Introduction
Behçet's disease (BD) is a chronic and
multi-systemic autoimmune disorder of unknown pathogenesis that is
associated with relapsing oral and genital ulcerations, ocular
manifestations and additional clinical manifestations in multiple
organ systems, including the skin, joints, central nervous system,
gastrointestinal tract and lungs. BD mainly occurs in young adults
between 20 and 40 years of age and is more common in males
(1). Vascular involvement with
underlying pathologic processes of vasculitis and perivascular
inflammatory infiltrates develops in 20–40% of patients with BD and
is considered the most serious complication, with a mortality rate
of 60% in patients with aortic aneurysm formation (2–4).
Arterial complications are reported to develop within 3.5–25 years
after the diagnosis of BD (5); thus,
early detection of vascular abnormalities is imperative to prolong
the survival of BD patients with vascular complications. CT has an
important role in diagnosing and assessing complications involving
different organs and systems. The present study provided a summary
of notable cardiovascular complications of BD focusing on the
characteristic imaging manifestations that should be noted by
clinicians and radiologists.
Materials and methods
Case selection
From January 2016 to May 2018, 361 patients were
diagnosed with BD at the Huadong Hospital affiliated to Fudan
University (Shanghai, China) based on diagnostic criteria suggested
by the International Study Group for BD (6). Due to lack of characteristic laboratory
and histopathologic findings, BD is diagnosed based on clinical
symptoms. The diagnostic criteria for BD include relapsing oral
ulceration plus 2 of the following: Recurrent genital ulceration,
ocular involvement, skin lesions and a positive pathergy test.
Patients with cancer, rheumatoid arthritis, diabetes and syphilis
were excluded. The patients underwent chest and abdominal
contrast-enhanced CT examination (100%), upper or lower extremity
vascular ultrasound (27.1%), MR angiography (23.5%) and cardiac
color Doppler ultrasound (100%). Among them, 45 patients (12.4%)
had confirmed vascular involvement as determined by CT angiography
(21, 91.3%), lower extremity vascular ultrasound (2, 8.7%) and MR
angiography (1, 4.3%). The patients' clinical background,
laboratory results, CT images and response to therapy (range of
follow-up time, 3–42 months) were assessed and analyzed. The
outcome endpoints during follow-up were death due to aneurysmal
rupture, severe hemoptysis and recurrence after surgery, including
post-operative perivalvular leakage, progression of aneurysm and
restenosis of the in-stent coronary artery. This study was
conducted retrospectively at one center, where the local ethical
institutional review board of Huadong Hospital (Shanghai, China)
waived the need for informed consent for this study.
Radiological examination
CT angiography was performed on the 45 patients with
a 64-slice multi-slice (MS)CT scanner (Sensation 64; Siemens
Medical Solutions). The scanning parameters were as follows:
Section thickness, 2.5 mm; tube voltage, 120 kVp; and tube current,
300 mA. A dual-source power injector (CT motion; Ulrich Medical)
was used for all examinations. After placing an 18-G intravenous
catheter through the right antecubital vein for all patients,
80–100 ml of the contrast medium iopromide (370 mg iodine/ml;
Bracco) was injected at a flow rate of 4.5–5.5 ml/sec based on the
body mass index and vein condition, followed by a 30- or 40-ml
saline chaser at the same flow rate. A region of interest was
placed in the ascending thoracic aorta and image acquisition was
automatically initiated once a selected threshold (120 Hounsfield
units) had been reached with bolus tracking. The venous phase was
obtained with a delay of between 90 and 120 sec after injection.
The patients' clinical background, laboratory results, CT images
and response to therapy were assessed and analyzed.
Imaging analysis
Images from all of the 45 patients were analyzed by
two radiologists (LQ and DBM) each with 7 years of diagnostic
experience using CT angiography, and their decisions were reached
by consensus. The location and number of aneurysms and other types
of lesions, including wall thickening without aneurysm formation
and thrombosis of the aorta, were measured and recorded for all
cases. The following characteristics of vascular aneurysms were
analyzed: Maximum diameter, length, wall thickness, border (clear
or unclear), luminal changes (dilated or normal), mural thrombus
(present or absent), cystic changes of the vessel wall (present or
absent), asymmetric bulging of the right part of the aortic wall
(RP-type) or uniform dilation, and calcific plaques (slight,
obvious or not significant).
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM Corp.) and GraphPad Prism (version 8.0; Graph-Pad
Software, Inc.). Values were expressed as either the mean ±
standard deviation or median with interquartile range after testing
the normality of variables using Shapiro-Wilk test. Subsequently,
data were compared between different groups by using Pearson's
chi-squared test for categorical variables, independent-samples
t-test for continuous variables with a normal distribution, and a
Mann-Whitney U-test for continuous variables with a skewed
distribution. Kaplan-Meier survival curves and log-rank test were
used for the survival outcomes of groups with and without aortic
and larger arterial aneurysms. Bivariate correlation tests were
performed to determine the dependence of the MSCT features of the
aneurysms and the ESR. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical presentation
The 45 BD patients with vascular lesions included 37
males and 8 females (Table I). The
median age was 40 years (total range, 25–73 years), The median
course of BD was 8 years (total range, 1–40 years), including
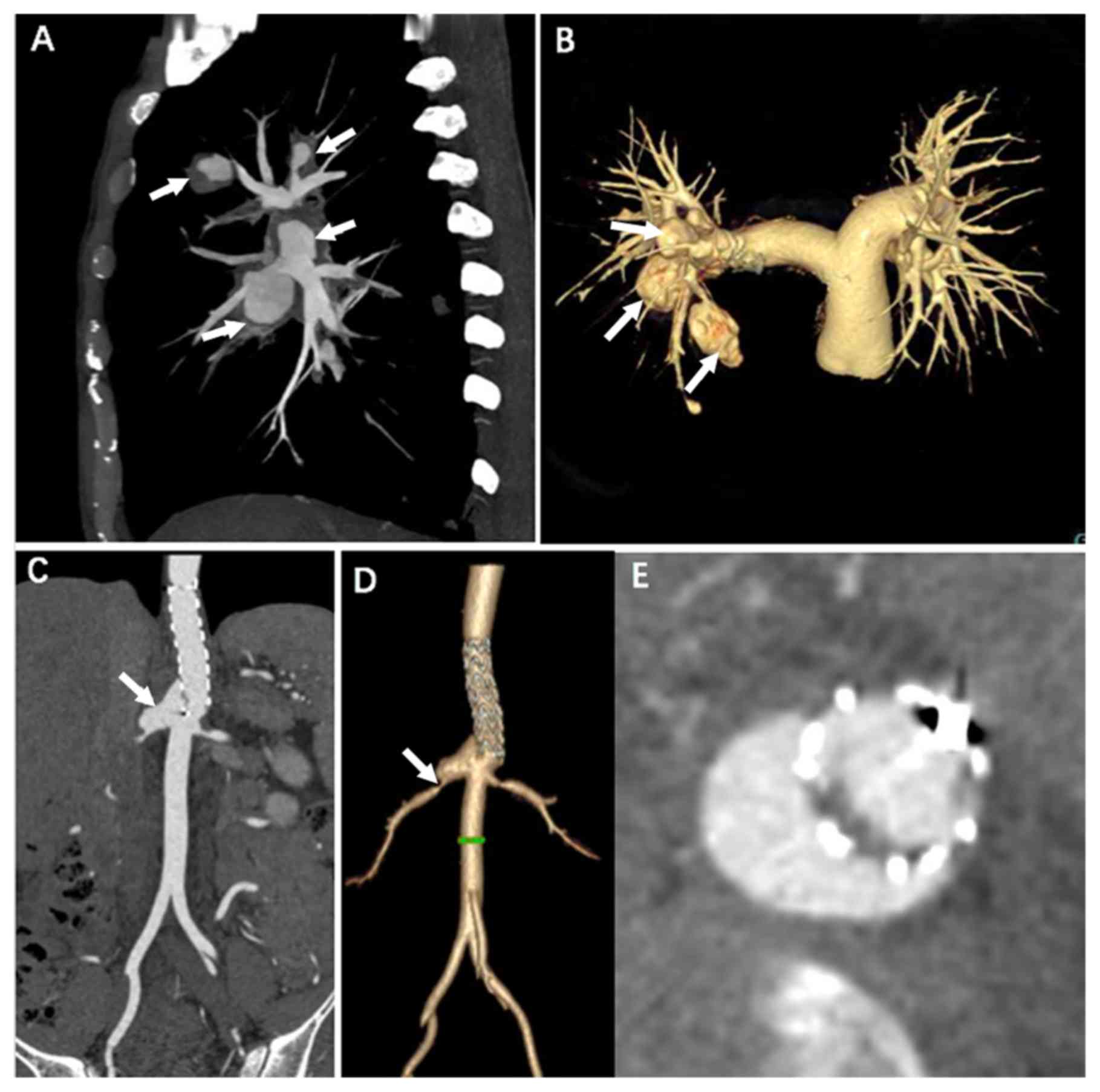
multiple pulmonary aneurysms found in a 30-year-old patient without
previous history of BD (Fig. 1A and
B). The median time to detect vascular complications after
onset of BD was 3 years (total range, 0–40 years).
Vasculitis-associated symptoms (Table
SI) included chest tightness and shortness of breath (22,
48.9%), abdominal pain (14, 31.1%), backache (4, 8.9%), left lower
extremity pain (8, 17.8%) and hoarseness (3, 6.7%). Certain
patients did not have any symptoms associated with vasculitis (10,
22.2%). One patient had a recurrent fever with cough, sputum and
relapsing hemoptysis. Among the 45 patients, 44 (97.8%) had an
elevated erythrocyte sedimentation rate (ESR) during
hospitalization, 22 patients (48.9%) had elevated C-reactive
protein (CRP) levels, white blood cell counts and D-dimer levels,
16 patients (35.6%) had elevated neutrophil counts, and 14 (31.1%)
and 6 patients (13.3%) had decreased hemoglobin levels and platelet
counts, respectively.
 | Table I.Clinical characteristics and
laboratory parameters of the patients by gender. |
Table I.
Clinical characteristics and
laboratory parameters of the patients by gender.
| Item | Total | Males | Females | Healthy range | P-value |
|---|
| Patients | 45 | 37
(82.2) | 8
(17.8) | – | 0.012 |
| Age (years) | 40
(25–73) | 40
(25–73) | 40
(30–70) | – | 0.037 |
| Onset age of BD
(years) | 11.7±11.4 | 30.7±9.0 |
28.5±10.3 | – | 0.085 |
| Detection time of
vascular complications after BD (years) | 3
(0–40) | 3
(0–40) | 4.5 (3–37) | – | 0.113 |
| Duration of BD
(years) | 8
(1–40) | 6.0 (1–40) |
22.3±19.2 | – | 0.223 |
| Clinical
presentation |
|
|
| – |
|
| Oral
ulcerations | 45 (100) | 37 (100) | 8
(100) | – | 0.408 |
| Genital
ulcerations | 31
(66.0) | 25
(67.6) | 6 (75) | – | 0.681 |
| Skin
lesions | 29
(64.4) | 25
(67.6) | 4 (50) | – | 0.347 |
|
Arthritis | 4
(8.9) | 2
(5.4) | 2 (10) | – | 0.077 |
| Ocular
disorders | 9 (20) | 5
(13.5) | 4 (50) | – | 0.019 |
|
Fever | 1
(2.2) | 1
(2.7) | n.d. | – | n.d. |
| Digestive
ulceration | 7
(15.6) | 3
(8.1) | 4 (50) | – | 0.003 |
| Heart
disorders | 8
(17.8) | 8
(21.6) | 0 | – | 0 |
| Laboratory
examination |
|
|
| – |
|
| ESR
(mm/h) | 15 (4–89) | 7
(4–89) | 16.0 (5.0–60) | 0-15/0-20 | 0.827 |
| CRP
(mg/l) | 8.8 (1.2–117) | 10.3 (1.2–117) | 3.75
(4.8–8.3) | <10 | 0.001 |
|
Leukocytes
(109/l) | 8.5
(4.6–12.4) | 10.80
(4.70–12.4) |
6.5±1.4 | 4-10 | 0.088 |
|
Neutrophils (%) | 58.1
(40.2–82.6) | 58.8
(47.3–82.6) |
51.6±9.4 | 50-70 | 0.011 |
|
Hemoglobin (g/l) | 135
(24.6–156) | 135
(24.6–156.0) |
134.8±10.4 |
110-160 | 0.651 |
| Thrombocytes
(109/l) | 176
(106.0–393.0) | 172.0
(106.0–393.0) | 184.3±6.6 | 100-300 | 0.161 |
| D-Dimer
elevation | 22 (48.9) | 20 (54.1) | 2 (5.3) | <0.2 | 0.136 |
BD at other sites
Except for vascular lesions, manifestations of BD in
other organs, including relapsing oral ulceration (100%), recurrent
genital ulceration (n=31, 66%), skin lesions including
epifolliculitis and erythema (n=29, 64.4%), ocular (n=9, 20%) and
joint (n=4, 8.9%) involvement, digestive ulceration (n=7, 15.6%)
and heart abnormalities (right ventricular thrombosis and
endocarditis; n=8, 17.8%) are listed in Table II. One patient had central nervous
system involvement with symptoms including a sudden inability to
speak, limb weakness and a right pupil unresponsive to light. A
brain MR examination indicated multiple abnormal signals in the
right frontal lobe, temporal lobe, bilateral occipital cortex and
brainstem.
 | Table II.Comparison of CT manifestations and
laboratory data between groups of aortic and larger arterial
aneurysms. |
Table II.
Comparison of CT manifestations and
laboratory data between groups of aortic and larger arterial
aneurysms.
| Item | Total (n=42) | Aorta (n=28) | Larger arteries
(n=14) | P-value |
|---|
| Diameter (mm) | 43.0 (13–118) |
46.6±13.1 | 31 (13–67) | 0.208 |
| Length (mm) | 43.0 (7–130) | 44.5
(32–130) | 26.0 (7–56.0) | 0.001 |
| Wall thickness
(mm) | 4.0 (3–14) | 4
(3–14) | 3 (1–4) | <0.001 |
| Number of organic
disorders | 4 (3–4) | 4
(3–4) | 4 (3–4) | 0.683 |
| RP type | 20 (47.6) | 18
(64.3) | 2 (14.2) | 0.003 |
| Unclear border | 28 (66.7) | 22
(78.6) | 6 (42.9) | 0.036 |
| Mural thrombus | 14 (33.3) | 4
(14.3) | 10 (71.4) | <0.001 |
| Calcific
plaques | 8 (19.0) | 4
(14.3) | 4 (28.6) | 0.357 |
| ESR (mm/h) | 18.0 (4.0–89) | 16.5 (5–89) | 20 (4–75) | 0.228 |
| CRP (mg/l) | 8.8 (1.2–117) | 9.5
(1.2–113.4) | 8.4 (3.7–41.5) | 0.155 |
| Leukocytes
(109/l) | 10.8 (4–17.3) | 10.2±4.6 | 7.1 (4.3–11.5) | 0.080 |
Significant differences were observed between
genders regarding age, ocular disorders, blood CRP and neutrophil
levels (P<0.05), as well as digestive ulceration (P<0.01). No
sex difference was observed regarding the onset age of BD and the
onset of vascular complications, incidence rate of organic
disorders other than those of the eyes and digestive tract, or the
leukocyte count.
Vascular aneurysms
A total of 42 aneurysms were identified in 38
patients. Comparison of CT features and laboratory data between two
groups with either aortic or larger artery aneurysms is provided in
Table II. A total of 14 patients
(14/45, 31.1%) had multiple vascular lesions, and 10 patients had
aneurysm and thrombosis (22.2%). A total of 28 aneurysms were
located in the aorta (28/42, 66.7%) including 20 lesions in the
thoracic aorta (20/28, 71.4%) and 8 in the abdominal aorta (8/28
cases, 28.6%). Among the patients with abdominal aortic aneurysms,
two were found with recurrent saccular pseudoaneurysm by CT
examination (Fig. 1C and D).
Furthermore, 14 aneurysms were located in large arteries (14/42,
33.3%), including the right brachiocephalic trunk (2/14, 14.3%),
right internal thoracic artery (1/14, 7.1%), right carotid artery
(2/14, 14.3%), left femoral artery (2/14, 14.3%), iliac arteries
(5/14, 35.7%) and right pulmonary artery (2/14, 14.3%). Of the 20
aneurysms in the thoracic aorta, 18 (90%) were located in the
aortic sinus, ascending aorta and aortic arch, and 2 (10%) were
located in the descending aorta. Of the 8 aneurysms in the
abdominal aorta, 6 lesions were located in the suprarenal abdominal
aorta and 2 were located in the infrarenal abdominal aorta. Of all
42 aneurysms, 14 lesions (33.3%) were located below the diaphragm
and 28 lesions (66.7%) were located above the diaphragm.
The diameter of the 42 aneurysms ranged from 13 to
118 mm (mean, 43 mm), and the length ranged from 7 to 130 mm (mean,
43 mm). The affected aortic or arterial walls were thickened in 34
aneurysms (81%), with an average maximum thickness of 4.0 mm
(range, 3–14 mm). Most aneurysmal walls (30/34, 88%) were
homogeneously enhanced during the late phase of contrast-enhanced
CT, while 4 of them (4/34, 11.8%) had irregular cystic changes in
the thickened walls. A total of 28 of the 42 aneurysms (66.7%) had
unclear borders and 14 (33.3%) had a mural thrombus (Table II). In 10 aneurysms (10/42, 23.8%),
atherosclerotic changes were present in the affected segments.
Furthermore, 20 aneurysms (20/42, 47.6%) had
asymmetric bulging of the right part of the aortic wall (RP-type).
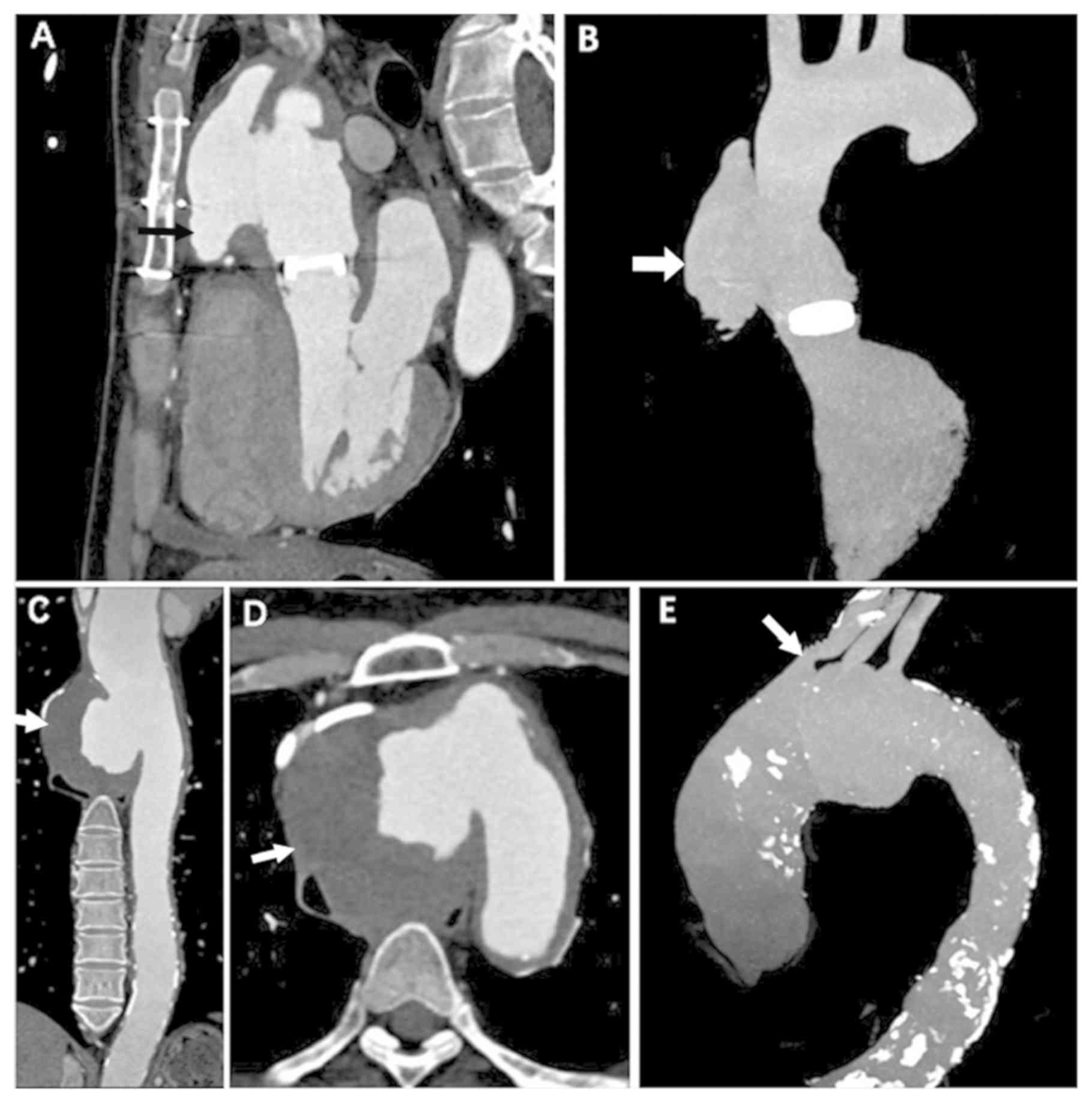
A large saccular pseudoaneurysm of RP-type was found in a patient
with 13 years of BD history (Fig. 2A and
B), whereas an asymmetric bulging of the aortic wall with mural
thrombus formation was found another patient (Fig. 2C-E). A total of 14 aneurysms were
located in the thoracic aorta (14/20, 70%), 5 were located in the
abdominal aorta (5/20, 25%) and 1 was located in the left iliac
artery (10%). All of the 14 thoracic aortic RP-type aneurysms were
located in the aortic sinus, ascending aorta and aortic arch. One
aneurysm located in the left femoral artery manifested as
asymmetric bulging of the left part of the arterial wall. In the
remaining 20 aneurysms, the aortic or arterial walls were
circumferentially enlarged.
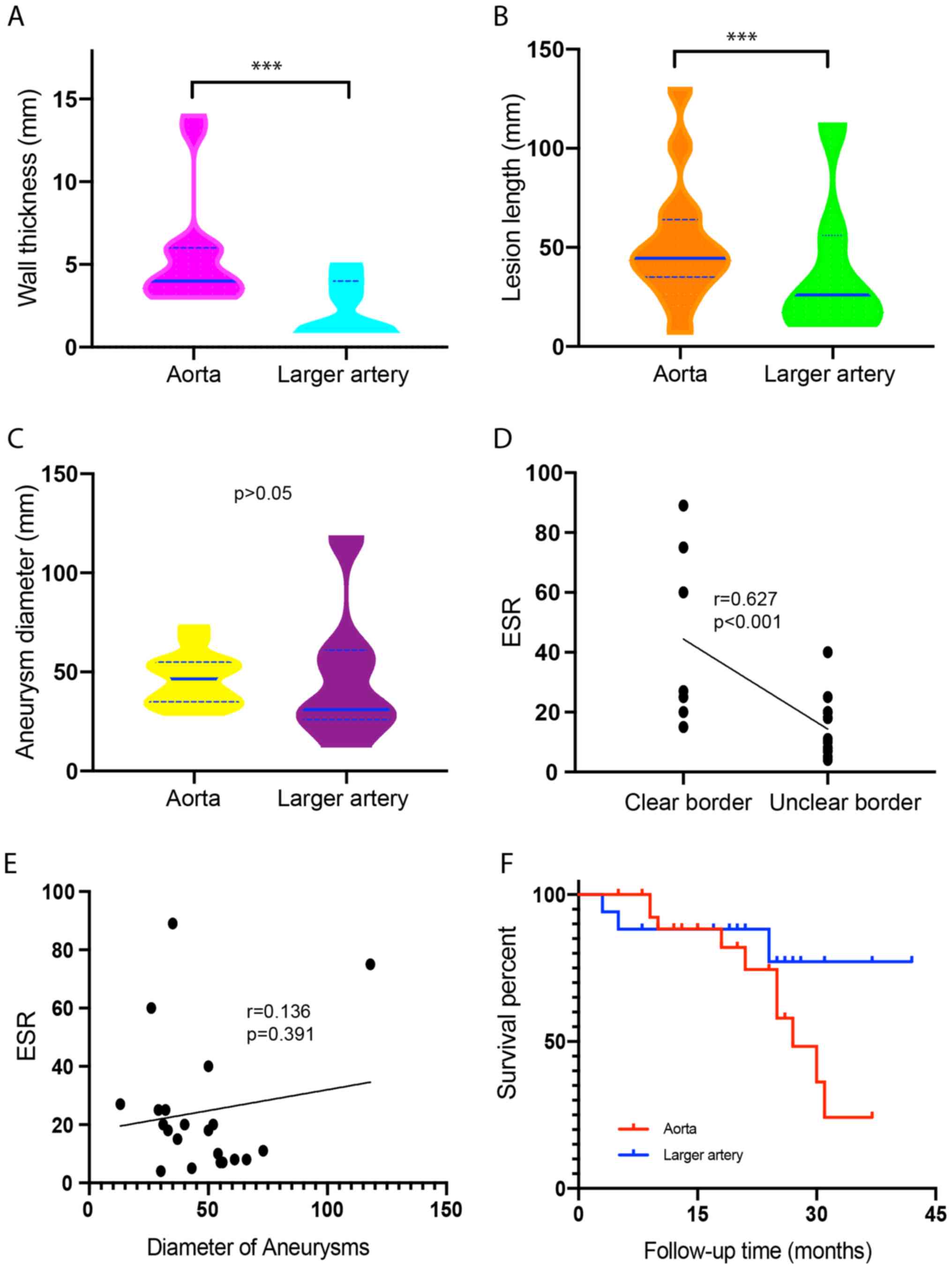
Comparison between groups classified by aortic and
larger arterial aneurysms (Table
II) revealed that aneurysms occurring in the aorta were more
likely to form a mural thrombus (P<0.001), have a thicker wall
(P<0.001, Fig. 3A) and unclear
borders (P=0.036), to be of the RP type (P=0.003) and have a longer
extension (P=0.001, Fig. 3B) than
those in larger arteries. No significant differences were observed
in aneurysmal diameter (Fig. 3C),
number of organic disorders, the presence of calcific plaques, as
well as the ESR, CRP and leukocytes.
Bivariate correlation tests were performed to
determine the dependence of the MSCT features of the aneurysms and
the ESR. A correlation was identified between the borders of the
aneurysmal wall and the ESR (correlation coefficient, 0.627;
P<0.001, Fig. 3D). However, the
correlations between the blood ESR levels and the diameter
(Fig. 3E), length, thickness,
presence of calcific plaque and presence of mural thrombus of the
aneurysmal walls were not significant.
Other types of vascular lesion
Of the 45 patients, 18 (40%) had arterial
thromboembolism. The majority of arteriovenous thromboemboli
occurred in larger arteries, including the bilateral pulmonary
arteries (8/18, 44.5%), bilateral renal arteries (2/14, 14.3%),
superior mesenteric artery (2/14, 14.3%), right subclavian artery
(1, 7.1%), subclavian artery (3/14, 21.4%) and coronary arteries
(2/14, 14.3%). A total of 7 of the 45 patients had unilateral
thrombosis of lower extremity veins (15.6%); 2 had inferior vena
cava thrombosis (4.5%) and 2 had pulmonary artery thrombus (4.5%).
All of those patients with vein thrombosis had severe stenosis
(5/11, 45.5%) and occlusion (6/11, 54.5%; Table SI).
Response to treatment and
follow-up
Of the 45 patients, 42 were treated with prednisone,
cyclophosphamide and monoclonal anti-tumor necrosis factor (TNF)
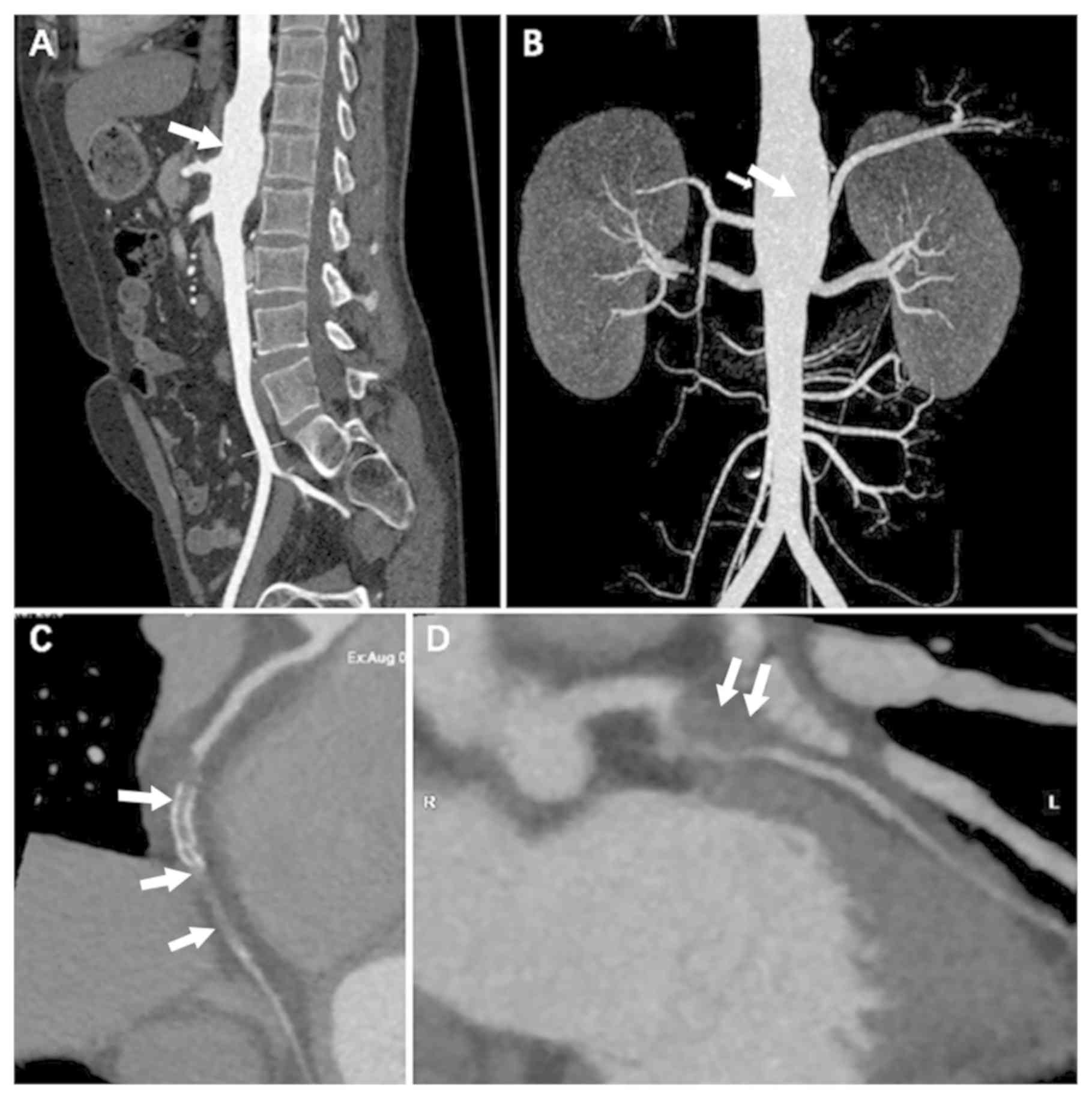
antibodies. Fig. 4A and B revealed
an aneurysm located in the abdominal aorta without a thickened wall
or mural thrombus formation, where no significant changes in the
aneurysm were observed by ultrasound in the 6-month follow-up after
medical treatment. Two patients refused medical treatment and one
died after 9 months due to thoracic aortic rupture. A total of 9
patients had aneurysm shrinkage after medical therapy as determined
by ultrasound. However, due to a lack of CT examinations during
follow-up, changes in the vascular walls of those patients were not
determined. In one case, diffuse occlusion of the stent and the
adjacent lumen of the right coronary artery, which exhibited
thickened vascular walls and unclear boundaries, were discovered by
coronary CT angiography at a 20-month follow-up after coronary
stent implantation (Fig. 4C and D).
A total of 21 patients underwent surgical treatments, including
endovascular graft exclusion (n=4), Bentall surgery (n=4), aortic
valve replacement (n=5), inferior vena cava filter placement (n=3)
and aneurysmectomy (n=5).
The follow-up duration ranged from 3 to 42 months
with a median follow-up period of 18 months. Kaplan-Meier survival
curves for the survival outcomes of the groups with and without
aortic and larger arterial aneurysms are provided in Fig. 3F. The outcome endpoints were death
due to aneurysmal rupture (n=2), massive hemoptysis (>500 ml/d,
n=1), recurrence after surgery including post-operative
perivalvular leakage (n=4), progression of the aneurysm (n=4) and
restenosis of the in-stent coronary artery (n=2). The two patients
who died of aneurysmal rupture displayed irregular cystic changes
in the aortic wall on reconstructed enhanced CT imaging. No overall
significant difference was observed between the two survival curves
(log-rank test, P=0.170), but there was significant difference
between the curves after 28 months of survival (P<0.01).
Discussion
The most significant complications of BD are
arterial involvement and aneurysm formation, which account for 12%
of the vascular complications (7).
The mortality rate associated with an untreated arterial aneurysm
is reported to be up to 60% and arterial manifestations are
reported to manifest 3.5–25 years after a diagnosis of BD (8). An initial precise diagnosis of vascular
involvement due to BD is difficult, particularly in the absence of
classical clinical manifestations, as it is a rare
complication.
The present study provided several predictors for
diagnosing and assessing the severity of vascular involvement
associated with BD based on the results obtained; most patients
with BD and vascular involvement were younger males, which is
consistent with the results of other studies (6,9,10). Serologically, an elevated ESR is a
known predictive parameter associated with the inflammatory
activity of aneurysms. In the present study, an unclear border of
the aneurysmal wall was the only radiologic predictor associated
with an elevated ESR. Vascular involvement associated with BD
mainly presents as aneurysms and thrombi. In the present cohort, BD
aneurysms had numerous characteristic features. The majority of BD
aneurysms were located in the aorta and above the diaphragm. They
were prone to having a large diameter and a homogeneously enhanced
thickened wall. Thoracic aortic lesions (in the aortic sinus,
ascending aorta and aortic arch) were commonly eccentric aneurysms
with bulging of the right wall and were likely to be associated
with mural thrombi. It may be speculated that the radiological
features of vascular involvement, including inflammatory aneurysms
and thrombi, are crucial features of a family of disorders
associated with BD and may help to establish a clinical diagnosis.
Pathologically, destruction of the medial layer, arterial
dilatation and fibrosis are signs of active vasculitis in BD and
are associated with perivascular infiltration of neutrophils,
lymphocytes and plasma cells in the medial layer and adventitia,
which induce fragmentation and splitting of the elastic fibers of
this layer (11–13).
An accurate diagnosis of BD vasculitis is usually
difficult to make only based on the initial clinical
manifestations, and aneurysms are usually detected during the
chronic stages of BD. In the present study, only 1 patient had
massive hemoptysis and pulmonary aneurysms as initial
manifestations. Pulmonary angiography revealed multiple saccular
dilated pulmonary aneurysms arising from the right pulmonary artery
with in situ thrombosis formation. Since BD-associated
pulmonary artery occlusion is induced by in situ thrombosis,
which differs from the pathogenesis of classical pulmonary
thromboembolic disorders, ‘pulmonary artery thrombosis’ should be
used for diagnosis instead of ‘pulmonary emboli’, and CT
angiography is the best radiological tool to assess pulmonary
involvement in BD (14). Cho et
al (9) reported that most BD
aneurysms originate from defects located in the posterior or
lateral walls. However, in the present study, the most common
pattern in patients with thoracic aortic aneurysms was asymmetric
bulging of the right part of the aortic wall. Previous studies
determined that, unlike in atheromatous aneurysms, the risk of
aneurysm rupture in patients with BD was not associated with the
maximum aneurysmal diameter (15,16). In
the present study, two patients died of an aortic aneurysm rupture
and CT angiography images revealed irregular cystic changes in the
thickened aortic wall. It may be speculated that cystic changes of
the wall may be associated with the risk of aneurysm rupture and
reflect inflammatory necrosis of the aortic wall, thus reducing
pressure resistance to blood flow shocks.
In the present study, another initial feature of
aneurysms associated with BD was the tendency for recurrent
symptoms and involvement of multiple sites. Aneurysms may occur in
various locations and simultaneously with arteriovenous thrombosis.
After stent-graft implantation, recurrent pseudoaneurysms are prone
to develop at the distal margins of aortic stent-grafts, and
perivalvular leakage may be present after Bentall surgery. However,
in larger arteries, thromboemboli are more likely to occur after
stent implantation than in the aorta. The explanation for this
observation may be that the stent-graft placement in actively
inflamed aortic walls and continuous mechanical irritation promote
pseudoaneurysm recurrence after aortic stent implantation. For
larger arteries, inflammatory infiltration of the wall after stent
implantation results in recurrent thromboembolism. Anastomotic and
intraluminal stenosis or occlusion may result from dysfunction of
the endothelium between the graft and arterial wall affected by BD
(17).
Aneurysms of BD require to be differentiated from
atherosclerotic aneurysms based on the following points: i) Patient
with BD aneurysm usually has a definite diagnosis and BD at a
chronic stage; ii) BD aneurysms frequently feature rapid
progression and have a greater risk of rupture, and consequently,
huge retroperitoneal hematoma or hemoperitoneum develop as initial
manifestations (9). iii) Multifocal
aneurysms are usually encountered during initial manifestation of
BD, and the majority of them exhibit asymmetric bulging of the
right side of the aortic wall, while concentric expansion of the
aortic wall is frequently seen in atherosclerotic aneurysms.
Medical therapy with cyclophosphamide and
corticosteroids has been recommended by the European League Against
Rheumatism for aortic and peripheral aneurysms (18). Medical therapy with cyclophosphamide
and corticosteroids is required, and monoclonal anti-TNF antibodies
should be considered in refractory cases. The primary management of
pulmonary artery aneurysms and thrombosis involves high-dose
glucocorticoids and cyclophosphamide. BD is the only acquired
disorder known to lead to the formation of pulmonary artery
aneurysms, and rupture of these aneurysms may cause massive
hemoptysis (19). In BD patients,
refractory venous thrombosis is thought to lead to inflammation of
vessel walls rather than hypercoagulability, which may result in
leg ulcers that are difficult to treat.
When assessing vascular aneurysms in BD patients,
arterial puncture for conventional catheter angiography should be
avoided due to the possibility of new aneurysm formation at the
puncture site. Compared to catheter angiography, CT angiography is
non-invasive and able to provide additional information regarding
the vessel walls and the presence of calcification and mural
thrombi. Furthermore, characteristic CT imaging features of
aneurysms may help to diagnose vascular involvement of BD and
assess its severity, particularly in the absence of the classical
clinical manifestations, which is helpful to establish a diagnosis
of BD and to assess the efficacies of medical and surgical
treatment (20).
Of note, the present study had several limitations.
First, due to its retrospective nature, not all of the patients
underwent CT angiographic examinations of the chest and abdomen.
Furthermore, BD patients with vascular complications require
multiple CT angiographic examination during follow-up before and
after treatment, and high dosage administration of contrast agent
increases the risk of contrast-induced nephropathy (21).
In conclusion, CT angiography is suitable to assess
vascular complications in patients with BD, especially of aneurysm
formation. In addition, the multiplanar capability of vascular
reconstruction by CT angiography is useful in demonstrating the
manifestations of the entire spectrum of aorta and its branches in
patients with BD.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 61976238), the Key Talents
Training Program of Huadong Hospital (grant no. HDGG2014011), the
Shanghai Hospital Development Center Program (grant no.
SHDC22015025), the Health Commission of Shanghai, Wise Information
Technology, Major Program of Medical Imaging (grant no.
2018ZHYL0103).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LQ and ML made substantial contributions to the
design of the research, and were involved in drafting and revising
the manuscript. DBM, JFC, MW and CL prepared the figures and
performed the data collection. YQH, XJ, XJG and WLW made
substantial contributions to the design and data analysis for the
work All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The institutional review board of Huadong Hospital
affiliated to Fudan University (Shanghai, China) approved this
retrospective study (approval no. 20160023) and written informed
consent for MSCT examinations was obtained from each participant
involved.
Patient consent for publication
This study was conducted retrospectively at one
center, and the local ethical institutional review board waived the
need for informed consent for this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tuzun H, Seyahi E, Arslan C, Hamuryudan V,
Besirli K and Yazici H: Management and prognosis of nonpulmonary
large arterial disease in patients with Behcet disease. J Vasc
Surg. 55:157–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uzun O, Erkan L, Akpolat I, Findik S,
Atici AG and Akpolat T: Pulmonary involvement in Behcet's disease.
Respiration. 75:310–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hiller N, Lieberman S, Chajek-Shaul T,
Bar-Ziv J and Shaham D: Thoracic manifestations of Behcet disease
at CT. Radiographics. 24:801–808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raz I, Okon E and Chajek-Shaul T:
Pulmonary manifestations in Behcet's syndrome. Chest. 95:585–589.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang SH and Zhang FX: Behcet's disease
with recurrent thoracic aortic aneurysm combined with femoral
artery aneurysm: A case report and literature review. J
Cardiothorac Surg. 12:792017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mat C, Yurdakul S, Sevim A, Özyazgan Y and
Tüzün Y: Behcet's syndrome: Facts and controversies. Clin Dermatol.
31:352–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JH, Chung JW, Joh JH, Song SY, Shin
SJ, Chung KS, Lee DY, Won JY and Kim SJ: Aortic and arterial
aneurysms in behcet disease: Management with stent-grafts-initial
experience. Radiology. 220:745–750. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reiter BP, Marin ML, Teodorescu VJ and
Mitty HA: Endoluminal repair of an internal carotid artery
pseudoaneurysm. J Vasc Interv Radiol. 9:245–248. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho YK, Lee W, Choi SI, Jae HJ, Chung JW
and Park JH: Cardiovascular Behcet disease: The variable findings
of rare complications with CT angiography and conventional
angiography and its interventional management. J Comput Assist
Tomogr. 32:679–689. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bilgin G, Sungur G and Kucukterzi V:
Systemic and pulmonary screening of patients with Behcet's disease
during periodic follow-up. Respir Med. 107:466–471. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishikawa S, Kawasaki A, Suzuki Y, Neya K,
Wada S, Kugawa S, Hayama T and Ueda K: Progression of abdominal
aortic aneurysm after endovascular stent-grafting in a patient with
Behcet's disease: Report of a case. Surg Today. 37:82–85. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SW, Lee DY, Kim MD, Won JY, Park SI,
Yoon YN, Choi D and Ko YG: Outcomes of endovascular treatment for
aortic pseudoaneurysm in Behcet's disease. J Vasc Surg. 59:608–614.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Emad Y, Abdel-Razek N, Gheita T, el-Wakd
M, el-Gohary T and Samadoni A: Multislice CT pulmonary findings in
Behcet's disease (report of 16 cases). Clin Rheumatol. 26:879–884.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Dai H, Ma Z, Yang Y and Liu Y:
Pulmonary involvement in patients with Behcet's disease: Report of
15 cases. Clin Respir J. 9:414–442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seyahi E: Behcet's disease: How to
diagnose and treat vascular involvement. Best Pract Res Clin
Rheumatol. 30:279–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goudard Y, Pierret C, de La Villéon B,
Mlynski A and de Kerangal X: In situ repair of a primary
Brucella-infected abdominal aortic aneurysm: Long-term follow-up.
Ann Vasc Sur. 27:241.e1–e5. 2013.
|
|
17
|
Tuzun H, Besirli K, Sayin A, Vural FS,
Hamuryudan V, Hizli N, Yurdakul S and Yazici H: Management of
aneurysms in Behcet's syndrome: An analysis of 24 patients.
Surgery. 121:150–156. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hatemi G, Christensen R, Bang D, Bodaghi
B, Celik AF, Fortune F, Gaudric J, Gul A, Kötter I, Leccese P, et
al: 2018 update of the EULAR recommendations for the management of
Behcet's syndrome. Ann Rheum Dis. 77:808–818. 2018.PubMed/NCBI
|
|
19
|
Ceylan N, Bayraktaroglu S, Erturk SM,
Savas R and Alper H: Pulmonary and vascular manifestations of
Behcet disease: Imaging findings. AJR Am J Roentgenol.
194:W158–W164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alkaabi JK and Pathare A: Pattern and
outcome of vascular involvement of Omani patients with Behcet's
disease. Rheumatol Int. 31:731–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Albrecht MH, Bickford MW, Nance JW Jr,
Zhang L, De Cecco CN, Wichmann JL, Vogl TJ and Schoepf UJ:
State-of-the-art pulmonary CT angiography for acute pulmonary
embolism. AJR Am J Roentgenol. 208:495–504. 2017. View Article : Google Scholar : PubMed/NCBI
|