Introduction
Pamidronate (Pami) is a type of bisphosphonate (BP)
agent, which is a pyrophosphate analogue, and successfully used in
the management of several bone diseases, including Paget's disease,
osteoporosis and hypercalcemia-associated cancer cell damage.
Additionally, BPs decrease bone pain or fractures in the elderly or
patients with cancer with cancer cells migrating to the bone
(1). In vitro studies show
that BPs, including clodronate, alendronate, zoledronate and Pami
(2–5), have an effect on cancer cells by
suppressing the proliferation and induction of apoptosis.
Interestingly, BPs inhibit bone metastasis and prolong the survival
rate in patients with cancer. A study on patients with estrogen
receptor (ER)-positive breast cancer indicated that zoledronic acid
in combination with a hormone antagonist improves disease-free
survival (6). Various studies
suggest that BPs could be an anticancer agent for patients with
prostate cancer (7,8). Furthermore, BPs have beneficial effects
on patients with prostate cancer who have cancer cells that migrate
to the bones. Moreover, Pami inhibits PC-3 prostate cancer cells
with high expression levels of Bcl-2 and anti-apoptotic protein
(2). BPs have been demonstrated to
have a high efficacy in cancer treatment in both in vitro
and in vivo studies, and the deep effects of Pami on other
cancer cells, including cholangiocarcinoma (CCA) cells, have been
revealed.
The proposed mechanisms of BPs on cancer cells are
indicated in several pathways and are important mechanisms in the
inhibition of cell growth and migration (9–11).
Furthermore, BPs decrease the gene-related cancer cell functions or
replication (12,13). Interestingly, one action of BPs is
elucidated by the reduction of enzymes in the mevalonate (MVA)
pathway, which reduces MVA products, such as farnesyl pyrophosphate
(FPP) and geranylgeranyl pyrophosphate (GGPP) (14,15) by
suppressing the farnesyl pyrophosphate synthase (FPPS) enzyme. FPP
and GGPP are crucial for producing intracellular proteins,
including Rho, Ras, Rap and Rac, which are involved in the
signaling pathways of normal and cancer functions (16). For tumor cells, MVA products can
control cell differentiation, growth, migration, invasion and
angiogenesis. Iguchi et al (2) have demonstrated that Pami inhibits
prostate cancer cell proliferation through decreasing the MVA
pathway and blocking Rap1, which is accompanied by the suppression
of Bcl-2 expression. This is consistent with the results of Zhang
et al (17), who demonstrated
that Pami showed less activity against cancer cells, with low
levels of MVA products, such as N-Ras or H-Ras. Therefore, the
inhibition of the MVA pathway may be useful for inhibiting cancer
cell growth and migration.
CCA, or bile duct cancer, is an aggressive cancer
and a significant public health problem in Southeast Asian
Countries, particularly in the North-Eastern part of Thailand.
Infection by Opisthorchis viverrini (OV) is the main risk
factor of CCA (17). OV infection
can induce long-term chronic inflammation. The accumulation of
numerous inflammatory cytokines and growth factors can lead to
uncontrollable cancer cell growth and metastasis (18). CCA is frequently identified at an
aggressive stage (stages III or IV) that is unsuitable for surgical
treatment. Furthermore, standard chemotherapeutic drugs display low
efficacy, high toxicity and high drug resistance (19). Therefore, novel anticancer agents,
such as BPs, with high efficiency for CCA and low toxicity are
urgently required.
In the present study, KKU-100 cells were used to
examine the effects of Pami on CCA cell death, migration and the
MVA pathway. To measure the cell death by sulforhodamine B (SRB),
colony formation and apoptosis, reactive oxygen species (ROS)
production, caspase-3 activity and flow cytometry assays were used.
To test the effects of Pami on MVA products, RT-qPCR and western
blotting were used. Finally, to assess the migration effects, wound
healing, Matrigel and gelatin zymography assays were used.
Materials and methods
Cell cultures and cell death
Human CCA KKU-100 cells were provided by the Faculty
of Medicine, Khon Kaen University. The cells were cultured in DMEM
supplemented with penicillin (100 U/ml), streptomycin (100 mg/ml)
and 10% FBS. Cell death was examined by SRB assays as previously
described (20). Cells were exposed
to various concentrations of 0–1,000 µM Pami or 0.25% dimethyl
sulfoxide (DMSO) for 24 to 48 h and then fixed with 10%
trichloroacetic acid (TCA), stained with 0.4% SRB and washed and
solubilized in 10 mM Tris base buffer. The optical density was
measured at 540 nm and compared with the control groups.
Colony formation
KKU-100 cell colony formation was examined using a
colony formation assay as previously described (20). Briefly, KKU-100 cells were exposed to
various concentration of 0–100 µM Pami or 0.25% DMSO for 24 h. The
DMEM medium was refreshed and cells were allowed to grow for 15
days. Next, the cells were washed with PBS buffer, fixed with
absolute methanol, stained with 0.5% crystal violet, washed and
dried. The colonies were counted and compared with the control
groups.
ROS generation
ROS production was assessed using a DHE-fluorescent
probe, as previously described (20). KKU-100 cells were exposed to various
concentrations of 0–250 µM Pami or 0.25% DMSO with 25 µM
DHE-fluorescent probes in the dark for 90 min. The fluorescent
intensity for the excitation wavelength was read at 518 nm and for
the emission wavelength at 605 nm. The intensity was calculated and
compared with the untreated groups.
Caspase-3 activity
Caspase-3 activity was examined with a caspase-3
assay kit, as previously described (20). Briefly, KKU-100 cells were exposed to
various concentrations of 0–250 µM Pami or 0.25% DMSO for 24 h and
then caspase-3 activity was determined. The caspase-3 activity was
calculated from the fluorescent intensity and compared with the
untreated groups.
Apoptosis
Apoptosis was tested by flow cytometry, as
previously described with some modifications (21). Briefly, KKU-100 cells were exposed to
various concentration of 0–250 µM Pami or 0.25% DMSO for 24 h,
stained with 5 µl propidium iodide (PI) and 5 µl Annexin V FITC and
then incubated for 15 min in the dark. Apoptosis was measured by
flow cytometry and compared with the control groups.
Wound healing
Migration was investigated using a wound healing
assay, as previously described (20). KKU-100 cells were scratched with 0.2
ml tips and incubated with various concentrations of 0–50 µM Pami
or 0.25% DMSO for 48 h. Then, the cancer cells were fixed and
stained with 0.5% crystal violet. The wound distance was calculated
and compared with the control groups.
Matrigel migration
Migration was tested with a Matrigel migration
assay, as previously described (22). KKU-100 cells were exposed to various
concentrations of 0–100 µM Pami or 0.25% DMSO for 24 h and, the
next day, the inserted well was removed, washed and stained with
0.5% crystal violet. The migration of the cells was compared with
the control groups.
Gelatin zymography
Migration was assessed by a gelatin zymography
assay, as previously described (23). KKU-100 cells were exposed to various
concentrations of 0–50 µM Pami or 0.25% DMSO for 24 h, the medium
was collected and the protein concentration was measured. The
protein was loaded onto 10% SDS-PAGE gels with 0.01% gelatin (w/v).
The gels were incubated with developing buffer overnight and
stained with 0.5% Coomassie brilliant blue R-250 until a clear band
was observed. The band density was calculated and compared with
that of the control groups.
Reverse transcription-quantitative
(RT-q)PCR
Gene expression levels were assessed by RT-qPCR, as
previously described (23,24). Briefly, KKU-100 cells were exposed to
250 µM Pami or 0.25% DMSO for 24 h. Afterwards, cellular RNA was
extracted and transcribed intro complementary DNA using the
iScript™ cDNA Synthesis kit (cat. no. 1708898; Bio-Rad
Laboratories, Inc.) at 42°C for 60 min. Rac1 and RhoA gene
expression were examined by RT-qPCR and primers for the target
genes are shown in Table I. A final
reaction volume of 20 µl was prepared containing SsoFast™ EvaGreen
Supermix with low Rox (Bio-Rad Laboratories, Inc.), and primers for
the target gene and the internal control β-actin. The PCR
conditions were as follows: Denaturation at 95°C for 3 min and
amplification by cycling 40 times at 95°C for 15 sec and 60°C for
30 sec. Expression was detected using a CFX96 Touch™ real-time PCR
Detection system (Bio-Rad Laboratories, Inc.). Differences in gene
expression levels were calculated using the 2−ΔΔCq
method (24) for relative
quantification and expressed as the fold change relative to the
untreated control. Assays assessing target gene expression were
performed in triplicate.
 | Table I.Primer sequences for Rac1, RhoA and
β-actin. |
Table I.
Primer sequences for Rac1, RhoA and
β-actin.
| Gene | Primer sequences
(5′-3′) |
|---|
| Rac1 | Forward:
ATGTCCGTGCAAAGTGGTATC |
|
| Reverse:
CTCGGATCGCTTCGTCAAACA |
| RhoA | Forward:
GGAAAGCAGGTAGAGTTGGCT |
|
| Reverse:
GGCTGTCGATGGAAAAACACAT |
| β-actin | Forward:
GTGACGTTGACATCCGTAAAGA |
|
| Reverse:
GCCGGACTCATCGTACTCC |
Western blotting
Protein expression levels were assessed by western
blotting, as previously described (23). Briefly, KKU-100 cells were exposed to
250 µM Pami or 0.25% DMSO for 24 h, the cell pellet was collected,
the cells were lysed and the protein concentration was measured.
The protein was loaded onto a 12% SDS-PAGE gel, which was blocked
and incubated with primary antibodies against Rac1 (1:1,000
dilution), RhoA (1:1,000 dilution) and beta-actin (1:2,500
dilution). Membranes were exposed to the secondary antibody
(1:2,500 dilution) the following day. The ECL substrate was used to
detect the band intensity and this was compared with the control
groups.
Statistical analysis
Data from three independent experiments were
statistically compared using Student's t-test and one-way ANOVA
followed by Tukey's post hoc test. The significance was set at 0.05
compared with the control by using SigmaStat software version 3.5
(Systat Software Inc.), and the IC50 values calculations
and statistical analyses were performed using Prism 5 software
(GraphPad Software, Inc.).
Results
Pami induces cell death and inhibits
colony formation
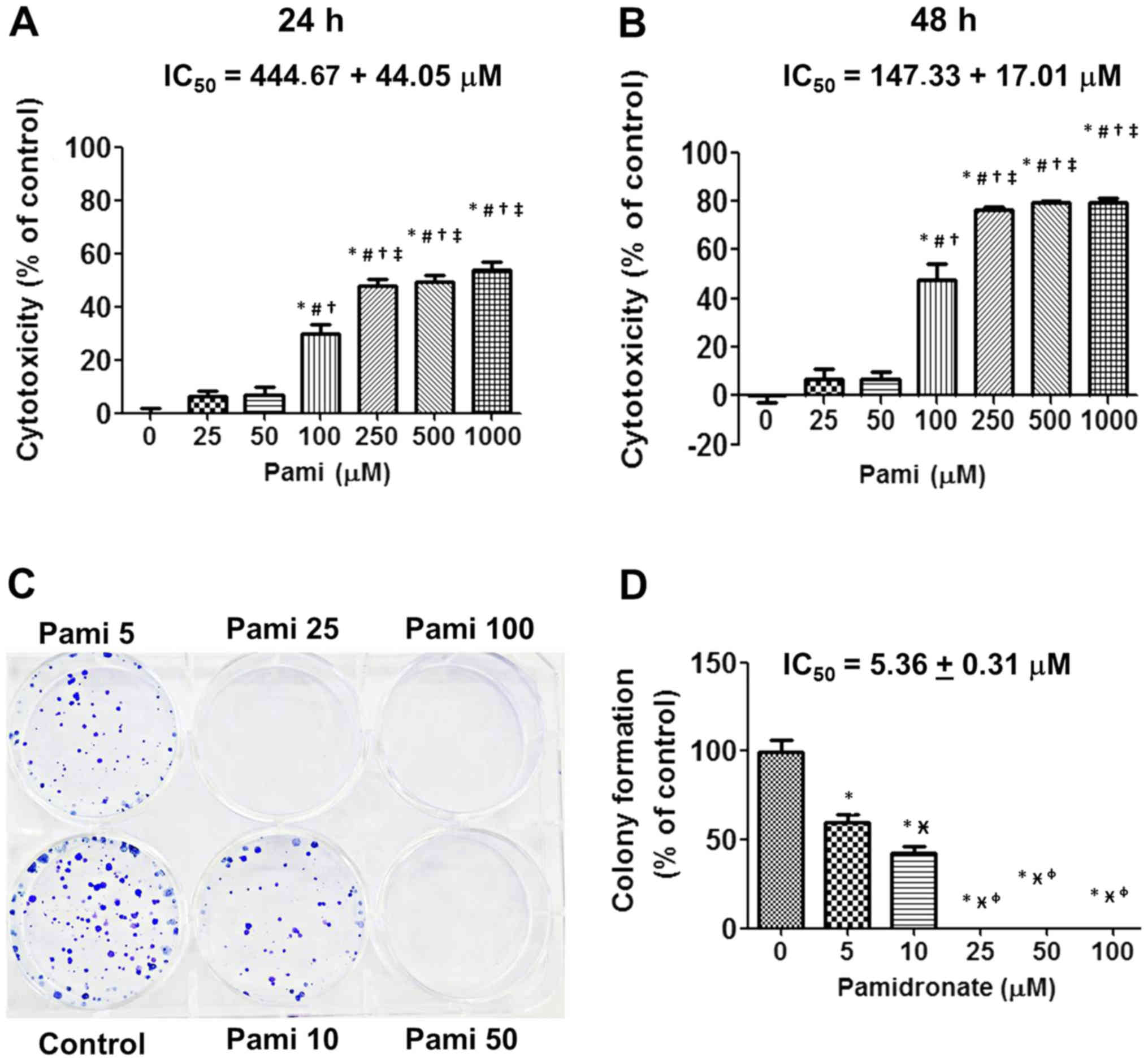
The results from the SRB assay indicated that Pami
suppressed CCA cells at 24 and 48 h (Fig. 1A and B). Notably, at doses between
100 and 1,000 µM Pami exhibited significant effects compared with
the control groups. Compared with the incubation time point, a
significant difference was identified in IC50 values of
444.67±44.05 µM for 24 h and IC50 values of 147.33±17.01
µM for 48 h for Pami effects. Accordingly, for growth inhibition,
colony formation was repressed in a concentration-dependent manner
with the lower concentration at IC50 values of 5.36±0.31
µM when compared with the growth inhibition (Fig. 1C and D).
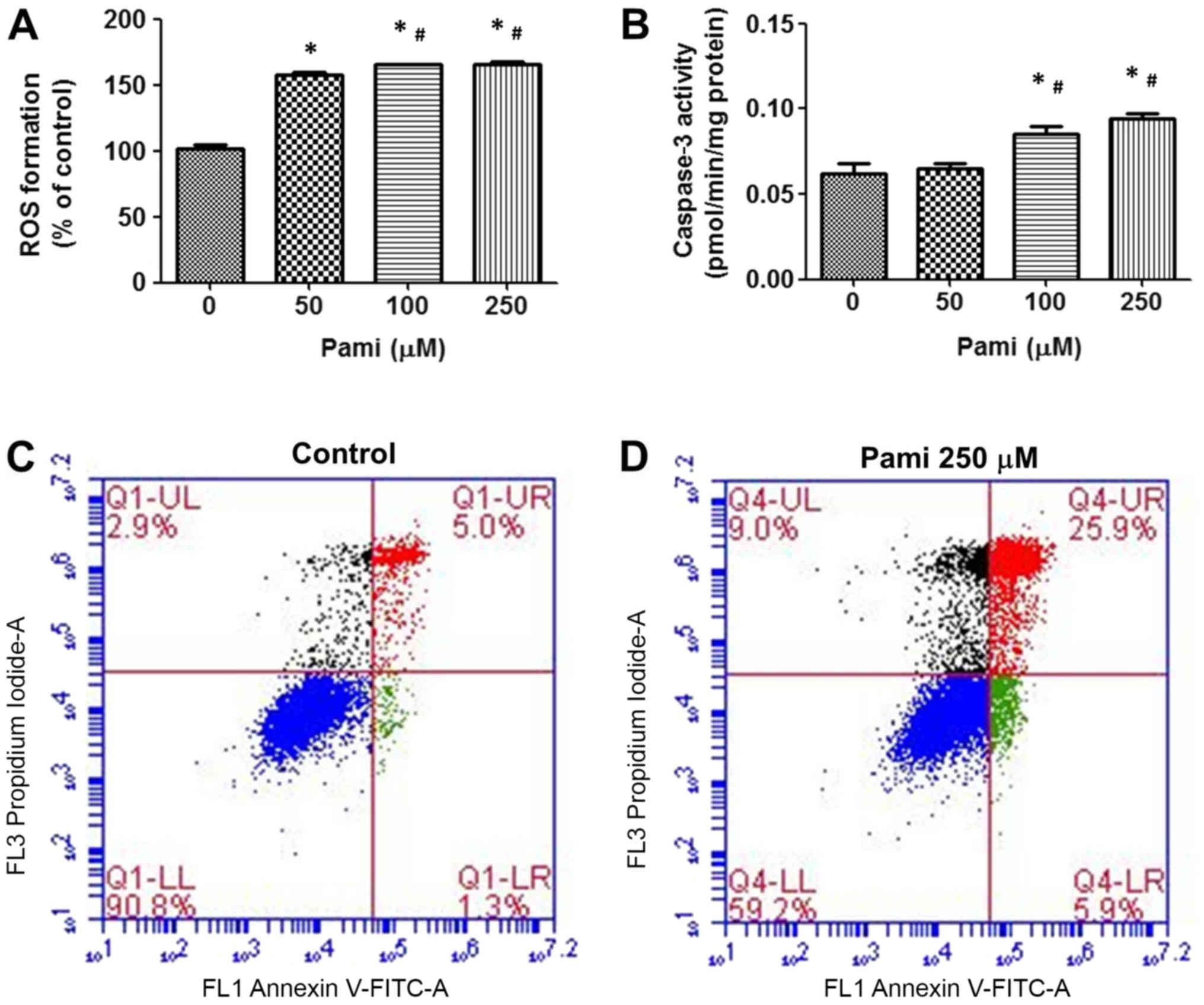
Pami induces cell apoptosis
The data indicated that Pami stimulated
intracellular ROS generation after incubation for 90 min (Fig. 2A) when compared with control group.
Correlating with the caspase-3 activity, Pami induced caspase-3
activity in a dose-dependent way at 24 h and showed significant
levels between the control groups at 100–250 µM (Fig. 2B). Testing cancer cell apoptosis by
flow cytometry, Pami caused significant induction of CCA apoptosis
and necrosis (Fig. 2C and D).
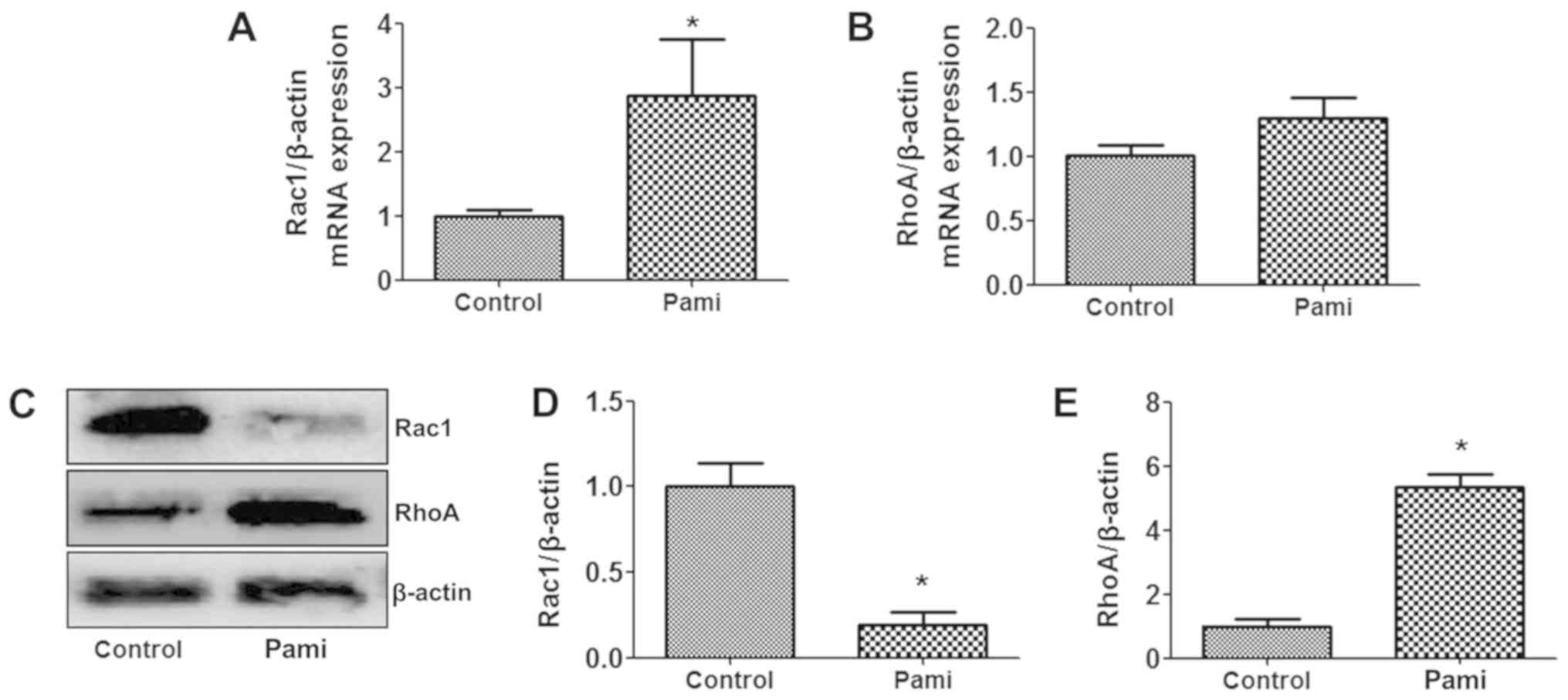
Pami decreases MVA product expression
levels
When exploring the Rac1 and RhoA gene expression
with RT-qPCR, after CCA cells were exposed to Pami, the Pami
effects modulated the Rac1 gene expression more than the
RhoA gene expression, and the Rac1 gene expression
was induced following incubation with Pami for 24 h, whereas
RhoA gene expression was not altered (Fig. 3A and B). When determining the protein
expression by western blotting, the Rac1 protein levels were
decreased at 24 h incubation, but RhoA protein levels were
increased (Fig. 3C-E).
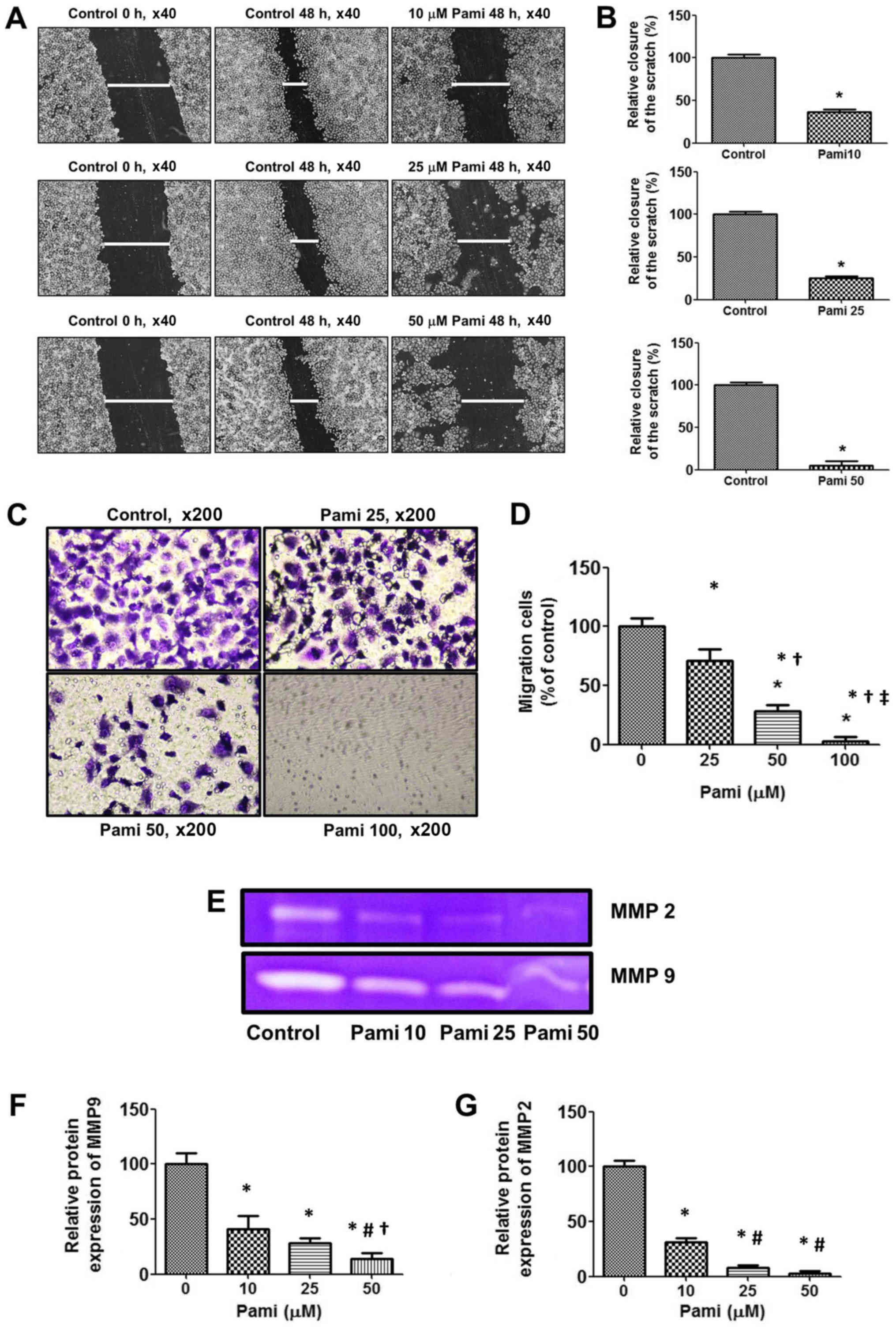
Pami reduces migration
The results showed that Pami blocked wound healing
effects after cells were scratched with a 0.2 ml pipette tip
(Fig. 4A and B) and then lead to the
inhibition of CCA cell migration (Fig.
4C and D) to the bottom of the Transwell chamber. Next, to
explore the mechanism associated with the blocking of the CCA
migration, a gelatin zymography assay was used. The results showed
that Pami strongly decreased both MMP2 and MMP9 expression levels,
which were secreted in the culture media, especially the MMP9
levels (Fig. 4E-G).
Discussion
The present study demonstrated that BPs can directly
suppress cancer cell growth, induce apoptosis and inhibit cell
migration, which was associated with the MVA pathway in CCA cell
lines. The MVA pathway serves a crucial role in the cancer process
and the biology that controls the intracellular pathway in tumors,
such as cell proliferation, growth, invasion and migration, as well
as resistance to chemotherapeutic agents. Therefore, the MVA
pathway is of interest if it can be inhibited or reduced by drugs
or natural products. The present study reported that Pami inhibited
CCA cell growth in SRB and colony formation assays, which was
accompanied by induction of CCA cell apoptosis as examined by ROS
generation, caspase-3 activity and apoptosis by flow cytometry.
Furthermore, Pami was revealed to modulate the MVA pathway and
suppress Rac1 protein expression levels 24 h after incubation.
Finally, Pami also decreased the CCA cell migration by inhibiting
wound healing and the invasion in the Matrigel assay, as well as
reducing MMP2 and MMP9 levels. The data demonstrated that Pami
acted against CCA cells with effects on growth inhibition,
apoptotic induction and migratory suppression. Pami may be useful
against CCA cells.
Pami suppressed the MVA pathway and downregulated
the downstream protein targets of MVA products, including Rac and
Rho, which control cell growth, proliferation and migration
(17). According to the results of
the present study, Pami modulated Rac1 and RhoA gene and protein
expression levels. Especially, Pami suppressed Rac1 protein
expression, which may have upregulated Rac1 gene expression at 24
h. Rac1 and RhoA are Rho GTPase family members that regulate actin
dynamics and cell proliferation (24). Rac1 regulates multiple signaling
pathways that control cytoskeleton organization, transcription and
cell growth (25). Direct inhibition
of Rac1 activity induces cell cycle arrest and apoptosis in human
breast cancer cells (26), as well
as CCA (27). Miller et al
(27) found that simvastatin is a
HMG-CoA reductase enzyme inhibitor that is important for inhibition
of the MVA pathway, it decreases Rac1 activity in CCA cells
(Mz-ChA-1 cells) and causes induction of CCA cell death by
increasing levels of cleaved caspase-3 and reducing p-ERK levels
(28). Similar to these results,
Pami suppressed Rac1 protein expression and further induced CCA
cell death and apoptosis by activating ROS formation and inducing
caspase-3 activity. However, our previous study showed that Pami
suppresses Rac1 and RhoA gene expression in MCF-7 breast cancer
cells following incubation for 24 h (data not shown). Therefore,
the bisphosphonate on the MVA products both in gene and protein
expression are required to explore the time and concentration for
each cancer cell type in further experiments. Data indicated that
the inhibition of the MVA pathway was associated with CCA cell
death and apoptosis. Further experiments are required to explore
the dose and incubation time associated with Rac1 and RhoA
levels.
Regarding CCA cell apoptosis, at a dose of 250 µM,
Pami caused a significant induction of apoptosis through ROS
formation and caspase-3 activity. The data revealed that Pami in
the dose range 50–250 µM induced ROS formation and also
significantly stimulated caspase-3 activity at doses of 100–250 µM.
In the present study, only 250 µM Pami was used to study apoptosis
by flow cytometry as it showed higher effects in terms of
activating CCA cell apoptosis. Riebeling et al (29) reported that 100 µM Pami stimulates
melanoma cell apoptosis through caspase-3 overexpression and DNA
fragmentation. Additionally, low doses of Pami (1–20 µM) reduce MVA
products, including Ras, RhoA and ROCK-1, and are associated with
the induction of cleaved caspase-3 and −9, and the reduction of
antiapoptotic protein Bcl-2 (30).
Pami exhibited high efficacy in accelerating CCA cell
apoptosis.
Regarding CCA cell metastasis, it was revealed that
Pami inhibited CCA cell migration in a dose-dependent manner by
reducing MMP2 and MMP expression levels. Metastasis is the
important step where tumor cells migrate from the original site to
a distant site and contribute to secondary tumors. Lai et al
(31) demonstrated that alendronate
may decrease MMP2 gene and protein expression and further
correlated with inhibiting the invasion of chondrosarcoma cells.
Furthermore, four types of BPs, such as zoledronate, clodronate,
Pami and alendronate, at non-cytotoxic doses suppressed MMP1, −2,
−3, −8, −9, −12, −13 and −20 (32)
as in these migration results. Interestingly, 10 µM Pami decreased
the invasion, migration and RhoA gene and protein expression in
breast cancer cells by downregulating the Serpin-A1 gene
(33). Similarly, Wada et al
(30) indicated that Pami can
suppress membrane RhoA and lead to inhibited hepatocellular
carcinoma (HCC) motility. Moreover, Pami potentiated the effects of
statins on the reduction of tumor cell adhesion to collagen IV and
fibronectin and suppression of the migration and invasiveness of
tumor cells (34). BPs can be
applied for antiinvasive and anti-metastatic activities, which may
inhibit cancer metastasis including CCA.
The current in vitro study presents a first
report of Pami efficacy on CCA cells by inhibiting growth, inducing
apoptosis and suppressing migration. Further in vivo studies
are essential to examine these effects and establish the safety and
efficacy of the Pami studied in this model before treating patients
with CCA.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
Thailand Research Fund (grant no. MRG6080071).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BB, LS and AP performed the experiments and analyzed
the data. BB and VK guided the experiments, designed the study and
wrote the manuscript. All authors gave final approval of the
version to be published. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fleisch H: Development of bisphosphonates.
Breast Cancer Res. 4:30–34. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iguchi K, Tatsuda Y, Usui S and Hirano K:
Pamidronate inhibits antiapoptotic bcl-2 expression through
inhibition of the mevalonate pathway in prostate cancer PC-3 cells.
Eur J Pharmacol. 641:35–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iguchi T, Miyakawa Y, Saito K, Nakabayashi
C, Nakanishi M, Saya H, Ikeda Y and Kizaki M: Zoledronate-induced S
phase arrest and apoptosis accompanied by DNA damage and activation
of the ATM/Chk1/cdc25 pathway in human osteosarcoma cells. Int J
Oncol. 31:285–291. 2007.PubMed/NCBI
|
|
4
|
Molinuevo MS, Bruzzone L and Cortizo AM:
Alendronate induces anti-migratory effects and inhibition of
neutral phosphatases in UMR106 osteosarcoma cells. Eur J Pharmacol.
562:28–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeisberger SM, Odermatt B, Marty C,
Zehnder-Fjällman AH, Ballmer-Hofer K and Schwendener RA:
Clodronate-liposome-mediated depletion of tumour-associated
macrophages: A new and highly effective antiangiogenic therapy
approach. Br J Cancer. 95:272–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gnant M, Mlineritsch B, Schippinger W,
Luschin-Ebengreuth G, Pöstlberger S, Menzel C, Jakesz R, Seifert M,
Hubalek M, Bjelic-Radisic V, et al: Endocrine therapy plus
zoledronic acid in premenopausal breast cancer. N Engl J Med.
360:679–691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asahi H, Mizokami A, Maeda Y, Komatsu K,
Koshida K and Namiki M: Bisphosphonate therapy for hormone
refractory prostate cancer with bone metastasis. J Urol.
169:281–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith MR, McGovern FJ, Zietman AL, Fallon
MA, Hayden DL, Schoenfeld DA, Kantoff PW and Finkelstein JS:
Pamidronate to prevent bone loss during androgen-deprivation
therapy for prostate cancer. N Engl J Med. 345:948–955. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boissier S, Ferreras M, Peyruchaud O,
Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaissé JM and
Clézardin P: Bisphosphonates inhibit breast and prostate carcinoma
cell invasion, an early event in the formation of bone metastases.
Cancer Res. 60:2949–2954. 2000.PubMed/NCBI
|
|
10
|
Iguchi K, Matsunaga S, Nakano T, Usui S
and Hirano K: Inhibition of caveolin-1 expression by incadronate in
PC-3 prostate cells. Anticancer Res. 26:2977–2981. 2006.PubMed/NCBI
|
|
11
|
Nishida S, Fujii Y, Yoshioka S, Kikuichi
S, Tsubaki M and Irimajiri K: A new bisphosphonate, YM529 induces
apoptosis in HL60 cells by decreasing phosphorylation of single
survival signal ERK. Life Sci. 73:2655–2664. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asahi H, Mizokami A, Miwa S, Keller ET,
Koshida K and Namiki M: Bisphosphonate induces apoptosis and
inhibits pro-osteoclastic gene expression in prostate cancer cells.
Int J Urol. 13:593–600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valenti MT, Bertoldo F, Dalle Carbonare L,
Azzarello G, Zenari S, Zanatta M, Balducci E, Vinante O and Lo
Cascio V: The effect of bisphosphonates on gene expression: GAPDH
as a housekeeping or a new target gene? BMC Cancer. 6:492006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oades GM, Senaratne SG, Clarke IA, Kirby
RS and Colston KW: Nitrogen containing bisphosphonates induce
apoptosis and inhibit the mevalonate pathway, impairing Ras
membrane localization in prostate cancer cells. J Urol.
170:246–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Virtanen SS, Väänänen HK, Härkönen PL and
Lakkakorpi PT: Alendronate inhibits invasion of PC-3 prostate
cancer cells by affecting the mevalonate pathway. Cancer Res.
62:2708–2714. 2002.PubMed/NCBI
|
|
16
|
Walker K and Olson MF: Targeting Ras and
Rho GTPases as opportunities for cancer therapeutics. Curr Opin
Genet Dev. 15:62–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang PL, Lun M, Siegelmann-Danieli N,
Blasick TM and Brown RE: Pamidronate resistance and associated low
ras levels in breast cancer cells: A role for combinatorial
therapy. Ann Clin Lab Sci. 34:263–270. 2004.PubMed/NCBI
|
|
18
|
Sripa B, Brindley PJ, Mulvenna J, Laha T,
Smout MJ, Mairiang E, Bethony JM and Loukas A: The tumorigenic
liver fluke Opisthorchis viverrini-multiple pathways to
cancer. Trends Parasitol. 28:395–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sookprasert A, Chindaprasert J and
Wirasorn K: Systemic therapy for locally advanced and metastatic
cholangiocarcinoma. Asian Pac J Cancer Prev. (Suppl 13):3–6.
2012.PubMed/NCBI
|
|
20
|
Buranrat B, Mairuae N and Kanchanarach W:
Cytotoxic and antimigratory effects of Cratoxy formosum extract
against HepG2 liver cancer cells. Biomed Rep. 6:441–448. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klungsaeng S, Kukongviriyapan V, Prawan A,
Kongpetch S and Senggunprai L: Cucurbitacin B induces
mitochondrial-mediated apoptosis pathway in cholangiocarcinoma
cells via suppressing focal adhesion kinase signaling. Naunyn
Schmiedebergs Arch Pharmacol. 392:271–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen H, Wang C, Qi M, Ge L, Tian Z, Li J,
Zhang M, Wang M, Huang L and Tang X: Anti-tumor effect of
Rhaponticum uniflorum ethyl acetate extract by regulation of
peroxiredoxin1 and epithelial-to-mesenchymal transition in oral
cancer. Front Pharmacol. 8:8702017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buranrat B, Mairuae N and Konsue A:
Cratoxy formosum leaf extract inhibits proliferation and migration
of human breast cancer MCF-7 cells. Biomed Pharmacother. 90:77–84.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bosco EE, Mulloy JC and Zheng Y: Rac1
GTPase: A ‘Rac’ of all trades. Cell Mol Life Sci. 66:370–374. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshida T, Zhang Y, Rivera Rosado LA, Chen
J, Khan T, Moon SY and Zhang B: Blockade of Rac1 activity induces
G1 cell cycle arrest or apoptosis in breast cancer cells through
downregulation of cyclin D1, survivin, and X-linked inhibitor of
apoptosis protein. Mol Cancer Ther. 9:1657–1668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miller T, Yang F, Wise CE, Meng F,
Priester S, Munshi MK, Guerrier M, Dostal DE and Glaser SS:
Simvastatin stimulates apoptosis in cholangiocarcinoma by
inhibition of Rac1 activity. Dig Liver Dis. 43:395–403. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kamigaki M, Sasaki T, Serikawa M, Inoue M,
Kobayashi K, Itsuki H, Minami T, Yukutake M, Okazaki A, Ishigaki T,
et al: Statins induce apoptosis and inhibit proliferation in
cholangiocarcinoma cells. Int J Oncol. 39:561–568. 2011.PubMed/NCBI
|
|
29
|
Riebeling C, Forsea AM, Raisova M, Orfanos
CE and Geilen CC: The bisphosphonate pamidronate induces apoptosis
in human melanoma cells in vitro. Br J Cancer. 87:366–371. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wada A, Fukui K, Sawai Y, Imanaka K, Kiso
S, Tamura S, Shimomura I and Hayashi N: Pamidronate induced
anti-proliferative, apoptotic, and anti-migratory effects in
hepatocellular carcinoma. J Hepatol. 44:142–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lai TJ, Hsu SF, Li TM, Hsu HC, Lin JG, Hsu
CJ, Chou MC, Lee MC, Yang SF and Fong YC: Alendronate inhibits cell
invasion and MMP-2 secretion in human chondrosarcoma cell line.
Acta Pharmacol Sin. 28:1231–1235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heikkilä P, Teronen O, Moilanen M,
Konttinen YT, Hanemaaijer R, Laitinen M, Maisi P, van der Pluijm G,
Bartlett JD, Salo T and Sorsa T: Bisphosphonates inhibit
stromelysin-1 (MMP-3), matrix metalloelastase (MMP-12),
collagenase-3 (MMP-13) and enamelysin (MMP-20), but not
urokinase-type plasminogen activator, and diminish invasion and
migration of human malignant and endothelial cell lines. Anticancer
Drugs. 13:245–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ponce-Cusi R and Calaf GM: Antitumor
activity of pamidronate in breast cancer cells transformed by low
doses of α-particles and estrogen in vitro. Int J Oncol.
46:2663–2669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Issat T, Nowis D, Legat M, Makowski M,
Klejman MP, Urbanski J, Skierski J, Koronkiewicz M, Stoklosa T,
Brzezinska A, et al: Potentiated antitumor effects of the
combination treatment with statins and pamidronate in vitro and in
vivo. Int J Oncol. 30:1413–1425. 2007.PubMed/NCBI
|