Introduction
Mucormycosis is a rare, atypical and
life-threatening zygomycosis that is mainly caused by
Rhizopus species, Mucor species, Cunninghamella
bertholletiae and Rhizomucor species (1–4).
Mucormycosis often coexists with other underlying conditions that
exhibit immunosuppression (1). Due
to the characteristics of invasion of blood vessels and thrombosis,
mucormysis usually exhibits rapid progression (3,4).
According to the anatomical localization, infection patterns could
include sinus, pulmonary, cutaneous, cerebral, gastrointestinal or
kidney-related and generalized disseminated patterns (1,4–6). Rhinocerebral and pulmonary mucormycosis
are the most common manifestation that occurs via air spore
inhalation (6). Disseminated
mucormycosis is mainly present in hematopoietic stem cell
transplant recipients, and usually has a high mortality rate with
>90% due to progressive invasive infection (1,7–9). The present study reports on a case of
cerebral disseminated mucormycosis in a patient with multiple
myeloma (MM) and secondary myelodysplastic syndrome (MDS),
confirmed by mycological culture and histopathological
examination.
Case report
In January 2013, a 68-year-old man visited Hangzhou
First People's hospital due to backache and a fever. The initial
laboratory tests showed hyperimmunoglobulin with immune globulin
(Ig) A at 2.64 g/l. The 24-h κ light chain level was 6,860 mg/24 h
and the serum β2-microglobulin level was 2,563 mg/l. In addition,
the serum albumin level was 38.9 g/l. Bone marrow smears revealed
13.5% abnormal plasma cells. MRI determined vertebral compression
fractures at T6 and T11. The pathological and flow cytometry
results of bone marrow specimens with plasma cell antigens was
CD117+CD38+CD138+CD56-cKappa+, and MM was diagnosed as Durie-Salmon
stage II phase A and International Staging System stage I phase
A.
The patient was administered chemotherapy including
vincristine with 0.5 mg per day (from the first day 4), doxorubicin
with 10 mg/m2 per day (10 mg/m2/d for days
1–4) and dexamethasone with 40 mg/day (days 1–4) on January 30,
2013. However, he responded poorly after two courses of
chemotherapy and refused to accept bortezomib treatment, so CyRd
chemotherapy comprising cyclophosphamide with 300 mg per
m2 per week (300 mg/m2/w for 4 weeks),
lenalidomide with 15 mg/day (from days 1–21) and dexamethasone with
40 mg per week (40 mg/w) was administered to him on 16 April, 2013.
After six courses of the CyRd regimen, the patient showed a very
good partial response. Thereafter, lenalidomide was used as
maintenance therapy with 15 mg/day; continuous medication for 21
days with an interval of 7 days in a 28-day-course treatment.
In May 2016, the patient was admitted to Affiliated
Hangzhou First People's hospital again due to disease progression
with fatigue and dizziness. Bone marrow pathology and chromosome
karyotyping revealed MM progression and secondary MDS. Therefore,
the patient was administered bortezomib (1.3 mg/m2/week)
combined with dexamethasone (40 mg; qw), but had a poor response.
Since the previous chemotherapy treatment, the patient had lost
weight and had been diagnosed with diabetes. However, the blood
glucose levels of the patient were not well controlled due to
incomplete compliance with insulin treatment.
On October 20, 2017, the patient visited the
hospital after suffering from chills and fever lasting 1 week. CT
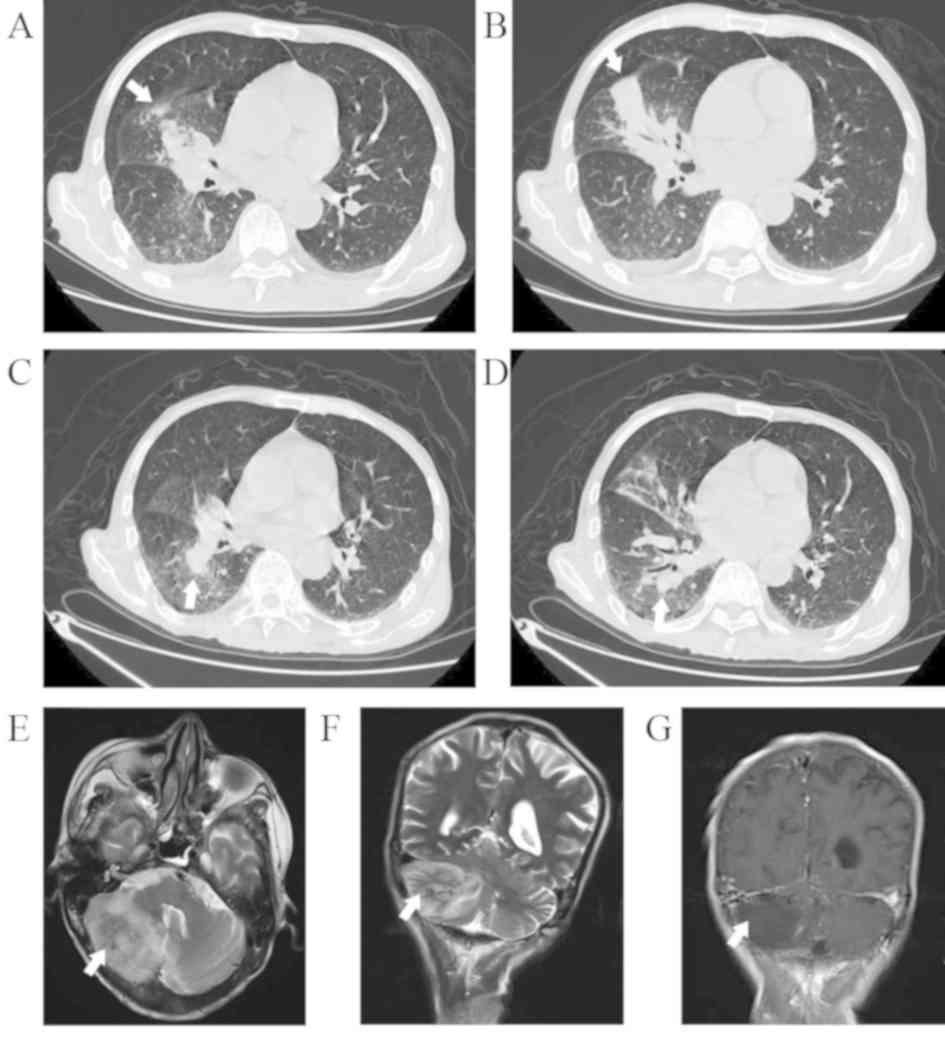
showed acute pneumonia in the right middle lung (Fig. 1A and B). The cranial MRI result was
normal. The results were negative with etiological separation and
culture, T-SPOT test for tuberculosis infection, the galactomannan
test for invasive pulmonary aspergillosis, normal cytomegalovirus
DNA and endotoxin levels. The level of 1–3-β-D glucan was 550.40
ng/l. Due to the antibiotic agents having minimal effect,
voriconazole (200 mg; twice per day) was empirically prescribed for
antifungal treatment in considering probable invasive
aspergillosis. The infection seemed to be controlled, as C-reactive
protein (CRP) level and body temperature were normal. On November
24, 2017, due to the progressive MM with a level of 24-h κ light at
19,548 mg/24 h, the patient underwent chemotherapy with reduced
doses of bortezomib (1.1 mg/m2/week), cyclophosphamide
(300 mg; qw) and dexamethasone (20 mg; qw) due to his low weight,
and the level of 24-h urine κ light chain was significantly reduced
to 2543 mg/24 h. Thereafter, the patient underwent outpatient
follow-up treatment.
During the period of outpatient follow-up treatment,
the patient independently reduced the oral dose of voriconazole due
to incomplete compliance. A period of 1 week later, fever presented
again with a dry cough. On December 19, 2017, the patient was
readmitted to Hangzhou First People's Hospital. The previous
infection in the right middle lung was improved according to
pulmonary CT images, but new infection sites had appeared in the
right lower lung (Fig. 1C and D).
MRI displayed certain abnormal signal lesions with new infection in
the right cerebellum (Fig. 1E-G). At
this time, combined therapy of voriconazole (200 mg, twice daily),
meropenem (2.0 g, three times daily) and linezolid (0.6 g, twice
daily) was started for the patient, but it was ineffective. After
10 days, the patient lost consciousness and was considered to
exhibit secondary occipital foramen with high intracranial
pressure. Immediately, emergency surgery was performed for
decompression, showing high tension in the cerebellum, and the
operation was conservative with an extremely low platelet count
(18×109/l). The brain tissue showed necrosis and was
obtained for histopathological and etiological examination. That
night, the patient was in a persistent coma, and subsequently died
due to myocardial infarction with ST-segment arch-back elevation in
the multi-lead anterior intermediate wall (cardiac troponin I, 7.8
ng/ml; creatine kinase-MB, 57 ng/ml). The patient's laboratory
tests and basic clinical data are presented in Table SI.
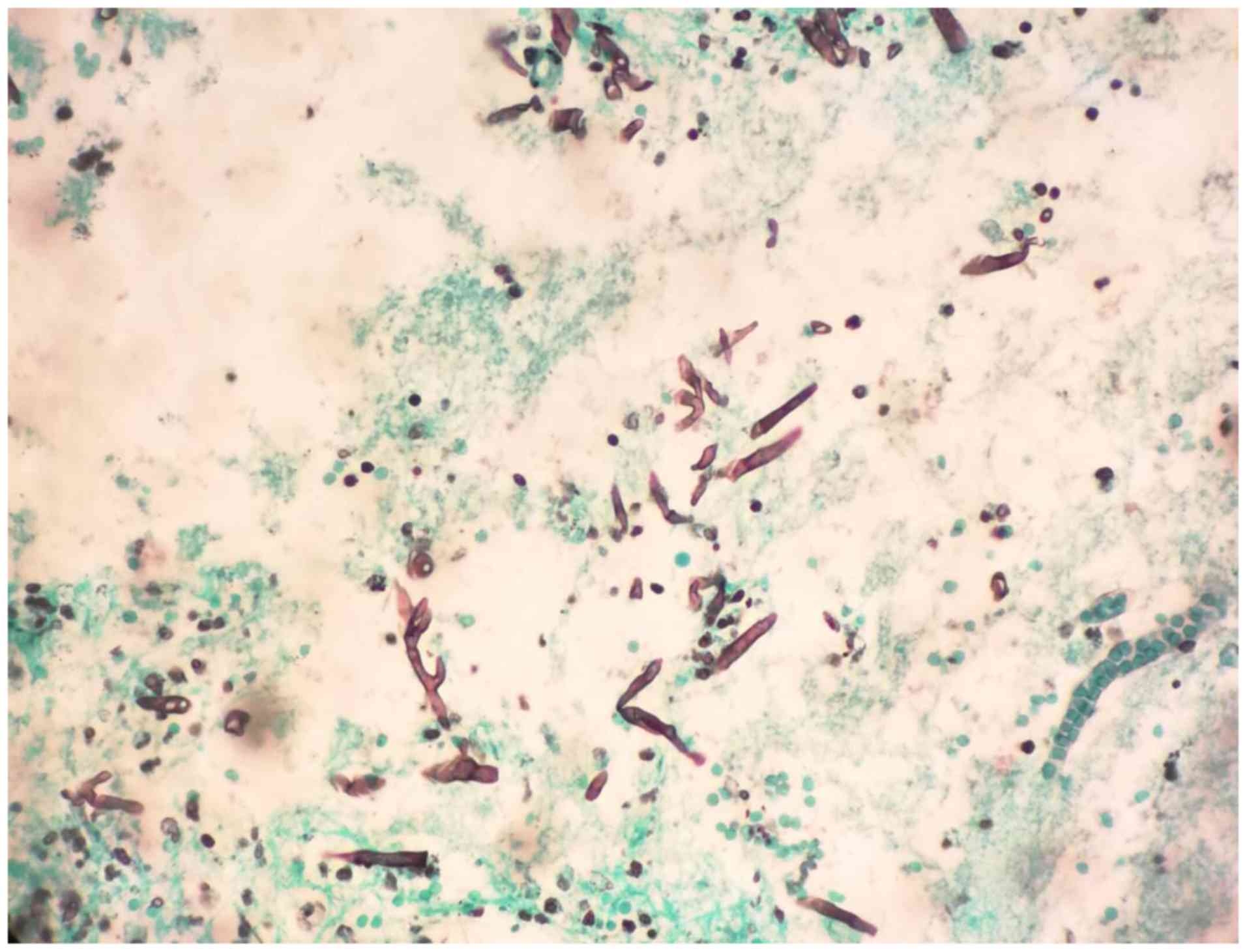
Histopathology demonstrated neutrophil infiltration
and large broad-based non-septate hyphae upon Gomori methenamine
silver staining, leading to a diagnosis of Mucor species
(Fig. 2). Mycological cultures were
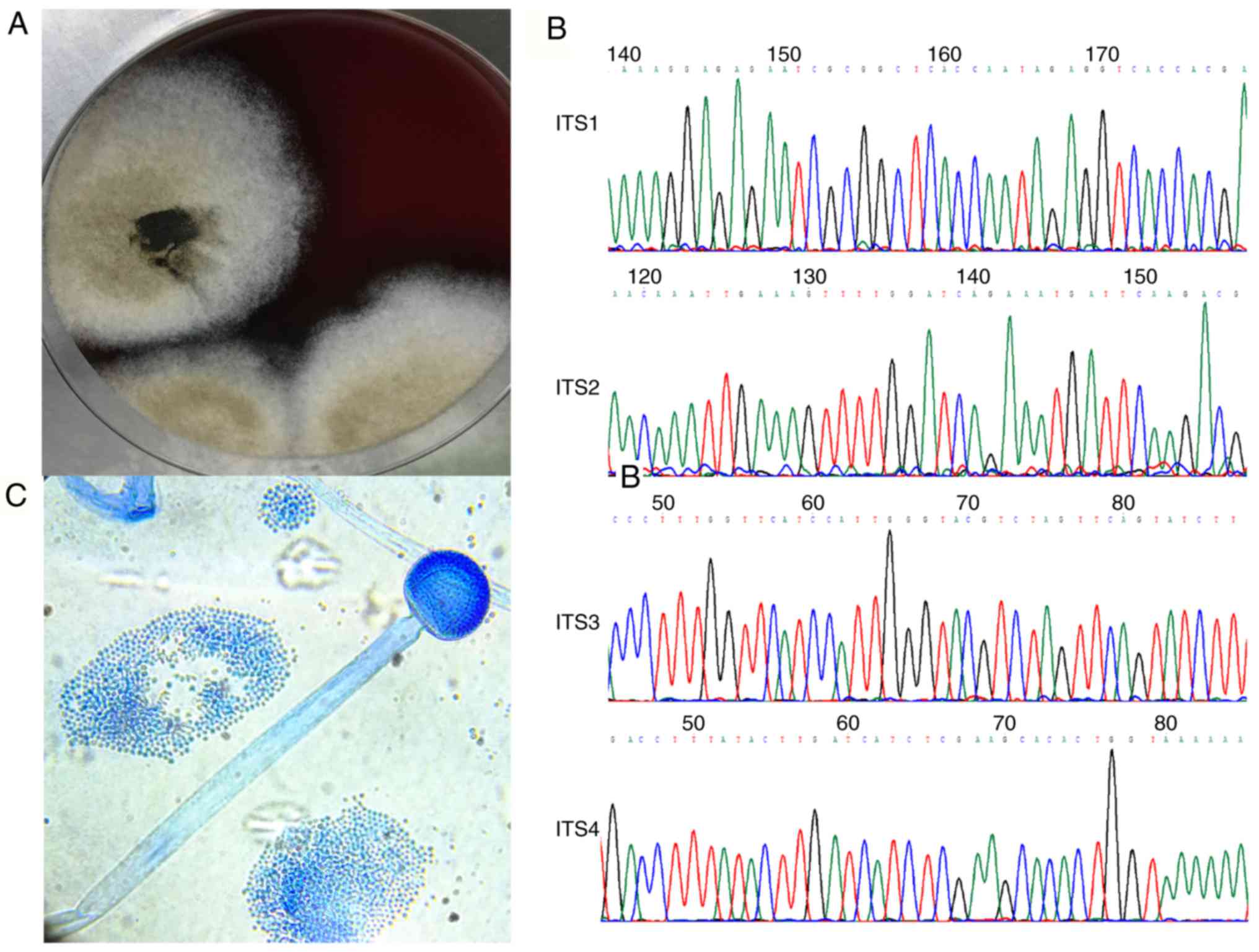
positive in three days with a loose filamentous fungal colony. Both
the colonial and microscopic features suggested Mucor
species (Fig. 3). The antifungal
susceptibility test was performed using the broth microdilution
method as previously documented (10,11), and
the results showed that the minimum inhibitory concentrations of
posaconazole and amphotericin B were 0.25 and 1 mg/l, respectively.
However, there was resistance to voriconazole and itraconazole,
according to the breakpoint of Aspergillus (Table I). Internal transcribed spacer region
sequencing confirmed the pathogenic basis of Rhizomucor
pusillus (Genbank numbers: MN061000 and MN061001in the database
of the National Center for Biotechnology Information; Fig. 3).
 | Table I.Minimum inhibitory concentrations of
different antifungal agents. |
Table I.
Minimum inhibitory concentrations of
different antifungal agents.
|
|
| Breakpoint |
|---|
|
|
|
|
|---|
| Antifungal
agentsa | MIC, mg/l | Susceptible | Intermediate | Resistant |
|---|
| Amphotericin B | 1 | ≤1 | 2 | ≥4 |
| Itraconazole | >256 | ≤1 | 2 | ≥4 |
| Posaconazole | 0.25 | ≤1 | 2 | ≥4 |
| Voriconazole | >256 | ≤1 | 2 | ≥4 |
| Anidulafungin | >8 | – | – | – |
| Micafungin | >8 | – | – | – |
| Caspofungin | >8 | – | – | – |
| 5-Fluorocytosine | >64 | – | – | – |
Discussion
In the present study, due to primary hematological
malignancy and long-term use of chemotherapy drugs, the patient had
additional high-risk factors for invasive fungal infections,
including diabetes, poor immunity and immunosuppression (4,6). The
patient likely contracted two different invasive fungal infections
in a short time. On October 20, 2017, the patient may have been
infected with a voriconazole-susceptible fungus without etiological
support, because the right middle lung imaging manifestations were
improved following voriconazole treatment, with the patient
displaying normal CRP levels and normal body temperature. On
December 19, 2017, the patient was readmitted to the hospital with
a probable new fungal infection that was secondary to voriconazole
treatment and was non-susceptible to voriconazole. However, when
the new infection site appeared in the right lower lung and brain,
the doctor incorrectly assumed the cause to be a secondary
infection, in addition to the first fungal infection, and failed to
identify a possible new Mucor infection. The present study
concluded that if the doctor had noticed the Mucor
infection, and if the patient had been appropriately treated with
posaconazole, the patient may have been cured of this
infection.
Although it is difficult to identify the etiological
diagnosis for patients with high-risk factors due to the limited
diagnostic methods (12,13) for a clinical presentation of invasive
fungal infection, it is helpful to use antifungal therapy
appropriately as an empirical treatment. The current report
revealed that voriconazole treatment may increase the risk of
Mucor infection within a short time in high-risk patients.
Previous studies determined that using voriconazole, especially in
patients with hematological malignancy and recipients of
hematopoietic stem cell transplants, is associated with an
increased incidence of mucormycosis (5,14).
Mucormycosis infection can occur as a breakthrough infection during
voriconazole treatment (15). The
patient presented in the present case report might have had a
breakthrough infection following treatment with voriconazole.
Mucormycosis is an aggressive and angioinvasive
fungal infection responsible for high morbidity and mortality
(6,13). Although the number of invasive
mucormycosis cases has increased in recent years due to the
increased prevalence of immunosuppression in the general population
(7,16,17), it
remains an uncommon infection (5,18).
Invasive mucormycosis often occurs in high-risk populations, such
as those with diabetes mellitus, hematological malignancies and
other immunocompromised states (1,4,19,20).
Even in immunocompetent populations, such as those with trauma,
burns or undergoing surgery, invasive mucormycosis may occur, as
documented in previous reports (7,12,18,19).
In the present report, a patient with MM and secondary MDS
demonstrated disseminated mucormycosis with cerebral involvement
during voriconazole treatment. For high-risk patients who undergo
voriconazole therapy, mucormycosis infection should be considered a
possibility.
Due to the angioinvasive ability of mucormycosis, it
can readily cause necrosis and thrombosis (21). The embolus spreads and is implanted
into other sites, and may develop into disseminated mucormycosis.
The central nervous system (CNS) is a common dissemination site
(1,3), and the rhino-orbito-cerebral type is
the predominant infection pattern (3,4).
Disseminated mucormycosis is a life-threatening disease with a high
mortality rate. Among 174 renal transplant recipients with
mucormycosis, disseminated patterns accounted for 14.4% of cases
and the mortality rate was 76.0%, compared with the overall
mortality rate of 42.5% (22). In a
retrospective investigation, 929 patients were diagnosed with
zygomycosis, and 283 had CNS involvement with a mortality rate of
62%, with all 25 patients with generalized disseminated infection
succumbing to the disease (7). In
contrast to the more common rhino-orbito-cerebral mucormycosis, the
infection site in the patient presented here was rare, as it
occurred in the cerebellum. Disseminated cerebellar mucormycosis
may have spread from the lung to the brain through the blood
circulation.
To date, no common reliable clinical serological
diagnostic method has been developed for mucormycosis (13,20).
Histopathological examination seems to be the gold standard, as
this method is able to the identify characteristic of non-septate
hyphae branching at a right angle. Autopsy is another reliable
invasive technique for tissue samples; however, the diagnosis rates
are low, even in high-risk patients (1,6).
Microbial culture is a time-consuming method with low sensitivity
(17). Additionally, DNA sequencing
targeting the region directly from tissue has provided a promising
method for pathogenic detection (23), but sequencing is challenging in a
basic laboratory setting. In most cases, high-risk patients with
mucormycosis are empirically treated with antifungal therapy
without histopathological and microbiological support. Thus, the
diagnosis of fungal infection not only depends on the empirical
work of the doctor, but is also supported by effective testing
technology in the laboratory (21).
Regarding mucormycosis therapy, amphotericin B and
amphotericin B lipid formulation are the most common antifungal
agents for mucormycosis (5,18). Posaconazole is a novel azole
antifungal for treating mucormycosis (5,24). A
previous study demonstrated that antifungal treatments combined
with surgical debridement are important therapies for mucormycosis
(17,25).
In conclusion, the present study described
disseminated mucormycosis in a patient with MM and secondary MDS.
Mucormycosis can be considered a breakthrough infection in
immunosuppressed patients who are receiving voriconazole therapy.
In addition to early diagnosis, appropriate initiation of
chemotherapy and surgical debridement are important.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Panpan Zhao from
the Pathology Department in the Hangzhou First People's Hospital
for providing the silver stain picture.
Funding
This work was supported by National Natural Science
Foundation of China (grant no. 81601799), and Hangzhou Science and
Technology Commission Social Development Project (grant no.
20180533B31).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article and its supplementary
information files.
Authors' contributions
QC and KC contributed to study design, clinical
information collection, data interpretation, literature search and
manuscript preparation. SXQ, XLH, PFS and KLW contributed to the
collection of clinical information, the literature search and case
analysis. SHW, LHX, MMW and XJW performed to the laboratory
examinations, the results analysis and interpretation. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from the patient.
Patient consent for publication
Informed consent for publication was obtained from
the son of the patient, due to the patient passing away.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Petrikkos G, Skiada A, Lortholary O,
Roilides E, Walsh TJ and Kontoyiannis DP: Epidemiology and clinical
manifestations of mucormycosis. Clin Infect Dis. 54 (Suppl
1):S23–S34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kauffman CA: Zygomycosis: Reemergence of
an old pathogen. Clin Infect Dis. 39:588–590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaezi A, Moazeni M, Rahimi MT, de Hoog S
and Badali H: Mucormycosis in Iran: A systematic review. Mycoses.
59:402–415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeong W, Keighley C, Wolfe R, Lee WL,
Slavin MA, Kong DCM and Chen SC: The epidemiology and clinical
manifestations of mucormycosis: A systematic review and
meta-analysis of case reports. Clin Microbiol Infect. 25:26–34.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kursun E, Turunc T, Demiroglu YZ, Aliskan
HE and Arslan AH: Evaluation of 28 cases of mucormycosis. Mycoses.
58:82–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Serris A, Danion F and Lanternier F:
Disease entities in mucormycosis. J Fungi (Basel). 5(pii): E232019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roden MM, Zaoutis TE, Buchanan WL, Knudsen
TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH,
et al: Epidemiology and outcome of zygomycosis: A review of 929
reported cases. Clin Infect Dis. 41:634–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andrey DO, Kaiser L, Emonet S, Erard V,
Chalandon Y and van Delden C: Cerebral Rhizomucor infection treated
by Posaconazole delayed-release tablets in an allogeneic stem cell
transplant recipient. Int J Infect Dis. 55:24–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xipell M, Losno RA, Garcia-Vidal C, Rovira
M, Alejo-Cancho I, Puig de la Bellacasa J, López M, Cardozo C,
Bodro M, Mensa J and Soriano A: Clinical features and outcome in
patients with mucormycosis in a tertiary hospital (2012–2016). Rev
Iberoam Micol. 35:162–166. 2018.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Posteraro B, Spanu T, Fiori B, De Maio F,
De Carolis E, Giaquinto A, Prete V, De Angelis G, Torelli R,
D'Inzeo T, et al: Antifungal susceptibility profiles of bloodstream
yeast isolates by Sensititre YeastOne over nine years at a large
Italian teaching hospital. Antimicrob Agents Chemother.
59:3944–3955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clinical and Laboratory Standards
Institute (CLSI) M38, . Reference method for broth dilution
antifungal susceptibility testing of filamentous Fungi. 3rd. CLSI;
Wayne, PA: 2017, https://clsi.org/standards/products/microbiology/documents/m38/November
30–2017
|
|
12
|
Kennedy KJ, Daveson K, Slavin MA, van Hal
SJ, Sorrell TC, Lee A, Marriott DJ, Chapman B, Halliday CL,
Hajkowicz K, et al: Mucormycosis in Australia: Contemporary
epidemiology and outcomes. Clin Microbiol Infect. 22:775–781. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Long B and Koyfman A: Mucormycosis: What
emergency physicians need to know? Am J Emerg Med. 33:1823–1825.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ville S, Talarmin JP, Gaultier-Lintia A,
Bouquié R, Sagan C, Le Pape P, Giral M and Morio F: Disseminated
mucormycosis with cerebral involvement owing to rhizopus
microsporus in a kidney recipient treated with combined liposomal
amphotericin B and posaconazole therapy. Exp Clin Transplant.
14:96–99. 2016.PubMed/NCBI
|
|
15
|
Chamilos G, Marom EM, Lewis RE, Lionakis
MS and Kontoyiannis DP: Predictors of pulmonary zygomycosis versus
invasive pulmonary aspergillosis in patients with cancer. Clin
Infect Dis. 41:60–66. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bitar D, Van Cauteren D, Lanternier F,
Dannaoui E, Che D, Dromer F, Desenclos JC and Lortholary O:
Increasing incidence of zygomycosis (mucormycosis), France,
1997–2006. Emerg Infect Dis. 15:1395–1401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cornely OA, Arikan-Akdagli S, Dannaoui E,
Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada
A, Akova M, et al: ESCMID and ECMM joint clinical guidelines for
the diagnosis and management of mucormycosis 2013. Clin Microbiol
Infect. 20 (Suppl 3):S5–S26. 2014. View Article : Google Scholar
|
|
18
|
Lin E, Moua T and Limper AH: Pulmonary
mucormycosis: Clinical features and outcomes. Infection.
45:443–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Liu B and Yan Y: Disseminated
mucormycosis (DM) after pneumonectomy: A case report. BMC Infect
Dis. 16:3372016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walsh TJ, Gamaletsou MN, McGinnis MR,
Hayden RT and Kontoyiannis DP: Early clinical and laboratory
diagnosis of invasive pulmonary, extrapulmonary and disseminated
mucormycosis (zygomycosis). Clin Infect Dis. 54 (Suppl 1):S55–S60.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paul SR and Gable PS: Mucormycosis as the
elusive cause of an aortic thrombus and tissue-obliterating
abscess. Case Rep Hematol. 2019:48421502019.PubMed/NCBI
|
|
22
|
Song Y, Qiao J, Giovanni G, Liu G, Yang H,
Wu J and Chen J: Mucormycosis in renal transplant recipients:
Review of 174 reported cases. BMC Infect Dis. 17:2832017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwen PC, Freifeld AG, Sigler L and
Tarantolo SR: Molecular identification of Rhizomucor pusillus as a
cause of sinus-orbital zygomycosis in a patient with acute
myelogenous leukemia. J Clin Microbiol. 43:5819–5821. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dannaoui E, Meletiadis J, Mouton JW, Meis
JF and Verweij PE; Eurofung Network, : In vitro susceptibilities of
zygomycetes to conventional and new antifungals. J Antimicrob
Chemother. 51:45–52. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Multani A, Reveron-Thornton R, Garvert DW,
Gomez CA, Montoya JG and Lui NS: Cut it out! Thoracic Surgeon's
approach to pulmonary mucormycosis and the role of surgical
resection in survival. Mycoses. 62:893–907. 2019. View Article : Google Scholar : PubMed/NCBI
|