Introduction
Diabetes mellitus (DM) is a metabolic disease
characterized by chronic hyperglycemia due to defects in insulin
secretion and/or action (1). The
global number of patients with DM was ~382 million in 2013, and is
predicted to reach ~592 million by 2035 (2). DM causes abnormal metabolism of sugar,
fat and protein, which can lead to serious complications such as
renal failure, ketoacidosis, peripheral neuropathy, blindness and
cerebral arteritis (3,4). Myocardial injury is a serious
complication of DM that is attracting research interest (5,6).
Myocardial injury induced by DM is primarily caused by inflammatory
reactions and hepatic lipid accumulation (7). Myocardial inflammation is a key
mechanism of the condition. Notably, anti-inflammatory drugs have
protective effects on diabetic myocardial injury (8), and may therefore be an effective
treatment.
Silent information regulator l (SIRT1) is a type of
nicotinamide adenosine dinucleotide dependent deacetylase, which
affects a variety of cellular processes and has an important role
in tissue damage and repair (9).
Recent studies have demonstrated that SIRT1 plays an important role
in inflammation and autoimmunity (10,11).
High mobility group box 1 (HMGB1) is a highly conserved
nucleoprotein, which affects a variety of biological processes,
including inflammatory diseases (12). HMGB1 in extracellular fluid also
serves a role as a damage-associated molecular pattern molecule in
the process of inflammation and cell migration (13). HMGB1 serves a role in the release of
pro-inflammatory cytokines (14).
Under inflammatory conditions, HMGB1 is passively released or
actively secreted into the extracellular environment from the
affected monocytes/macrophages (14), while NF-κB is activated and takes
part in the inflammatory response (15). It has been identified that HMGB1
participates in various pathophysiological signaling pathways
triggered by the diabetic environment; therefore, it is a promising
molecular target for the treatment of DM (16).
Chrysophanol (CHR), an anthraquinone isolated from
rhubarb, belongs to the anthraquinone family, which also contains
emodin, aloe emodin, rhein and physcion (17,18). CHR
has been demonstrated to have multiple beneficial pharmacological
effects, such as anti-inflammatory (19), antitumor (20), antiviral (21) and antiproliferative effects (22). To the best of our knowledge, there
has been no previous research into whether CHR has therapeutic
effects on diabetic myocardial injury in db/db mice.
Therefore, the aim of the present study was to evaluate the ability
of CHR to attenuate diabetic myocardial injury.
Materials and methods
Animal study
A total of 48 male spontaneously diabetic mice
(C57BL/KsJ-db/db mice; age, 6–8 weeks; weight, 35–40
g) and 12 male wild-type C57BLKS/J mice (age, 6–8 weeks; weight,
20–22 g) were obtained from the Model Animal Research Center of
Nanjing University. Mice were maintained under standard laboratory
condition, with free access to food and water and housed prior to
experiments in an animal room under standard conditions (23±2°C;
60±10% humidity; 12 h light/dark cycle). All the experimental
procedures were approved by and performed in accordance with
Nantong Medical University.
Experimental design
Following 1 week of feeding and adaptation, the mice
were divided randomly into five groups (each n=12): i) Wild, in
which wild-type C57BLKS/J mice were orally administered normal
saline; ii) C57BL/KsJ-db/db control group, in which
C57BL/KsJ-db/db mice were orally administered normal
saline; iii) metformin, in which C57BL/KsJ-db/db mice
were intragastrically administered metformin (Sigma-Aldrich; Merck
KGaA; 100 mg/kg/day); iv) CHR, 50 mg/kg, in which
C57BL/KsJ-db/db mice were intragastrically
administered CHR (50 mg/kg/day; 98% purity; National Institutes for
Food and Drug Control); and v) CHR, 100 mg/kg/day, in which
C57BL/KsJ-db/db mice were administered CHR by gavage
at a dose of 100 mg/kg/day. All treatments were provided for 28
consecutive days. The CHR concentrations of 50 and 100 mg/kg/day
were selected for use in the present study according to a
preliminary experiment (data not shown). At the conclusion of the
experiment, the mice were anesthetized with ketamine/xylazine (100
and 10 mg/kg respectively, 0.1 ml/25 g body weight) by
intraperitoneal injection, and then ~0.8-ml blood samples were
collected from the orbital plexus of the eyes. The blood samples
were centrifuged at 3,000 × g for 10 min at 4°C and the serum was
collected. Blood serum was collected for subsequent hematological
or biochemical assays. Death of the mice was verified by the
complete cessation of the heartbeat and breathing, and
disappearance of reflexes. In addition, heart tissue was
immediately removed, rinsed with physiological saline solution and
then stored at −80°C prior to further analysis. Sections of the
heart were fixed in 10% (v/v) neutral buffered formalin for
hematoxylin and eosin (H&E) staining.
Oral glucose tolerance test
(OGTT)
On day 29 day after the initiation of treatment, the
OGTT was performed. Mice were fasted overnight and subsequently
received glucose (2 g/kg) by gavage at 8:00 a.m. Blood glucose
concentrations at different time points (0, 30, 60, 90 and 120 min)
following glucose administration were evaluated using a glucose
analyzer (SureStep®; Lifescan, Inc.).
Biochemical measurements
Creatine kinase (CK), lactate dehydrogenase (LDH),
total triglyceride (TG) and total cholesterol (TC) levels in the
serum were measured using commercially available standard kits
(cat. nos. A032, A020-2, A110-1 and A111-1, respectively; Nanjing
Jiancheng Bioengineering Institute Co., Ltd.) according to the
manufacturer's instructions. The insulin concentration in serum was
determined using an insulin ELISA kit (cat. no. 7544-MR; R&D
Systems, Inc.).
Determination of tumor necrosis factor
(TNF)-α, interleukin (IL)-1β and IL-6 levels in serum and heart
tissues
Levels of cytokines in the serum and heart, namely
IL-6, IL-1β and TNF-α, were analyzed using commercially available
ELISA kits (cat. nos. M6000B, MLB00C and MTA00B, respectively;
R&D Systems, Inc.) in accordance with the manufacturer's
instructions. The optical density (OD) of each well was read at 450
nm, and the concentration of the inflammatory cytokine was
quantified with reference to a standard curve.
Histological examination
Heart tissues were carefully removed and fixed in
10% (v/v) formalin at room temperature for 48 h, then embedded in
paraffin wax. Samples were cut into 4-µm sections and stained with
H&E (Nanjing Jiancheng Bioengineering Institute). Following
dehydration with 80, 90 and 100% ethanol and n-butanol, the
myocardial tissue was waxed in a 60°C wax box and then embedded in
paraffin. Tissue sections (5-µm) were dried at 45°C and obtained
from each paraffin block. The sections were heated at 60°C for 1 h
and dewaxed with xylene. Following hydration, the sections were
stained with 0.5% H&E at room temperature for 5 min, dehydrated
with gradient ethanol, cleared with xylene and mounted with neutral
gum. Optical microscopy (Olympus Corporation) was used to examine
pathological changes of the heart tissue (magnification, ×200).
Immunohistochemistry
The expressions of HMGB1 and phosphorylated
(p)-NF-κB p65 in the heart tissues were evaluated using
immunohistochemistry staining. The heart tissues were embedded in
paraffin and sectioned. Then, the paraffin sections were
deparaffinized in xylene, rehydrated by ethanol and incubated with
3% hydrogen peroxide. Heart tissues samples were blocked at room
temperature with 3% BSA (Beijing Solarbio Science & Technology
Co., Ltd.) and incubated with HMGB1(1:1,00, cat. no. ab79823;
Abcam) and phosphorylated (p)-NF-κB p65 (1:1,00, cat. no. ab86299;
Abcam) at 4°C overnight. Samples were then washed three times with
PBS, treated with horseradish peroxidase goat anti-rabbit IgG
secondary antibody (1:200; cat. no. WLA037a; Wanleibio Co., Ltd.)
for 20 min at 37°C and washed three times with PBS. Samples were
then stained at room temperature for 2 min with 0.05%
3-3′diaminobenzidine (DAB) and a total of 10 fields were randomly
selected from each sample were observed using a microscope
(magnification, ×200, Olympus Corporation).
Western blot analysis
Heart tissue was homogenized, washed with PBS and
lysed in radioimmunoprecipitation assay buffer (Beyotime Institute
of Biotechnology). The protein concentration was determined using
an Enhanced Bicinchoninic Acid Protein Assay kit (Beyotime
Institute of Biotechnology). An equal amount of protein (~50 µg)
was loaded per lane and separated via SDS-PAGE (Mini-Protean
3®; Bio-Rad Laboratories, Inc.) on a 10% gel. Proteins
were then transferred onto a polyvinylidene difluoride membrane
(EMD Millipore; Merck KGaA) and blocked with 5% skim milk at room
temperature for 2 h. Membranes were incubated overnight at 4°C with
primary antibodies against SIRT1 (1:1,000; cat. no. ab110304;
Abcam), HMGB1 (1:1,000; cat. no. ab79823; Abcam), NF-κB p65
(1:1,000; cat. no. ab16502; Abcam), phosphorylated (p)-NF-κB p65
(1:1,000; cat. no. ab86299; Abcam), NF-κB inhibitor-α (IκBα;
1:1,000; cat. no. ab32518; Abcam), p-IκBα (1:1,000; cat. no.
ab24783; Abcam) and GAPDH (1:2,000; cat. no. ab181602; Abcam).
Membranes were then incubated with secondary antibody, horseradish
peroxidase-labeled mouse anti-rabbit IgG (1:5,000; cat. no. 5127,
Cell Signaling Technology, Inc.) or horseradish peroxidase-labeled
rabbit anti-mouse IgG (1:5,000; cat. no. 58802, Cell Signaling
Technology, Inc.), at room temperature for 2 h. Protein bands were
visualized using an enhanced chemoluminescence staining detection
kit (Bio-Rad Laboratories, Inc.) and densitometry was performed
with Bandscan 5.0 software (Glycomix Ltd.). The expression of
target protein was normalized to that of GAPDH.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Differences between multiple groups were evaluated by one-way
analysis of variance with Tukey's multiple comparison test as the
post hoc test using GraphPad Prism software 6.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
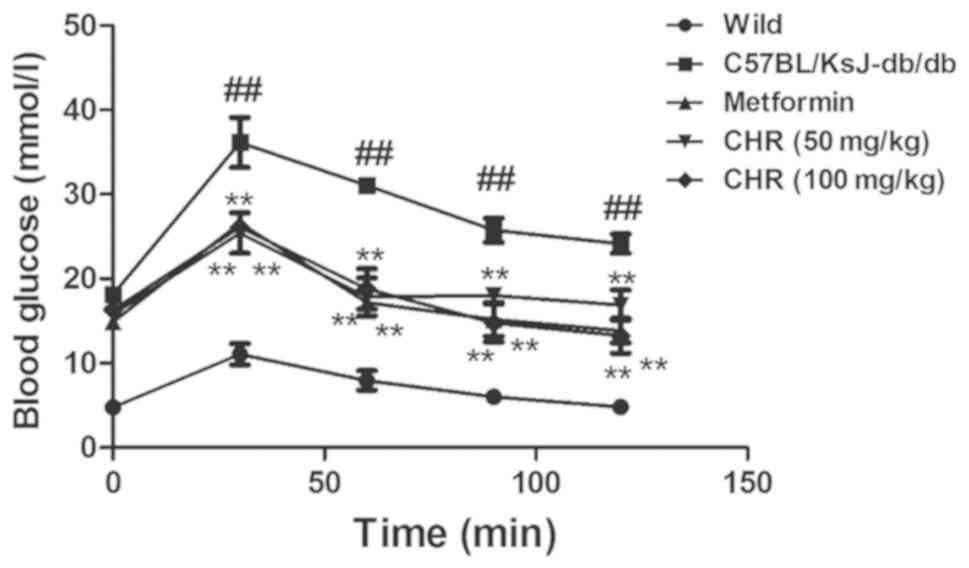
CHR reduces blood glucose levels in a
diabetic mouse model
The C57BL/KsJ-db/db group displayed
significantly higher blood glucose levels compared with the wild
group (Fig. 1). Following treatment
with CHR (50 and 100 mg/kg), the blood glucose levels of the
C57BL/KsJ-db/db mice were significantly lower than
those of the C57BL/KsJ-db/db mice treated with
saline. Metformin (100 mg/kg) similarly decreased the blood glucose
concentration of the C57BL/KsJ-db/db mice.
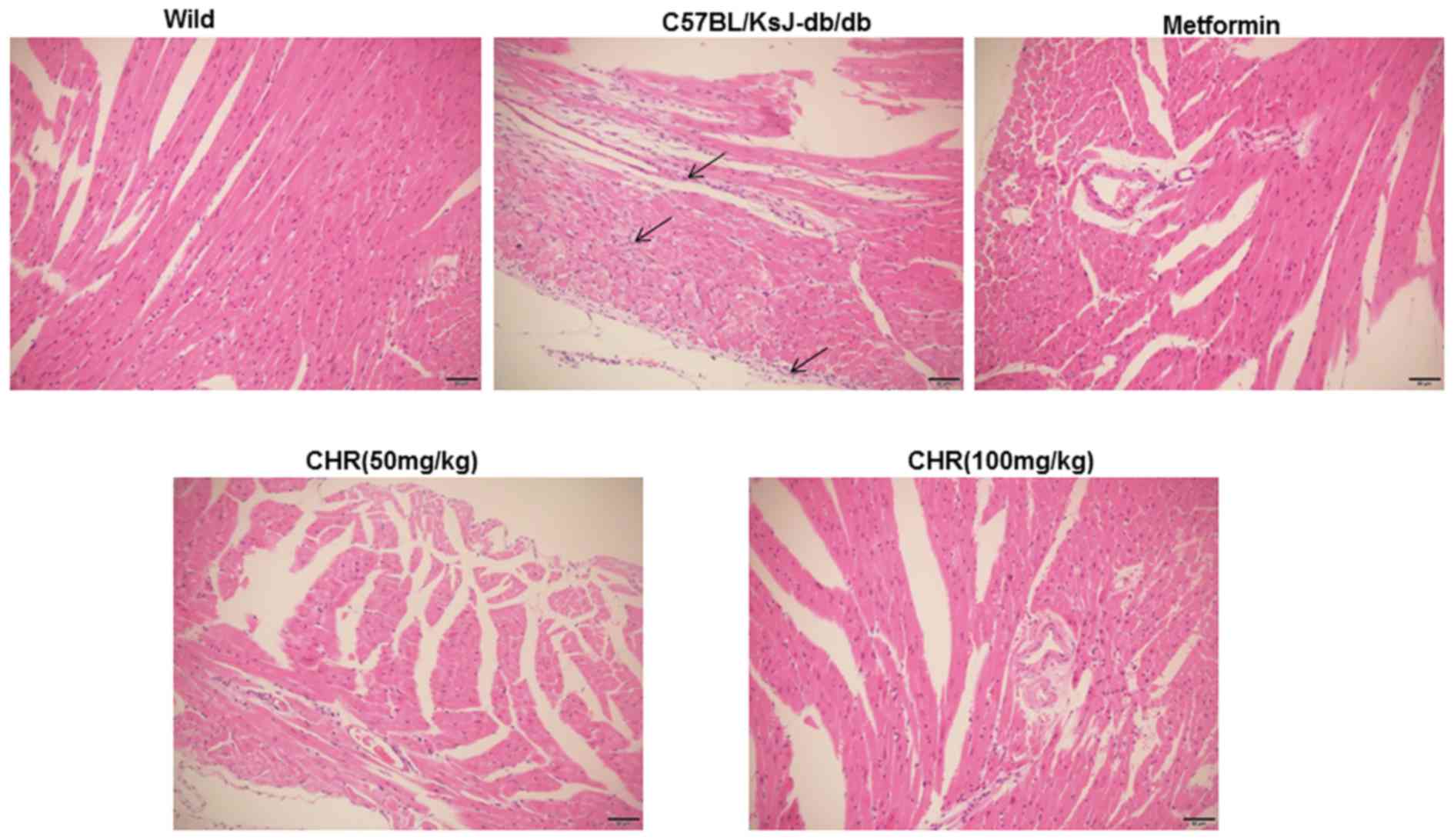
CHR attenuates myocardial pathological
changes in a diabetic mouse model
In the wild group, the myocardial cell membranes
were intact, the myofibril structure was balanced, and adjacent
myofibrils were continuous (Fig. 2).
By contrast, a large number of inflammatory cells were observed in
the hearts of the C57BL/KsJ-db/db group, myocardial
cells were swollen or denatured, myocardial necrosis was evident,
and no striations were visible (Fig.
2). CHR or metformin treatment markedly attenuated the
pathological changes observed in the diabetic mouse model,
especially in the high dose CHR group (Fig. 2).
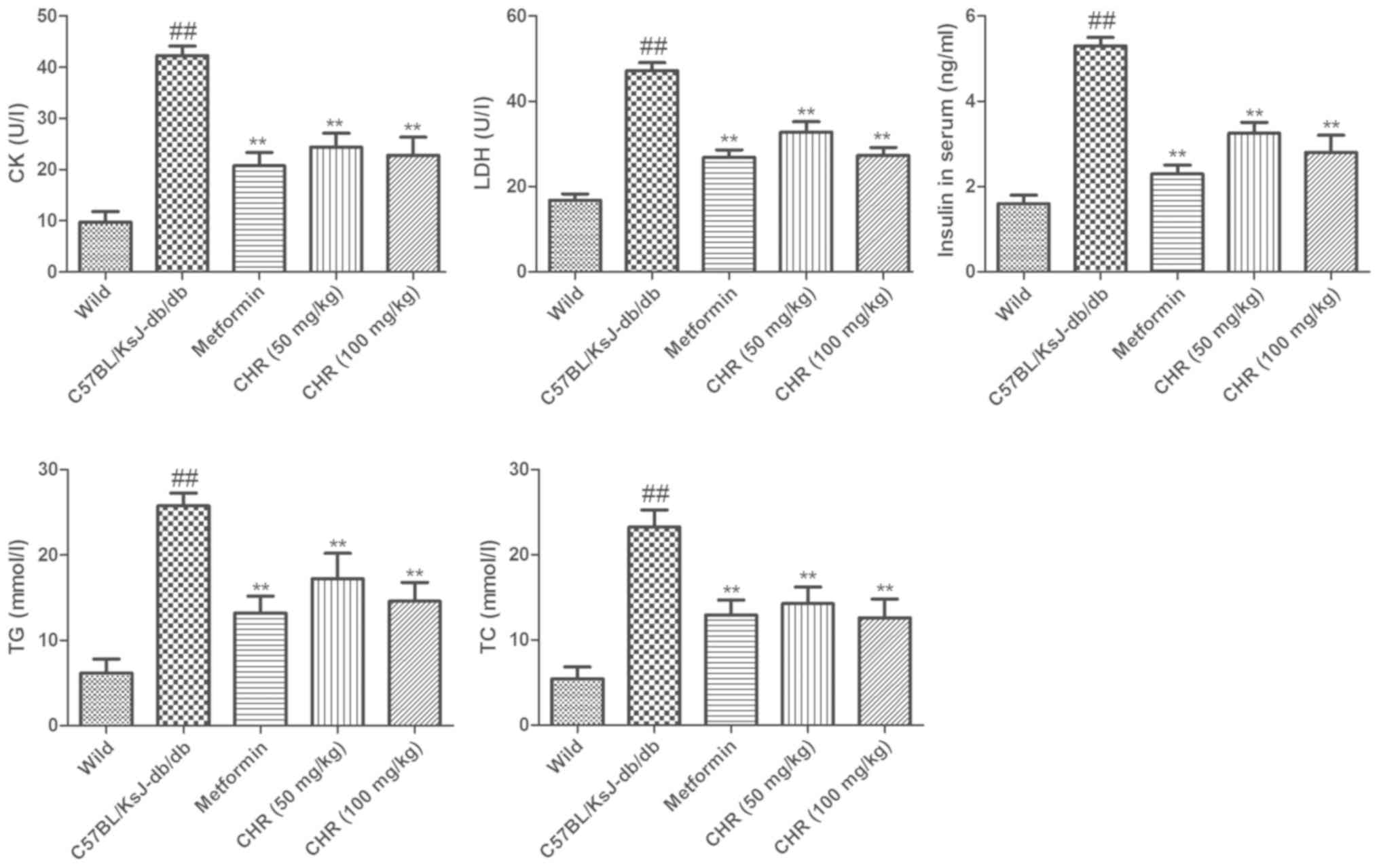
CHR reduces CK, LDH, insulin, TG and
TC levels in serum
The serum TG and TC levels of
C57BL/KsJ-db/db mice were significantly higher
compared with those of the wild group (Fig. 3). Treatment with CHR (50 or 100
mg/kg) or metformin significantly attenuated the DM-induced
increases in TG and TC levels. To explore the influence of CHR on
heart function, the CK and LDH activities and serum insulin levels
of the mice were also measured. In the
C57BL/KsJ-db/db group, the serum insulin, CK and LDH
levels were significantly increased compared with those of the wild
group (Fig. 3). Treatment with CHR
(50 or 100 mg/kg) or metformin significantly attenuated the
DM-induced increases in insulin, CK and LDH levels (Fig. 3).
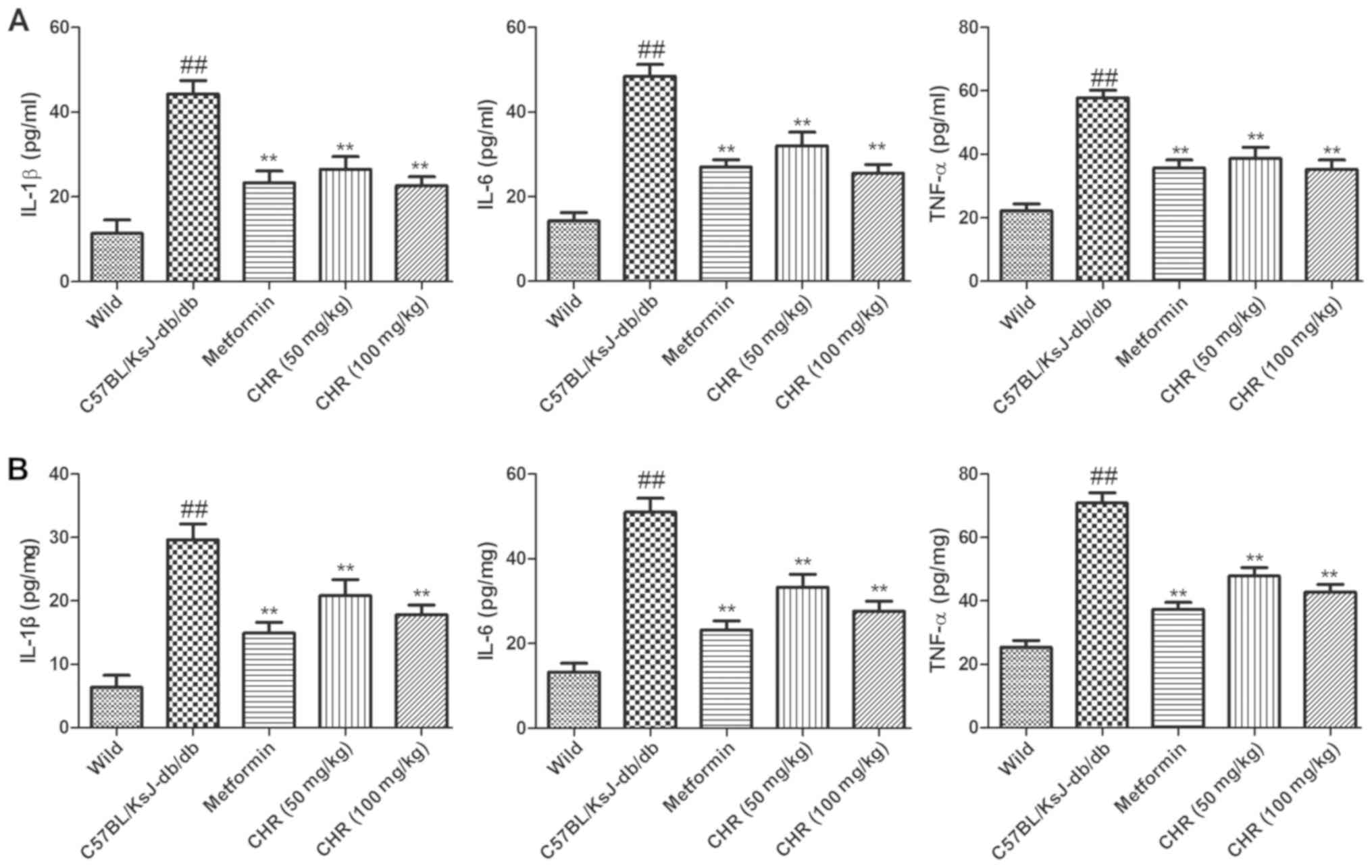
CHR reduces inflammatory cytokine
levels in serum and the heart
In order to explore the influence of CHR on the
inflammatory response, TNF-α, IL-6 and IL-1β levels in the serum
and heart tissues of the mice were determined. The results
demonstrated that inflammatory cytokine levels in the serum and
heart tissues of C57BL/KsJ-db/db mice were
significantly higher compared with those of the wild-type control
mice (Fig. 4). However, treatment
with CHR (50 or 100 mg/kg) or metformin significantly suppressed
the levels of inflammatory cytokines in the serum and heart tissues
of the C57BL/KsJ-db/db mice (Fig. 4).
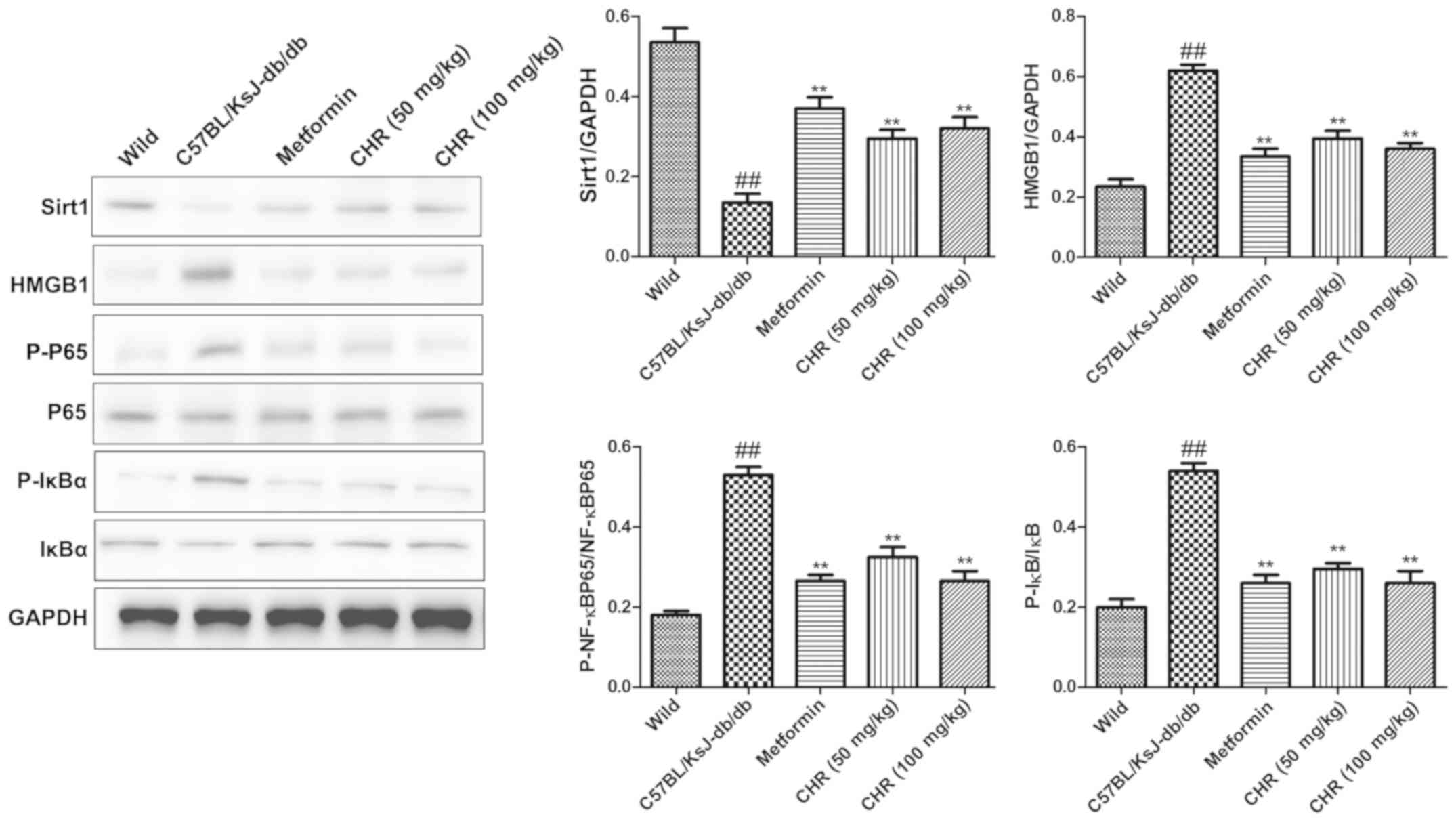
CHR increases SIRT1 expression and
inhibits the HMGB1/NF-κB signaling pathway in a diabetic mouse
model
SIRT1 and the HMGB1/NF-κB signaling pathway
participate in the regulation of inflammation (23,24).
Western blot analysis was used to explore whether SIRT1 and the
HMGB1/NF-κB signaling pathway participate in the effect of CHR on
heart inflammation. HMGB1, p-NF-κB p65 and p-IκB were significantly
upregulated while SIRT1 was downregulated in the hearts of
C57BL/KsJ-db/db mice (Fig.
5). CHR (50 and 100 mg/kg) and metformin (100 mg/kg)
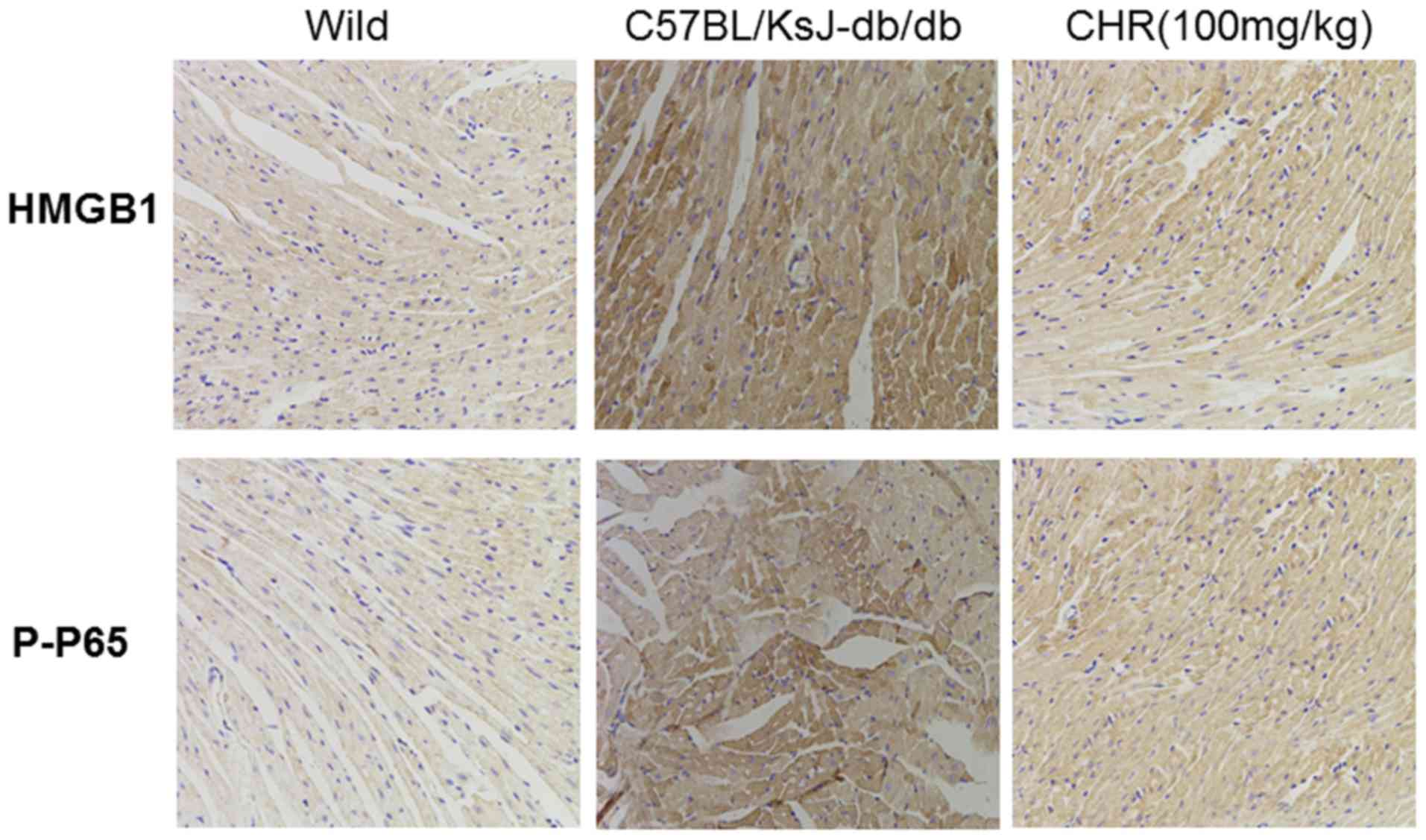
significantly attenuated these changes (Fig. 5). Additionally, immunohistochemistry
demonstrated that the expression of HMGB1 and p-NF-κB in heart
tissue was higher in C57BL/KsJ-db/db mice than in
their wild-type counterparts, and that treatment with 100 mg/kg CHR
markedly decreased HMGB1 and p-NF-κB expression in
C57BL/KsJ-db/db mice (Fig.
6).
Discussion
DM is characterized by hyperglycemia, insulin
deficiency, insulin resistance and pathology in many organs,
including nerves, the liver and glomeruli (25). DM accelerates atherosclerotic
diseases and affects the heart, brain and lower limb arteries
(26). DM and insulin resistance are
important factors in DM-induced heart injury. In the present study,
spontaneously diabetic mice demonstrated abnormal OGTT performance
and lipid profiles, and elevated serum insulin, CK and LDH levels.
The results indicated that CHR and metformin attenuated the heart
injury of the diabetic mice. No significant dose-dependent effects
of CHR treatment were observed when comparing low dose (50 mg/kg)
and high dose (100 mg/kg) groups, which might be due to the 28-day
CHR treatment duration being too short (27). Future study will involve increasing
the duration of observation to 8–20 weeks to better analyze the
dose-dependent effects of CHR. The blood glucose results were
consistent with the observed heart histological changes, which
confirmed the beneficial effect of CHR on heart injury. Therefore,
the present findings suggest that CHR is an effective cardiac
protection agent. In order to elucidate the protective effect of
CHR on diabetic heart injury, the possible molecular mechanism was
investigated. The present study determined that the TNF-α, IL-6 and
IL-1β levels in the serum and heart tissue of
C57BL/KsJ-db/db mice were higher than those in the
wild-type mice, which suggests that inflammation was increased
during DM. Treatment with CHR significantly suppressed the
inflammatory cytokine levels in the serum and heart tissue of the
C57BL/KsJ-db/db mice. Inflammatory cytokines have
important roles in the initiation and development of heart injury
(28), as they affect the
infiltration of white blood cells in the liver and amplify the
damage to the heart (29). IL-6 has
a pathological effect on chronic inflammation, myocardial
infarction and rheumatoid arthritis (30). The present study demonstrated that
CHR has the ability to reduce these cytokine levels, which suggests
that the anti-inflammatory effects of CHR may participate in
protecting the heart.
In order to explore the anti-inflammatory molecular
mechanism of CHR on heart injury, the roles of inflammation-related
SIRT1 and the HMGB1/NF-κB signaling pathway were investigated.
HMGB1 is a key factor during inflammation in aseptic and
infection-associated reactions (31). It has common characteristics with
cytokines, acts on the surface receptors of immune cells, and
induces the expression of inflammatory factors and the further
release of HMGB1, thereby promoting an inflammatory cascade
reaction (32). When heart injury
occurs, HMGB1 is passively released from damaged cells or actively
secreted from activated immune cells. HMGB1 activates NF-κB and
induces the expression of pro-inflammatory genes (33). It has been demonstrated that SIRT1
inhibits the transcriptional activity of NF-κB through
deacetylation of the p65 subunit, thereby reducing the production
and activation of inflammatory cytokines (34). Therefore, CHR might exert beneficial
activities by attenuating inflammatory responses through
downregulation of the HMGB1/NF-κB pathway via the upregulation of
SIRT1. Decreased inflammatory responses promote the activation and
recruitment of inflammatory cells, leading to the initiation of
inflammation (35). In the present
study, western blot analysis demonstrated that HMGB1, p-NF-κB p65
and p-IκB protein levels in the heart tissue of
C57BL/KsJ-db/db mice were significantly increased
compared with those in wild-type mice, and SIRT1 expression levels
in the heart tissue of C57BL/KsJ-db/db mice were
lower compared with those in wild-type mice. CHR intervention
significantly reversed these changes, suggesting that the
anti-inflammatory effect of CHR may occur via regulation of SIRT1
and inhibition of the HMGB1/NF-κB pathway.
In conclusion, the present study successfully
demonstrated the protective effect of CHR against DM-induced heart
damage. The possible mechanisms underlying the protective effect of
CHR may be associated with the attenuation of inflammation of the
heart via regulation of SIRT1 and inhibition of the HMGB1/NF-κB
signaling pathway. The present findings provide preliminary
evidence suggesting the potential of CHR as a therapeutic drug for
DM-induced heart injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PX, JZ, AZ and LL contributed to conception and
design of the study. HC, WT, NZ, ZY and MG contributed to
acquisition, analysis and interpretation of the data and writing
the manuscript. PX, JZ, MG and QW carried out the animal
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All the experimental procedures were approved by and
perfor-med in accordance with Nantong Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mäkimattila S, Virkamäki A, Groop PH,
Cockcroft J, Utriainen T, Fagerudd J and Yki-Järvinen H: Chronic
hyperglycemia impairs endothelial function and insulin sensitivity
via different mechanisms in insulin-dependent diabetes mellitus.
Circulation. 94:1276–1282. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adeyemi DO, Ukwenya VO, Obuotor EM and
Adewole SO: Anti-hepatotoxic activities of Hibiscus sabdariffa L.
in animal model of streptozotocin diabetes-induced liver damage.
BMC Complement Altern Med. 14:2772014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dias AS, Porawski M, Alonso M, Marroni N,
Collado PS and González-Gallego J: Quercetin decreases oxidative
stress, NF-kappaB activation, and iNOS overexpression in liver of
streptozotocin-induced diabetic rats. J Nutr. 135:2299–2304. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matafome P, Nunes E, Louro T, Amaral C,
Crisóstomo J, Rodrigues L, Moedas AR, Monteiro P, Cipriano A and
Seiça R: A role for atorvastatin and insulin combination in
protecting from liver injury in a model of type 2 diabetes with
hyperlipidemia. Naunyn Schmiedebergs Arch Pharmacol. 379:241–251.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wellen KE and Hotamisligil GS:
Inflammation, stress, and diabetes. J Clin Invest. 115:1111–1119.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Finkel T, Deng CX and Mostoslavsky R:
Recent progress in the biology and physiology of sirtuins. Nature.
460:587–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai T, Wen X, Wu D, Su G, Gao Y, Chen C,
Wu W, Lv Y, Chen Z, Lv Q, et al: SIRT1 protects against urban
particulate matter-induced airway inflammation. Int J Chron
Obstruct Pulmon Dis. 14:17412019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JH, Moon JH, Lee YJ and Park SY:
SIRT1, a Class III histone deacetylase, regulates LPS-induced
inflammation in human keratinocytes and mediates the
anti-inflammatory effects of hinokitiol. J Invest Dermatol.
137:1257–1266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harris HE, Andersson U and Pisetsky DS:
HMGB1: A multifunctional alarmin driving autoimmune and
inflammatory disease. Nat Rev Rheumatol. 8:195–202. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang H, Wang H, Chavan SS and Andersson U:
High mobility group box protein 1 (HMGB1): The prototypical
endogenous danger molecule. Mol Med 1(Suppl 21). S6–S12. 2015.
View Article : Google Scholar
|
|
14
|
Kornblit B, Munthe-Fog L, Madsen HO, Strøm
J, Vindeløv L and Garred P: Association of HMGB1 polymorphisms with
outcome in patients with systemic inflammatory response syndrome.
Crit Care. 12:R832008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xueyang D, Zhanqiang M, Chunhua M and Kun
H: Fasudil, an inhibitor of Rho-associated coiled-coil kinase,
improves cognitive impairments induced by smoke exposure.
Oncotarget. 7:78764–78772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Komers R: Rho kinase inhibition in
diabetic kidney disease. Br J Clin Pharmacol. 76:551–559.
2013.PubMed/NCBI
|
|
17
|
Lu CC, Yang JS, Huang AC, Hsia TC, Chou
ST, Kuo CL, Lu HF, Lee TH, Wood WG and Chung JG: Chrysophanol
induces necrosis through the production of ROS and alteration of
ATP levels in J5 human liver cancer cells. Mol Nutr Food Res.
54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SJ, Kim MC, Lee BJ, Park DH, Hong SH
and Um JY: Anti-Inflammatory activity of chrysophanol through the
suppression of NF-kappaB/caspase-1 activation in vitro and in vivo.
Molecules. 15:6436–6451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wen Q, Mei L, Ye S, Liu X, Xu Q, Miao J,
Du S, Chen D, Li C and Li H: Chrysophanol demonstrates
anti-inflammatory properties in LPS-primed RAW 264.7 macrophages
through activating PPAR-γ. Int Immunopharmacol. 56:90–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shiezadeh F, Mousavi SH, Amiri MS,
Iranshahi M, Tayarani-Najaran Z and Karimi G: Cytotoxic and
apoptotic potential of rheum turkestanicum janisch root extract on
human cancer and normal cells. Iran J Pharm Res. 12:811–819.
2013.PubMed/NCBI
|
|
21
|
Chang SJ, Huang SH, Lin YJ, Tsou YY and
Lin CW: Antiviral activity of Rheum palmatum methanol extract and
chrysophanol against Japanese encephalitis virus. Arch Pharm Res.
37:1117–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhi-Yun LI, Ming Z, Wei JI, et al:
Protective effect of chrysophanol on hypoxic injury of rat adrenal
medulla pheochromocytoma cells. Chin J Cerebrovascular Dis.
9:418–427. 2012.(In Chinese).
|
|
23
|
Zhang J, Yang S, Chen F, Li H and Chen B:
Ginkgetin aglycone ameliorates LPS-induced acute kidney injury by
activating SIRT1 via inhibiting the NF-κB signaling pathway. Cell
Biosci. 7:442017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi Z, Zhang Y, Qi S, Ling L, Gui L, Yan L,
Lv J and Li Q: Salidroside inhibits HMGB1 acetylation and release
through upregulation of SirT1 during inflammation. Oxid Med Cell
Longev. 2017:98215432017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brownlee M: Biochemistry and molecular
cell biology of diabetic complications. Nature. 414:813–820. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gerrity RG, Natarajan R, Nadler JL and
Kimsey T: Diabetes-induced accelerated atherosclerosis in swine.
Diabetes. 50:1654–1665. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He Q, Pu J, Yuan A, Yao T, Ying X, Zhao Y,
Xu L, Tong H and He B: Liver X receptor agonist treatment
attenuates cardiac dysfunction in type 2 diabetic db/db mice.
Cardiovasc Diabetol. 13:1492014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bujak M and Frangogiannis NG: The role of
IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp
(Warsz). 57:165–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohsuzu F: The roles of cytokines,
inflammation and immunity in vascular diseases. J Atheroscler
Thromb. 11:313–321. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6:a0162952014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang H and Tracey KJ: Targeting HMGB1 in
inflammation. Biochim Biophys Acta. 1799:149–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Andersson U and Tracey KJ: HMGB1 Is a
therapeutic target for sterile inflammation and infection. Annu Rev
Immunol. 29:139–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kauppinen A, Suuronen T, Ojala J,
Kaarniranta K and Salminen A: Antagonistic crosstalk between NF-kB
and SIRT1 in the regulation of inflammation and metabolic
disorders. Cell Signal. 25:1939–1948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Salem ML, Hossain MS and Nomoto K:
Mediation of the immunomodulatory effect of beta-estradiol on
inflammatory responses by inhibition of recruitment and activation
of inflammatory cells and their gene expression of TNF-alpha and
IFN-gamma. Int Arch Allergy Immunol. 121:235–245. 2000. View Article : Google Scholar : PubMed/NCBI
|