Introduction
Diabetes is a highly prevalent chronic metabolic
disease, affecting >400 million individuals worldwide, which
often causes delayed or impaired wound healing (1), representing a major health concern and
a heavy socioeconomic burden. According to incomplete statistics,
20% of patients with diabetes suffer from foot ulcers and >20%
of patients require amputations (2–4). The
rate of amputation is markedly higher among diabetic patients
compared with the general population (2,5). Wound
healing in diabetes is delayed and several therapeutic approaches
are ineffective (6,7). The etiology of diabetic foot ulcers is
complex. Age, sex, vascular disease, infection, blood pressure and
smoking may affect the progression of diabetic foot ulcers, and the
majority of studies have reported that the pathogenesis of diabetic
foot ulcers is closely associated with ischemia, neuropathy and
infection (8,9). Angiopathy (9), particularly in vascular diseases of the
lower extremities, is the earliest and most common complication
leading to the development of diabetic foot ulcers. Additionally,
diabetic microangiopathy is a risk factor for diabetic foot. Due to
long-term hyperglycemia, diabetic patients accumulate a large
number of advanced glycation end-products in vivo, resulting
in endothelial cell damage and apoptosis, thickening of the intimal
vascular wall, luminal stenosis or obstruction (10). Furthermore, endothelial cell damage
promotes platelet adhesion, erythrocyte aggregation and
microthrombosis, leading to insufficient irrigation of the affected
limb, with ensuing ischemia, hypoxia and eventually diabetic ulcer
formation or aggravation of diabetic foot ulcers (10–12).
Wound healing is a complex biological process that
may be divided into three stages: Inflammatory response, cell
differentiation and proliferation, and tissue repair (13). Numerous factors may delay the wound
healing process, including the inhibition of cytokine production by
fibroblasts and inflammatory cells. During the early stages of
wound healing, the overexpression of inflammatory factors, such as
interleukin (IL)-6 and tumor necrosis factor (TNF)-α (14,15),
severely impairs the formation of granulation tissue and further
delays wound healing. However, a number of studies have revealed
that growth factors, including vascular endothelial growth factor
(VEGF) (2,4,16),
epidermal growth factor (17,18) and
transforming growth factor-β (TGF-β) (19,20),
serve an important role in the promotion of wound healing.
A number of treatment methods have been developed to
promote wound healing in diabetic foot ulcers, including vascular
reconstruction, negative pressure treatment, stem cell
transplantation therapy, hyperbaric oxygen therapy and tissue
engineering technology, among others (21–25).
Although these treatment methods have improved the wound healing of
diabetic foot ulcers, their efficacy is unsatisfactory. Hence, an
increasing number of topical treatments have been developed,
particularly involving debridement methods including surgical,
biological, and dressing debridement, and have been widely applied
for the treatment of diabetic patients in the clinical setting
(26). Debridement eliminates
necrotic tissue, decreases chronic inflammatory factor levels,
increases cytokine secretion, promotes the growth of granulation
tissue and reduces the absorption of toxins during necrosis tissue
decomposition and degradation (27–29).
Therefore, debridement is widely used in the clinical setting to
promote wound healing in patients with diabetes (30). In addition, ultrasonic debridement
may promote the repair of various injuries, including those of the
bone, tendon, muscle, cartilage and ligament (31–33).
The aim of the present study was to determine
whether low-frequency ultrasound accelerates wound healing and
tissue regeneration in diabetic rats, and to investigate its
effects on the expression of VEGF, TGF-β1, IL-6 and TNF-α.
Materials and methods
Animals
A total of 45 female Wistar rats, weighing 250–300
g, were purchased from the Laboratory Animal Center of North
Sichuan Medical College. They were fed for 1 week at 18-2°C in 12 h
light/dark cycle with access to food and water ad libitum,
and a humidity of 50–60%. The animals were handled humanely
according to the guidelines provided in the Guide for the Care and
Use of Laboratory Animals, published by the National Institute of
Health (34). The rats were
anesthetized by intraperitoneal administration of 2.25%
pentobarbital sodium (45 mg/kg). All animal procedures were
approved by the Ethics Committee of Affiliated Hospital of North
Sichuan Medical College [approval no. 2016ER(A)022].
Streptozotocin (STZ)-induced diabetic
rat model and experimental groups
A diabetic rat model was established in all Wistar
rats using STZ, as described previously (35–37).
Rats were intraperitoneally injected with 1% STZ (60 mg/kg)
following anesthesia. A total of 1 week after STZ injection, the
blood glucose levels of all rats were >16.7 mmol/l. Rats were
subsequently placed in a prone position on a fixed plate, where a
circular area (3.0 cm in diameter) was marked on the skin of the
back. A skin incision was created and cleaned with iodine. The
diabetic animals were then randomly divided into three groups
(n=15) according to the different treatments administered: the
untreated control group; the ultrasound (US) treatment group and
the common treatment group. In rats of the US group, the wound was
cleaned with normal saline and treated with low-frequency US
(frequency, 1 MHz; sound intensity, 1.0 W/cm2) for 15
min. The working sound intensity range of low-frequency US is
adjustable from 0.1–1.1 W/cm2, with a frequency of 1 MHz
and a repetition frequency of 1 KHz with continuous waves,
accounting for 20% of the air ratio. The skin wound was then
irradiated with an US sound intensity of 1.0 W/cm2 once
per day for 21 days. The sterile head of the ultrasound machine was
connected to the ultrasonic debridement machine, with a saline bag
used as the washing solution. When atomized water drops appeared on
the machine head subsequent to first use, the front of the machine
head was tilted to contact the wound surface at a 45° angle. The
irradiated wound surface was then moved at a constant and slow
speed. After horizontal scanning, the irradiated wound surface was
vertically scanned to ensure irradiation of all wound surfaces. In
the common treatment group, the wound was cleaned with normal
saline alone once per day (35–37).
Estimation of the wound healing
rate
All rats were observed on day 7, 14 and 21 after
wound formation. The area of the wound was recorded to calculate
the rate of wound healing as follows: Wound healing
rate=(1-remaining-wound area/initial wound area) ×100% (38).
Histological analysis
A biopsy sample was obtained from the wound edge on
day 7, 14 and 21 to determine the pathological changes occurring
within the wound. Part of the biopsy specimens was fixed in 4%
paraformaldehyde at 4°C for 48 h, embedded in paraffin sectioned (4
µm) and stained with hematoxylin for 3–8 min and eosin for 1–3 min
(H&E) at room temperature to examine the pathological changes.
The remaining part of the tissue was frozen at −70°C to extract
total RNA from rat skin.
Immunohistochemical (IHC)
analysis
IHC semi-quantification analysis was performed as
described previously (39), using a
horseradish peroxidase-3,3′-diaminobenzidine (HRP-DAB) staining kit
(Beyotime Institute of Biotechnology). Tissue sections from the
different groups were blocked by 3% H2O2 at room temperature for 15
min, and then incubated with the following primary antibodies for 1
h: anti-rabbit VEGF (Abcam; cat. no. ab11939, 1:100) and
anti-rabbit TGF-β1 (Abcam; cat. no. ab92486; 1:100) at 4°C. Each
antibody was diluted in PBS. Subsequently, samples were incubated
with biotinylated goat anti-rabbit antibodies (Abcam; cat. no.
ab6721; 1:100) for 30 min at room temperature. The specific binding
of the secondary to primary antibodies was visualized using HRP for
the enzymatic conversion of the chromogenic substrate DAB into a
brown precipitate. The sections were mounted, cleared,
cover-slipped, and examined under a fluorescence microscope
(magnification, ×100). The scale bar was 100 µm.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from rat skin using the
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Samples were then reverse
transcribed using the Bestar™ qPCR RT kit (Takara Bio, Inc.). The
RT conditions were 37°C for 15 min and 98°C for 5 min. qPCR was
performed using the ABI Prism 7500 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with the
SYBR® Premix Ex Taq™ kit (Takara Bio, Inc.). PCR
amplification conditions were as follows: denaturing at 95°C for 30
sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 1 min.
GAPDH was used for normalization. Data are presented as fold
difference and were calculated using the 2−ΔΔCq relative
expression method (40). The primers
used were as follows: VEGF forward 5′-TCCAGGAGTACCCCGATGA-3′ and
reverse, 5′-CTCCGCTCTGAACAAGGCT-3′; TGF-β1 forward
5′-TAAGGCTCGCCAGTCCCC-3′ and reverse,
5′-GGTTTTGTCATAGATTGCGTTGTT-3′; TNF-α forward
5′-CTTCTCATTCCTGCTCGTGG-3′ and reverse, 5′-TCCGCTTGGTGGTTTGC-3′;
IL-6 forward, 5′-GCCTTCTTGGGACTGATGTTG-3′ and reverse,
5′-GCTCTGAATGACTCTGGCTTTG-3′; GAPDH forward,
5′-TGAACGGGAAGCTCACTGG-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) and determined
using a bicinchoninic acid assay kit (Beyotime Institute of
Biotechnology) in accordance with the manufacturer's protocol.
Subsequently, 20 mg of the cell lysate was separated via 10%
SDS-PAGE and transferred to PVDF membranes (EMD Millipore). The
membranes were then blocked with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) for 2 h at room temperature and
incubated with the following primary antibodies obtained from Abcam
overnight at 4°C: Anti-rabbit VEGF (Abcam; cat. no. ab11939;
1:500), anti-rabbit TGF-β1 (Abcam; cat. no. ab92486; 1:1,000),
anti-mouse TNF-α (Abcam; cat. no. ab9739; 1:1,000), anti-rabbit
IL-6 (Abcam; cat. no. ab208113; 1:500) and anti-rabbit GAPDH
(Abcam; cat. no. ab9385; 1:1,000). The immune complexes were then
immunoblotted with horseradish peroxidase-conjugated anti-mouse or
anti-rabbit IgG antibodies (Beijing ComWin Biotech Co., Ltd;
1:2,000). Immunodetection was performed using enhanced
chemiluminescence reagents (Fdbio Science) by Image J 1.8.0
(National Institutes of Health).
Statistical analysis
Data were analyzed using SPSS 23.0 software (IBM
Corp.), and were presented as the mean ± standard deviation. Data
were analyzed using an unpaired Student's t-test and one-way ANOVA
with Bonferroni's post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
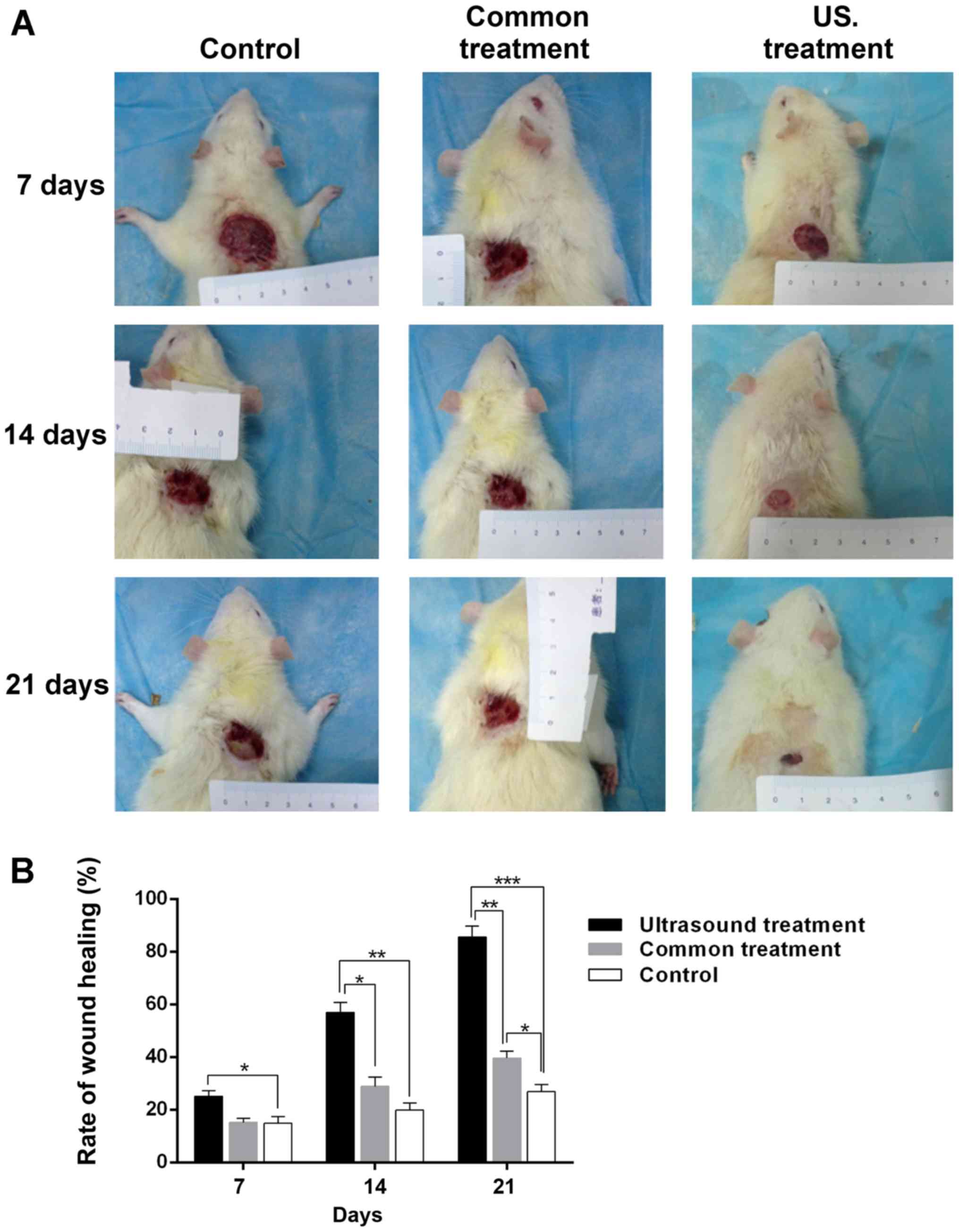
Low-frequency US treatment accelerates
wound healing in diabetic rats
After each post-operative treatment, the width of
the wound was measured every 7 days, and the rate of wound healing
was calculated with the aforementioned formula. The results
demonstrated that, compared with the control group, the size of the
wound area was significantly decreased in the US treatment group
(Fig. 1A). As presented in Fig. 1B, the wound healing rate in the US
treatment group was higher compared with the control (25.12±2.06
vs. 14.89±2.53%; P<0.05) on the 7th day. However, as the
treatment time was prolonged, the difference in wound healing rate
became markedly higher between the US treatment group and the
control group (14th day, 56.98±3.76 vs. 19.91±2.72%; P<0.01;
21st day, 85.62±4.16 vs. 26.89±2.64%; P<0.001). In addition, the
wound healing rate in the US treatment group was higher compared
with the common treatment group at day 7, 14 and 21 post-treatment
(14th day, 56.98±3.76 vs. 28.86±3.52%; P<0.05, 21st day,
85.62±4.16 vs. 39.63±2.54%; P<0.01). Furthermore, the wound
healing rate of diabetic rats treated with normal saline for 21
days was significantly higher compared with the control group
(39.63±2.54 vs. 26.89±2.64%; P<0.05). The results indicated that
low-frequency US treatment accelerates wound healing in diabetic
rats.
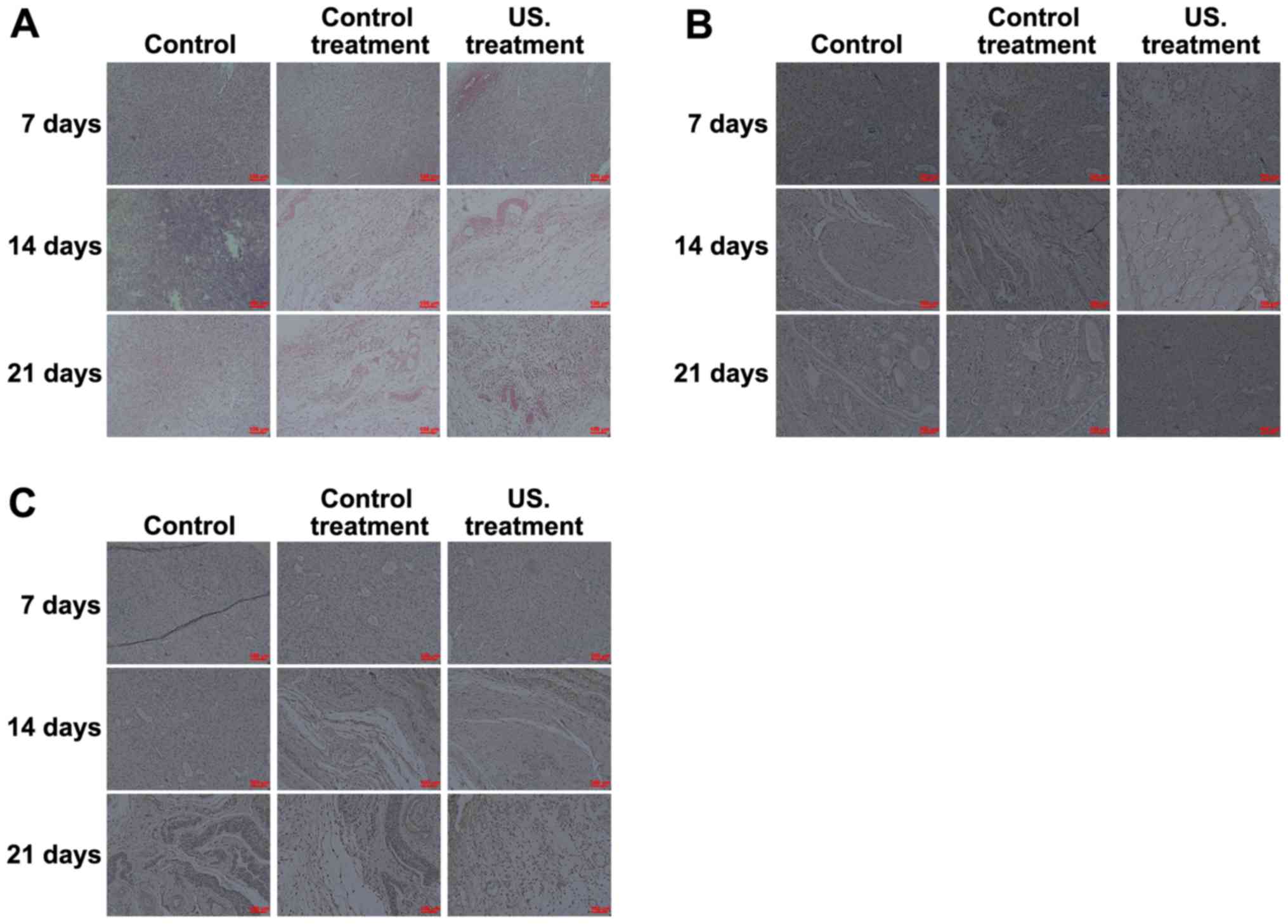
Histological and IHC analysis
To determine the effects of low frequency US
treatment on diabetic rat wound healing, H&E staining was
performed to investigate the pathological changes that occurred in
all treatment groups. The results obtained from the biopsy
specimens collected from the wound margin revealed a marked
inflammatory response occurring on the 7th day. As the treatment
time was prolonged, the inflammatory response decreased, and when
compared with the control, more fibroblasts, collagen fibers and
neovascularization were observed in the rats of the US treatment
group on days 14 and 21 (Fig. 2A).
IHC analysis was performed to determine the role of VEGF (Fig. 2B) and TGF-β1 (Fig. 2C) in the wound healing process of
diabetic rats. Following treatment at all times, the results
indicated that the expression of TGF-β1 and VEGF was significantly
increased in the US treatment group compared with the control
group. Expressions were also significantly increased in the US
group compared with the common treatment group, and TGF-β1 and VEGF
levels in the common treatment group were higher compared with the
control group. Furthermore, as the treatment time prolonged, the
expression of TGF-β1 and VEGF in the wound of US treated rats
decreased on the 14th day, and the expression of TGF-β1 and VEGF
then increased on the 21st day (Fig. 2B
and C).
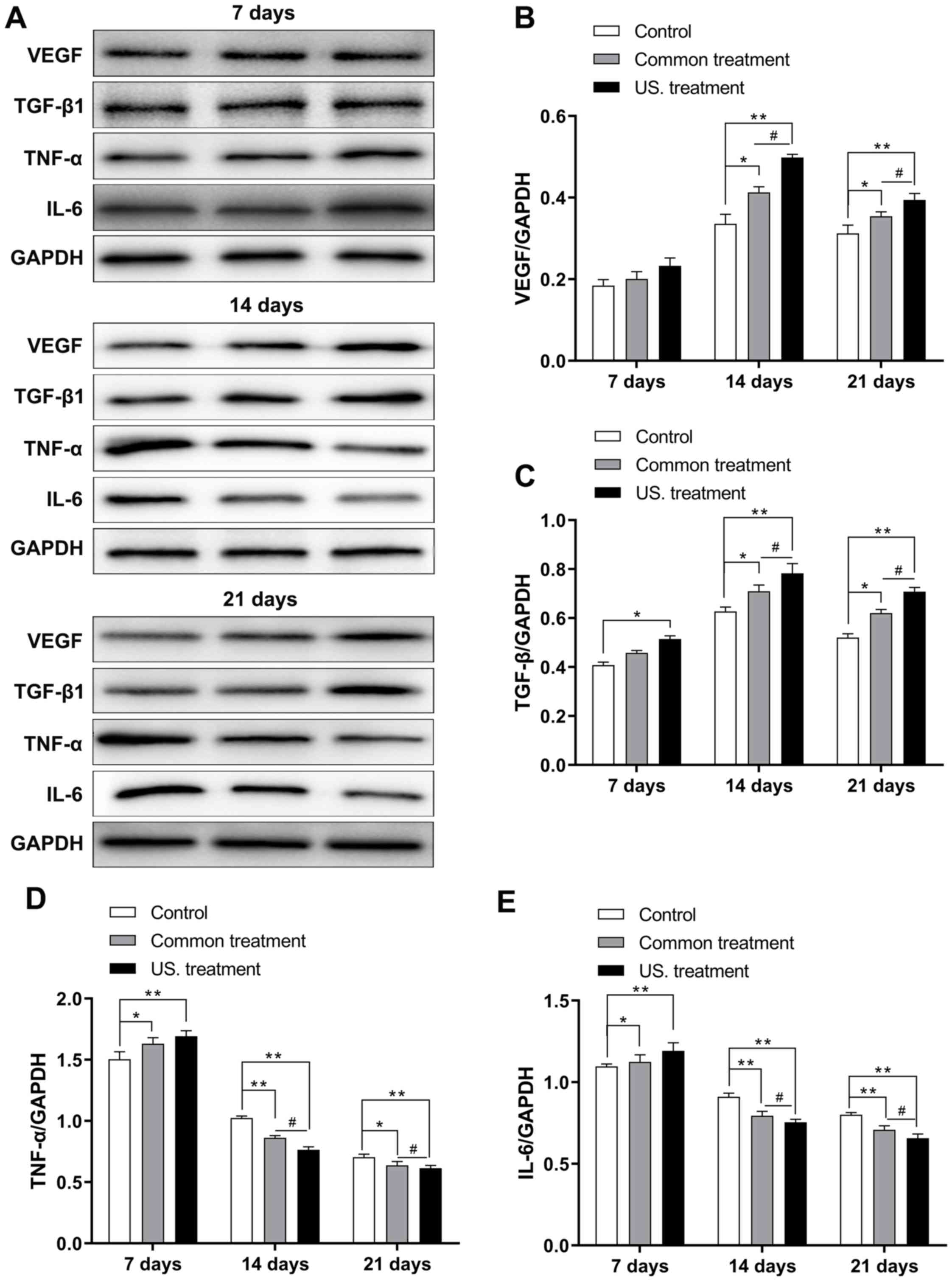
Low frequency US treatment upregulates
the expression of TGF-β1 and VEGF in diabetic rats
To further investigate the difference in expression
of TGF-β1 and VEGF in diabetic rats after receiving treatment, the
mRNA and protein expression of these molecules was assessed in the
wounds of diabetic rats via RT-qPCR and western blotting,
respectively. The results revealed that on the 7th day, mRNA
expression did not exhibit any marked difference among the three
groups, except for the TGF-β1. TGF-β1 mRNA levels did not exhibit
any marked differences among the three groups and TGF-β1 protein
expression in the US group was significantly increased when
compared with the control group (P<0.05). However, the mRNA
(Fig. 3) and protein (Fig. 4) expression of VEGF in the US group
were significantly increased on the 14th day (P<0.01 in both
mRNA and protein) and 21st day (P<0.05 in the mRNA and P<0.01
in the protein) compared with the control group (Figs. 3A and 4A
and B); the mRNA (Fig. 3) and
protein (Fig. 4) expression of
TGF-β1 in the US group were significantly increased on the 14th day
(P<0.01 in both mRNA and protein) and 21st day (P<0.05 in the
mRNA and P<0.01 in the protein) compared with the control group
(Fig. 3B and Fig. 4A-C);, and the common treatment group
(P<0.05; Fig. 3A and B and
Fig. 4B and C). In addition, the
mRNA and protein levels of TGF-β1 and VEGF in the common treatment
group were increased when compared with the control group
(P<0.05). Furthermore, as treatment time prolonged, the mRNA and
protein levels of TGF-β1 and VEGF were markedly increased on the
14th day compared with the 7th day. Levels then decreased on the
21st day compared with the 14th day.
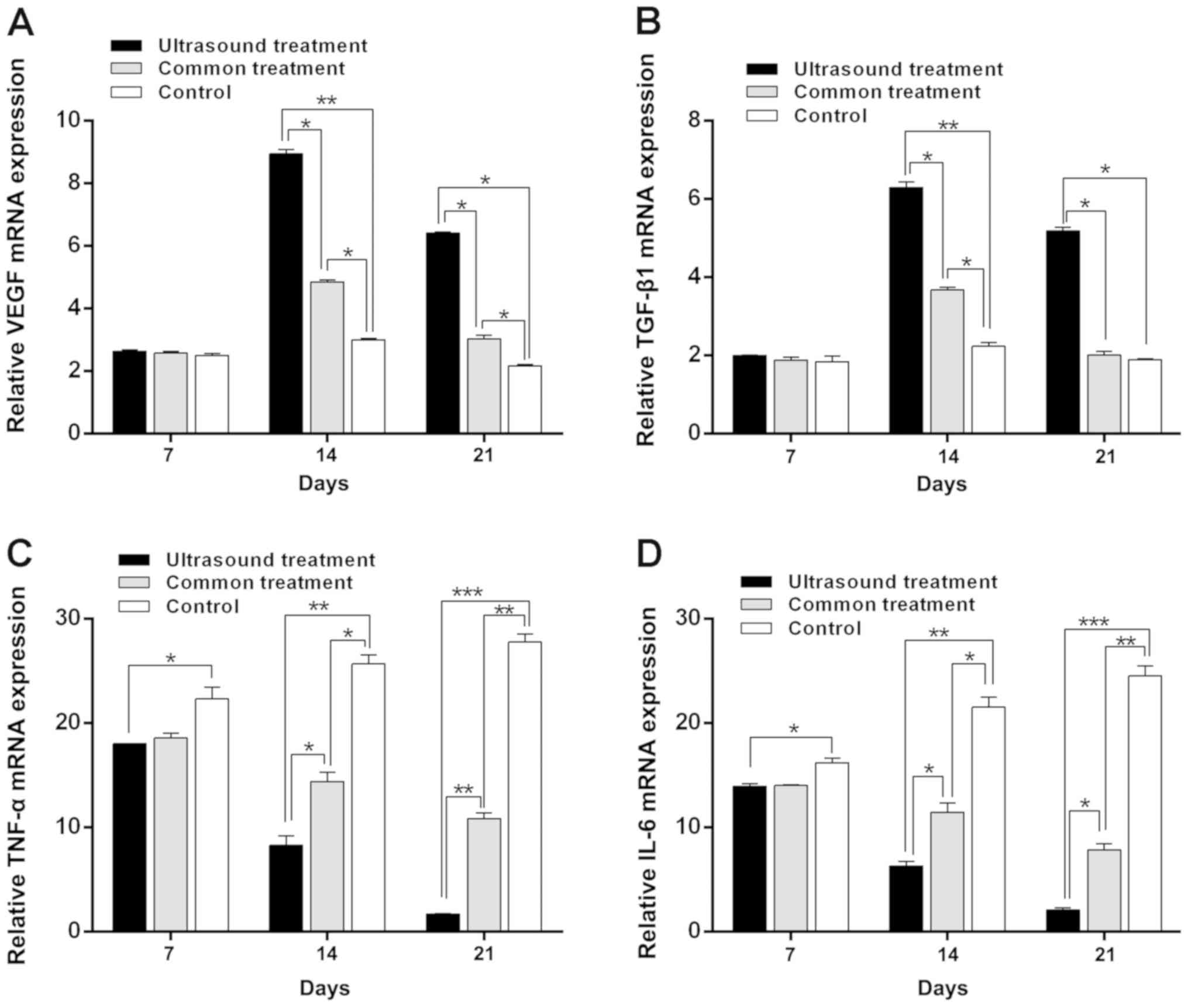 | Figure 3.Reverse transcription-quantitative
PCR results of VEGF, TGF-β1, TNF-α and IL-6 in each group following
treatment. The expression of (A) VEGF and (B) TGF-β1 in the US
group was significantly increased on the 14th day and 21st day
compared with the control. Furthermore, as treatment time
prolonged, the expression of (C) TNF-α and (D) IL-6 were gradually
reduced in the US group, with the expression being lowest on the
21st day. *P<0.05, **P<0.01 and ***P<0.001 as indicated.
VEGF, vascular endothelial growth factor; TGF-β1, transforming
growth factor-β1; TNF-α, tumor necrosis factor-α; IL-6,
interleukin-6; US, ultrasound. |
Low-frequency US treatment suppresses
the inflammatory response of diabetic rats
To confirm the effect of low-frequency US treatment
on the inflammatory response of rats receiving treatment during the
wound healing process, levels of IL-6 and TNF-α were determined via
RT-qPCR and western blotting. As the treatment time was prolonged,
the mRNA (Fig. 3) and protein
(Fig. 4) levels of IL-6 and TNF-α in
the US treatment group were reduced. The results were similar for
the common treatment group. However, the mRNA and protein
expression levels of IL-6 and TNF-α in the control group were
increased (Figs. 3C and D and
4D and E). Additionally, the mRNA
and protein levels of IL-6 and TNF-α in the US treatment group were
lower compared with those in the control group on the 7 and 14th
day (7th day, P<0.05 in both TNF-α and IL-6; 14th day, P<0.01
in both TNF-α and IL-6). The mRNA and protein levels of IL-6 and
TNF-α were the lowest compared with the control group on the 21st
day (P<0.001, Figs. 3C and D and
4D and E). Furthermore, the mRNA
expression of IL-6 and TNF-α in the US treatment group were
significantly reduced compared with the common treatment group at
day 14 (P<0.05 in both TNF-α and IL-6) and 21 (P<0.01 in the
TNF-α, and P<0.05 in the IL-6; Fig.
3C and D); the protein expression of IL-6 and TNF-α in the US
treatment group were significantly reduced compared with the common
treatment group at day 14 and 21 (P<0.05 in both TNF-α and IL-6;
Fig. 4D and E).
Discussion
Diabetic patients often suffer from diabetic foot
ulcers, occasionally requiring amputation, which severely affects
patient health and poses a major socioeconomic burden to patients'
families and society. Despite several interesting and promising
experimental results, their application in the clinical setting has
not been satisfactory to date. Hence, a novel and effective
therapeutic method is urgently required. With advances in medical
technology, previous studies have indicated that US treatment may
promote the repair of various injuries, including bone, tendon,
muscle, cartilage and ligament injuries (31–33). The
results of the current study demonstrated that the wound healing
rate of diabetic rats in the US treatment group (85.62±4.16%) was
higher compared with that in the other groups at 21 days after
treatment, indicating that low-frequency US may enhance
epithelialization and granulation tissue formation, thereby
accelerating wound healing in diabetic rats. These effects may be
mediated by decreasing the inflammatory response and promoting the
production of growth factors.
The process of wound healing may be divided into
inflammatory response, cell differentiation, cell proliferation and
tissue repair stages (13). TNF-α
and IL-6 serve a dual role by promoting as well as hindering wound
healing (41). During the early
stages of the inflammatory response, TNF-α and IL-6 promote the
chemotaxis of inflammatory cells to remove necrotic tissues and
pathogens, and promote the production and secretion of various cell
growth factors including VEGF, basic fibroblast growth factor and
IL-8 (14,15), further accelerating the
differentiation and proliferation of various repair cells,
extracellular matrix formation and neovascularization, ultimately
promoting wound healing (42).
However, the continuation of the inflammatory response and
overexpression of TNF-α and IL-6 may cause the accumulation of
harmful substances and severely impair granulation tissue formation
and wound healing (43). The results
of the present study demonstrated that the expression of IL-6 and
TNF-α in US treatment group on the 7th, 14th and 21st day decreased
with the time. However, the expression levels of IL-6 and TNF-α in
the control group were markedly increased compared with those
determined on the first day. Additionally, as the treatment time
was prolonged, the expression of IL-6 and TNF-α in US treatment
group was gradually downregulated. These results indicated that
low-frequency US markedly inhibited the expression of IL-6 and
TNF-α in diabetic rats after treatment for 7 days and that their
expression was gradually downregulated with increased treatment
duration.
A number of studies have demonstrated that
angiogenesis is the physiological basis of wound repair, which is
regulated by cytokines via different signaling pathways that
promote or inhibit wound healing (37,38).
VEGF is a soluble factor that is one of the key regulators of
angiogenesis, which binds to the VEGF receptor to induce the
proliferation and migration of vascular endothelial cells via
autocrine and paracrine pathways, ultimately regulating
neovascularization (2,4,16,44).
Additionally, TGF-β1 serves a key role in wound healing by
mediating the chemotaxis of inflammatory cells, the differentiation
and proliferation of fibroblasts, and the production and
degradation of collagen and extracellular matrix (19,20,45).
Decreased expression and dysfunction of TGF-β1 and its receptors
may hamper wound healing. In the present study, the results of IHC
examination indicated that the expression of TGF-β1 and VEGF was
significantly increased in the US group compared with the control
group. The highest result was observed on day 14 of US treatment,
which subsequently decreased by day 21. To further investigate
whether low-frequency US promoted the expression of TGF-β1 and
VEGF, RT-qPCR and western blotting were performed. The results
demonstrated that the mRNA expression of TGF-β1 and the mRNA and
protein expressions VEGF did not differ significantly among the
three groups on day 7. However, compared with the control group,
the expression of TGF-β1 and VEGF in the US treatment group were
significantly increased on days 14 and 21. The results confirmed
that low-frequency US increased the expression of TGF-β1 and VEGF
after treatment for 14 days, further promoting wound healing.
In conclusion, the present study indicated that
low-frequency US increased the expression of TGF-β1 and VEGF,
induced the proliferation and differentiation of vascular
endothelial cells and fibroblasts, and regulated the process of
neovascularization in a diabetic rat model. Furthermore, it also
reduced the expression of IL-6 and TNF-α after treatment for 7 days
and regulated and suppressed the abnormal inflammatory response,
thereby accelerating wound healing in diabetic rats. Although the
different effects of radiation on diabetic wound healing have been
verified, the underlying mechanism of low frequency US in wound
healing should be elucidated in following pharmacodynamic
experiments.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Scientific
Research Foundation of the Education Department of Sichuan
Province, China (grant no. 15ZB0186) and the Research and
Development Program of North Sichuan Medical College (grant no.
CBY13-A-ZP01).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LC conceived the current study, analyzed the
majority of the data and wrote the initial draft of the manuscript.
QZ, XC, JW and LW refined the study design, performed additional
analyses and finalized the manuscript.
Ethics approval and consent to
participate
The present study was approved by IRB of the
Affiliated Hospital of North Sichuan Medical College Approval
Notice (file no. 2016 ER(A)022).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Cho NH, Shaw JE, Karuranga S, Huang Y, da
Rocha Fernandes JD, Ohlrogge AW and Malanda B: IDF Diabetes Atlas:
Global estimates of diabetes prevalence for 2017 and projections
for 2045. Diabetes Res Clin Pract. 138:271–281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuo YR, Wang CT, Cheng JT, Kao GS, Chiang
YC and Wang CJ: Adipose-derived stem cells accelerate diabetic
wound healing through the induction of autocrine and paracrine
effects. Cell Transplant. 25:71–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zahedi A and Ebrahimi M: Statistical
comments on ‘The effect of foot exercise on wound healing in type 2
diabetes patients with a foot ulcer’. J Wound Ostomy Continence
Nurs. 45:2982018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li G, Zou X, Zhu Y, Zhang J, Zhou L, Wang
D, Li B and Chen Z: Expression and influence of matrix
metalloproteinase-9/tissue inhibitor of metalloproteinase-1 and
vascular endothelial growth factor in diabetic foot ulcers. Int J
Low Extrem Wounds. 16:6–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernando ME, Seneviratne RM, Tan YM,
Lazzarini PA, Sangla KS, Cunningham M, Buttner PG and Golledge J:
Intensive versus conventional glycaemic control for treating
diabetic foot ulcers. Cochrane Database Syst Rev. CD107642016.
|
|
6
|
Corrêa MG, Gomes CM, Marques MR, Casati
MZ, Nociti FH Jr and Sallum EA: Histometric analysis of the effect
of enamel matrix derivative on the healing of periodontal defects
in rats with diabetes. J Periodontol. 84:1309–1318. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dumville JC, Deshpande S, O'Meara S and
Speak K: Hydrocolloid dressings for healing diabetic foot ulcers.
Cochrane Database Syst Rev. CD0090992012.PubMed/NCBI
|
|
8
|
Berlanga-Acosta J, Fernández-Montequín J,
Valdés-Pérez C, Savigne-Gutiérrez W, Mendoza-Marí Y, García-Ojalvo
A, Falcón-Cama V, García Del Barco-Herrera D, Fernández-Mayola M,
Pérez-Saad H, et al: Diabetic foot ulcers and epidermal growth
factor: Revisiting the local delivery route for a successful
outcome. Biomed Res Int. 2017:29237592017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lefrancois T, Mehta K, Sullivan V, Lin S
and Glazebrook M: Evidence based review of literature on detriments
to healing of diabetic foot ulcers. Foot Ankle Surg. 23:215–224.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brantley JN and Verla TD: Use of placental
membranes for the treatment of chronic diabetic foot ulcers. Adv
Wound Care (New Rochelle). 4:545–559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Catrina SB and Zheng X: Disturbed hypoxic
responses as a pathogenic mechanism of diabetic foot ulcers.
Diabetes Metab Res Rev. 32 (Suppl 1):S179–S185. 2016. View Article : Google Scholar
|
|
12
|
Adeghate J, Nurulain S, Tekes K, Fehér E,
Kalász H and Adeghate E: Novel biological therapies for the
treatment of diabetic foot ulcers. Expert Opin Biol Ther.
17:979–987. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferroni L, Gardin C, De Pieri A, Sambataro
M, Seganfreddo E, Goretti C, Iacopi E, Zavan B and Piaggesi A:
Treatment of diabetic foot ulcers with Therapeutic Magnetic
Resonance (TMR®) improves the quality of granulation
tissue. Eur J Histochem. 61:28002017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan L, Hou Z and Gao Y: Efficacy of
combined treatment with vacuum sealing drainage and recombinant
human epidermal growth factor for refractory wounds in the
extremities and its effect on serum levels of IL-6, TNF-α and IL-2.
Exp Ther Med. 15:288–294. 2018.PubMed/NCBI
|
|
15
|
Basso FG, Pansani TN, Turrioni AP, Soares
DG, de Souza CC and Hebling J: Tumor necrosis factor-α and
interleukin (IL)-1β, IL-6, and IL-8 impair in vitro migration and
induce apoptosis of gingival fibroblasts and epithelial cells,
delaying wound healing. J Periodontol. 87:990–996. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J, Ni M, Liu X, Ren Z and Zheng Z:
Curcumol promotes vascular endothelial growth factor
(VEGF)-mediated diabetic wound healing in streptozotocin-induced
hyperglycemic rats. Med Sci Monit. 23:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon D, Yoon D, Cha HJ, Lee JS and Chun W:
Enhancement of wound healing efficiency mediated by artificial
dermis functionalized with EGF or NRG1. Biomed Mater. 13:450072018.
View Article : Google Scholar
|
|
18
|
Zhengcai-Lou, Zihan-Lou and Yongmei-Tang:
Comparative study on the effects of EGF and bFGF on the healing of
human large traumatic perforations of the tympanic membrane.
Laryngoscope. 126:E23–E28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fekrazad R, Sarrafzadeh A, Kalhori KAM,
Khan I, Arany PR and Giubellino A: Improved wound remodeling
correlates with modulated TGF-beta expression in skin diabetic
wounds following combined red and infrared photobiomodulation
treatments. Photochem Photobiol. 94:775–779. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abdoli A, Maspi N and Ghaffarifar F: Wound
healing in cutaneous leishmaniasis: A double edged sword of IL-10
and TGF-β. Comp Immunol Microbiol Infect Dis. 51:15–26. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karam RA, Rezk NA, Abdel Rahman TM and Al
Saeed M: Effect of negative pressure wound therapy on molecular
markers in diabetic foot ulcers. Gene. 667:56–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lopes L, Setia O, Aurshina A, Liu S, Hu H,
Isaji T, Liu H, Wang T, Ono S, Guo X, et al: Stem cell therapy for
diabetic foot ulcers: A review of preclinical and clinical
research. Stem Cell Res Ther. 9:1882018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen CY, Wu RW, Hsu MC, Hsieh CJ and Chou
MC: Adjunctive hyperbaric oxygen therapy for healing of chronic
diabetic foot ulcers: A randomized controlled trial. J Wound Ostomy
Continence Nurs. 44:536–545. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asadi MR, Torkaman G, Hedayati M,
Mohajeri-Tehrani MR, Ahmadi M and Gohardani RF: Angiogenic effects
of low-intensity cathodal direct current on ischemic diabetic foot
ulcers: A randomized controlled trial. Diabetes Res Clin Pract.
127:147–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mills JL: Lower limb ischaemia in patients
with diabetic foot ulcers and gangrene: Recognition, anatomic
patterns and revascularization strategies. Diabetes Metab Res Rev.
32 (Suppl 1):S239–S245. 2016. View Article : Google Scholar
|
|
26
|
Blume P and Wu S: Updating the diabetic
foot treatment algorithm: Recommendations on treatment using
advanced medicine and therapies. Wounds. 30:29–35. 2018.PubMed/NCBI
|
|
27
|
Tardivo JP, Serrano R, Zimmermann LM,
Matos LL, Baptista MS, Pinhal MAS and Atallah ÁN: Is surgical
debridement necessary in the diabetic foot treated with
photodynamic therapy? Diabet Foot Ankle. 8:13735522017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Campitiello F, Mancone M, Corte AD,
Guerniero R and Canonico S: An evaluation of an ultrasonic
debridement system in patients with diabetic foot ulcers: A case
series. J Wound Care. 27:222–228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Volpe P, Marcuccio D, Stilo G, Alberti A,
Foti G, Volpe A, Princi D, Surace R, Pucci G and Massara M:
Efficacy of cord blood platelet gel application for enhancing
diabetic foot ulcer healing after lower limb revascularization.
Semin Vasc Surg. 30:106–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uysal S, Ozturk AM, Tasbakan M, Simsir IY,
Unver A, Turgay N and Pullukcu H: Human myiasis in patients with
diabetic foot: 18 cases. Ann Saudi Med. 38:208–213. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Sousa ACT, da Rocha ÍBP, de Carvalho
AFM, de Freitas Coelho NPM, Feitosa MCP, Barros EML, Arisawa EALS
and de Amorim MRL: Comparative study between low level laser and
therapeutic ultrasound in second intention ulcers repair in mice. J
Lasers Med Sci. 9:134–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao AH, Hung CR, Chen HK and Chiang CP:
Ultrasound-mediated EGF-coated-microbubble cavitation in dressings
for wound-healing applications. Sci Rep. 8:83272018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Murphy CA, Houghton P, Brandys T, Rose G
and Bryant D: The effect of 22.5 kHz low-frequency contact
ultrasound debridement (LFCUD) on lower extremity wound healing for
a vascular surgery population: A randomised controlled trial. Int
Wound J. 15:460–472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakamoto Y, Hojo M, Kosugi Y, Watanabe K,
Hirose A, Inomata A, Suzuki T and Nakae D: Comparative study for
carcinogenicity of 7 different multi-wall carbon nanotubes with
different physicochemical characteristics by a single
intraperitoneal injection in male Fischer 344 rats. J Toxicol Sci.
43:587–600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Winter GD: Effect of air exposure and
occlusion on experimental human skin wounds. Nature. 200:378–379.
1963. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Johnson LE, Larsen M and Perez MT: Retinal
adaptation to changing glycemic levels in a rat model of type 2
diabetes. PLoS One. 8:e554562013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muhammad AA, Arulselvan P, Cheah PS, Abas
F and Fakurazi S: Evaluation of wound healing properties of
bioactive aqueous fraction from Moringa oleifera lam on
experimentally induced diabetic animal model. Drug Des Devel Ther.
10:1715–1730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang XN, Ma ZJ, Wang Y, Sun B, Guo X, Pan
CQ and Chen LM: Angelica Dahurica ethanolic extract improves
impaired wound healing by activating angiogenesis in diabetes. PLoS
One. 12:e01778622017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jerić M, Vukojević K, Vuica A and
Filipović N: Diabetes mellitus influences the expression of NPY and
VEGF in neurons of rat trigeminal ganglion. Neuropeptides.
62:57–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan SF, Yan SD, Ramasamy R and Schmidt AM:
Tempering the wrath of RAGE: An emerging therapeutic strategy
against diabetic complications, neurodegeneration, and
inflammation. Ann Med. 41:408–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun M, He Y, Zhou T, Zhang P, Gao J and Lu
F: Adipose extracellular matrix/stromal vascular fraction gel
secretes angiogenic factors and enhances skin wound healing in a
murine model. Biomed Res Int. 2017:31057802017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Patel S, Maheshwari A and Chandra A:
Biomarkers for wound healing and their evaluation. J Wound Care.
25:46–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsai JL, Lee YM, Pan CY and Lee AY: The
novel VEGF121-VEGF165 fusion attenuates angiogenesis and drug
resistance via targeting VEGFR2-HIF-1α-VEGF165/Lon signaling
through PI3K-AKT-mTOR pathway. Curr Cancer Drug Targets.
16:275–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ishii T, Uchida K, Hata S, Hatta M, Kita
T, Miyake Y, Okamura K, Tamaoki S, Ishikawa H and Yamazaki J: TRPV2
channel inhibitors attenuate fibroblast differentiation and
contraction mediated by keratinocyte-derived TGF-β1 in an in vitro
wound healing model of rats. J Dermatol Sci. 90:332–342. 2018.
View Article : Google Scholar : PubMed/NCBI
|