Introduction
Menopause is defined as the end of a woman's
reproductive period, associated with the decreased production of
estradiol and progesterone by the ovaries (1). With increased life expectancies
worldwide, it is estimated that by 2025, the number of
post-menopausal women will reach 1.1 billion (2). The gradual or sudden cessation of
ovarian follicular function can manifest in a number of
psychological, somatic and urogenital menopause symptoms that have
a significant impact on the quality of life of women (3). These severity of climacteric complaints
is routinely determined by using the Kupperman Menopause Index
(KMI) and the menopause rating scale (MRS) (4,5) that
evaluate menopause-related somato-vegetative, psychological and
urogenital symptoms (6).
Insomnia is considered one of the most common
symptoms of menopause, occurring in over half of climacteric women
(7–11). Chronic poor sleep has the most
profound negative effect on the quality of life of menopausal women
(12–14), and can also lead to the development
and exacerbation of cardiovascular and metabolic diseases (15). Therefore, addressing emerging sleep
symptoms during menopause may significantly improve the overall
health and wellbeing of women.
Systemic estrogen/progestin menopausal hormone
therapy (MHT) is one of the most common treatments used to
counteract menopausal symptoms, such as hot flashes, night sweats
and urogenital atrophy (16).
However, in women with a previous history of breast cancer,
conventional estrogen/progestin MHT may increase the risk of
recurrence. This risk may be reduced by the use of progestogens,
and among these, is the synthetic steroid tibolone, whose
metabolites have estrogenic, progestogenic and androgenic
properties (17). The effect of MHT
on sleep quality remains poorly understood. Several randomized
clinical trials have demonstrated that estrogen/progestin MHT
results in slightly improved sleep quality in menopausal women with
vasomotor symptoms (18,19). However, significant heterogeneity in
the selection criteria of the trial participants and formulations
of MHT make it is difficult to determine whether the same effect
could be achieved in general population of menopausal women
(19).
Sleep disturbances can be either evaluated by
objective methods such as polysomnography, or by using
patient-filled questionnaires, such as the self-rated Pittsburgh
Sleep Quality Index (PSQI) that assesses sleep quality and
disturbances over a time interval (20,21). A
systematic review by Devine et al demonstrated that
questionnaires filled by the patient are an accurate tool for
predicting the quality of sleep, and evaluating the efficacy of
different treatments (22).
In this study we use PSQI to assess the effects of
estrogen/progestin and progestogen MHT on sleep quality, and
further examined the effects of MHT on the overall quality of life
of women experiencing menopausal symptoms.
Subjects and methods
Study design and participants
The Ethics Committee of the Maternity and Child
Health Care of Zaozhuang approved this retrospective medical record
review (approval no. 2016006). From the history reports of the
patients who were enrolled in this study, all patients or patient
carers signed informed consents. A total of 342 women newly
attending the outpatient clinic who were naïve to MHT treatment
were enrolled in this study. The menopausal status of the
participants was assessed by their self-reported menstrual history.
Menopause was determined as the cessation of menstruation with 12
months of amenorrhea. All participants were required to fill in a
demographic form, as well as detailed medical history, including
the characteristics of menopause, urogenital and metabolic
disorders, and any previous history of oncological diseases. The
study was approved by the ethics review board of the hospital and
each participant provided their written informed consent prior to
participation.
MHT
All participants enrolled in this study received
MHT. Women with no family history of breast cancer and mammography
scores <3 received femoston (estradiol/dydrogesterone 2/10 mg,
Abbott laboratories) according to the established standard
protocol. Briefly, 1 red tablet, containing 2 mg estradiol was
administered daily for the first 14 days, and 1 yellow tablet,
containing 2 mg estradiol and 10 mg dydrogesterone was administered
daily for the following 14 days of each 28-day cycle orally. A
subgroup of 14 participants with a family history of cancer and
mammography scores ≥3, received tibolone (Livial®;
Organon), 2.5 mg daily.
Determination of sleep quality and
severity of menopausal symptoms
Perceived sleep quality was measured and scored with
the PSQI, as previously described (23). A total score >5 indicates
insufficient sleep quality; a PSQI ≤5 indicates good sleepers,
while a PSQI >5 corresponds to poor sleepers. The assessment of
menopausal symptoms was performed using the KMI and the MRS that
measure the severity of age-/menopause-related complaints by rating
a profile of symptoms and their impact on health-related quality of
life.
Statistical analysis
All variables are expressed as the means ± standard
deviation. Follow-up data at each time interval was compared using
a one-way analysis of variance test (ANOVA) followed by Tukey's
honestly significant difference (HSD) test. Values of P<0.05
were considered to indicate statistically significant
differences.
Results
This study comprised of 342 women with an average
age (SD) of 50.3 (5.6) years. The baseline demographic and medical
characteristics for all the subjects are summarized in Table I. The mean weight of the participants
was 56.5 (7.3) kg and the average age at the onset of menses was
14.3 (1.6) years. The majority of women enrolled in this study
(95.9%) had no family history of breast disease. In addition, the
majority of women did not suffer from or were treated for cervical,
endometrial or ovarian cancer prior to exhibiting menopausal
symptoms (100, 99.7 and 100%, respectively). The majority of women
did not have a confirmed diagnosis of endometriosis and uterine
fibroids (99.4 and 95.9% respectively), and had not undergone any
type of urogenital surgical interventions (98.8%). The majority of
participants also had no previous history of hypertension (93.9%),
diabetes (98.8%), lipid metabolism disorders (94.2%), gallbladder
disease (94.4%) or thyroid dysfunction (90.6%).
 | Table I.Demographic characteristics and
medical history of the participants. |
Table I.
Demographic characteristics and
medical history of the participants.
| Demographics | No. of
participants | Mean | (±SD) |
|---|
| Age | 342 | 50.3 | 5.6 |
| Weight (kg) | 325 | 56.5 | 7.3 |
| Age at 1st
period | 181 | 14.3 | 1.6 |
|
| Medical
history | No. of
participants | Yes (%) | No (%) |
|
| Dysmenorrhea | 182 | 66 (36.8) | 115 (63.2) |
| Stress before
period | 182 | 33 (18.1) | 149 (81.9) |
| Gynecological
surgery | 342 | 54 (15.8) | 288 (84.2) |
| Family history of
breast disease | 342 | 14 (4.1) | 328 (95.9) |
| History of
endometrial cancer prior to onset of menopausal symptoms | 342 | 0 (0.0) | 342 (100.0) |
| Treated for cervical
cancer prior to onset of menopausal symptoms | 342 | 1 (0.3) | 341 (99.7) |
| History of ovarian
cancer prior to onset of menopausal symptoms | 342 | 0 (0.0) | 342 (100.0) |
| Confirmed diagnosis
of endometriosis | 342 | 2 (0.6) | 340 (99.4) |
| Surgical
interventions for uterine fibroids | 342 | 14 (4.1) | 328 (95.9) |
| Gynecological benign
surgery - other surgeries | 342 | 4 (1.2) | 338 (98.8) |
| Tumor (current) | 342 | 0 (0.0) | 342 (100.0) |
| History of
hypertension | 342 | 21 (6.1) | 321 (93.9) |
| History of
diabetes | 342 | 4 (1.2) | 338 (98.8) |
| History of lipid
metabolism disorder | 342 | 20 (5.8) | 322 (94.2) |
| History of
gallbladder disease | 342 | 19 (5.6) | 323 (94.4) |
| History of thyroid
disease | 342 | 32 (9.4) | 310 (90.6) |
| History of other
diseases | 342 | 20 (5.8) | 322 (94.2) |
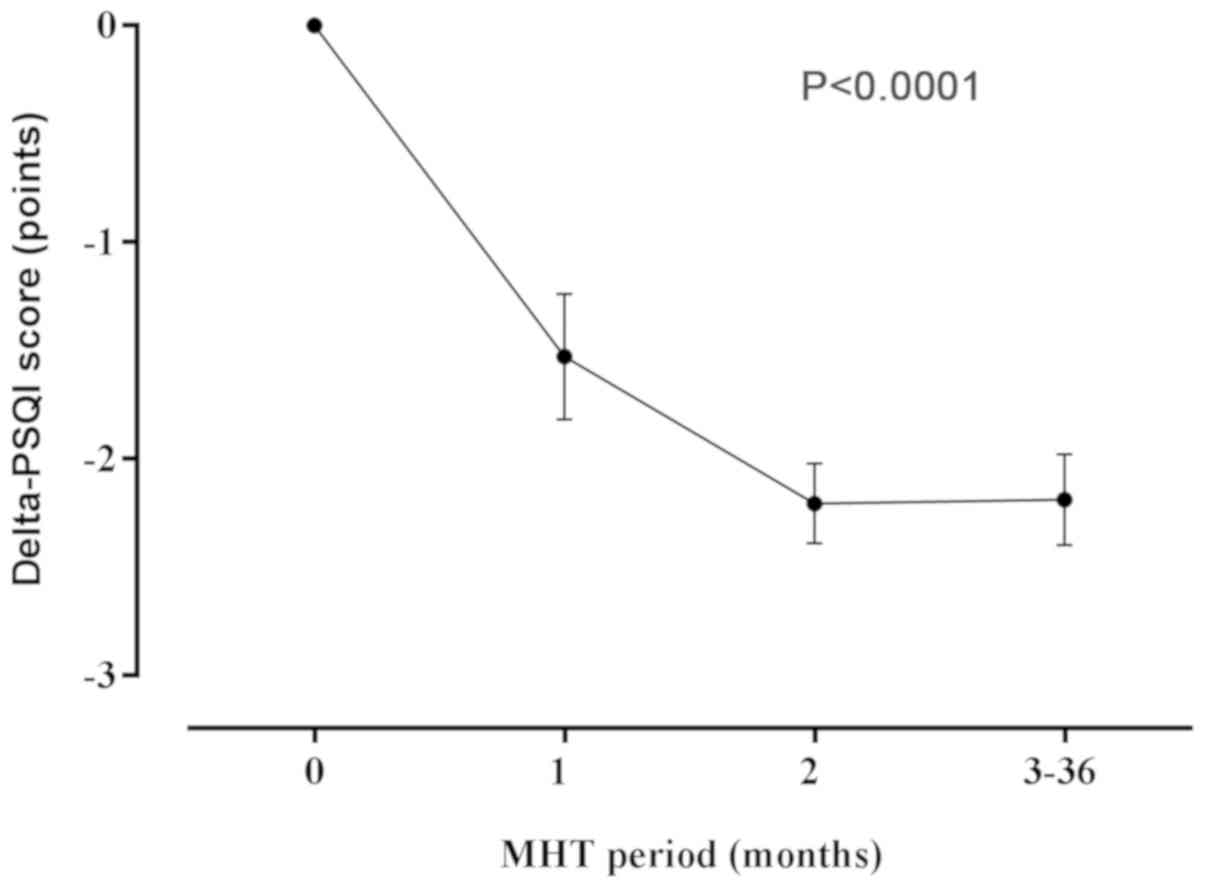
At the start of the study (baseline), the mean PSQI
score of the participants was 9.16 (4.84) points, indicative of a
poor sleep quality (Table II). At 1
month after the commencement of beginning MHT, the participants
reported significantly improved sleep quality as indicated by the
reduction in the PSQI global sleep quality score (decrease of 1.53
points; P<0.0001), as compared to the baseline score. The PSQI
score further decreased at 2 months after the commencement of MHT
(2.21 points, P<0.0001), and continued to remain significantly
below baseline scores throughout the 3 years of continuous
treatment (P<0.0001). After 3 years of MHT, the participants
continued to maintain a mean decrease (SD) of 2.19 (0.21) points
(1.3-fold) as compared to the baseline score (Fig. 1).
 | Table II.Comparison of PSQI scores of the
study participants at baseline and follow-up assessments. |
Table II.
Comparison of PSQI scores of the
study participants at baseline and follow-up assessments.
| MHT period | No. of
participants | PSQI mean
(±SD) | Delta-PSQI mean
(±SD) | One-way ANOVA
(P-value) | Tukey's HSD
post-hoc testa
(P-value) |
|---|
| 0 days
(baseline) | 342 | 9.16 (4.84) | 0 | N/A | – |
| 1st month | 111 | 7.63 (4.58) | 1.53 (0.29) |
| – |
| 2nd month | 108 | 6.97 (4.50) | 2.21 (0.187) |
| <0.0001 |
| 3rd month | 93 | 7.02 (4.01) | 2.16 (0.2) |
| <0.0001 |
| >3 months | 171 | 6.95 (4.32) | 2.21 (0.221) |
| <0.0001 |
| >6 months | 113 | 6.97 (4.33) | 2.19 (0.191) | <0.0001 | <0.0001 |
| >9 months | 84 | 7.37 (4.41) | 1.79 (0.139) |
| <0.0001 |
| >1 year | 134 | 6.90 (4.17) | 2.28 (0.19) |
| <0.0001 |
| >2 years | 76 | 6.71 (4.56) | 2.45 (0.161) |
| <0.0001 |
| >3 years | 79 | 6.90 (4.76) | 2.26 (0.6) |
| <0.0001 |
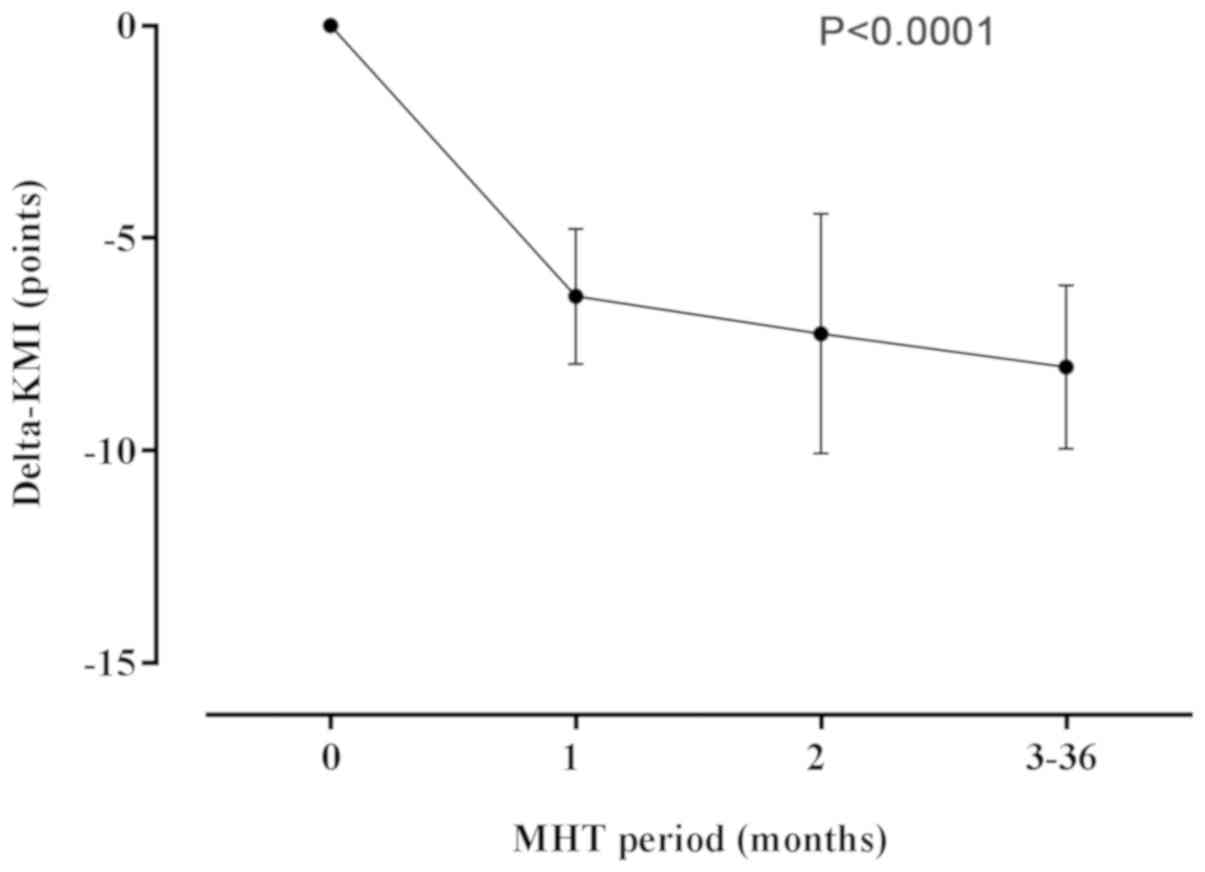
The mean (SD) KMI score of the participants at the
baseline was 18.63 (9.86) (Table
III). We observed a 6.37 (1.59) point decrease in the score
during the 1st month of MHT (P<0.0001). The KMI scores continued
to gradually decline throughout the course of hormone therapy,
reaching an average of 9.9 (7.33), with a decrease of 8.73 (1.92)
points compared to the baseline score (P<0.0001; Fig. 2).
 | Table III.Changes in KMI scores of the
participants receiving MHT. |
Table III.
Changes in KMI scores of the
participants receiving MHT.
| MHT period | No. of
participants | Mean (±SD) | One-way ANOVA
(P-value) | Tukey's HSD
post-hoc testa
(P-value) |
|---|
| 0 days
(baseline) | 342 | 18.63 (9.86) |
| – |
| 1st month | 111 | 12.26 (8.09) |
| <0.0001 |
| 2nd month | 107 | 11.38 (6.86) |
| <0.0001 |
| 3rd month | 92 | 12.14 (7.60) |
| <0.0001 |
| >3 months | 169 | 11.16 (7.38) |
| <0.0001 |
| >6 months | 113 | 9.78
(7.59) | <0.0001 | <0.0001 |
| >9 months | 84 | 10.67 (8.59) |
| <0.0001 |
| >1 year | 133 | 9.99
(7.37) |
| <0.0001 |
| >2 years | 76 | 10.52 (8.40) |
| <0.0001 |
| >3 years | 79 | 9.90
(7.33) |
| <0.0001 |
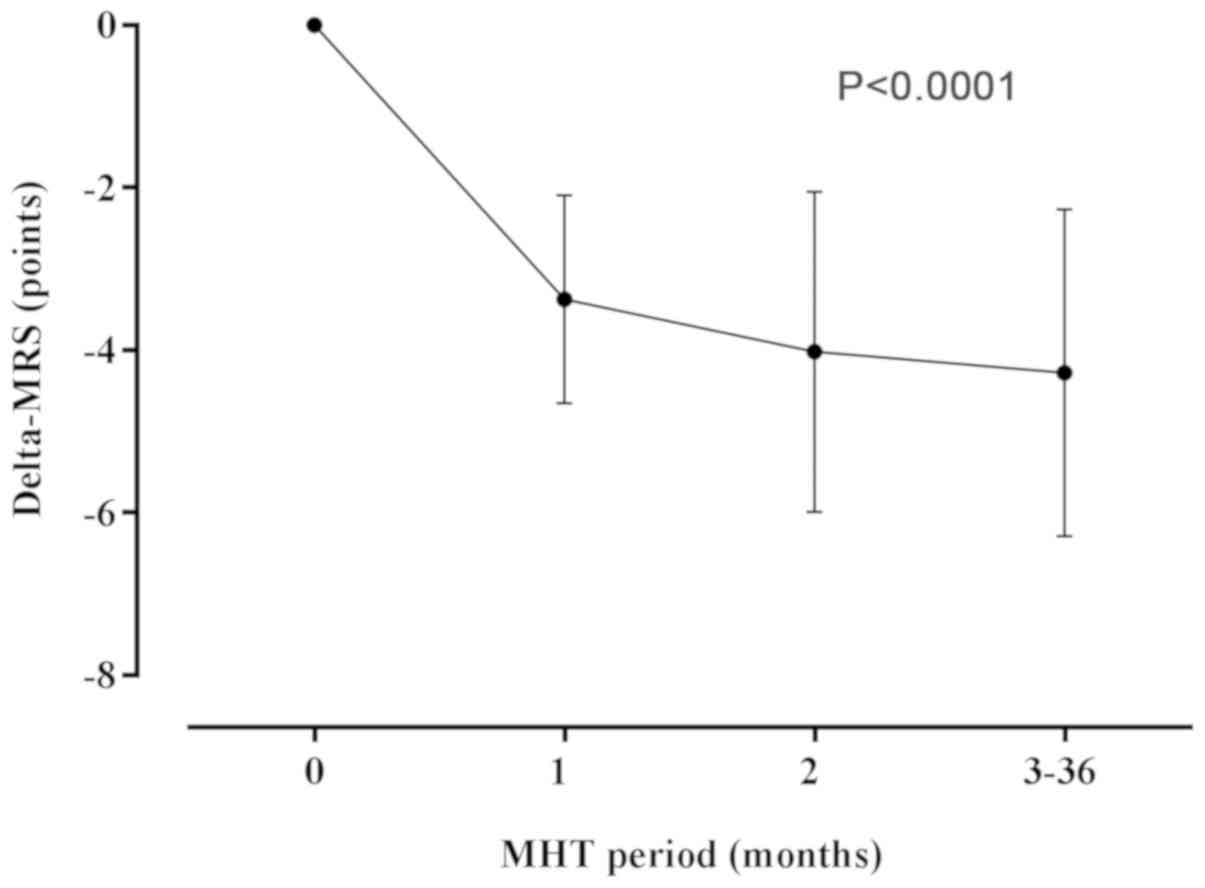
There was a significant [3.56 (1.05) points
(P<0.0001)] decrease in the mean MRS scores of the participants
during the 1st month of MHT as compared to baseline (Fig. 3). Subsequent hormone treatment did
not result in further significant changes in the MRS scores
(Fig. 3), and the average score at 3
years after the commencement of MHT was 4.7 (4.6), as compared to
8.66 (6.17) at baseline, an equivalent of 3.96 (2.01)-point
decrease (P<0.0001; Table IV and
Fig. 3).
 | Table IV.Changes in MRS scores of the in
participants receiving MHT. |
Table IV.
Changes in MRS scores of the in
participants receiving MHT.
| MHT period | No. of
participants | Mean (±SD) | One-way ANOVA
(P-value) | Tukey's HSD
post-hoc testa
(P-value) |
|---|
| 0 days
(baseline) | 342 | 8.66 (6.17) |
| – |
| 1st month | 111 | 5.10 (4.67) |
| <0.0001 |
| 2nd month | 107 | 4.64 (4.20) |
| <0.0001 |
| 3rd month | 92 | 4.93 (4.26) |
| <0.0001 |
| >3 months | 169 | 4.93 (4.32) |
| <0.0001 |
| >6 months | 113 | 4.22 (3.83) | <0.0001 | <0.0001 |
| >9 months | 84 | 4.32 (5.68) |
| <0.0001 |
| >1 year | 133 | 3.47 (3.35) |
| <0.0001 |
| >2 years | 76 | 4.04 (4.09) |
| <0.0001 |
| >3 years | 79 | 4.70 (4.60) |
| <0.0001 |
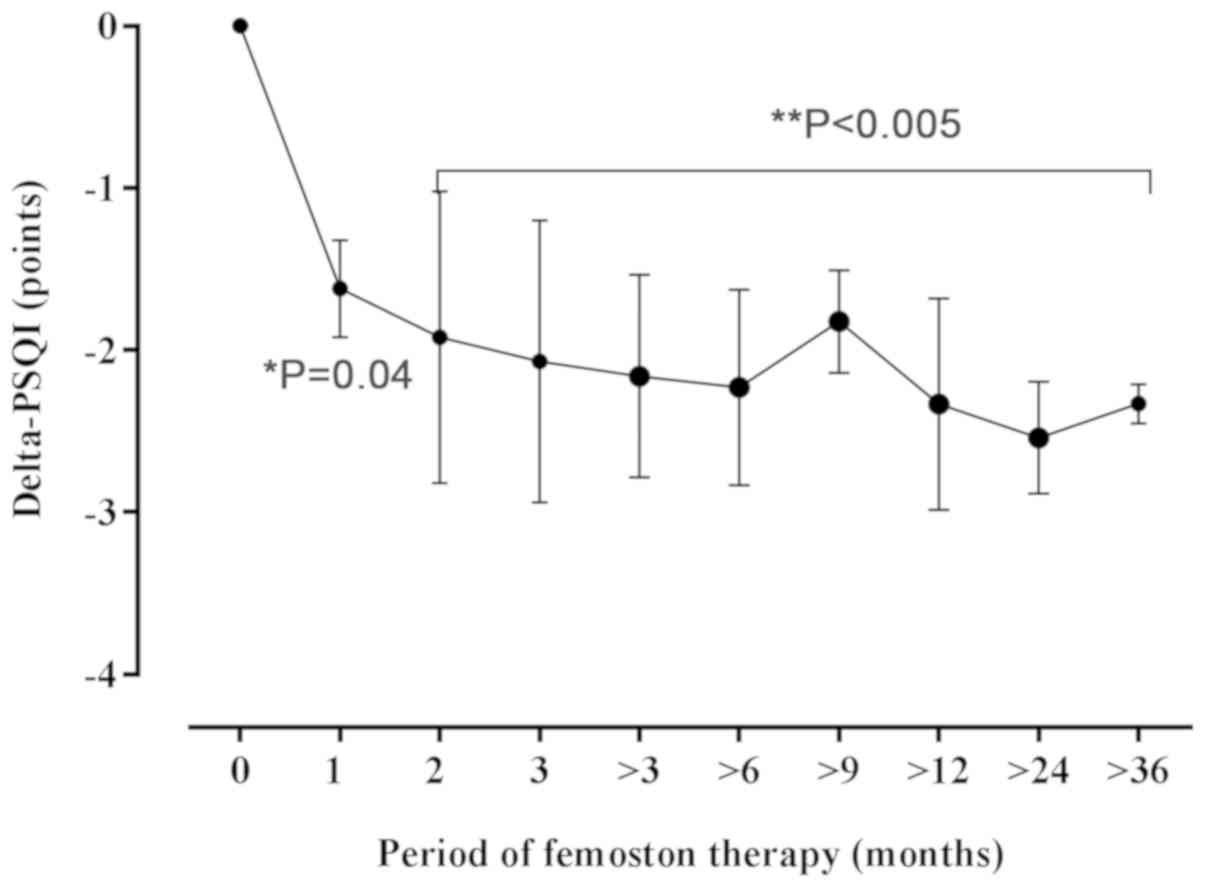
We then evaluated whether different MHT regimens
(femoston or tibolone) had a different impact on the changes in the
PSQI scores. In the subgroup of participants receiving femoston,
the baseline mean PSQI score was 9.14 (4.84) points (Table V). During the 1st and 2nd months of
MHT, the PSQI score improved significantly, exhibiting a decrease
of 1.62 (1.01) (P=0.04), and 2.26 (0.72) points (P=0.0002)
respectively, comparing to baseline (Fig. 4). There was no further significant
decrease in the PSQI score, maintaining a stable 2.28 (0.67)-points
difference compared to the initial score prior to the commencement
of MHT (P<0.005).
 | Table V.Changes in PSQI scores of patients
receiving femoston. |
Table V.
Changes in PSQI scores of patients
receiving femoston.
| MHT period | No. of
participants | Mean (±SD) | One-way ANOVA
(P-value) | Tukey's HSD
post-hoc testa
(P-value) |
|---|
| 0 days
(baseline) | 328 | 9.14 (4.84) |
| – |
| 1st month | 105 | 7.52 (4.50) |
| 0.04 |
| 2nd month | 105 | 6.88 (4.47) |
|
0.0002 |
| 3rd month | 88 | 7.07 (3.98) |
|
0.0047 |
| >3 months | 166 | 6.90 (4.30) |
| <0.0001 |
| >6 months | 110 | 6.85 (4.31) | <0.0001 |
0.0001 |
| >9 months | 76 | 7.30 (4.46) |
|
0.0417 |
| >1 year | 129 | 6.84 (4.15) |
| <0.0001 |
| >2 years | 72 | 6.61 (4.52) |
|
0.0006 |
| >3 years | 78 | 6.81 (4.72) |
|
0.0015 |
In the small subgroup of 14 participants with a
previous history of breast cancer, who were receiving tibolone MHT,
no significant effect of the hormonal therapy was observed on the
PSQI scores (P=0.956; Table
VI).
 | Table VI.Changes in PSQI scores of
participants receiving tibolone. |
Table VI.
Changes in PSQI scores of
participants receiving tibolone.
| MHT period | N | Mean (±SD) | One-way ANOVA
(P-value) | Tukey's HSD
post-hoc testa
(P-value) |
|---|
| 0 days
(baseline) | 14 | 9.57
(5.00) |
| – |
| 1st month | 6 | 9.50
(5.96) |
| 1.09 |
| 2nd month | 3 | 10.33 (5.51) |
| 1 |
| 3rd month | 5 | 6.20
(4.92) |
| 0.93 |
| >3 months | 5 | 8.80
(5.02) |
| 1 |
| >6 months | 3 | 10.00 (4.58) | 0.956 | 1 |
| >9 months | 8 | 8.00
(4.17) |
| 0.99 |
| >1 year | 5 | 8.60
(4.83) |
| 1 |
| >2 years | 4 | 8.25
(3.95) |
| 1 |
| >3 years | 1 | 14 (N/A) | – | – |
Discussion
In the present study, we examined the effects of
hormone replacement therapy on self-reported menopausal symptoms in
general, and the quality of sleep of post-menopausal women in
particular. The mechanisms through which estrogen/progestin
therapies may improve sleep quality are not yet well known. A
previous study using rodents suggested that estrogen consolidates
circadian sleep-wake rhythms in female rats (24). Alternatively, the effects of
estradiol on sleep/wake cycles may be explained by a reduction in
prostaglandin synthesis in the ventrolateral preoptic nucleus of
the hypothalamus (25). It is also
possible that estrogen therapy may improve sleep quality
indirectly, by relieving nocturnal hot flashes (26). In this study, it was found that MHT
induced a rapid decrease in the PSQI scores as early as 1 month
after the commencement of therapy. Sleep improvement further
continued through the 2nd month of MHT, resulting in a >1.3-fold
decrease in the PSQI score compared to the baseline assessment.
Notably, after the initial improvement of the PSQI scores achieved
after 2 months of MHT, there was no further significant improvement
in sleep quality, with the PSQI scores maintaining the same
decrease of 2.19 points compared to baseline.
In this study, the dynamics of sleep quality
improvement observed in participants receiving MHT, coincided with
a similar pattern of overall improvement in menopausal symptoms, as
indicated by the KMI and MRS scores. It was demonstrated, that
similar to the PSQI scores, patients reported the most significant
decrease in the severity of menopausal symptoms during the first
months of MHT. A major improvement was reported by the 2nd month of
therapy. Prolonged treatment did not result in any further
alleviation of symptoms, although it rather helped to maintain the
KMI and MRS scores that were decreased during the initial months of
hormone replacement therapy. While the precise mechanisms through
which menopausal transition affect sleep quality remain unclear,
previous studies have demonstrated that symptoms, such as nocturnal
hot flashes, mood disorders and sleep-disordered breathing are the
main contributors to a poor sleep quality (9,27–29). It
is possible, therefore, that the immediate partial relief of these
symptoms by hormone replacement therapy may have led to a similarly
rapid improvement in the sleep quality of the participants.
There is still limited evidence as regards the
effects of a conventional estrogen/progestin MHT, such as femoston,
on sleep quality. It has been demonstrated that progesterone may
have a sedative effect (30), and
estrogen is able to increase overall sleep time, and decrease sleep
onset latency (31), and awakening
after sleep onset (32). In this
study, femoston was found to have a marked and rapid effect on
self-reported sleep quality, with the PSQI score decreasing
1.32-fold during the first 2 months of the treatment. This decrease
was maintained throughout the 3 years of the study period,
suggesting that estrogen/progestin MHT results in a stable
improvement of sleep quality. Of note, MHT with tibolone did not
improve sleep quality in a small group of participants with a
previous history of breast disease. Tibolone is a synthetic steroid
that has progestogenic, androgenic, and estrogenic effects, shown
to improve insomnia via stimulation of the production and release
of β-endorphin (33,34). It is possible that the lack of an
effect of tibolone on sleep quality in this study is related to an
insufficient number of participants receiving this MHT. Better
designed, larger clinical studies are thus required to examine the
efficiency of tibolone in improving the sleep quality of menopausal
women.
In conclusion, this study found that hormone
replacement therapy had an immediate and long-lasting positive
effect on sleep quality and on the general well-being of women
experiencing menopausal symptoms. While a conventional
estrogen/progestin MHT with femoston resulted in a marked
improvement of sleep quality, tibolone treatment had no effect on
the PSQI score. Further clinical studies with larger patient groups
are required to compare the effects of both MHT regiments on sleep
quality.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL and XY conceived and designed the study. CL, LW
and XS provided the study materials or patient data and were
responsible for the collection and assembly of the data, data
analysis and interpretation. CL was involved in the writing of the
manuscript. XY was involved in the editing of the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Maternity and Child
Health Care of Zaozhuang approved this retrospective medical record
review (approval no. 2016006).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jehan S, Masters-Isarilov A, Salifu I,
Zizi F, Jean-Louis G, Pandi-Perumal SR, Gupta R, Brzezinski A and
McFarlane SI: Sleep Disorders in Postmenopausal Women. J Sleep
Disord Ther. 4:42015.
|
|
2
|
Al-Safi ZA and Santoro N: Menopausal
hormone therapy and menopausal symptoms. Fertil Steril.
101:905–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Potthoff P, Heinemann LA, Schneider HP,
Rosemeier HP and Hauser GA: The Menopause Rating Scale (MRS II):
Methodological standardization in the German population. Zentralbl
Gynakol. 122:280–286. 2000.(In German). PubMed/NCBI
|
|
4
|
Schneider HP, Heinemann LA, Rosemeier HP,
Potthoff P and Behre HM: The Menopause Rating Scale (MRS):
Reliability of scores of menopausal complaints. Climacteric.
3:59–64. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kupperman HS, Blatt MH, Wiesbader H and
Filler W: Comparative clinical evaluation of estrogenic
preparations by the menopausal and amenorrheal indices. J Clin
Endocrinol Metab. 13:688–703. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tao M, Shao H, Li C and Teng Y:
Correlation between the modified Kupperman Index and the Menopause
Rating Scale in Chinese women. Patient Prefer Adherence. 7:223–229.
2013.PubMed/NCBI
|
|
7
|
Mitchell ES and Woods NF: Symptom
experiences of midlife women: Observations from the Seattle Midlife
Women's Health Study. Maturitas. 25:1–10. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai JN, Chen HJ, Chen CM, Chen PC and Wang
JD: Quality of life and climacteric complaints amongst women
seeking medical advice in Taiwan: Assessment using the WHOQOL-BREF
questionnaire. Climacteric. 9:119–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dennerstein L, Dudley EC, Hopper JL,
Guthrie JR and Burger HG: A prospective population-based study of
menopausal symptoms. Obstet Gynecol. 96:351–358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawada T, Yosiaki S, Yasuo K and Suzuki S:
Population study on the prevalence of insomnia and insomnia-related
factors among Japanese women. Sleep Med. 4:563–567. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan HA, Wu MH, Hsu CC, Yao BL and Huang
KE: The perception of menopause among women in Taiwan. Maturitas.
41:269–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shade MY, Berger AM, Dizona PJ, Pozehl BJ
and Pullen CH: Sleep and health-related factors in overweight and
obese rural women in a randomized controlled trial. J Behav Med.
39:386–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen JH, Waite LJ and Lauderdale DS:
Marriage, Relationship Quality, and Sleep among U.S. Older Adults.
J Health Soc Behav. 56:356–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lampio L, Saaresranta T, Engblom J, Polo O
and Polo-Kantola P: Predictors of sleep disturbance in menopausal
transition. Maturitas. 94:137–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luyster FS, Strollo PJ Jr, Zee PC and
Walsh JK; Boards of Directors of the American Academy of Sleep
Medicine and the Sleep Research Society, : Sleep: A health
imperative. Sleep (Basel). 35:727–734. 2012. View Article : Google Scholar
|
|
16
|
Lobo RA: Where are we 10 years after the
Women's Health Initiative? J Clin Endocrinol Metab. 98:1771–1780.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Formoso G, Perrone E, Maltoni S, Balduzzi
S, D'Amico R, Bassi C, Basevi V, Marata AM, Magrini N and Maestri
E: Short and long term effects of tibolone in postmenopausal women.
Cochrane Database Syst Rev. 2012:CD0085362012.
|
|
18
|
Bruyneel M: Sleep disturbances in
menopausal women: Aetiology and practical aspects. Maturitas.
81:406–409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cintron D, Lipford M, Larrea-Mantilla L,
Spencer-Bonilla G, Lloyd R, Gionfriddo MR, Gunjal S, Farrell AM,
Miller VM and Murad MH: Efficacy of menopausal hormone therapy on
sleep quality: Systematic review and meta-analysis. Endocrine.
55:702–711. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Backhaus J, Junghanns K, Broocks A,
Riemann D and Hohagen F: Test-retest reliability and validity of
the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom
Res. 53:737–740. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mollayeva T, Thurairajah P, Burton K,
Mollayeva S, Shapiro CM and Colantonio A: The Pittsburgh sleep
quality index as a screening tool for sleep dysfunction in clinical
and non-clinical samples: A systematic review and meta-analysis.
Sleep Med Rev. 25:52–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Devine EB, Hakim Z and Green J: A
systematic review of patient-reported outcome instruments measuring
sleep dysfunction in adults. Pharmacoeconomics. 23:889–912. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buysse DJ, Reynolds CF III, Monk TH,
Berman SR and Kupfer DJ: The Pittsburgh Sleep Quality Index: A new
instrument for psychiatric practice and research. Psychiatry Res.
28:193–213. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwartz MD and Mong JA: Estradiol
modulates recovery of REM sleep in a time-of-day-dependent manner.
Am J Physiol Regul Integr Comp Physiol. 305:R271–R280. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mong JA, Devidze N, Goodwillie A and Pfaff
DW: Reduction of lipocalin-type prostaglandin D synthase in the
preoptic area of female mice mimics estradiol effects on arousal
and sex behavior. Proc Natl Acad Sci USA. 100:15206–15211. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Polo-Kantola P, Erkkola R, Helenius H,
Irjala K and Polo O: When does estrogen replacement therapy improve
sleep quality? Am J Obstet Gynecol. 178:1002–1009. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohayon MM: Severe hot flashes are
associated with chronic insomnia. Arch Intern Med. 166:1262–1268.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shahar E, Redline S, Young T, Boland LL,
Baldwin CM, Nieto FJ, O'Connor GT, Rapoport DM and Robbins JA:
Hormone replacement therapy and sleep-disordered breathing. Am J
Respir Crit Care Med. 167:1186–1192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krystal AD, Edinger J, Wohlgemuth W and
Marsh GR: Sleep in peri-menopausal and post-menopausal women. Sleep
Med Rev. 2:243–253. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Manber R and Armitage R: Sex, steroids,
and sleep: A review. Sleep. 22:540–555. 1999.PubMed/NCBI
|
|
31
|
Schiff I, Regestein Q, Tulchinsky D and
Ryan KJ: Effects of estrogens on sleep and psychological state of
hypogonadal women. JAMA. 242:2405–4. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Landis CA and Moe KE: Sleep and menopause.
Nurs Clin North Am. 39:97–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Genazzani AR, Facchinetti F, Ricci-Danero
MG, Parrini D, Petraglia F, La Rosa R and D'Antona N:
Beta-lipotropin and beta-endorphin in physiological and surgical
menopause. J Endocrinol Invest. 4:375–378. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taskin O, Gökdeniz R, Yalcinoglu A, Buhur
A, Burak F, Atmaca R and Ozekici U: Placebo-controlled cross-over
study of effects of tibolone on premenstrual symptoms and
peripheral beta-endorphin concentrations in premenstrual syndrome.
Hum Reprod. 13:2402–2405. 1998. View Article : Google Scholar : PubMed/NCBI
|