaneous nephrolithotomy: Are there any predictive
factors? Urology. 66:38–40. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng G, Zhao Z, Wan S, Khadgi S, Long Y,
Zhang Y, Cao G and Yang X: Failure of initial renal arterial
embolization for severe post-percutaneous nephrolithotomy
hemorrhage: A multicenter study of risk factors. J Urol.
190:2133–2138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Resorlu B, Kara C, Ozyuvali E and Unsal A:
Percutaneous nephrolithotomy in hypertensive patients with
different sizes of instruments. Acta Chir Belg. 111:228–231. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Desai J and Solanki R: Ultra-mini
percutaneous nephrolithotomy (UMP): One more armamentarium. BJU
Int. 112:1046–1049. 2013.PubMed/NCBI
|
|
23
|
Desai J, Zeng G, Zhao Z, Zhong W, Chen W
and Wu W: A novel technique of ultra-mini-percutaneous
nephrolithotomy: Introduction and an initial experience for
treatment of upper urinary calculi less than 2 cm. Biomed Res Int.
2013:4907932013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akman T, Binbay M, Sari E, Yuruk E,
Tepeler A, Akcay M, Muslumanoglu AY and Tefekli A: Factors
affecting bleeding during percutaneous nephrolithotomy: Single
surgeon experience. J Endourol. 25:327–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patterson DE, Segura JW, LeRoy AJ, Benson
RC Jr and May G: The etiology and treatment of delayed bleeding
following percutaneous lithotripsy. J Urol. 133:447–451. 1985.
View Article : Google Scholar : PubMed/NCBI
|